How To Understand The Human Body
|
|
|
- Aleesha Rice
- 3 years ago
- Views:
Transcription
1 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A whale is easily recognized by from and size. Nonliving forms vary in form and size, such as a single water droplet to an entire ocean. 2. Chemical composition a. Primarily made of Carbon (C), Hydrogen(H), Oxygen(O), Nitrogen(N) b. Complex organization but all living things are made of one or more Cells 3. Metabolism - Metabolism is the sum of all of the chemical reactions that occur in a cell. For example, the ability to breakdown energy from the environment (e.g. food) into useable forms of energy is metabolism and a characteristic of life. 4. Irritability - The ability to respond to the environment. e.g. a plant gradually turning its leaves towards the window with the sunlight. 5. Homeostasis - Balance; Equilibrium; The ability to maintain constancy internally. 6. Growth and life cycle 7. Reproduction occurs to propagate the species with all characteristic traits being inherited from the parents. C. Human Uniqueness - Obviously humans meet the criteria of a living organism. In this course, we narrow down the field of biology to humans. How are humans different to other living things? 1. Environmental alterations - Humans can build cities and houses. Certainly other living creatures, such as bees, can alter their environment as well. 2. Speech - Communication is NOT unique to humans, but our ability to articulate and speak is quite advanced in the animal kingdom.
2 3. Tools - Our ability to make and use tools is extraordinary. However, other species use tools, but not to the same extent as humans. (Birds can use a small twig to reach insects in a hole in a tree.) 4. Social organization & cultural heritage - Although other animal colonies have social organization, none is to the extent that humans organize into families, government, with a strong cultural heritage. 5. Reasoning; abstract concepts;- This ability it the primary trait that sets humans apart from all other living things. In fact, the scientific name of humans, Homo sapiens, means "Knowing one". II. Biology as a Science A. Dynamic - Science is ever-changing as new discoveries are made. B. Scientific Method - You need to understand how scientists approach problems. 1. Question/Problem - e.g. Does DDT pesticide affect the formations of any part of a bird's egg? 2. Hypothesis - Based on a scientist's background knowledge, the scientist proposes a reasonable answer to the problem. A hypothesis is an educated prediction. e.g. Because eagle populations declined when DDT was widely used, DDT must weaken the eggs. 3. Experiment; Collection of data - Next, a scientist must prove or disprove the hypothesis. This involves experimentation, when possible. For example, the scientist would set up an experiment exposing one group of birds with DDT in their feed and examine all eggs for integrity and shell consistency and another group as a control group. The control group makes the experiment meaningful, because it places the birds in exact conditions, but without the variable being tested. In this example, the control group lives in similar conditions, but is being fed birdseed without the DDT. Next the scientist collects data and results. In some cases, experimentation is not possible, such as studying the feeding habits of a dinosaur. In this case, one cannot run a dinosaur experiment. Instead, scientists must rely on available fossils and compare them to living creatures to investigate their question.
3 4. Conclusion - Theory / Principle - After all data is collected and analyzed a conclusion may be reached. The conclusion may or may not support the hypothesis. The conclusion explains natural phenomena. If the conclusion withstands the test of time and other experimentation, it may be "upgraded" to a theory. With further support through testing and time, a theory may be "upgraded" to a principle. III. Levels of organization in the human body A. Chemicals - The chemical level is the simplest level of organization in the body. Examples include water (H2O), glucose, carbon dioxide. B. Cells - If you combine chemicals for a common function, you reach the cellular level. Examples include muscle cells and neurons (nerve cells). The cell is the basic unit of life. C. Tissues - If you combine cells together for a common function, such as lining an intestine, you reach the tissue level of organization. For example, many rectangular shaped cells line the intestine for the function of absorption of nutrients. This is a tissue - specifically epithelial tissue. There are four categories of tissues in the body: Epithelium, Connective tissue, Nervous tissue and Muscle tissue. These will all be studied later in the course. D. Organs - If you combine tissues together for a common function, you reach the organ level. For example, epithelium plus muscle tissue, plus connective tissue comprise the intestine, which is an organ. E. Systems - A system is several organs combined for a common function. The mouth, esophagus, stomach, intestine, pancreas, and liver all combine for the function of digesting food, and thus make up the digestive system. F. Organism - All systems together comprise a human being. IV. Chemistry - There is such a close relationship between biology and chemistry when studying the human body; you must know the basic terminology used in chemistry. A. Atom - Chemicals are made of tiny, tiny particles called atoms. Atoms cannot be altered by any chemical reaction. Atoms are the smallest chemical units of matter -
4 whether it is living or nonliving. For example, if you could take gold and chop it into tiny, tiny particles that all still have the properties of gold, those would be gold atoms. 1. Subatomic Particles - Physicists have been able to blast atoms in to even tinier particles, called subatomic particles. Three different particles are fundamental to the structure of an atom. a. Neutron - A particle with no electrical charge. b. Proton - A particle with a positive charge, as in a hydrogen ion: H +. c. Electron - A particle with a negative charge. These light particles orbit around the neutrons and protons. For example, the chloride ion (Cl - ) has one more electron than it has protons, and therefore carries a negative charge. 2. Ions - Ions are atoms (or molecules) with a positive or a negative charge. B. Element - Periodic tables are chemical charts showing all of the different elements known. Each element has certain characteristic properties unique to that element (e.g. color, density, mass) Carbon, chlorine, nitrogen, oxygen, are all examples of elements. Elements contain only one kind of atom. For example, carbon only contains carbon atoms. C. Compound - If one combines different elements, a compound is made. For example two hydrogens combine to one oxygen, via a chemical affinity termed a chemical bond and a compound, H2O or water is formed. Another example is NaCl which is salt. D. Molecule - A chemical structure containing more than one atom is a molecule. The atoms may be the same or different. For example, if two oxygens combine, you make oxygen molecule, O2. By definition, all compounds must also be molecules as well. E. Water - Water accounts for about 2/3 of our body, and is the single most important constituent in the body. Thus it is important to investigate some features of water. Water can break apart in a reversible reaction, into and hydrogen ions and hydroxide ions: {H2O <--> H + + OH - } The levels of hydrogen ions in the body are so important, they are expressed in a logarithmical abbreviation termed ph. It represents the amount, or concentration, of Hydrogen ions.
5 1. ph scale - This scale ranges from 0-14 and measures the relative hydrogen ion concentrations. A ph of 7 is neutral, and the solution would have as many hydrogen ions as hydroxide ions. Distilled water has a ph of 7. Your body has a ph slightly above 7. Readings of 7-14 indicate fewer hydrogen ions, and more hydroxide ions. These substances are bases. For example, drain cleaner has a very high ph, about 13, and is a strong, strong base. Bleach has a ph of about 9.5 and is a weaker base than drain cleaner. Readings on the ph scale of 0-7 indicate more hydrogen ions and fewer hydroxide ions. These substances are acids. The lower the ph reading, the stronger the acid. For example, hydrochloric acid has a ph near one, and is a very strong acid. Coffee has a ph near 5 and is a weak acid. 2. Acids - Acids are substances which release hydrogen when mixed with water. ph less than Bases (= alkaline) - Bases are substances which release hydroxide ions when mixed with water. ph more than 7. F. Organic Compounds - Organic compounds are based upon carbon and predominate in the human body. 1. Carbohydrates - These organic molecules are sources of energy and are comprised of sugars and starches. A common example of a simple sugar in the body is glucose. 2. Lipids - These organic compounds are fats and oils. A substance is considered a fat if it is solid at room temperature and an oil if it is liquid at room temperature. Lipids are great energy sources, as they are packed with calories. They are also important in the structure of cell membranes and in the synthesis (making) of some hormones. 3. Proteins - Proteins are quite important in the structure of the body. The skeleton and muscles are primarily made of protein. Your blood contains proteins. Proteins are important in fighting off disease - immunity. Proteins are made of simpler molecules, called amino acids, linked together. Proteins are important in structure and function of nearly every body part. Another example of a protein in your body is an enzyme. Structurally enzymes are proteins. Their job is to speed up chemical reactions in the body.
6 4. Nucleic acids - These organic compounds comprise the molecules necessary for inheritance. a. DNA - These important three letters stand for deoxyribose nucleic acid. DNA makes up your genes which carry all of the inheritance information in your body. 1. DNA fingerprinting - With the exception of identical twins, everyone's DNA is slightly different. This information helps find persons committing violent crimes. 2. Genetic code - The Human Genome Project is a federally funded project to unravel the human genetic code. This information can be useful in detection and treatment of genetically-based diseases. This information is important in genetic engineering in which the genetic code of cells is altered (such as crossing two different fruit varieties, or inserting the human gene for insulin into laboratory bacteria that grow and reproduce rapidly). b. RNA - This nucleic acid stands for ribose nucleic acid and is responsible for reading and carrying out the instructions of the DNA in the cell. c. ATP - This high energy compound is adenosine triphosphate. It is the cell's energy source. Food must be converted into ATP in order for cells to be able to use it.
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
The chemical reactions inside cells are controlled by enzymes. Cells may be specialised to carry out a particular function.
 12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Unit I: Introduction To Scientific Processes
 Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Topic 3: Nutrition, Photosynthesis, and Respiration
 1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Enzymes: Practice Questions #1
 Enzymes: Practice Questions #1 1. Compound X increases the rate of the reaction below. Compound X is most likely A. an enzyme B. a lipid molecule C. an indicator D. an ADP molecule 2. The equation below
Enzymes: Practice Questions #1 1. Compound X increases the rate of the reaction below. Compound X is most likely A. an enzyme B. a lipid molecule C. an indicator D. an ADP molecule 2. The equation below
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Essentials of Human Anatomy & Physiology 11 th Edition, 2015 Marieb
 A Correlation of Essentials of Human Anatomy Marieb To the Next Generation Science Standards Life A Correlation of, HS-LS1 From Molecules to Organisms: Structures and Processes HS-LS1-1. Construct an explanation
A Correlation of Essentials of Human Anatomy Marieb To the Next Generation Science Standards Life A Correlation of, HS-LS1 From Molecules to Organisms: Structures and Processes HS-LS1-1. Construct an explanation
BIO 137: CHAPTER 1 OBJECTIVES
 BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
The Excretory and Digestive Systems
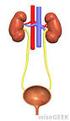 The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
6023-1 - Page 1. Name: 4) The diagram below represents a beaker containing a solution of various molecules involved in digestion.
 Name: 6023-1 - Page 1 1) Which one of the following situations indicates a serious organ system malfunction? A) Mitochondria stop functioning in a unicellular organism exposed to pollutants. B) White blood
Name: 6023-1 - Page 1 1) Which one of the following situations indicates a serious organ system malfunction? A) Mitochondria stop functioning in a unicellular organism exposed to pollutants. B) White blood
B2 Revision. Subject Module Date Biology B2 13 TH May (am)
 B2 Revision Subject Module Date Biology B2 13 TH May (am) Useful websites www.aqa.org.uk This website contains the specifications that we follow and also has a large number of past papers and mark schemes
B2 Revision Subject Module Date Biology B2 13 TH May (am) Useful websites www.aqa.org.uk This website contains the specifications that we follow and also has a large number of past papers and mark schemes
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
tissues are made of cells that work together, organs are )
 Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Name Date Period. Keystone Review Enzymes
 Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Topic 4: Digestion and Nutrition
 Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Biopharmaceuticals and Biotechnology Unit 2 Student Handout. DNA Biotechnology and Enzymes
 DNA Biotechnology and Enzymes 35 Background Unit 2~ Lesson 1 The Biotechnology Industry Biotechnology is a process (or a technology) that is used to create products like medicines by using micro-organisms,
DNA Biotechnology and Enzymes 35 Background Unit 2~ Lesson 1 The Biotechnology Industry Biotechnology is a process (or a technology) that is used to create products like medicines by using micro-organisms,
MCAS Biology. Review Packet
 MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
Chapter 48. Nutrients in Food. Carbohydrates, Proteins, and Lipids. Carbohydrates, Proteins, and Lipids, continued
 Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Human Anatomy & Physiology General
 Human Anatomy & Physiology General Biology is the study of life but, what exactly is life? how are living things different from nonliving things eg. a human from a rock eg. a a human from a robot eg. a
Human Anatomy & Physiology General Biology is the study of life but, what exactly is life? how are living things different from nonliving things eg. a human from a rock eg. a a human from a robot eg. a
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
CHAPTER 9 BODY ORGANIZATION
 CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
Photosynthesis and Cellular Respiration. Stored Energy
 Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
Plant and Animal Cells
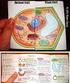 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Determination of Specific Nutrients in Various Foods. Abstract. Humans need to consume food compounds such as carbohydrates, proteins, fats,
 Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Cell and Membrane Practice. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
H.W. 1 Bio 101 Prof. Fournier
 H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
7 Answers to end-of-chapter questions
 7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
THE LIVING CELL. Cells also have variety of shapes. Plant cells are often rectangular or polygonal, while egg cells are usually spherical.
 THE LIVING CELL A Tour of the cell The cell is the smallest and the basic unit of structure of all organisms. There are two main types or categories of cells: prokaryotic cells and eukaryotic cells. Prokaryotic
THE LIVING CELL A Tour of the cell The cell is the smallest and the basic unit of structure of all organisms. There are two main types or categories of cells: prokaryotic cells and eukaryotic cells. Prokaryotic
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Reproductive System & Development: Practice Questions #1
 Reproductive System & Development: Practice Questions #1 1. Which two glands in the diagram produce gametes? A. glands A and B B. glands B and E C. glands C and F D. glands E and F 2. Base your answer
Reproductive System & Development: Practice Questions #1 1. Which two glands in the diagram produce gametes? A. glands A and B B. glands B and E C. glands C and F D. glands E and F 2. Base your answer
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Additional Science. Biology BL2FP. (Jun15BL2FP01) General Certificate of Secondary Education Foundation Tier June 2015.
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Additional Science Unit Biology B2 Biology Unit Biology B2 General Certificate
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Additional Science Unit Biology B2 Biology Unit Biology B2 General Certificate
Which of the following can be determined based on this model? The atmosphere is the only reservoir on Earth that can store carbon in any form. A.
 Earth s Cycles 1. Models are often used to explain scientific knowledge or experimental results. A model of the carbon cycle is shown below. Which of the following can be determined based on this model?
Earth s Cycles 1. Models are often used to explain scientific knowledge or experimental results. A model of the carbon cycle is shown below. Which of the following can be determined based on this model?
Investigating cells. Cells are the basic units of living things (this means that all living things are made up of one or more cells).
 SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
North Bergen School District Benchmarks
 Grade: 10,11, and 12 Subject: Anatomy and Physiology First Marking Period Define anatomy and physiology, and describe various subspecialties of each discipline. Describe the five basic functions of living
Grade: 10,11, and 12 Subject: Anatomy and Physiology First Marking Period Define anatomy and physiology, and describe various subspecialties of each discipline. Describe the five basic functions of living
Element of same atomic number, but different atomic mass o Example: Hydrogen
 Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Sketch the model representation of the first step in the dissociation of water. H 2. O (l) H + (aq) + OH- (aq) + H 2. OH - (aq) + H 3 O+ (aq)
 Lesson Objectives Students will: Create a physical representation of the autoionization of water using the water kit. Describe and produce a physical representation of the dissociation of a strong acid
Lesson Objectives Students will: Create a physical representation of the autoionization of water using the water kit. Describe and produce a physical representation of the dissociation of a strong acid
Hole s Human Anatomy and Physiology Eleventh Edition. Mrs. Hummer Hanover Area Jr./Sr. High School. Chapter 1 Introduction to Anatomy and Physiology
 Hole s Human Anatomy and Physiology Eleventh Edition Mrs. Hummer Hanover Area Jr./Sr. High School Chapter 1 Introduction to Anatomy and Physiology 1 Chapter 1 Introduction to Human Anatomy and Physiology
Hole s Human Anatomy and Physiology Eleventh Edition Mrs. Hummer Hanover Area Jr./Sr. High School Chapter 1 Introduction to Anatomy and Physiology 1 Chapter 1 Introduction to Human Anatomy and Physiology
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems
 Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Cells & Cell Organelles
 Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
UNIT 2 PRACTICE EXAM (Part 1: General Chemistry)
 UIT 2 PRACTICE EXAM (Part 1: General Chemistry) 1. Which would be the best definition of an ionic bond? a. The attraction between the partial positive region of one molecule and the partial negative region
UIT 2 PRACTICE EXAM (Part 1: General Chemistry) 1. Which would be the best definition of an ionic bond? a. The attraction between the partial positive region of one molecule and the partial negative region
Get It Right. Answers. Chapter 1: The Science of Life. A biologist studies all living things.
 Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
STUDY GUIDE AGRICULTURAL SCIENCES GRADE 11
 STUDY GUIDE AGRICULTURAL SCIENCES GRADE 11 A publication of Impak Onderwysdiens (Pty) Ltd Copyright reserved. Apart from any fair dealing for the purpose of research, criticism or review as permitted under
STUDY GUIDE AGRICULTURAL SCIENCES GRADE 11 A publication of Impak Onderwysdiens (Pty) Ltd Copyright reserved. Apart from any fair dealing for the purpose of research, criticism or review as permitted under
Examination One. Biology 101. Dr. Jaeson T. Fournier
 Examination One Biology 101 Dr. Jaeson T. Fournier Examination Instructions: Answers are to be indicated on a scantron. Keep your work protected! This helps prevent dishonesty. The instructor will not
Examination One Biology 101 Dr. Jaeson T. Fournier Examination Instructions: Answers are to be indicated on a scantron. Keep your work protected! This helps prevent dishonesty. The instructor will not
Genetic material of all living organisms. Biology - 100
 Genetic material of all living organisms. Biology - 100 This antibiotic is made from a fungus that was first discovered growing on an orange and it became the first antibiotic to treat infection. Biology
Genetic material of all living organisms. Biology - 100 This antibiotic is made from a fungus that was first discovered growing on an orange and it became the first antibiotic to treat infection. Biology
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Lesson 3: Blood glucose
 Lesson 3: Blood glucose Inquiry Focus: How does the body deliver the energy in food to its parts? Student Learning Objectives: By the end of the lesson, students will be able to do the following: Describe
Lesson 3: Blood glucose Inquiry Focus: How does the body deliver the energy in food to its parts? Student Learning Objectives: By the end of the lesson, students will be able to do the following: Describe
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Todays Outline. Metabolism. Why do cells need energy? How do cells acquire energy? Metabolism. Concepts & Processes. The cells capacity to:
 and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
Regents Biology REGENTS REVIEW: PROTEIN SYNTHESIS
 Period Date REGENTS REVIEW: PROTEIN SYNTHESIS 1. The diagram at the right represents a portion of a type of organic molecule present in the cells of organisms. What will most likely happen if there is
Period Date REGENTS REVIEW: PROTEIN SYNTHESIS 1. The diagram at the right represents a portion of a type of organic molecule present in the cells of organisms. What will most likely happen if there is
Respiration occurs in the mitochondria in cells.
 B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
An introduction to the biochemistry of diet.
 An introduction to the biochemistry of diet. SEPA BioScience Montana Module 3 Introduction: The following provides a basic introduction to the biochemistry of three major nutritional components of your
An introduction to the biochemistry of diet. SEPA BioScience Montana Module 3 Introduction: The following provides a basic introduction to the biochemistry of three major nutritional components of your
Pre-requisites: Successful completion of 4th grade science and the 4th grade science assessment.
 Throughout each unit, assessments are incorporated into lessons. These assessments are activities that occur within the context of each lesson providing the guidelines for assessing students' progress.
Throughout each unit, assessments are incorporated into lessons. These assessments are activities that occur within the context of each lesson providing the guidelines for assessing students' progress.
Fundamentals of Anatomy & Physiology Course Outline, Objectives and Accreditation Information
 201 Webster Building 3411 Silverside Road Wilmington, DE 19810 Phone: 1-888-658-6641 Fax: 1-302-477-9744 learn@corexcel.com www.corexcel.com Course Outline, Objectives and Accreditation Information Chapter
201 Webster Building 3411 Silverside Road Wilmington, DE 19810 Phone: 1-888-658-6641 Fax: 1-302-477-9744 learn@corexcel.com www.corexcel.com Course Outline, Objectives and Accreditation Information Chapter
The Living Cell from the Biology: The Science of Life Series. Pre-Test
 1 Pre-Test Directions: Answer each question TRUE OR FALSE. 1. The instructions for making proteins are stored in molecules of DNA. 2. Proteins are made in the nucleus. 3. All cells are surrounded by a
1 Pre-Test Directions: Answer each question TRUE OR FALSE. 1. The instructions for making proteins are stored in molecules of DNA. 2. Proteins are made in the nucleus. 3. All cells are surrounded by a
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
CHAPTER 2 : CELL AS THE BASIC UNIT OF LIFE
 CHAPTER 2 : CELL AS THE BASIC UNIT OF LIFE Parts of microscope : An instrument that magnifies minute objects so they can be seen easily. It is one of the most important tools of science. Physicians and
CHAPTER 2 : CELL AS THE BASIC UNIT OF LIFE Parts of microscope : An instrument that magnifies minute objects so they can be seen easily. It is one of the most important tools of science. Physicians and
8-3 The Reactions of Photosynthesis Slide 1 of 51
 8-3 The of Photosynthesis 1 of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 2 of 51 Inside a Chloroplast Chloroplasts
8-3 The of Photosynthesis 1 of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 2 of 51 Inside a Chloroplast Chloroplasts
Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes.
 LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
7-5.5. Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including:
 7-5.5 Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including: NaCl [salt], H 2 O [water], C 6 H 12 O 6 [simple sugar], O 2 [oxygen
7-5.5 Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including: NaCl [salt], H 2 O [water], C 6 H 12 O 6 [simple sugar], O 2 [oxygen
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
