LECTURE 7: METABOLISM
|
|
|
- Berniece Hart
- 7 years ago
- Views:
Transcription
1 LECTURE 7: METABOLISM OVERVIEW In order for our body to maintain homeostasis and do work, it needs to breakdown organic molecules to extract energy from them and it needs to synthesize new molecules to support maintenance, growth and repair. Metabolism is the sum of all chemical reactions in the body's cells to support life and maintain homeostasis. Series of chemical reactions known as metabolic pathway occur within cell. Via metabolic pathways cells continuously breakdown organic molecules to extract energy from them, and then use this energy to do work and synthesize new organic molecules, maintain homeostasis, muscle contraction and other function. In general metabolism may be divided into two categories: catabolism; and anabolism. Catabolic reactions break down organic substrates to release energy i.e. catabolic reactions breakdown large complex molecules into smaller and simpler molecules, for example breakdown of glucose to two pyruvic acids. Anabolic reactions use energy to form new chemical bonds, and formation of new chemical bonds means synthesis of new organic molecules i.e. anabolic reactions assemble large complex molecules from small simple molecules (for example to synthesize DNA, RNA, protein). New organic molecules are synthesized to support growth, to perform structural maintenance and repair, to produce a secretion such as hormone. Nutrient pool are all the organic substrates that can be used for energy generation or synthesis of new molecules. CARBOHYDRATE METABOLISM Cells can breakdown any available organic substrate from the nutrient pool to obtain energy. However nearly all organisms rely on the breakdown of glucose as a preferred sustrate to provide energy to their cells, because proteins and lipids are more important as structural component of cells and also they are not easy to metabolize (breakdown). Overview of Charbohydrate Digestion and Absorption Food is any substance consumed to provide nutritional support for the body to maintain homeostasis and support growth. The food we eat may contain all or any of the following organic and Metabolism Sum of all biochemical reactions inside cell Metabolic Pathway Series of chemical reactions Organic Molecules 1. Amino Acids 2. Monosaccharide 3. Lipids e.g. Triglyceride Catabolism To break large molecules into smaller molecules Releases energy Anabolism To make large molecules from smaller molecules Uses energy Energy Released from Catabolism Used in anabolism Used by cells for contraction, locomotion, active transport Synthesis of New Molecules Examples are DNA, RNA, and protein. They are synthesized to support growth, structural maintenance, and secretion Macronutrients (Nutrients Before Digestions) Carbohydrates Proteins Lipids Micronutrients (Nutrients after complete Digestions) Monosaccharaides Amino Acids Fatty Acid and Glycerol Carbohydrates Monosaccharaides = Glucose, Fructose, Galactose Disaccharide = Sucrose, Lactose, Maltose Polysaccharide = Starch. Glycogen, Cellulose.. incorganic moleucles: Proteins; Carbohydrates, Lipida; Water; Minerals; Vitamins. The organic compounds of food (lipids, charbohydrates and proteins) are large (macromolecules or also called macronutrients) and often insoluble. Therefore organic compounds are broken down to their constiteunt subunits (fatty acids & glycerole, monosaccharides, amino acids) before they can be absorbed and transported into the cell. Lets follow journey of Carbohydrates from mouth to the cell
2 Parotid Gland Sublingual Gland Submandibula Gland Site In the oral cavity food is mixed with saliva, and saliva contains amylase that begins breakdown of carbohydrates Mucous Cells Parietal Cells Chief Cells G Cells Gastric Pit Gastric Gland What happens? In the oral cavity salivary glands produce saliva. Saliva contains enzyme Amylase. Amylase breakdown Carbohydrate to oligosaccharide and disaccharide Salivary enzyme continues to digest carbohydrates from the meal, until the ph falls below 4.5 Gastric juices which contain HCl (produced by the parietal cells of gastric pit), drops the ph below 4.5 and inactivates salivary amylase maltase splits bonds between the two glucose molecules of the disaccharide called maltose. From the stomach chyme arrives in the first portion of small intestine called Duodenum. Prior to absorption, disaccharides and trisaccharides are fragmented into monosaccharides (simple sugars) by brush border enzymes of the intestinal microvilli. The brushborder of small intestine produces the following enzymes: maltase, Sucrase and Lactase. The pancreas is primarily an exocrine organ, producing digestive enzymes and buffers. Pancreatic juice (about 1000 ml per day) of pancreas is an alkaline mixture of digestive enzymes, water, and ions. Pancreatic juice is released into duodenum when chyme arrives Sucrase breaks the disaccharide sucrose into glucose and fructose. Lactase hydrolyzes the disaccharide lactose into a molecule of glucose and one of galactose. The pancreas releases Biocaronates into duodenum to neutralize the acidic ph of chyme that arrived from the stomach. The pancreas also releases pancreatic juis that contan several enzymes including Amylase Amylase breakdown carbohydrates. At the brushborder of intestine disaccharides are hydrolyzed into monosaccharide (glucoses, fructose, galactos) and then they are absorbed and transported to the liver (mostly) and skeletal muscle cells. Liver distributes glucose to other cells throughout the body as needed, and also store excess glucose by converting it to glycogen. NOTE: Cells of the nervous system most require continuous supply of glucose Digestion of carbohydrate begin at oral cavity and ends at the brush border of jejunum (the second portion of small intestine). As carbohydrates are broken down into monosaccharide, they are transported across the plasma membrane (at the brush border of the intestinal cell) into the cell, and then from cell monosaccharides are transported into the interstitial fluid, and then from the interstitail fluid they diffues into the capillaries. Hepatic portal vein dilivers monosaccharides into the liver. The liver releases glucose into the circulation as needed and converts extra glucose that is not needed for immediate energy into glycogen. Glycogenesis Syntheis of glycogen from excess glucose Conversion of excess glucose to glycogen for storage Glycogenolysis breakdown of glycogen to glucose Conversion of glycogen to glucose when blood glucose level is low
3 The liver releases glucose into the blood (to maintain normal glucose level of 90mg/dL). When glucose level (e.g. after meals) in the blood increases, the pancrease produces insuline which stimulate carrier proteins (located on the cell membrane) to transport glucose into cells. Once glucose is inside the cell, it can be broken down and used as a source of energy or it can be converted to glycogen and stored for later use or it can be converted into other organic molecules such as ribose or glycerole. If the cell requires immediate energy, then glucose (a 6-carbon molecule) is broken down into two 3-carbon molecules of pyruvate via glycolysis. Glycolysis GLYCOLYSIS Glycolysis is the breakdown of glucose to two pyruvic acid molecules. The process of glycolysis consists of series of chemical reactions that breaks down glucose into two pyruvates and generates 2ATP and 2NADH. In the presence of oxygen each pyruvate molecule can subsequently enter mitochondria to generate more energy via Krebs cycle and electron transport system. Figure above: The process of glycolysis occurs in the cytoplasm, produces a net of 2 ATP, uses or consumes 2 ATP,
4 The process of glycolysis is an anaerobic process that consist of series of chemical steps as follow: 1. As soon as glucose enters the cell, a phosphate is added to carbon number 6 of glucose, and the new molecule is called glucose 6 phosphate. In this step ATP is converted to ADP (one phosphate of ATP is removed and added to carbon number 6 of glucose). 2. Glucose 6 phosphate goes through the second phosphorylation reaction and a phosphate is added to Carbone number 1. The new molecule produced as a result is called Fructose 1,6 Bisphosphate 3. The Fructose 1,6 bisphosphate will split into two 3 carbon molecule: a. Glyceraldehyde 3 phosphate b. Dihydroxyacetone 4. Glyceraldehyde goes through series of chemical reactions and becomes pyruvate. During the first step NADH is generated form NAD, during the second step one ATP is generated from one ADP, during the third step water molecule is produced, and during the fourth step one ATP is generated from one ADP molecule. 5. Dihydroxyacetone phosphate is converted to glyceraldehyde and will follow the same series of chemical reactions and will be converted to pyruvic acid.
5 SUMMERY GLYCOLYSIS Input Molecules Output Molecules 1 molecule of Glucose 2 molecules of Pyruvic 2 ATP (Steps 1 and 2) Acid 4 ADP (Steps 5 and 7) 2 ADP (Steps 1 and 2) 2 NAD (Step 4) 4 ATP (Steps 5 and 7) 2 NADH (step 4) PYRUVIC ACID CROSS ROADS Products of Glycolysis (pyruvic acid) can follow anaerobic or aerobic pathways. In the present of oxygen pyruvate is oxidized to acetyl-coa, which then enters the citric acid cycle. In the absence of oxygen pyruvate is reduced to lactate. In anaerobic respiration pyruvate is reduced and NAD is generated. During extensive exercise, besides being fatigued, your muscles feel heavy. This happens due to buildup of lactic acid as a result of anaerobic respiration. Why? ATP is needed to continue the exercise; therefore body breaks down glucose to pyruvic acid. So pyruvate builds up in the muscle cells, and the oxidizing agent used in glycolysis (NAD + ) is being converted to NADH. Because of the limited "pool" of NAD + in the cells, NADH has to be reoxidized back to NAD. In the absence of oxygen the only way to convert NADH to NAD + is to reduce pyruvate to lactate. In aerobic respiration pyruvate enters mitochondria and is converted to Acetyl CoA. In the presence of oxygen, pyruvate is oxidized to acetyl- CoA. In the process CO 2 is released. Oxygen is the final electron acceptor in aerobic respiration. Goal of anaerobic respiration To reduce pyruvate and generate NAD. Why? In the absence of oxygen this is the only way to generate NAD. Why NAD? So cell could generate ATP via glycolysis in the absence of oxygen. NAD is one of the input molecules in glycolysis i.e. NAD is the oxidizing agent used in glycolysis Oxidation Loss of electron or hydrogen Reduction Gain of electron or hydrogen MITOCHONDRIA In the presence of oxygen Pyruvate enters mitochondria and is converted to Acetyl CoA. In the mitochondria pyruvate (a 3 carbon molecule) is oxidized to acetyl-coa (a 2 carbon molecule), by NAD +. In this step pyruvate combine with coenzyme A to form acetyl coenzyme A. Acetyl CoA enters the Krebs cycle to generate NADH, FADH 2 and ATP Input Molecules Pyruvic Acid NAD Oxygen CoA Output Molecules Acetyl CoA NADH CO 2
6 KREBS CYCLE The Krebs cycle also known as the tricarboxylic acid cycle (TCA cycle), and the citric acid cycle is a series of enzyme-catalyzed chemical reactions as part of cellular respiration. 1. Acetyl CoA will transfer the acetyl group to oxaloacetic acid (a 4 carbon molecule) and CoA will become free. The union of oxaloacetic acid (4 carbon molecule) and acetyl group (two carbon molecule) will generate a 6 carbon molecule called citric acid. The free coenzyme A will be reused by another pyruvic acid to make acetyl CoA. 2. Citric acid will go through number of steps (e.g. citric acid will be converted to isocetric acid, and then isocetric acid will be converted to α-ketoglutaric acid and so on) and eventually will become oxaloacetic acid 3. One molecule of glucose generates two pyruvate, and two pyruvate generates two acetyl CoA, therefore TCA runs twice to breakdown one molecule of glucose. In one round of TCA 3 molecules of NADH, 1 molecule of FADH 2, 1 molecule of ATP and 2 molecules of CO 2 are produced. ELECTRON TRANSPORT SYSTEM The electron transport system occurs in the cristae of the mitochondria, where a series of cytochromes (cell pigments) and coenzymes exist. These cytochromes and coenzymes act as carrier molecules and transfer molecules. They accept high-energy electrons and pass the electrons to the next molecule in the system. Within mitochondria, the hydrogen atoms are used to generate ATP through the process of oxidative phosphorylation. Electron transport system involves a series of steps: Phosphorylation Attachment of high energy phosphate group to ADP, to produce ATP Oxidative Transfer of electrons
7 1. Coenzyme (NAD and FAD) strips a pair of hydrogen atoms from a substrate molecule during the citric acid cycle 2. NADH and FADH 2 deliver hydrogen atoms to coenzymes embedded in the inner membrane of a mitochondrion. Each hydrogen atom consists of an electron (e - ) and a hydrogen ion (H + ). At the inner mitochondrial membrane, the coenzymes release the proton into the mitochondrial matrix and transfer the electron to first cytochrome of the series of cytochromes embedded in the inner mitochondrial membrane. 3. Electrons are passed along the electron transport system, losing energy in a series of small steps (first cytochrome passes electron to the second and second to the third) down the electron transport chain. The final acceptor of electron is oxygen i.e. when the electron is passed down the chain and gets to the final cytochrome, then from the final cytochrome, the electron is passed to the oxygen. When oxygen accepts electron, its charge becomes negative. 4. As electrons are passed from one cytochrome to the next cytochrome, 2 hydrogen are pumped into the mitochondrial inter-membrane space. 5. As electrons are passed from cytochrome to cytochrome, one NADH provides enough energy to pump 6 hydrogen in to the mitochondrial inter-membrane space, and one FADH2 provides enough energy to pump 6 hydrogen. 6. Diffusion of hydrogen ions from the mitochondrial inter-membrane space back to the matrix through the ATP Synthase produces ATP. For every 2 hydrogen that passes through the ATP synthase, one ADP is converted to one ATP 7. As hydrogen ions enter mitochondrial matrix, they react with oxygen ions to form water molecule
8 8. The diagram bellow shows all the steps. The difference between NADH and FADH2 is that FADH2 skips the first cytochrome and passes the electron to the second cytochrome, therefore instead of pumping out 6 hydrogen, only 4 hydrogen are pumped. 1 NADH = 3ATP 1 FADH2 = 2 ATP SUMMARY OF ETS Coenzymes (NAD and FAD) deliver hydrogen atoms from the citric acid cycle to the ETS. Each hydrogen atom consists of an electron (e - ) and hydrogen ion (H + ). There are proteins (called cytochromes) embedded at the inner mitochondrial membrane. This sequence of embedded proteins on the inner mitochondrial membrane is ETS. Coenzyme take the electrons from the molecules of citric acid cycle and give it first protein of ETS. The first cytochrome passes electron to the second, which passes them to the third, and so on down the ETS. As electron is passed from one cytochrome to the next down the ETS, enough energy is released to pump hydrogen ions from the mitochondrial matrix into the mitochondrial intermembrane space. Electron passed down the ETS from one NADH pumps 6 hydrogen, and electron from one FADH2 pumps 4 hydrogen. Concentration of hydrogen increases in the intermembrane space and hydrogen diffuses back into the mitochondrial matrix through a channel called ATP synthase. For every pair (two) hydrogen that diffuses into the mitochondrial matrix via ATP synthase, one ATP will be generated. NOTE: 1NADH pumps 6 hydrogen, every 2 hydrogen = 1 ATP, so 1NADH = 3 ATP; 1FADH2 pumps 4 hydrogen, every 2 hydrogen = 1ATP, 1FADH2 = 2 ATP
9 SUMMARY OF ENERGYT PRODUCTION SUMMARY OF THE PROCESSES Gluconeogenesis: Glucose is synthesized from smaller 3 carbon molecules. This process is stimulated by the hormone Glucagon produced by pancreas Glycolysis: Glucose is broken down into two pyruvate Glycogenolysis: Glycogen is broken down to glucose. This process is stimulated by Glucagon
10 Glycogenesis: glycogen is synthesized from glucose. This step is stimulated by hormone Insulin produced by pancreas Oxidative Decarboxylation: oxidation of pyruvate to acetyl CoA OVERVIEW OF LIPID DIGESTION, ABSORPTION AND TRANSPORT Site Parotid Gland Sublingual Gland What happens? Mechanical processing in the mouth breaks material into smaller chunks. Salivary Lipase attacks triglycerides, breaking them down into monoglycerides and free fatty acids. Submandibula Gland Mucous Cells Parietal Cells Chief Cells G Cells Gastric Pit Gastric Gland Lipids are not water soluble, thus mixing of chyme in the stomach creates large droplets of lipids. Salivary lipase continues to break down triglycerides. However because lipase is water soluble, it can only attack triglycerides that are on the surface of those droplets. The arrival of lipids in the duodenum stimulates the secretion of the hormones CCK. CCK (cholecystokinin) Stimulates gall bladder to release bile. Stimulation of gallbladder leads to the ejection of bile from gall bladder into the duodenum. Gallbladder Most dietary lipids are not water soluble. Mechanical processing in the stomach creates large fat drops. Pancreatic lipase is water soluble, so the enzymes can interact with lipids only at the surface of a lipid droplet. Store and modify bile bile is produced by the liver and delivered for storage to the gallbladder. Bile salts break the fat droplets apart in a process called emulsification. Emulsification breaks large fat droplets into smaller ones and increases the surface area of fat droplet accessible to enzymatic attack. Secretion of pancreatic enzymes are controlled by hormones of small intestine such as CCK and Secretin. Lipase is released by the pancreas into the duodenum and do most of the digestive work in the small intestine. Pancreatic lipase breaks down triglycerides into a mixture of free fatty acids, glycerole and monoglycerides. Interaction of free fatty acids and glycerol with bile salt forms micelles (bile salt and lipid complex). Micelle contact the intestinal epithelium. Because fatty acids, monoglycerides are lipid soluble they easily diffuse across the plasma membrane in the intestinal mucosa. Once fatty acids and monoglycerides are inside the cells, they enter the endoplasmic reticulum, where they are resynthesized into triglycerides. Triglycerides, combined with cholesterol and phospholipids and then they are coated with proteins, creating a complex called chylomicrons (complex of lipids and proteins or lipoproteins). The protein coat makes them water soluble and facilitates exocytosis. The intestinal cell transports chylomicron into the interstitial fluid via exocytosis. Because of their large size chylomicrons cannot diffuse into the capillaries, but they can diffuse into the lacteals of lymphatic system which has large gaps and lack basal lamina. Chylomicrons are delivered by the thoracic duct of lymphatic vessels to the blood stream. Content of thoracic duct empties into the left subclavian vein and enters bloodstream.
11 Chylomicron is destined for the liver, however on its way to the liver they will encounter lipoprotein lipase and some of the triglycerides (TG) present in chylomicrons will be hydrolyzed by lipoprotein lipase of the capillary walls. This will result in an overall reduction in the size of the chylomicron as TG is removed, and the reduced size chylomicron is called chylomicron remnant. Fatty acids and monoglyceride that are released by lipoprotein lipase will diffuse out of capillary into the interstitial fluid. Fatty acids and monoglycerides are: absorbed by skeletal muscle cells and metabolized to generate ATP; absorbed by adipose cells (fat cells) to synthesize triglyceride for storage. Chylomicron remnant arrives at the liver. Liver cells modify content of chylomicron remnant by adding more TG to it, and the new lipoprotein complex is called very low density lipoprotein (VLDL). Liver releases VLDL into the circulation to deliver triglycerides (TG) to the cells i.e. ) VLDL are Lipoproteins, containing triglycerides manufactured in the liver, and are transported to peripheral tissues. At the capillaries TG of VLDL is hydrolyzed, and the lipoprotein complex becomes intermediate density lipoprotein (IDL). IDL is delivered back to the liver. At the liver cholesterol is added to the IDL and the surface proteins are altered. The new lipoprotein complex called low density lipoprotein (LDL) is released back into the circulation to deliver cholesterol to peripheral tissue. At the peripheral tissue LDL is absorbed by the cells, and then LDL is broken down by lysosome and cholesterol is released in the cell. The cell uses cholesterol for membrane synthesis, hormone synthesis and various other ways. The excess cholesterol that is not used by the cell diffuses out of the cell and enters bloodstream. High density lipoproteins (HDLs) that are released by the liver absorb the excess cholesterol that was not used by the cell. HDL delivers the cholesterol back to the liver i.e. HDL are lipoproteins, carrying mostly cholesterol and phospholipids from peripheral tissues to the liver. In the liver cholesterols are extracted from HDL, and then cholesterols are: packaged to make new LDL so it could be transported back to peripheral tissue; used to synthesize bile salt or excreted with bile. The healthiest ratio of cholesterol is to have high number of HDL (good cholesterol) and low number of LDL (bad cholesterol).
12 LIPID CATABOLISM OR LIPOLYSIS During lipid hydrolysis or catabolism, triglycerides are broken down into 3 fatty acids and glycerol. Glycerol is converted into pyruvate and fatty acids are broken down into fragments of 2 carbons by a process called betaoxidation. The process of beta-oxidation occurs through the sequential removal of 2-carbon units by oxidation at the beta-carbon position of the fatty acyl-coa molecule. Lipolysis Break down of Lipids into pieces that can be converted into pyruvate. For example triglycerides are split into glycerol and fatty acids Triglyceride is broken down to glycerol and 3 fatty acids Glycerol In Cytosol glycerol is converted to pyruvate Triglyceride 3 Fatty Acids Fatty acids enter mitochondria, and inside mitochondria fatty acids are broken down via beta oxidation Each round of beta-oxidation produces one mole of NADH, one mole of FADH 2 and one mole of acetyl-coa. The acetyl-coa, the end product of each round of beta-oxidation, then enters the TCA cycle, where it is further oxidized to CO 2 with the concomitant generation of three moles of NADH, Beta-Oxidation one mole of FADH 2 and one mole of ATP. The NADH and FADH 2 generated Breakdown of fatty acids into during the fat oxidation and acetyl-coa oxidation in the TCA cycle then can fragments of 2 carbons enter the respiratory pathway for the production of ATP. The first step of beta-oxidation requires binding of Coenzyme A bind to fatty acid. This step requires one ATP. This reaction will prepare fatty acid for beta oxidation and generate a fatty acid attached to CoA
13 The first round of beta oxidation will generate one NADH, one FADH2 and one Acetyl CoA. Fatty acid will be shortened by 2 carbons. Acetyl CoA will enter TCA cycle and generate 3NADH, 1FADH and 1GTP. 3NADH = 9ATP, 1FADH2 = 2ATP, and GTP = 1ATP. Non-essential fatty acids Fatty acids that can be synthesized NADH and FADH2 will enter the ETS and generate ATP: 1NADH = 3ATP; 1FADH2 = 2ATP Essential fatty acids Fatty acids that cannot be synthesized and must be included in diet Linoleic acid, arachidonic acid, and linolenic acid are examples of essential fatty acids SUMMARY OF BETA OXIDATION Beta oxidation is the process that breaks down fatty acids into two-carbon fragments that can be metabolized by the TCA cycle, which yields large amount of ATP. The input molecules of beta oxidation is Coenzyme A, NAD, and FAD i.e. beta oxidation requires coenzyme A, NAD, and FAD. The output molecules of beta oxidation are Acetyl CoA, NADH, and FADH2. The process of beta oxidation occurs in the mitochondria, NADH and FADH2 enters the ETS (electron transport system) and Acetyl CoA enters the Krebs cycle. Lipids provide energy for cells with modest energy demands and for skeletal muscle when energy demands are low. LIPID ANABOLISM OR LIPOGENESIS Fatty acid synthesis involves different and distinct set of reaction sequences from that of beta oxidation. Therefore we cannot make every amino acid that we break. For examples due to lack of enzymes we cannot synthesize linolenic acid (omega 3 fatty acid) or linoleic acid (omega 6 fatty acid). Yet these fatty acids are needed for synthesis of hormones such as prostaglandin. Therefore these fatty acids that cannot be synthesized by the body cell are called essential fatty acids.
14 Synthesis of non-essential fatty acids (fatty acids that can be made by the body cells) and steroids begin with acetyl-coa i.e. Lipogenesis generally begins with acetyl-coa. Because acetyl CoA can be the product of carbohydrate catabolism and amino acid catabolism, thus fatty acids can be made from any organic molecule. Pyruvate can be converted to glycerol. Because pyruvate can be made from glucose, thus glucose can be converted to glycerol via pyruvate. PROTEIN METABOLISM Proteins have very complex structure, so protein digestion is complex and time consuming. See the digestive system lecture for details of protein digestion. After complete digestion of protein, amino acids diffuse into the epithelial cells of the intestine, and then epithelial cells release amino acids into the interstitial fluid. From interstitial fluid amino acids enter intestinal capillaries and then they are transported to the liver by hepatic portal veins. Liver uses the amino acids to synthesize plasma proteins. Amino acids that can be catabolized (broken down) and used for gluconeogenesis when other sources of glucose are not available i.e. amino acids from may be used for synthesis of new proteins, or may be burned for energy.
15 An amino acid molecule contains amino group (red circle in figure bellow), a carboxyl group (blue circle) and functional group (rectangle R) Before amino acids can be oxidized for energy (used for energy), they must have the amine group removed, a process called deamination. The deaminated amino acid molecule is converted to pyruvic acid, or a Krebs cycle ketoacid intermediate. Deaminated amino acids may also be reconverted to glucose and contribute to gluconeogenesis. The ammonium ion (by product of deamination) is very toxic, thus it is quickly combined with carbon dioxide and urea is synthesized (liver has all the enzymes for the urea synthesis) i.e. the most abundant nitrogenous waste in blood is urea, which is produced by the combination of ammonia (by product of protein metabolism) with carbon dioxide. Liver cells and other cells can synthesize the non-essential amino acids by transamination or amination. In an amination reaction, an ammonium ion (NH + 4 ) is used to form an amino group that is attached to a molecule, yielding an amino acid. In transamination, an amino group from an amino acid is transferred to an organic acid (non-amino acid molecule), and the attachment of amino group to the non-amino acid molecule will produce a new amino acid. For example the amino group of an amino acid is transferred to a keto acid, and keto acid becomes an amino acid. The process of deamination produces ammonia (toxic substance) that will be converted to urea by the liver and then excreted by the kidneys.
16 VITAMINS AND MINERALS Vitamins are organic elements and minerals are inorganic compounds. Both are required in very small quantities, but they play an essential role in metabolic pathways. Most vitamins must be obtained from the diet, but the body can synthesize some of them from precursors called provitamins. Vitamins are classified as watersoluble or fat-soluble. Water-soluble vitamins are absorbed with water from the small intestine, dissolve freely in body fluids, and are quickly excreted i.e. we cannot store water soluble vitamins thus we have to continuously take water-soluble vitamins with our diet. Water-soluble vitamins are ascorbic acid (vitamin C) and the B vitamins. Fat-soluble vitamins are incorporated into lipid micelles in the small intestine and are absorbed with dietary lipids. Fat soluble vitamins include vitamin K,D,A, and E. Function of Vitamins Vitamin A is a component of the visual pigments and promotes proteoglycan synthesis and epithelial maintenance. Vitamin D promotes calcium absorption and bone mineralization. Vitamin K is essential to prothrombin synthesis and blood clotting. Vitamins A and E are antioxidants, like vitamin C. Ascorbic acid promotes hemoglobin synthesis, collagen synthesis, and sound connective tissue structure and is also an antioxidant. B vitamins function as coenzymes or parts of coenzymes, assisting in electron transfer.
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Chapter 25: Metabolism and Nutrition
 Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline
 AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
Summary of Metabolism. Mechanism of Enzyme Action
 Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
CHAPTER 15: ANSWERS TO SELECTED PROBLEMS
 CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
The correct answer is d C. Answer c is incorrect. Reliance on the energy produced by others is a characteristic of heterotrophs.
 1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Lactic Acid Dehydrogenase
 Lactic Acid Dehydrogenase Pyruvic Acid Dehydrogenase Complex Pyruvate to ACETYL coa CC CoA + CO 2 Mitochondria 3 carbon Pyruvate to 2 carbon ACETYL Coenzyme A Pyruvate Acetyl CoA + CO 2 + NADH + H + CO2
Lactic Acid Dehydrogenase Pyruvic Acid Dehydrogenase Complex Pyruvate to ACETYL coa CC CoA + CO 2 Mitochondria 3 carbon Pyruvate to 2 carbon ACETYL Coenzyme A Pyruvate Acetyl CoA + CO 2 + NADH + H + CO2
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Essentials of Anatomy and Physiology, 5e (Martini/Nath) Chapter 17 Nutrition and Metabolism. Multiple-Choice Questions
 Essentials of Anatomy and Physiology, 5e (Martini/Nath) Chapter 17 Nutrition and Metabolism Multiple-Choice Questions 1) The sum of all of the biochemical processes going on within the human body at any
Essentials of Anatomy and Physiology, 5e (Martini/Nath) Chapter 17 Nutrition and Metabolism Multiple-Choice Questions 1) The sum of all of the biochemical processes going on within the human body at any
008 Chapter 8. Student:
 008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Copyright 2010 Pearson Education, Inc. Chapter Twenty Three 1
 23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
Special organ structures and functions conduct these tasks through the successive parts of the overall system.
 Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
OVERVIEW OF LIPID METABOLISM
 VERVIEW F LIPID METABLISM Date: September 20, 2005 * Time: 8:00 am 8:50 am * Room: G202 Biomolecular Building Lecturer: Steve Chaney 515A Mary Ellen Jones Building stephen_chaney@med.unc.edu 9663286 *Please
VERVIEW F LIPID METABLISM Date: September 20, 2005 * Time: 8:00 am 8:50 am * Room: G202 Biomolecular Building Lecturer: Steve Chaney 515A Mary Ellen Jones Building stephen_chaney@med.unc.edu 9663286 *Please
1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because:
 Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
Harvesting Energy: Glycolysis and Cellular Respiration. Chapter 8
 Harvesting Energy: Glycolysis and Cellular Respiration Chapter 8 Overview of Glucose Breakdown The overall equation for the complete breakdown of glucose is: C 6 H 12 O 6 + 6O 2 6CO 2 + 6H 2 O + ATP The
Harvesting Energy: Glycolysis and Cellular Respiration Chapter 8 Overview of Glucose Breakdown The overall equation for the complete breakdown of glucose is: C 6 H 12 O 6 + 6O 2 6CO 2 + 6H 2 O + ATP The
The Human Digestive System
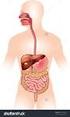 The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
Cellular Respiration and Fermentation
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
Chapter 15 Lecture Notes: Metabolism
 Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
Chapter 7 Cellular Respiration
 Phases of aerobic cellular respiration 1. Glycolysis 2. Transition or Acetyl-CoA reaction 3. Krebs cycle 4. Electron transport system Chapter 7 Cellular Respiration These phases are nothing more than metabolic
Phases of aerobic cellular respiration 1. Glycolysis 2. Transition or Acetyl-CoA reaction 3. Krebs cycle 4. Electron transport system Chapter 7 Cellular Respiration These phases are nothing more than metabolic
Chapter 48. Nutrients in Food. Carbohydrates, Proteins, and Lipids. Carbohydrates, Proteins, and Lipids, continued
 Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Integration of Metabolism
 I. Central Themes of Metabolism 1. ATP is the universal energy carrier. Integration of Metabolism Bryant Miles 2. ATP is generated by the oxidation of metabolic fuels Glucose Fatty Acids Amino Acids 3.
I. Central Themes of Metabolism 1. ATP is the universal energy carrier. Integration of Metabolism Bryant Miles 2. ATP is generated by the oxidation of metabolic fuels Glucose Fatty Acids Amino Acids 3.
Photosynthesis takes place in three stages:
 Photosynthesis takes place in three stages: Light-dependent reactions Light-independent reactions The Calvin cycle 1. Capturing energy from sunlight 2. Using energy to make ATP and NADPH 3. Using ATP and
Photosynthesis takes place in three stages: Light-dependent reactions Light-independent reactions The Calvin cycle 1. Capturing energy from sunlight 2. Using energy to make ATP and NADPH 3. Using ATP and
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
The Digestive System. You are what you eat!
 The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
The Vertebrate (mostly human) Digestive System
 The Vertebrate (mostly human) Digestive System Mouth - mastication, lubrication, digestion Pharynx and Esophagus - swallowing Stomach - some digestion Small intestine - most digestion and absorption Large
The Vertebrate (mostly human) Digestive System Mouth - mastication, lubrication, digestion Pharynx and Esophagus - swallowing Stomach - some digestion Small intestine - most digestion and absorption Large
Todays Outline. Metabolism. Why do cells need energy? How do cells acquire energy? Metabolism. Concepts & Processes. The cells capacity to:
 and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
Overview of Lipid Metabolism
 Overview of Lipid Metabolism Learning Objectives By the end of this lecture the students should be able to understand: Classification of Lipids The digestion, absorption and utilization of dietary lipids
Overview of Lipid Metabolism Learning Objectives By the end of this lecture the students should be able to understand: Classification of Lipids The digestion, absorption and utilization of dietary lipids
AP Bio Photosynthesis & Respiration
 AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
Chapter 9 Cellular Respiration
 Chapter 9 Cellular Respiration Electrons carried in NADH Mitochondrion Glucose Glycolysis Pyruvic acid Krebs Cycle Electrons carried in NADH and FADH 2 Electron Transport Chain Cytoplasm Mitochondrion
Chapter 9 Cellular Respiration Electrons carried in NADH Mitochondrion Glucose Glycolysis Pyruvic acid Krebs Cycle Electrons carried in NADH and FADH 2 Electron Transport Chain Cytoplasm Mitochondrion
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Weds 5/20/15. Membranes - finish last lecture outline. Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system
 Membranes - finish last lecture outline Weds 5/20/15 Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system structures and functions // accessory organs mechanism
Membranes - finish last lecture outline Weds 5/20/15 Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system structures and functions // accessory organs mechanism
Chapter 15 Digestive System.
 Chapter 15 Digestive System. I. The Gastrointestinal Tract. a. The digestive system mechanically and chemically breaks down food into molecules that can be absorbed into the bloodstream or lymph. Residues
Chapter 15 Digestive System. I. The Gastrointestinal Tract. a. The digestive system mechanically and chemically breaks down food into molecules that can be absorbed into the bloodstream or lymph. Residues
1. Explain the difference between fermentation and cellular respiration.
 : Harvesting Chemical Energy Name Period Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second to look at the big picture. Photosynthesis and cellular
: Harvesting Chemical Energy Name Period Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second to look at the big picture. Photosynthesis and cellular
Chapter 7 Active Reading Guide Cellular Respiration and Fermentation
 Name: AP Biology Mr. Croft Chapter 7 Active Reading Guide Cellular Respiration and Fermentation Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second
Name: AP Biology Mr. Croft Chapter 7 Active Reading Guide Cellular Respiration and Fermentation Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Cellular Respiration An Overview
 Why? Cellular Respiration An Overview What are the phases of cellular respiration? All cells need energy all the time, and their primary source of energy is ATP. The methods cells use to make ATP vary
Why? Cellular Respiration An Overview What are the phases of cellular respiration? All cells need energy all the time, and their primary source of energy is ATP. The methods cells use to make ATP vary
Chapter 14- RESPIRATION IN PLANTS
 Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
Photosynthesis (CO 2 + H 2 O C 6 H 12 O 6 + O 2 )
 The vital role of A This is the energy-rich compound that is the source of energy for all living things. It is a nucleotide, comprising a 5C sugar (ribose); an organic base (adenosine); and 3 phosphate
The vital role of A This is the energy-rich compound that is the source of energy for all living things. It is a nucleotide, comprising a 5C sugar (ribose); an organic base (adenosine); and 3 phosphate
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman
 SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
Digestive System Functions
 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 9 Review Worksheet Cellular Respiration
 1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
Chapter 17 Digestive System. Alimentary Canal. Movements of the Tube
 Chapter 17 Digestive System Functions of Digestive System ingestion mechanical digestion chemical digestion propulsion absorption defecation Consists of the alimentary canal and accessory organs 1 Alimentary
Chapter 17 Digestive System Functions of Digestive System ingestion mechanical digestion chemical digestion propulsion absorption defecation Consists of the alimentary canal and accessory organs 1 Alimentary
1. Enzymes. Biochemical Reactions. Chapter 5: Microbial Metabolism. 1. Enzymes. 2. ATP Production. 3. Autotrophic Processes
 Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
How Cells Release Chemical Energy Cellular Respiration
 How Cells Release Chemical Energy Cellular Respiration Overview of Carbohydrate Breakdown Pathways Photoautotrophs make ATP during photosynthesis and use it to synthesize glucose and other carbohydrates
How Cells Release Chemical Energy Cellular Respiration Overview of Carbohydrate Breakdown Pathways Photoautotrophs make ATP during photosynthesis and use it to synthesize glucose and other carbohydrates
Cellular Respiration & Metabolism. Metabolism. Coupled Reactions: Bioenergetics. Cellular Respiration: ATP is the cell s rechargable battery
 Cellular Respiration & Metabolism Metabolic Pathways: a summary Metabolism Bioenergetics Flow of energy in living systems obeys: 1 st law of thermodynamics: Energy can be transformed, but it cannot be
Cellular Respiration & Metabolism Metabolic Pathways: a summary Metabolism Bioenergetics Flow of energy in living systems obeys: 1 st law of thermodynamics: Energy can be transformed, but it cannot be
- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.
![- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells. - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.](/thumbs/40/21014327.jpg) Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Absorption and Transport of Nutrients
 Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Chapter 9 Mitochondrial Structure and Function
 Chapter 9 Mitochondrial Structure and Function 1 2 3 Structure and function Oxidative phosphorylation and ATP Synthesis Peroxisome Overview 2 Mitochondria have characteristic morphologies despite variable
Chapter 9 Mitochondrial Structure and Function 1 2 3 Structure and function Oxidative phosphorylation and ATP Synthesis Peroxisome Overview 2 Mitochondria have characteristic morphologies despite variable
Cellular Respiration Stage 4: Electron Transport Chain
 Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP Life takes
Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP Life takes
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Anabolic and Catabolic Reactions are Linked by ATP in Living Organisms
 Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Digestive system Review
 Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Topic 4: Digestion and Nutrition
 Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
-Loss of energy -Loss of hydrogen from carbons. -Gain of energy -Gain of hydrogen to carbons
 Cellular Respiration- Equation C6H12O6 + 6O2 6CO2 +6H20 and energy -The energy is released from the chemical bonds in the complex organic molecules -The catabolic process of releasing energy from food
Cellular Respiration- Equation C6H12O6 + 6O2 6CO2 +6H20 and energy -The energy is released from the chemical bonds in the complex organic molecules -The catabolic process of releasing energy from food
ATP accounting so far ELECTRON TRANSPORT CHAIN & CHEMIOSMOSIS. The Essence of ETC: The Electron Transport Chain O 2
 accounting so far The final stage of cellular respiration: ELECTRON TRANSPORT CHAIN & CHEMIOSMOSIS Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to extract more energy than 4! There
accounting so far The final stage of cellular respiration: ELECTRON TRANSPORT CHAIN & CHEMIOSMOSIS Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to extract more energy than 4! There
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
VII. Metabolism Overview and Digestion. A. Introduction
 VII. Metabolism verview and Digestion A. Introduction 1. All living organisms require a constant supply of energy a. Basic problem (1) Living organisms are working against natural tendency for things to
VII. Metabolism verview and Digestion A. Introduction 1. All living organisms require a constant supply of energy a. Basic problem (1) Living organisms are working against natural tendency for things to
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Chem 306 Chapter 21 Bioenergetics Lecture Outline III
 Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Outline Digestive System
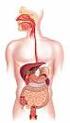 Outline Digestive System The Digestive System Digestive System Lecture Packet 19 Chapter 15 I. Function II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine,
Outline Digestive System The Digestive System Digestive System Lecture Packet 19 Chapter 15 I. Function II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine,
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT
 CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
Chapter 15 Digestion and Nutrition
 Chapter 15 Digestion and Nutrition Digestive System: Digestion refers to the mechanical and chemical breakdown of foods so that nutrients can be absorbed by cells. Consists of the canal which is all of
Chapter 15 Digestion and Nutrition Digestive System: Digestion refers to the mechanical and chemical breakdown of foods so that nutrients can be absorbed by cells. Consists of the canal which is all of
Digestive System Notes
 Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
The diagram below summarizes the effects of the compounds that cells use to regulate their own metabolism.
 Regulation of carbohydrate metabolism Intracellular metabolic regulators Each of the control point steps in the carbohydrate metabolic pathways in effect regulates itself by responding to molecules that
Regulation of carbohydrate metabolism Intracellular metabolic regulators Each of the control point steps in the carbohydrate metabolic pathways in effect regulates itself by responding to molecules that
CITRIC ACID (KREB S, TCA) CYCLE
 ITRI AID (KREB S, TA) YLE Date: September 2, 2005 * Time: 10:40 am 11:30 am * Room: G202 Biomolecular Building Lecturer: Steve haney 515A Mary Ellen Jones Building stephen_chaney@med.unc.edu 9663286 *Please
ITRI AID (KREB S, TA) YLE Date: September 2, 2005 * Time: 10:40 am 11:30 am * Room: G202 Biomolecular Building Lecturer: Steve haney 515A Mary Ellen Jones Building stephen_chaney@med.unc.edu 9663286 *Please
SMALL AND LARGE INTESTINE SECRETIONS
 SMALL AND LARGE INTESTINE SECRETIONS Objectives At the end of lecture student should be able to know, Digestive system Digestive system secretions Small intestine Component of small intestine Intestinal
SMALL AND LARGE INTESTINE SECRETIONS Objectives At the end of lecture student should be able to know, Digestive system Digestive system secretions Small intestine Component of small intestine Intestinal
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
The Excretory and Digestive Systems
 The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
Copyright 2000-2003 Mark Brandt, Ph.D. 54
 Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain.
 Lipid Metabolism 1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain. 2. How can excess acetyl CoA trapped in the mitochondria, be utilized as a substrate for fatty
Lipid Metabolism 1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain. 2. How can excess acetyl CoA trapped in the mitochondria, be utilized as a substrate for fatty
(Woods) Chem-131 Lec-19 09-4 Lipids 1. Lipids:
 (Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Classifying Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, p sphingolipids, p and cholesterol
(Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Classifying Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, p sphingolipids, p and cholesterol
Chapter 2 Digestion and Absorption Chapter Outline
 Chapter 2 Digestion and Absorption Chapter Outline I. Anatomy of the Digestive Tract A. The Digestive Organs 1. Mouth to the Esophagus 2. Esophagus to the Stomach 3. The Small Intestine 4. The Large Intestine
Chapter 2 Digestion and Absorption Chapter Outline I. Anatomy of the Digestive Tract A. The Digestive Organs 1. Mouth to the Esophagus 2. Esophagus to the Stomach 3. The Small Intestine 4. The Large Intestine
I The THREE types of LIPIDS
 LECTURE OUTLINE Chapter 5 The Lipids: Fats, Oils, Phospholipids and Sterols I The THREE types of LIPIDS A. Triglycerides (fats & oils)- the MAJOR type of lipid in food and humans. 1. 2 parts of triglyceridesa)
LECTURE OUTLINE Chapter 5 The Lipids: Fats, Oils, Phospholipids and Sterols I The THREE types of LIPIDS A. Triglycerides (fats & oils)- the MAJOR type of lipid in food and humans. 1. 2 parts of triglyceridesa)
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BCOR 011 Exam 2, 2004
 BCOR 011 Exam 2, 2004 Name: Section: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. According to the first law of thermodynamics, A. the universe
BCOR 011 Exam 2, 2004 Name: Section: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. According to the first law of thermodynamics, A. the universe
Digestion. What we ll cover. Main stages of digestion. Digestion: A Closer Look. A Tour of the Human Digestive System. Mechanical digestion
 Digestion What we ll cover What are the digestive system structures and their functions? Where does carbohydrate, protein and fat digestion and absorption occur? What are the 3 accessory organs of digestion?
Digestion What we ll cover What are the digestive system structures and their functions? Where does carbohydrate, protein and fat digestion and absorption occur? What are the 3 accessory organs of digestion?
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps):
 Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps): 1) How many ATP molecules are produced for each glucose molecule used in fermentation?
Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps): 1) How many ATP molecules are produced for each glucose molecule used in fermentation?
Lipids. Classifying Lipids
 (Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, sphingolipids, and cholesterol (together with proteins)
(Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, sphingolipids, and cholesterol (together with proteins)
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
8. Be able to label a diagram of an earthworm. Know the function of each of the major parts of the earthworm.
 Review for Unit Test: The Digestive System 1. Know the meaning of these terms: heterotrophs digestion peristalsis microvilli autotrophs chemical digestion chyme lacteal intracellular digestion mechanical
Review for Unit Test: The Digestive System 1. Know the meaning of these terms: heterotrophs digestion peristalsis microvilli autotrophs chemical digestion chyme lacteal intracellular digestion mechanical
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Name Date Period. Keystone Review Enzymes
 Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Metabolism Poster Questions
 Metabolism Poster Questions Answer the following questions concerning respiration. 1. Consider the mitochondrial electron transport chain. a. How many hydrogen ions can be pumped for every NADH? b. How
Metabolism Poster Questions Answer the following questions concerning respiration. 1. Consider the mitochondrial electron transport chain. a. How many hydrogen ions can be pumped for every NADH? b. How
GI TRACT ORGANS ACCESSORY ORGANS
 Digestive System GI TRACT ORGANS Oral cavity Oropharynx Esophagus Stomach Small intestine Large Intestine Anus ACCESSORY ORGANS Salivary glands Pancreas Liver Gall bladder GI TRACT LAYERS Mucosa Submucosa
Digestive System GI TRACT ORGANS Oral cavity Oropharynx Esophagus Stomach Small intestine Large Intestine Anus ACCESSORY ORGANS Salivary glands Pancreas Liver Gall bladder GI TRACT LAYERS Mucosa Submucosa
