DENTAL ADMISSION TESTING PROGRAM Sample Test Items
|
|
|
- Charity Newton
- 8 years ago
- Views:
Transcription
1 DENTAL ADMISSION TESTING PROGRAM Sample Test Items Dental Admission Testing Program 211 East Chicago Avenue, Suite 600 Chicago, Illinois American Dental Association These sample test items are reprinted for distribution in 2007 by the American Dental Association American Dental Association. All rights reserved. You may not reproduce or transmit, by any means or for any purpose, this publication, or any part of it, in print, electronic or other format without prior express written permission from the American Dental Association.
2 DENTAL ADMISSION TEST PREPARATION MATERIALS How does one prepare for the DAT? There are no shortcuts to the process of learning, and these test preparation materials are not designed to provide the applicant with an opportunity to bypass the extensive process of absorbing basic information through class participation and months of study. These test preparation materials contain samples of the four tests used in the Dental Admission Testing Program. These are available to DAT applicants as a means of discovering possible areas of weakness in their comprehension of subjects covered by the test. They will also enable the candidates to become familiar with the types of material included in the test as well as with the general coverage and format of the various parts of the test battery. The entire DAT takes 4 hours and 45 minutes (including a 15-minute optional tutorial and break). In the real DAT, the time limit will be indicated in the upper right hand corner on the computer screen. Therefore, you will need to pace yourself as you proceed through each test in the Dental Admission Test. If you have time remaining for a section of the test, you can review your responses. When time expires, the computer screen will move to the next test or optional break period. The structure of the test is given below. You are encouraged to review the DAT Tutorial at under Step 4 before taking the actual DAT. The tutorial provides some sample items and information on navigating through the test. Dental Admission Test Components of the Test Number of Items Time Allowance Optional Tutorial 15 minutes Survey of the Natural Sciences minutes Perceptual Ability Test minutes Optional Break 15 minutes Reading Comprehension Test 50 - across three reading passages 60 minutes Quantitative Reasoning Test minutes The Survey of the Natural Sciences is a test of achievement. The content is limited to those areas covered by an entire first-year course in biology, general chemistry, and organic chemistry. The examination is comprised of 100 items: 40 biology items, 30 general chemistry items, and 30 organic chemistry items. Since separate sub-scores will be given for each of the three science sections, you should pace yourself through each section. A periodic table is available in the front of the Sample Test Items booklet and will be available as an Exhibit button on the actual DAT. The Perceptual Ability Test includes various types of nonverbal visual acuity items. There are six sections to the Perceptual Ability Test. One section covers two-dimensional perception, while the other sections cover three-dimensional perception. It is important that you read and understand the instructions at the beginning of each section. You must pace yourself so that you complete all six sections of the Perceptual Ability Test within the given time frame. You are not permitted to use measuring devices (i.e., pencils and fingers) while taking the Perceptual Ability Test. The Reading Comprehension Test consists of three reading passages, each with 16 to 17 items. The reading passages are scientific in nature and may reflect topics covered in dental school. Candidates are encouraged to read each passage before attempting to answer the corresponding questions. On the actual DAT, the Reading Comprehension Test is presented in a split-screen format with the items presented on the upper half of the screen, while the reading passage is presented in a scrollable format on the lower half of the screen. One reading passage is provided in the Sample Test Items. The Quantitative Reasoning Test measures your ability to reason with numbers, to manipulate numerical relationships, and deal intelligently with quantitative materials. On the actual DAT, a basic calculator will be available as a pop-up image for the Quantitative Reasoning Test only.
3 PERIODIC TABLE OF THE ELEMENTS 2000 American Dental Association. All rights reserved.
4 This examination is comprised of 100 items: Biology (1-40), General Chemistry (41-70), and Organic Chemistry (71-100) 1. Organisms that obtain their energy from light can be termed A. autotrophic. B. holotrophic. C. chemotrophic. D. heterotrophic. E. heliotrophic. 2. Fermentation A. produces pyruvic acid as an end product. B. yields less energy per mole of glucose than aerobic respiration. C. occurs only in the presence of oxygen. D. prevents glycolysis from occurring. E. converts ethanol to glucose. 3. In respiration, oxygen A. combines with lactic acid to form pyruvic acid. B. acts as a cofactor for glycolytic enzymes. C. yields energy in the form of ATP as it is passed down the respiratory chain. D. acts as an acceptor for electrons (and protons), forming water. E. combines directly with carbon, forming CO An enzyme is added to an aqueous solution of ATP, DNA, albumen, fat and glycogen; the reaction mixture is incubated for 10 minutes. If an analysis of the mixture reveals the presence of all of the above compounds plus glucose, it can be concluded that the enzyme hydrolyzed some of the A. albumen. B. fat. C. glycogen. D. ATP. E. DNA. 5. What cellular organelles would you expect to be absent from fungi? A. Mitochondria B. Lysosomes C. Ribosomes D. Golgi bodies E. Chloroplasts 6. Intracellular organelles that participate in metabolic oxidations involving H 2 O 2 are called A. centrioles. B. endoplasmic granules. C. peroxisomes. D. lysosomes. E. macrobodies. 1
5 7. The two daughter cells formed by mitosis and cytokinesis have A. half the number of chromosomes present in the parent cell. B. half the number of the chromosomes present in the parent cell if this parent cell is found in the testicular or ovarian tissue. C. the same number of chromosomes present in the parent cell. D. twice the number of chromosomes present in the parent cell. E. a variable number of chromosomes so that an exact prediction cannot be made. 8. Starch, cellulose and glycogen are all A. proteins. B. linked internally by hydrogen bonds. C. water soluble. D. polymers of glucose. E. nucleic acids. 9. Each of the following cell organelles have a membranous structure EXCEPT one. Which one is the EXCEPTION? A. Golgi complex B. Centrioles C. Mitochondria D. Lysosomes E. Endoplasmic reticulum 10. In anaerobic glycolysis in muscle cells, one mole of glucose is oxidized to A. six moles of carbon dioxide. B. two moles of acetic aid. C. two moles of lactic acid. D. two moles of acetyl CoA. E. two moles of carbon dioxide and six moles of water. 11. The movement of water soluble molecules through cell membranes, from higher to lower concentrations, by attachment to a carrier protein, describes A. diffusions. B. osmosis. C. pinocytosis. D. active transport. E. facilitated diffusion. 12. As far as their products are concerned, all biosynthetic reactions in living cells result in A. a more ordered state, therefore a decrease in entropy. B. a more ordered state, therefore an increase in entropy. C. energy released in the form of ATP. D. energy made available for motion. E. a more ordered state with no entropy change. 13. Which is the smallest organelle in the cell? A. Golgi body B. Nucleus C. Mitochondrion D. Ribosome E. Chloroplast 14. For a given diameter of an axon, one factor which increases the velocity of a nerve impulse is A. the length of the axon. B. the ploidy of the nucleus. C. the density of mitochondria along the axon. D. maximal stimulation of the neuron. E. the presence of a myelin sheath. 2
6 15. Which chiefly stimulates action of the respiratory center? A. Carbon dioxide in the blood B. Relaxin C. Lack of oxygen in the blood D. Inflation of the alveolus E. Vagus nerve 16. The term motor unit refers to A. an entire muscle. B. a single muscle fiber. C. all the muscle fibers innervated by one nerve fiber. D. all the motor nerves in one muscle. E. all the sliding filaments of actin and myosin in one muscle fiber. 17. The human heart beat is initiated within the A. sinus venosus. B. Hensen s node. C. conus arteriosus. D. artio-ventricular node. E. sino-atrial node. 18. In the nephron of the kidney, filtration occurs between A. Bowman s capsule and Henle s loop. B. the glomerulus and Bowman s capsule. C. the proximal tubule and Henle s loop. D. Henle s loop and the vasa recta. E. the peritubular network and the convoluted tubules. 19. The addition of potassium iodide as a nutritional supplement to common table salt would most directly affect the function of which of these glands? A. Thyroid B. Sweat glands C. Adrenal cortex D. Kidneys E. Parathyroid 20. Each of the following is synthesized by the anterior lobe of the pituitary gland of vertebrates EXCEPT one. Which one is the EXCEPTION? A. Thyrotropic hormone B. Adrenocorticotropic hormone C. Follicle-stimulating hormone D. Growth hormone E. Oxytocin 21. Clotting of human blood A. requires that hemoglobin be present. B. results from fibrin joining globulin. C. is a result of platelets releasing fibrinogen. D. depends on the formation of fibrin from fibrinogen. E. is accelerated when Ca 2+ is removed. 22. At some stage of development, all chordates have A. a pharynx, a vertebral column, and a notochord. B. pharyngeal pouches, a notochord, and a dorsal tubular nerve cord. C. pharyngeal pouches, a notochord, and a ventral nerve cord. D. pharyngeal pouches, vertebral column, and a dorsal tubular nerve cord. E. a pharynx and an ectodermally derived, solid nerve cord. 3
7 23. Organisms that have the characteristics of radial symmetry, water vascular system, a spiny skin, and are found exclusively in a marine habitat would be in which phylum? A. Annelida B. Chordata C. Cnidaria D. Porifera E. Echinodermata 24. Of the following, which group of invertebrates is apparently most closely related to primitive vertebrates? A. Annelida B. Mollusca C. Cnidaria D. Arthropoda E. Echinodermata 25. Under the five-kingdom classification, members of the kingdom Monera are generally separated from the members of all the other kingdoms by having A. heterotrophic nutrition versus autotrophic nutrition. B. unicellular organization versus multicellular organization. C. microscopic size versus macroscopic size. D. prokaryotic cells versus eukaryotic cells. E. parasite-host relationship versus predator-prey relationship. 26. A segment of DNA with the sequence GGCATTAGG would be transcribed into a messenger RNA segment with the sequence A. CCGUAAUCC. B. AATGCCGTT. C. CCGTAATCC. D. AAUGCCGUU. E. CCGTUUTGG. 27. Assuming no linkage, how many different kinds of gametes can be produced by an organism with the genotype AaBbcc? A. 32 B. 16 C. 8 D. 6 E Which statement concerning alleles is true for diploid organisms? 1. At most only two alleles occur at a given locus in an organisms genome. 2. Alleles occupy an identical locus in homologous chromosomes. 3. Alleles of a given gene usually occur on non-homologous chromosomes. 4. A single chromosome usually carries two alleles of each gene. A. 4 B. 1 and 2 C. 3 D. 1, 2 and 4 E. 3 and In watermelons, the unlinked genes for green color (G) and for short length (S) are dominant over alleles for striped color (g) and long length (s). Predict the phenotypes and their ratios for the cross Ggss x ggss. A. All green short B. 1:2:1 green short: striped long: striped short C. All striped long D. 1:1:1:1 green short: striped short: green long: striped long E. 1:1 green short: striped long 4
8 30. Sexual and asexual reproduction usually differ in A. the ability of the new offspring to reproduce. B. the rate at which mutations occur. C. the amount of genotypic variation between parent and offspring. D. the viability of offspring. E. whether or not natural selection can occur. 31. In human beings, color blindness is controlled by an X-linked recessive allele. In a cross involving this X-linked trait, the male parent has normal color vision, but the female parent is a carrier. What are the chances (in %) that a male offspring will inherit color blindness? A. 10 B. 25 C. 50 D. 75 E Consider a pair of homologous human chromosomes. In this pair one would expect A. them to be genetically identical. B. one chromosome to carry dominant alleles and the other recessive alleles. C. one chromosome to have been inherited from the mother and the other from the father. D. the two chromosomes to synapse during mitotic prophase. E. them to have different shapes. 33. Embryonic induction is a process in which A. embryonic tissues influence adjacent tissues to differentiate. B. an unfertilized egg is induced to develop. C. genes are transferred from one developing tissue to another. D. resting potentials are induced in neurons of embryos. E. the maternal parent induces expression of recessive genes in embryos. 34. Which statement is true of the archenteron? A. The cavity of the archenteron is called the blastocoele. B. The cavity of the archenteron represents the beginning of the primitive gut. C. The archenteron is formed during blastula formation. D. The cavity of the archenteron represents the first cavity of the developing heart. E. The archenteron is formed by a closing of the neural tube. 35. Of the germ layers comprising the early human embryo, which one forms most of the central nervous system? A. Ectoderm B. Mesoderm C. Endoderm D. Notochord E. Dermis 5
9 36. Of the following, the rate and type of cleavage occurring after fertilization would be most affected by the A. amount and distribution of yolk. B. number of chromosomes. C. thickness of the cell membrane. D. temperature. E. thickness of the zona pellucida. 37. The long-term natural process by which a pond eventually becomes a terrestrial community is referred to as A. environmental disruption. B. habitat development. C. organic evolution. D. ecological succession. E. desertification. 38. The initial step in the speciation process often involves A. inbreeding within the species. B. geographical separation of populations. C. intraspecific-random mating. D. the inheritance of acquired characteristics. E. a Hardy-Weinberg equilibrium. 39. A complex behavioral response to a specific cue or releaser, which is exhibited by all members of the species as a stereotyped response to the same stimulus, is known as a A. conditioned response. B. fixed-action pattern. C. reflex. D. kinesis. E. taxis. 40. Each of the following changes the frequency of alleles in a population EXCEPT one. Which one is the EXCEPTION? A. Mutation B. Natural selection C. Immigration D. Random interbreeding E. Genetic drift 41. A 49-gram sample of sulfuric acid, H 2 SO 4 (98 g mol -1 ) contains A. 1 mol of S atoms. B grams of O. C. 2.0 grams of H. D. 2 moles of O atoms. E. 1 mole of molecules. 42. If 1 mole of N 2 and 1 mole of H 2 are mixed and allowed to react according to the equation N 2 + 3H 2 2NH 3. What is the maximum number of moles of NH 3 that could be produced? A. 2/3 B. 3/2 C. 2/1 D. 1/2 E. 1/1 43. A flask weighs 95 g when empty. When filled with 200 ml of a certain liquid, the weight is 328 g. What volume (in milliliters) would 1,000 g of the liquid occupy? 6
10 44. If 3.00 g of a nitrogen-oxygen compound is found to contain 2.22 g of oxygen, what is the percentage of nitrogen in the compound? 46. When the volume of a gas is decreased at constant temperature, the pressure increases because the molecules A. move faster. B. move slower. C. become heavier. D. become lighter. E. strike a unit area of the container more often. 47. Water has a higher boiling point than compounds of a similar molecular weight. Which best explains this phenomenon? 45. A 10.0 liter sample of oxygen at 100 C and 1 atm is cooled to 27 C and expanded until the pressure is 0.5 atm. Find the final volume of the oxygen. A. Extensive hydrogen bonding exists between water molecules. B. One of the natural isotopes of hydrogen, deuterium, is present in sufficient quantities to significantly raise the boiling point. C. Water is a polar covalent compound. D. Van der Waals forces exist between individual water molecules. E. Water is largely dissociated leading to large electrostatic forces between individual water molecules. 48. A substance is non-conducting as a solid and melts at 750 C. The melt conducts electricity. The solid is observed to be soluble in water. This substance would be best classified as A. molecular. B. ionic. C. macromolecular. D. metallic. E. polymeric. 7
11 49. How many grams of NaOH (40 g mol -1 ) are there in 250 ml of 0.4 M NaOH solution? 52. If 25 ml of 0.5 M NaOH neutralizes 35 ml of a monoprotic acid, which is the molarity of the acid? A. 0.1 B. 0.4 C. 4 D. 10 E Which will be the final volume when 400 ml of 0.6 M HCl is diluted to 0.5 M HCl? 53. In which reaction is H 2 O considered to be acting as an acid? 51. During a titration it was determined that ml of a M Ce 4+ solution was required to react completely with ml of a M Fe 2+ solution. Which reaction occurred? A. Ce Fe 2+ + H 2 O 3Fe 3+ + CeO - + 2H + B. 2Ce 4+ + Fe 2+ Fe Ce 3+ C. Ce 4+ + Fe 2+ Fe 3+ + Ce 3+ D. Ce Fe 2+ 2Fe 3+ + Ce 2+ E. Ce Fe 2+ 2Fe 4+ + Cs e - A. Zn(s) + 2H 3 O + Zn 2+ + H 2 (g) + H 2 O B. HCl(g) + H 2 O H 3 O + + Cl - C. HC 2 H 3 O 2 + H 2 O H 3 O + + C 2 H 3 O - 2 D. NH 3 + H 2 O NH OH - E. NH 3 + H 3 O + NH H2 O 54. At constant temperature when the following reactions involving gases are at equilibrium, which reaction shifts to the right if the pressure is increased? A. 2H 2 + O 2 2H 2 O B. 2NH 3 N 2 + 3H 2 C. 2SO 3 2SO 2 + O 2 D. 2NO N 2 + O 2 E. 2CO 2 2CO + O 2 8
12 55. For the equilibrium Ag 2 SO 4 (s) 2Ag + 2- (aq) + SO 4 (aq) The solubility product expression (K sp ) is A. 2[Ag + 2- ][SO 4 ] B. [Ag + ][SO 2- ]/[Ag 2 SO 4 ] 4 C. [Ag + 2- ][SO 4 ] D. 2[Ag + ] 2 [SO 2- ]/[Ag 2 SO 4 ] 4 E. [Ag + ] 2 2- [SO 4 ] 56. The concentrations of silver ion and chloride ion in an aqueous solution in equilibrium with solid silver chloride are 1.0 x 10-6 M. What is the value of K sp for AgCl equal to? A. (2.0 x 10-6 )(1.0 x 10-6) B. C. 1.0 x 10-6 D. 1(1.0 x 10-6 ) E. (1.0 x 10-6 ) Determine the heat in kcal/mol available from the oxidation of one mole of glucose (C 6 H 12 O 6 ). C 6 H 12 O 6 (s) + 6O 2 (g) 6CO 2 (g) + 6H 2 O(1) The heats of formation are: Substance C 6 H 12 O 6 (s) -297 CO 2 (g) -94 H 2 O(l) -68 H o f, kcal/mol A B. 6(-94) + 6(-68) + 1(-297) C. 6(-94) + 6(-68) - 1(-297) D. 1(-297) - 6(-94) - 6(-68) E Which process is accompanied by a decrease in entropy? A. Sublimation of carbon dioxide B. Evaporation of water C. Freezing of water D. Shuffling a deck of cards E. Heating a balloon filled with a gas 59. If a solution which is 0.50 M in compound X decomposes for 5.0 minutes at an average rate of M min -1, the new concentration of X will be A M. B M. C M. D M. E M. 9
13 60. For the reaction A + B C, the experimentally determined rate of formation of C is given by: Rate = k[a][b] 2. Doubling the concentration of B will A. quadruple the initial reaction rate. B. double the initial reaction rate. C. have no effect on the initial rate. D. halve the initial reaction rate. E. reduce the rate to one-fourth its initial value. 61. The following is a spontaneous oxidationreduction reaction: 2- Cr 2 O H + + 6I - 2Cr 3+ +7H 2 O + 3I 2 Which is the best reducing agent? 2- A. Cr 2 O 7 B. H + C. I 2 D. H 2 O E. I Given the following half-cell reactions: Cl 2 (g) + 2e - 2Cl - (aq) E = +1.36v 63. In which two compounds does nitrogen have the same oxidation number? A. N 2 O 3 and HNO 3 B. N 2 O 5 and HNO 3 C. NO 2 and N 2 O 3 D. N 2 O 4 and HNO 2 E. HNO 2 and NH Which species is linear? A. H 2 O B. H 2 Se C. SO 2 D. ClO 2 E. CO The three common isotopes of oxygen: 16 O, 17 O, 18 O, have A. the same atomic number and an equal number of protons. B. the same mass number and an equal number of neutrons. C. the same atomic number and an equal number of neutrons. D. the same mass number and an equal number of protons. E. the same mass number and an equal number of electrons. Cu 2+ (aq) + 2e - Cu(s) E = +0.34v What is the value of E for the following reaction? Cu 2+ (aq) + 2Cl - (aq) Cu(s) + Cl 2 (g) A v B v C v D v E v 10
14 66. A Lewis structure for the NO - 3 ion is Including this structure, the total number of ground state resonance structures for this ion is A. 1. B. 2. C. 3. D. 4. E The electronic configuration of a particular neutral atom is 1s 2 2s 2 2p 6 3s 2 3p 2. What is the number of unpaired electrons in this atom? A. 1 B. 2 C. 3 D. 4 E Which property increases with atomic number among the representative elements of period two? 70. In the reaction shown below a nitrogen nucleus containing six neutrons emits a positron. What is the second product of the balanced reaction? N e A. N 7 14 B. N 7 14 C. C 6 13 D. C 6 13 E. O A characteristic feature of the S N 2 reaction mechanism is that A. it follows first-order kinetics. B. it produces stereochemical inversion of configuration. C. there is no rate-determining step. D. steric factors have little influence on the reaction rate constant. E. collision of three or more particles is required. A. Atomic radius B. Electronegativity C. Metallic character D. Normal boiling point E. Melting temperature 69. Which pair would give the bond with the most ionic character? A. Al and S B. P and O C. B and Br D. C and S E. Li and O 11
15 72. Which alkyl bromide will most readily undergo S N 2 reaction with NaOH? 74. How does the energy content of the transition state of a chemical reaction compare with that of the reactants and products? A. It is greater than products but less than reactants. B. It is greater than reactants but less than products. C. It is equal to both reactants and products. D. It is less than both reactants and products. E. It is greater than both reactants and products. 75. Which intermediate is most likely to be involved in the reaction shown below? Cl CH 3 CH=CHCH 3 +HCl CH 3 CH 2 CHCH Ethane reacts with chlorine in the presence of heat or ultraviolet light to give chloroethane. heat or CH 3 CH 3 + Cl 2 CH 3 CH 2 Cl + HCl UV light Which is an intermediate in this reaction? 12
16 If partitioned between equal volumes of ether and water, which would show the greatest preference for the water layer? A. CH 3 CH 2 CH 2 CH 2 CH 3 B. CH 3 CH 2 CH 2 CH 2 CH 2 Cl C. CH 3 CH 2 CH 2 CH 2 CH 2 OH D. HO CH 2 CH 2 CH 2 CH 2 CH 2 OH OH OH OH E. HO CH 2 CH CH CH CH 2 OH 79. Which structure below is an important resonance form of 77. A strong infrared absorption band between 1,750 and 1,700 cm-1 ( µ) indicates the presence of 13
17 80. Which of the structures below is chiral? 82. Which conformation of 1, 4- dibromocyclohexane is the most stable? 81. What are the following? A. Structural isomers B. Enantiomers C. Diastereomers D. Identical compounds E. Meso compounds 14
18 83. Which structure represents a trans (E) isomer? 85. The reduction of a ketone A. an aldehyde first, then a primary alcohol. B. a primary alcohol. C. a secondary alcohol. D. a tertiary alcohol. E. a carboxylic acid. 86. What is the major product of the following reaction? 84. Which is the IUPAC name for this compound? A. 4-methyl-l-hexene B. 4-ethyl-1-pentene C. 2-ethyl-4-pentene D. sec-butyl propylene E. 3-methyl-5-hexene 15
19 87. In the reaction, 89. Which reaction is an example of a free radical chain termination step? the major product is 90. What is the product of the reaction shown below? 88. The reaction of CH 3 CH 2 MgBr with O CH 3 CCH 3 followed by hydrolysis with dilute aqueous acid gives 16
20 91. Which could be used in the following conversion? 93. The two Bronsted-Lowry bases in the equilibrium below are HOAc + NaCN HCN + NaOAc A. LiAlH 4 B. CrO 3, H + C. SOCl 2 D. PBr 3 E. H 3 O + A. HoAc + NACN B. HOAc + NaOAc C. NaCN + NaOAc D. NaCN + HCN E. HOAc + HCN 94. The most acidic type of hydrogen in the following compound is 92. Which of the alkenes shown below reacts with ozone to give the products shown? A. a B. b C. c D. d E. e 17
21 95. The conjugate acid of p-aminophenol 96. The structure below is shown without complete geometrical detail. What is the correct assignment of bond angles? A. a = 90 b = 90 B. a = 180 b = C. a = 120 b = 120 D. a = 180 b = 120 E. a = 180 b = Which is stabilized by resonance? 18
22 98. Which of the following is aromatic? 99. Treatment of benzoic acid with thionyl chloride followed by addition of ethanol gives which as the major product? 19
23 100. What is the final product (B) of the sequence below? 20
24 24
25 25
26 26
27 27
28 28
29 29
30 30
31 31
32 32
33 33
34 34
35 35
36 36
37 37
38 38
39 39
40 40
41 41
42 42
43 43
44 44
45 45
46 46
47 47
48 48
49 49
50 50
51 51
52 Reading Comprehension Test RCT - Test Number 52 Time limit: 15 minutes (Actual Test will contain three passages and the time limit will be 60 minutes) IONIZING RADIATION: RISK AND BENEFIT X radiation is a form of energy which was discovered by the German physicist, Wilhelm Conrad Roentgen in Like visible light, radiowaves, and microwaves, X-rays belong to a group of radiations known as the electromagnetic spectrum. Electromagnetic radiations are comprised of units of pure energy called photons or quanta. Unlike corpuscular, or particular, radiations which are composed of subatomic particles, electromagnetic radiations have no mass or weight. Subatomic particles that can be involved in corpuscular radiations include the alpha particle or helium radical, the beta particle or electron, neutrons and protons. Corpuscular radiations can cause ionization; however, for the purposes of the present discussion only electromagnetic radiations capable of causing ionization will be considered. All photons of electromagnetic radiation travel in direct lines in a wave motion at the speed of 300,000 kilometers per second. Many of our conceptual ideas about wave motion are the result of our sensory experience with the transverse waves which occur in water and in the stretched string of a musical instrument. It is a pity in some way that the same term, wave, is given to both this transverse wave form and the oscillatory movement which is propagated along the direction of travel by electromagnetic radiations. This oscillatory movement, or longitudinal wave propagation, can be seen when a coiled spring is tapped sharply at one end, and as such this is a good paradigm for electromagnetic wave motion. Whereas for transverse waves the wavelength is between successive crests, the wavelength for electromagnetic radiations is the distance between successive areas of compression. This distance can vary enormously and electromagnetic radiations of different wavelengths have different properties. At one end of the spectrum there are very long wavelengths. Electromagnetic radiation of long wavelength is used in the transmission of radio messages. At the other end of the spectrum are the short wavelength radiations such as gamma radiations, which arise from naturally occurring unstable elements, and X- rays which are similar in property to gamma radiations, but are man-made by bombarding a target material with electrons in an X-ray tube. For gamma and x radiations the wavelengths are so small that they are measured in Angstrom units, where an Angstrom unit is 1/100,000,000 centimeter. The shorter the wavelength, the higher the energy and penetrating power of the photon, and (as all electromagnetic radiations travel at the speed of light) the higher the frequency of waves. X-ray wavelengths used in diagnostic radiology range from approximately 0.1 to 0.5 Angstroms. At such wavelengths the radiation has sufficient energy to cause ionization of atoms and molecules. If such atoms or molecules are within living systems, there is the potential for biological harm. This is the reason for the paradox that X-rays can cause cancer, can be used to help in diagnosis of disease, and in high doses can be used to destroy cancer cells. Consideration of the potential benefits of an activity is involved in the decision of risk acceptability. In diagnostic radiology, the riskbenefit equation is difficult to estimate. Risk is generally given in units of equivalent radiation dose, while the benefit is expressed in such terms as saved or disease cured. Gibbs and his fellow workers have noted that estimates of risk whole-body exposure, which is not generally the case for the diagnostic use of 1
53 x radiation. Moreover, they indicated that it has not yet been possible to define the value of a life saved in units of dose equivalence. Because of these uncertainties, diagnostic radiation is to be regarded as a potentially noxious agent. Hence radiological examination should be carried out only if it is likely that the information obtained will be useful for the clinical management of the patient. Undoubtedly ionizing radiation in high doses can be harmful. The first report of patient injury from a diagnostic radiological procedure, namely skin burns, was made within a few months of Roentgen s discover of the X-ray. In that case the exposure time was one hour, but it is impossible to estimate the dose received. As early as 1902 the first case of cancer attributed to radiation injury was reported in the literature. Nonetheless, the magnitude of the risk (or even if there is a risk) from the small doses of x radiation presently employed for diagnostic purposes is still undetermined. Various accidents, such as the recent reactor incident at Chernobyl in the Soviet Union, knowledge gained from follow-up studies on survivors from the atom bomb explosions in Hiroshima and Nagasaki at the end of World War II, and experiments subjecting various plant and animal species to ionizing radiation indicate that radiation bioeffects can be divided into two basic types where relatively high doses of radiation are concerned. One category of effects requires a threshold dose can be met before detectable change occurs. Such effects are termed non-stochastic, and are primarily a result of cell death. Examples are the acute radiation syndrome and the development of cataracts. On the other hand, stochastic effect show statistical probability of occurrence as a function of dose, but no threshold cut off for the effect. Examples of stochastic effects are carcinogenesis and genetic mutations. The problem in evaluating the risk of cancer or mutation in human populations due to the diagnostic use of x radiation is that there is no known method to distinguish between disease resulting from the radiation and that which is spontaneous or due to other factors in environment. The only way to assess the magnitude of the risk would be to determine the excess incidence of cancer or mutations in an irradiated population. Where the excess incidence is expected to be small, extremely large populations and long periods of observation are required. Land, for example, suggested that the risk of breast cancer from mammography is numerically so small when compared to the spontaneous incidence of one in 13 for breast cancer in U.S. women, that the epidemiologic methods of evaluation would require a population of at least 60 million women followed from age 35 until death. Half of them would receive mammograms and the other half, the control group, would not. It goes without saying, that such a study would take at least 40 years to conduct and would be so prohibitively expensive that it is not likely to be carried out. Similar considerations apply to the evaluation of risks from small doses of ionizing radiation of all human cancers and mutations. Hence, it has been common practice to use quantitative estimates and interpolations from observations of human and animal populations exposed to large radiation doses, when attempting to make numeric estimates of the risks to humans from low doses of ionizing radiation. In view of the uncertainty surrounding possible risks from the diagnostic use of X-rays, the International Commission on Radiological Protection has originated the concept of keeping exposure levels as low as reasonably achievable. This concept has been summarized in cryptic acronym form as the ALARA Principle. The three key ways of minimizing exposure to radiation are minimizing the duration of exposure, maximizing the distance from the source, and using barriers such as leaded clothing or screens. Diagnostic X-ray production occurs only when the X-ray tube is energized, and this is only necessary when radiographs are being exposed. The time that the X-ray tube is energized can be reduced by using fast image receptors, and by 2
IB Chemistry. DP Chemistry Review
 DP Chemistry Review Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant Assessment statement Apply the mole concept to substances. Determine the number of particles and the amount
DP Chemistry Review Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant Assessment statement Apply the mole concept to substances. Determine the number of particles and the amount
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Unit I: Introduction To Scientific Processes
 Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Indiana's Academic Standards 2010 ICP Indiana's Academic Standards 2016 ICP. map) that describe the relationship acceleration, velocity and distance.
 .1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
.1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
MCAS Biology. Review Packet
 MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY II FINAL EXAM REVIEW
 Name Period CHEMISTRY II FINAL EXAM REVIEW Final Exam: approximately 75 multiple choice questions Ch 12: Stoichiometry Ch 5 & 6: Electron Configurations & Periodic Properties Ch 7 & 8: Bonding Ch 14: Gas
Name Period CHEMISTRY II FINAL EXAM REVIEW Final Exam: approximately 75 multiple choice questions Ch 12: Stoichiometry Ch 5 & 6: Electron Configurations & Periodic Properties Ch 7 & 8: Bonding Ch 14: Gas
Amount of Substance. http://www.avogadro.co.uk/definitions/elemcompmix.htm
 Page 1 of 14 Amount of Substance Key terms in this chapter are: Element Compound Mixture Atom Molecule Ion Relative Atomic Mass Avogadro constant Mole Isotope Relative Isotopic Mass Relative Molecular
Page 1 of 14 Amount of Substance Key terms in this chapter are: Element Compound Mixture Atom Molecule Ion Relative Atomic Mass Avogadro constant Mole Isotope Relative Isotopic Mass Relative Molecular
Southeastern Louisiana University Dual Enrollment Program--Chemistry
 Southeastern Louisiana University Dual Enrollment Program--Chemistry The Southeastern Dual Enrollment Chemistry Program is a program whereby high school students are given the opportunity to take college
Southeastern Louisiana University Dual Enrollment Program--Chemistry The Southeastern Dual Enrollment Chemistry Program is a program whereby high school students are given the opportunity to take college
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Chapter 14 Solutions
 Chapter 14 Solutions 1 14.1 General properties of solutions solution a system in which one or more substances are homogeneously mixed or dissolved in another substance two components in a solution: solute
Chapter 14 Solutions 1 14.1 General properties of solutions solution a system in which one or more substances are homogeneously mixed or dissolved in another substance two components in a solution: solute
Symmetric Stretch: allows molecule to move through space
 BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
Review - After School Matter Name: Review - After School Matter Tuesday, April 29, 2008
 Name: Review - After School Matter Tuesday, April 29, 2008 1. Figure 1 The graph represents the relationship between temperature and time as heat was added uniformly to a substance starting at a solid
Name: Review - After School Matter Tuesday, April 29, 2008 1. Figure 1 The graph represents the relationship between temperature and time as heat was added uniformly to a substance starting at a solid
Exam 4 Practice Problems false false
 Exam 4 Practice Problems 1 1. Which of the following statements is false? a. Condensed states have much higher densities than gases. b. Molecules are very far apart in gases and closer together in liquids
Exam 4 Practice Problems 1 1. Which of the following statements is false? a. Condensed states have much higher densities than gases. b. Molecules are very far apart in gases and closer together in liquids
INFRARED SPECTROSCOPY (IR)
 INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
Name Date Period. 2. When a molecule of double-stranded DNA undergoes replication, it results in
 DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
5. Which temperature is equal to +20 K? 1) 253ºC 2) 293ºC 3) 253 C 4) 293 C
 1. The average kinetic energy of water molecules increases when 1) H 2 O(s) changes to H 2 O( ) at 0ºC 3) H 2 O( ) at 10ºC changes to H 2 O( ) at 20ºC 2) H 2 O( ) changes to H 2 O(s) at 0ºC 4) H 2 O( )
1. The average kinetic energy of water molecules increases when 1) H 2 O(s) changes to H 2 O( ) at 0ºC 3) H 2 O( ) at 10ºC changes to H 2 O( ) at 20ºC 2) H 2 O( ) changes to H 2 O(s) at 0ºC 4) H 2 O( )
Candidate Style Answer
 Candidate Style Answer Chemistry A Unit F321 Atoms, Bonds and Groups High banded response This Support Material booklet is designed to accompany the OCR GCE Chemistry A Specimen Paper F321 for teaching
Candidate Style Answer Chemistry A Unit F321 Atoms, Bonds and Groups High banded response This Support Material booklet is designed to accompany the OCR GCE Chemistry A Specimen Paper F321 for teaching
Science Tutorial TEK 6.9C: Energy Forms & Conversions
 Name: Teacher: Pd. Date: Science Tutorial TEK 6.9C: Energy Forms & Conversions TEK 6.9C: Demonstrate energy transformations such as energy in a flashlight battery changes from chemical energy to electrical
Name: Teacher: Pd. Date: Science Tutorial TEK 6.9C: Energy Forms & Conversions TEK 6.9C: Demonstrate energy transformations such as energy in a flashlight battery changes from chemical energy to electrical
* Is chemical energy potential or kinetic energy? The position of what is storing energy?
 Biology 1406 Exam 2 - Metabolism Chs. 5, 6 and 7 energy - capacity to do work 5.10 kinetic energy - energy of motion : light, electrical, thermal, mechanical potential energy - energy of position or stored
Biology 1406 Exam 2 - Metabolism Chs. 5, 6 and 7 energy - capacity to do work 5.10 kinetic energy - energy of motion : light, electrical, thermal, mechanical potential energy - energy of position or stored
Chemistry 151 Final Exam
 Chemistry 151 Final Exam Name: SSN: Exam Rules & Guidelines Show your work. No credit will be given for an answer unless your work is shown. Indicate your answer with a box or a circle. All paperwork must
Chemistry 151 Final Exam Name: SSN: Exam Rules & Guidelines Show your work. No credit will be given for an answer unless your work is shown. Indicate your answer with a box or a circle. All paperwork must
Aqueous Solutions. Water is the dissolving medium, or solvent. Some Properties of Water. A Solute. Types of Chemical Reactions.
 Aqueous Solutions and Solution Stoichiometry Water is the dissolving medium, or solvent. Some Properties of Water Water is bent or V-shaped. The O-H bonds are covalent. Water is a polar molecule. Hydration
Aqueous Solutions and Solution Stoichiometry Water is the dissolving medium, or solvent. Some Properties of Water Water is bent or V-shaped. The O-H bonds are covalent. Water is a polar molecule. Hydration
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT
 CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Carbon Hydrogen Oxygen Nitrogen
 Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Respiration occurs in the mitochondria in cells.
 B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Chemistry Assessment Unit AS 1
 Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2011 Chemistry Assessment Unit AS 1 assessing Basic Concepts in Physical and Inorganic Chemistry [AC111]
Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2011 Chemistry Assessment Unit AS 1 assessing Basic Concepts in Physical and Inorganic Chemistry [AC111]
Cell and Membrane Practice. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Chem 1A Exam 2 Review Problems
 Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
The chemical reactions inside cells are controlled by enzymes. Cells may be specialised to carry out a particular function.
 12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
Topic 3: Nutrition, Photosynthesis, and Respiration
 1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
Chapter 1 The Atomic Nature of Matter
 Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models
 EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
Chemical Reactions in Water Ron Robertson
 Chemical Reactions in Water Ron Robertson r2 f:\files\courses\1110-20\2010 possible slides for web\waterchemtrans.doc Properties of Compounds in Water Electrolytes and nonelectrolytes Water soluble compounds
Chemical Reactions in Water Ron Robertson r2 f:\files\courses\1110-20\2010 possible slides for web\waterchemtrans.doc Properties of Compounds in Water Electrolytes and nonelectrolytes Water soluble compounds
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Page 1. 6. Which hydrocarbon is a member of the alkane series? (1) 1. Which is the structural formula of methane? (1) (2) (2) (3) (3) (4) (4)
 1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Name Class Date. In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Name: Class: Date: 2 4 (aq)
 Name: Class: Date: Unit 4 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) The balanced molecular equation for complete neutralization of
Name: Class: Date: Unit 4 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) The balanced molecular equation for complete neutralization of
7. 1.00 atm = 760 torr = 760 mm Hg = 101.325 kpa = 14.70 psi. = 0.446 atm. = 0.993 atm. = 107 kpa 760 torr 1 atm 760 mm Hg = 790.
 CHATER 3. The atmosphere is a homogeneous mixture (a solution) of gases.. Solids and liquids have essentially fixed volumes and are not able to be compressed easily. have volumes that depend on their conditions,
CHATER 3. The atmosphere is a homogeneous mixture (a solution) of gases.. Solids and liquids have essentially fixed volumes and are not able to be compressed easily. have volumes that depend on their conditions,
Chapter 1: Moles and equations. Learning outcomes. you should be able to:
 Chapter 1: Moles and equations 1 Learning outcomes you should be able to: define and use the terms: relative atomic mass, isotopic mass and formula mass based on the 12 C scale perform calculations, including
Chapter 1: Moles and equations 1 Learning outcomes you should be able to: define and use the terms: relative atomic mass, isotopic mass and formula mass based on the 12 C scale perform calculations, including
Science Standard Articulated by Grade Level Strand 5: Physical Science
 Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
ATOMS. Multiple Choice Questions
 Chapter 3 ATOMS AND MOLECULES Multiple Choice Questions 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv)
Chapter 3 ATOMS AND MOLECULES Multiple Choice Questions 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv)
stoichiometry = the numerical relationships between chemical amounts in a reaction.
 1 REACTIONS AND YIELD ANSWERS stoichiometry = the numerical relationships between chemical amounts in a reaction. 2C 8 H 18 (l) + 25O 2 16CO 2 (g) + 18H 2 O(g) From the equation, 16 moles of CO 2 (a greenhouse
1 REACTIONS AND YIELD ANSWERS stoichiometry = the numerical relationships between chemical amounts in a reaction. 2C 8 H 18 (l) + 25O 2 16CO 2 (g) + 18H 2 O(g) From the equation, 16 moles of CO 2 (a greenhouse
Chemistry B11 Chapter 4 Chemical reactions
 Chemistry B11 Chapter 4 Chemical reactions Chemical reactions are classified into five groups: A + B AB Synthesis reactions (Combination) H + O H O AB A + B Decomposition reactions (Analysis) NaCl Na +Cl
Chemistry B11 Chapter 4 Chemical reactions Chemical reactions are classified into five groups: A + B AB Synthesis reactions (Combination) H + O H O AB A + B Decomposition reactions (Analysis) NaCl Na +Cl
I. ACID-BASE NEUTRALIZATION, TITRATION
 LABORATORY 3 I. ACID-BASE NEUTRALIZATION, TITRATION Acid-base neutralization is a process in which acid reacts with base to produce water and salt. The driving force of this reaction is formation of a
LABORATORY 3 I. ACID-BASE NEUTRALIZATION, TITRATION Acid-base neutralization is a process in which acid reacts with base to produce water and salt. The driving force of this reaction is formation of a
Sketch the model representation of the first step in the dissociation of water. H 2. O (l) H + (aq) + OH- (aq) + H 2. OH - (aq) + H 3 O+ (aq)
 Lesson Objectives Students will: Create a physical representation of the autoionization of water using the water kit. Describe and produce a physical representation of the dissociation of a strong acid
Lesson Objectives Students will: Create a physical representation of the autoionization of water using the water kit. Describe and produce a physical representation of the dissociation of a strong acid
Plant and Animal Cells
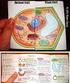 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Prentice Hall. Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition. High School. High School
 Prentice Hall Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition High School C O R R E L A T E D T O High School C-1.1 Apply established rules for significant digits,
Prentice Hall Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition High School C O R R E L A T E D T O High School C-1.1 Apply established rules for significant digits,
Chapter 3: Stoichiometry
 Chapter 3: Stoichiometry Key Skills: Balance chemical equations Predict the products of simple combination, decomposition, and combustion reactions. Calculate formula weights Convert grams to moles and
Chapter 3: Stoichiometry Key Skills: Balance chemical equations Predict the products of simple combination, decomposition, and combustion reactions. Calculate formula weights Convert grams to moles and
Chemistry 51 Chapter 8 TYPES OF SOLUTIONS. A solution is a homogeneous mixture of two substances: a solute and a solvent.
 TYPES OF SOLUTIONS A solution is a homogeneous mixture of two substances: a solute and a solvent. Solute: substance being dissolved; present in lesser amount. Solvent: substance doing the dissolving; present
TYPES OF SOLUTIONS A solution is a homogeneous mixture of two substances: a solute and a solvent. Solute: substance being dissolved; present in lesser amount. Solvent: substance doing the dissolving; present
Infrared Spectroscopy 紅 外 線 光 譜 儀
 Infrared Spectroscopy 紅 外 線 光 譜 儀 Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample (nondestructive method). The amount of light absorbed
Infrared Spectroscopy 紅 外 線 光 譜 儀 Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample (nondestructive method). The amount of light absorbed
Acids and Bases. Chapter 16
 Acids and Bases Chapter 16 The Arrhenius Model An acid is any substance that produces hydrogen ions, H +, in an aqueous solution. Example: when hydrogen chloride gas is dissolved in water, the following
Acids and Bases Chapter 16 The Arrhenius Model An acid is any substance that produces hydrogen ions, H +, in an aqueous solution. Example: when hydrogen chloride gas is dissolved in water, the following
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
The Empirical Formula of a Compound
 The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
Cellular Respiration An Overview
 Why? Cellular Respiration An Overview What are the phases of cellular respiration? All cells need energy all the time, and their primary source of energy is ATP. The methods cells use to make ATP vary
Why? Cellular Respiration An Overview What are the phases of cellular respiration? All cells need energy all the time, and their primary source of energy is ATP. The methods cells use to make ATP vary
Energy. Mechanical Energy
 Principles of Imaging Science I (RAD119) Electromagnetic Radiation Energy Definition of energy Ability to do work Physicist s definition of work Work = force x distance Force acting upon object over distance
Principles of Imaging Science I (RAD119) Electromagnetic Radiation Energy Definition of energy Ability to do work Physicist s definition of work Work = force x distance Force acting upon object over distance
Chemistry. The student will be able to identify and apply basic safety procedures and identify basic equipment.
 Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Cells & Cell Organelles
 Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
tissues are made of cells that work together, organs are )
 Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.
 CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
Chapter 9 Review Worksheet Cellular Respiration
 1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
Given these characteristics of life, which of the following objects is considered a living organism? W. X. Y. Z.
 Cell Structure and Organization 1. All living things must possess certain characteristics. They are all composed of one or more cells. They can grow, reproduce, and pass their genes on to their offspring.
Cell Structure and Organization 1. All living things must possess certain characteristics. They are all composed of one or more cells. They can grow, reproduce, and pass their genes on to their offspring.
W1 WORKSHOP ON STOICHIOMETRY
 INTRODUCTION W1 WORKSHOP ON STOICHIOMETRY These notes and exercises are designed to introduce you to the basic concepts required to understand a chemical formula or equation. Relative atomic masses of
INTRODUCTION W1 WORKSHOP ON STOICHIOMETRY These notes and exercises are designed to introduce you to the basic concepts required to understand a chemical formula or equation. Relative atomic masses of
Cell Unit Practice Test #1
 ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
Element of same atomic number, but different atomic mass o Example: Hydrogen
 Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
The correct answer is d C. Answer c is incorrect. Reliance on the energy produced by others is a characteristic of heterotrophs.
 1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
Evolution of Metabolism. Introduction. Introduction. Introduction. How Cells Harvest Energy. Chapter 7 & 8
 How ells Harvest Energy hapter 7 & 8 Evolution of Metabolism A hypothetical timeline for the evolution of metabolism - all in prokaryotic cells!: 1. ability to store chemical energy in ATP 2. evolution
How ells Harvest Energy hapter 7 & 8 Evolution of Metabolism A hypothetical timeline for the evolution of metabolism - all in prokaryotic cells!: 1. ability to store chemical energy in ATP 2. evolution
Introduction, Noncovalent Bonds, and Properties of Water
 Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
http://faculty.sau.edu.sa/h.alshehri
 http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
Chetek-Weyerhaeuser High School
 Chetek-Weyerhaeuser High School Anatomy and Physiology Units and Anatomy and Physiology A Unit 1 Introduction to Human Anatomy and Physiology (6 days) Essential Question: How do the systems of the human
Chetek-Weyerhaeuser High School Anatomy and Physiology Units and Anatomy and Physiology A Unit 1 Introduction to Human Anatomy and Physiology (6 days) Essential Question: How do the systems of the human
Stoichiometry. 1. The total number of moles represented by 20 grams of calcium carbonate is (1) 1; (2) 2; (3) 0.1; (4) 0.2.
 Stoichiometry 1 The total number of moles represented by 20 grams of calcium carbonate is (1) 1; (2) 2; (3) 01; (4) 02 2 A 44 gram sample of a hydrate was heated until the water of hydration was driven
Stoichiometry 1 The total number of moles represented by 20 grams of calcium carbonate is (1) 1; (2) 2; (3) 01; (4) 02 2 A 44 gram sample of a hydrate was heated until the water of hydration was driven
Introduction to Chemistry. Course Description
 CHM 1025 & CHM 1025L Introduction to Chemistry Course Description CHM 1025 Introduction to Chemistry (3) P CHM 1025L Introduction to Chemistry Laboratory (1) P This introductory course is intended to introduce
CHM 1025 & CHM 1025L Introduction to Chemistry Course Description CHM 1025 Introduction to Chemistry (3) P CHM 1025L Introduction to Chemistry Laboratory (1) P This introductory course is intended to introduce
CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH
 1. Is H 3 O + polar or non-polar? (1 point) a) Polar b) Non-polar CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH 2. The bond strength is considerably greater in HF than in the other three hydrogen halides
1. Is H 3 O + polar or non-polar? (1 point) a) Polar b) Non-polar CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH 2. The bond strength is considerably greater in HF than in the other three hydrogen halides
Introduction to the Cell: Plant and Animal Cells
 Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes.
 LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
Concept 1. The meaning and usefulness of the mole. The mole (or mol) represents a certain number of objects.
 Chapter 3. Stoichiometry: Mole-Mass Relationships in Chemical Reactions Concept 1. The meaning and usefulness of the mole The mole (or mol) represents a certain number of objects. SI def.: the amount of
Chapter 3. Stoichiometry: Mole-Mass Relationships in Chemical Reactions Concept 1. The meaning and usefulness of the mole The mole (or mol) represents a certain number of objects. SI def.: the amount of
INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION. 37 74 20 40 60 80 m/e
 CHM111(M)/Page 1 of 5 INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION SECTION A Answer ALL EIGHT questions. (52 marks) 1. The following is the mass spectrum
CHM111(M)/Page 1 of 5 INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION SECTION A Answer ALL EIGHT questions. (52 marks) 1. The following is the mass spectrum
Forensic Science Standards and Benchmarks
 Forensic Science Standards and Standard 1: Understands and applies principles of scientific inquiry Power : Identifies questions and concepts that guide science investigations Uses technology and mathematics
Forensic Science Standards and Standard 1: Understands and applies principles of scientific inquiry Power : Identifies questions and concepts that guide science investigations Uses technology and mathematics
In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and oxygen. [Include any charges or partial charges.
 Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Chemistry: Chemical Equations
 Chemistry: Chemical Equations Write a balanced chemical equation for each word equation. Include the phase of each substance in the equation. Classify the reaction as synthesis, decomposition, single replacement,
Chemistry: Chemical Equations Write a balanced chemical equation for each word equation. Include the phase of each substance in the equation. Classify the reaction as synthesis, decomposition, single replacement,
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
How To Understand The Science Of Inquiry
 7th Grade Science Curriculum Overview Philosophy and Common Beliefs Science Curriculum Philosophy Statement Northbrook/Glenview District 30 utilizes a rigorous science curriculum built on essential questions,
7th Grade Science Curriculum Overview Philosophy and Common Beliefs Science Curriculum Philosophy Statement Northbrook/Glenview District 30 utilizes a rigorous science curriculum built on essential questions,
Chapter 6. Solution, Acids and Bases
 Chapter 6 Solution, Acids and Bases Mixtures Two or more substances Heterogeneous- different from place to place Types of heterogeneous mixtures Suspensions- Large particles that eventually settle out
Chapter 6 Solution, Acids and Bases Mixtures Two or more substances Heterogeneous- different from place to place Types of heterogeneous mixtures Suspensions- Large particles that eventually settle out
PART I: Neurons and the Nerve Impulse
 PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
SCH 4C1 Unit 2 Problem Set Questions taken from Frank Mustoe et all, "Chemistry 11", McGraw-Hill Ryerson, 2001
 SCH 4C1 Unit 2 Problem Set Questions taken from Frank Mustoe et all, "Chemistry 11", McGraw-Hill Ryerson, 2001 1. A small pin contains 0.0178 mol of iron. How many atoms of iron are in the pin? 2. A sample
SCH 4C1 Unit 2 Problem Set Questions taken from Frank Mustoe et all, "Chemistry 11", McGraw-Hill Ryerson, 2001 1. A small pin contains 0.0178 mol of iron. How many atoms of iron are in the pin? 2. A sample
1. When the following equation is balanced, the coefficient of Al is. Al (s) + H 2 O (l)? Al(OH) 3 (s) + H 2 (g)
 1. When the following equation is balanced, the coefficient of Al is. Al (s) + H 2 O (l)? Al(OH) (s) + H 2 (g) A) 1 B) 2 C) 4 D) 5 E) Al (s) + H 2 O (l)? Al(OH) (s) + H 2 (g) Al (s) + H 2 O (l)? Al(OH)
1. When the following equation is balanced, the coefficient of Al is. Al (s) + H 2 O (l)? Al(OH) (s) + H 2 (g) A) 1 B) 2 C) 4 D) 5 E) Al (s) + H 2 O (l)? Al(OH) (s) + H 2 (g) Al (s) + H 2 O (l)? Al(OH)
Unit 6 The Mole Concept
 Chemistry Form 3 Page 62 Ms. R. Buttigieg Unit 6 The Mole Concept See Chemistry for You Chapter 28 pg. 352-363 See GCSE Chemistry Chapter 5 pg. 70-79 6.1 Relative atomic mass. The relative atomic mass
Chemistry Form 3 Page 62 Ms. R. Buttigieg Unit 6 The Mole Concept See Chemistry for You Chapter 28 pg. 352-363 See GCSE Chemistry Chapter 5 pg. 70-79 6.1 Relative atomic mass. The relative atomic mass
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
The Cell Teaching Notes and Answer Keys
 The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
