CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-II
|
|
|
- Aileen Wilkinson
- 7 years ago
- Views:
Transcription
1 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-II
2 LECTURE OUTLINE By the end of the lecture, the student should know: The formation of the ring structure. The glycosidic linkage. The functions and biological importance of polysaccharides.
3 THE FORMATION OF THE RING STRUCTURE Sugar molecules that have five or six carbons are flexible. If a six member sugar forms a ring between Carbon 1 and Carbon 5, it is called a pyranose ring If a six member sugar forms a ring between Carbon 1 and Carbon 4 it is called a furanose ring. (Also if the ring is formed between Carbon 2 and 5 in keto sugars)
4
5 The flexible carbons in monosaccharides like glucose or fructose bring Aldehyde or Ketone Group close to the OH group of the same molecule and form a ring structure. If this ring is formed by an aldehyde group, it is called Hemiacetal ring or if it is formed by a keto group, it is called Hemi Ketal Ring
6
7
8 The aldehyde group comes in close proximity to the hydroxyl group. The H of the hydroxyl group is transferred to the oxygen of the aldehyde group. The oxygen from the hydroxyl group forms a bond with the carbon of the aldehyde group.
9 GLYCOSIDES Glycosides are compounds in which: A Monosaccharide is attached to an Alcoholic group of a second compound By Glycosidic Linkage. Glycosidic Linkage is Defined as an: Acetal Linkage Between Carbonyl Carbon of a Monosaccharide and Hydoxyl Group of an Another Compound.
10 METHYL GLUCOSIDE
11 IN GLYCOSIDES OTHER COMPOUND MAY OR MAY NOT BE A MONOSACCHARIDE When the alcoholic compound in a Glycoside is a Non-Carbohydrate it is called Aglycon. In methyl Glucoside Methyl group is an Aglycon.
12 H- C------O H-C-OH OH-C-H H-C-OH H-C-OH CH2OH IN METHYL GLUCOSIDE METHYL GROUP IS AN AGLYCON CH 3 Aglycon Glycon Methyl Glucoside
13 GLYCOSIDES Carbonyl Carbon of a Monosaccharide is attached, by an Acetal linkage, to an Alcoholic group of a second compound.(acetal is an organic molecule where two separate oxygen atoms are single bonded to a central oxygen atom) H- C=O H-C-OH OH-C-H H-C-OH H-C-OH CH2OH OH CH 3 Methyl Glucoside
14 HEMIACETAL AND ACETAL
15 GLYCOSIDIC LINKAGE BEING FORMED BETWEEN TWO SUGARS
16 GLYCOSIDIC LINKAGE BEING FORMED BETWEEN TWO SUGARS
17 Types of Glycosidic Linkages In the formation of disaccharides, the type of glycosidic linkage formed is O Glycosidic Linkage. There are other glycosidic linkages as well which include S-glycosidic bonds, N- Glycosidic bonds.
18 POLYSACHHARIDES Most of the Carbohydrates found in nature occur in the form of high molecular weight complex compounds called POLYSACCHARIDES. Composed of ten or more monosaccharides or their derivatives. Monomer units are linked by the glycosidic (acetal) linkages. On Hydrolysis yield a large number of Monosaccharide units or their derivatives.
19 POLYSACCHARIDE MAY BE CLASSIFIED INTO TWO GROUPS When polysaccharides are composed of a single type of monosaccharide building block, they are termed: Homo polysaccharides or (Homo Glycans). Polysaccharides composed of more than one type of monosaccharide are termed: Hetero polysaccharide or (Hetero Glycans).
20 HOMOPOLYSACCHARIDES: POLYSACCHARIDES MAY BE COMPOSED OF A SINGLE TYPE OF MONOSACCHARIDES G G G G G G G G G Or it may be composed of more than one type of Units AG GA AG GA AG GA AG GA
21
22 HOMOPOLYSACCHARIDES Polysaccharides which are composed of Similar Types of Monosaccharide Units. Common examples are: Starch. Glycogen. Cellulose. Dextrin.
23 TWO TYPES OF GLYCOSIDIC LINKAGES IN GLUCOSE POLYMERS: 1-4, Glycosidic Linkage. 1-6, Glycosidic Linkage (Branch Point)
24 STARCH:A COMPLEX GLUCOSE POLYMER It is a homopolysaccharide comprising of molecules of glucose joined together by alpha 1,4 and alpha 1,6 linkages. It is made up of two polysaccharide units. AMYLOSE AMYLOPECTIN
25 Amylose: Straight Chain of Glucose molecules. Having Only 1-4, Glycosidic Linkages. Amylopectin: Complexed & highly Branched Polymer of Glucose. Having Both 1-4 & 1-6, Linkages. Linear Segment with 1-4 Linkage. 1-6, Linkages at Branch Points.
26 ALPHA 1-4 LINKAGE
27 ALPHA 1-6 LINKAGE
28 AMYLOSE; UNBRANCHED GLUCOSE POLYMER Straight chain of Glucose molecules. Several thousands of Glucose units (In the range of 300 and 3000). 1 4 Glycosidic bonds. 1 4 Reducing End Non-reducing End
29 AMYLOPECTIN; COMPLEXED & HIGHLY BRANCHED POLYMER OF GLUCOSE. Having Both 1-4 (Linear Segment) & 1-6, Linkages (Branch Points). Branching takes place with (1 6) bonds occurring every 24 to 30 glucose units. Formed of 2,000 to 200,000 glucose units.
30 STARCH IS A COMPLEX OF TWO TYPES OF GLUCOSE POLYMERS Unbranched highly coiled Amylose Highly branched uncoiled Amylopectin
31 STARCH; MIXTURE OF TWO COMPLEX CARBOHYDRATES Amylose Forms about 25% of starch molecule. Unbranched Highly coiled Amylopectin Forms about 75% of starch molecule. Highly branched Uncoiled.
32 STARCH; DIETARY SOURCES In human diet Starch is commonly obtained from: Cereals (Rice, Wheat, and Corn) Potatoes and sweet potato. Fruits such as Banana.
33 STARCH: BIOLOGICAL SIGNIFICANCE Major form of stored carbohydrate in plant cells. Plants store starch within specialized organelles called Amyloplasts. When energy is needed for cell work, the plant hydrolyzes the starch releasing the glucose. Humans also have enzymes to hydrolyze (Digest) starch.
34 Principle dietary carbohydrate. In terms of human nutrition, starch is by far the most important of the polysaccharides. It constitutes more than half the carbohydrates even in many affluent diets, and much more in poorer diets. Major source of Glucose (Metabolic fuel).
35 DEXTRINS ARE PARTIALLY HYDROLYSED STARCH Intermediate products of hydrolysis of starch by acids or Amylase. Similar to starch but less complex and having lower molecular weight. Dextrin occurs in all starch producing parts of plants as an intermediate of starch synthesis or break down.
36 GLYCOGEN Polymer of about 60,000 Glucose residues. Structure similar to Starch, but is even more compact & highly branched. This compactness allows large amounts of energy to be stored in a small volume, with little effect on cellular osmolarity. Glycogen is the analogue of starch in plants, and is commonly referred to as animal starch.
37 GLYCOGEN; BIOLOGICAL SIGNIFICANCE Major form of storage of carbohydrates (energy store) in animal liver and muscles Primary short term energy storage. Forms an energy reserve that can be quickly mobilized to meet a sudden need for glucose (Energy)
38 ONLY THE LIVER GLYCOGEN IS THE SOURCE OF BLOOD GLUCOSE AND MADE AVAILABLE TO OTHER ORGANS Although the total amount of Glycogen in muscles exceeds that in liver, Only the stores in the liver can be made accessible to other organs. Muscle glycogen provides Glucose (Metabolic fuel) to muscles only during exercise.
39 CELLULOSE DISTRIBUTION Most abundant organic compound in the earth's biosphere is in cellulose. Cellulose is the primary structural component of green plants. The primary cell wall of green plants is made of cellulose. Wood is largely cellulose, while paper and cotton are nearly pure cellulose.
40 CELLULOSE STRUCTURE; STRAIGHT, UNBRANCHED AND UNCOILED CHAIN OF GLUCOSE Glucose Polymer: 500 to 5000 Glucose units. Glucose units linked by β(1 4)-glycosidic bonds.
41 CELLULOSE; BIOLOGICAL SIGNIFICANCE It is a structural polysaccharide. The peculiar long fiber like structure contributes to their high tensile strength. Due to high tensile strength it is important in cell walls, where they provide rigidity to plant cells.
42 NUTRITIONAL VALUE OF CELLULOSE IS INSIGNIFICANT IN HUMAN BEINGS Humans and many other animals lack an enzyme (Cellulase) to break the betalinkages, so they do not digest cellulose. In the diets of humans Cellulose functions as roughage and is eliminated largely unchanged.
43 CELLULOSE; CLINICAL SIGNIFICANCE Cellulose is not digestible by humans and is often referred to as 'Dietary fiber' or 'Roughage. Roughage acts as a bulking agent that increases the intestinal motility and helps propagating intestinal contents. Dietary Cellulose therefore prevents Constipation.
44 HETROPOLYSACCHARIDES MAY BE CLASSIFIED IN THREE MAJOR GROUPS Mucopolysaccharides. Mucilages Hemicellulose
45 MUCOPOLYSACCHARIDES Composed of: Amino Sugars and Uronic Acids. Important examples are: Hyaluronic Acid Chondrotin Heparin.
46 HYALURONIC ACID AND CHONDROTIN ARE IMPORTANT COMPONENTS OF INTERCELLULAR MATRIX Intercellular Matrix is the organic material filled in the intercellular spaces (So Called Ground substance). This organic matter is composed mainly of: Collagen (Protein) and Mucopolysaccharides.
47 INTERCELLULAR MATRIX (GROUND SUBSTANCE) PLAYS MANY IMPORTANT AND VITAL ROLES: Cementing and strengthening substance for the tissue cells. Protective Barrier for the tissues. Holds the extracellular water and electrolytes in a homogenous distribution in the body. Intercellular Matrix is the Protective Barrier for the tissues. Any living or non living injurious substance when penetrates the tissue, they have to pass through the ground substance to reach the cells. The ground substance prevents the penetration of bacteria or any injurious agent in the tissue.
48 MAJOR MUCOPOLYSACCHARIDES OF GROUND SUBSTANCE ARE: Hyaluronic acid in soft tissues Chondrotin in hard tissues.
49 HYALURONIC ACID Long chain of alternate molecules of: Glucuronic Acid And N Acetyl Glucosamine. Hyaluronic Acid is Principally Found in Ground Substance Of the Soft Tissues e.g. Skin, Muscles, Liver and Synovial Fluid.
50 BIOLOGICAL IMPORTANCE OF ACID. HYALURONIC Being the component of ground substance It has a significant contribution in all the vital functions of Intercellular Matrix. Lubrication of Joints. Hyaluronic acid present in the synovial joint spaces acts as lubricant and shock absorber.
51 CHONDROTIN Mainly present in the Ground Substance of Bone and Cartilage. Polymer of N Acetyl Galactoseamine and Glucuronic Acid. They help in compressibility of cartilage and weight bearing.
52 HEPARIN Polymer of: Glucosamine Sulphate and Glucuronic Acid Sulphate. o IT is produced by mast cells.
53 HEPARIN IS A NATURAL ANTICOAGULANT Heparin is a natural anticoagulant for the blood as it prevents the unnecessary and harmful intravascular coagulation of blood. Widely used as an anticoagulant drug. Secretion and action of Heparin as an Anticoagulant is one of Haemodynamic mechanisms which are responsible for the smooth blood flow.
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
Structure of proteins
 Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Topic 4: Digestion and Nutrition
 Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Photo Cell Resp Practice. A. ATP B. oxygen C. DNA D. water. The following equation represents the process of photosynthesis in green plants.
 Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
NUTRIENTS: THEIR INTERACTIONS
 NUTRIENTS: THEIR INTERACTIONS TEACHER S GUIDE INTRODUCTION This Teacher s Guide provides information to help you get the most out of Nutrients: Their Interactions. The contents in this guide will allow
NUTRIENTS: THEIR INTERACTIONS TEACHER S GUIDE INTRODUCTION This Teacher s Guide provides information to help you get the most out of Nutrients: Their Interactions. The contents in this guide will allow
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Digestive System Functions
 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
7 Answers to end-of-chapter questions
 7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Chapter 48. Nutrients in Food. Carbohydrates, Proteins, and Lipids. Carbohydrates, Proteins, and Lipids, continued
 Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
THE HISTORY OF CELL BIOLOGY
 SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
Cells, tissues and organs
 Chapter 8: Cells, tissues and organs Cells: building blocks of life Living things are made of cells. Many of the chemical reactions that keep organisms alive (metabolic functions) take place in cells.
Chapter 8: Cells, tissues and organs Cells: building blocks of life Living things are made of cells. Many of the chemical reactions that keep organisms alive (metabolic functions) take place in cells.
Fatty Acids carboxylic acids
 Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Biofuel Feedstocks and Production
 Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. 2. (U4C1L4:G13) Another name for fiber in a diet is.
 Cadet Name: Date: 1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. A) True B) False 2. (U4C1L4:G13) Another name for fiber in a diet is. A) vegetables B) laxative C) fruit D)
Cadet Name: Date: 1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. A) True B) False 2. (U4C1L4:G13) Another name for fiber in a diet is. A) vegetables B) laxative C) fruit D)
Chapter 4. Chemical Energy
 hapter 4 hemical Energy Perhaps the most convenient form in which to store energy is chemical energy. The foods we eat, combined with the oxygen we breathe, store energy that our bodies extract and convert
hapter 4 hemical Energy Perhaps the most convenient form in which to store energy is chemical energy. The foods we eat, combined with the oxygen we breathe, store energy that our bodies extract and convert
GLUCOSAMINE, CHONDROITIN, AND MSM
 GLUCOSAMINE, CHONDROITIN, AND MSM Compiled by Campbell M Gold (2009) (This material was compiled from various unverified sources) CMG Archives http://campbellmgold.com IMPORTANT The health information
GLUCOSAMINE, CHONDROITIN, AND MSM Compiled by Campbell M Gold (2009) (This material was compiled from various unverified sources) CMG Archives http://campbellmgold.com IMPORTANT The health information
Topic 3: Nutrition, Photosynthesis, and Respiration
 1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
O 2. What is anaerobic digestion?
 What is anaerobic digestion? Microbial degradation of organic material under anaerobic conditions Ubiquitous, naturally-occurring process Occurs in swamps, hydric soils, landfills, digestive tracks of
What is anaerobic digestion? Microbial degradation of organic material under anaerobic conditions Ubiquitous, naturally-occurring process Occurs in swamps, hydric soils, landfills, digestive tracks of
Solid Phase Peptide Synthesis
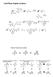 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.
![- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells. - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.](/thumbs/40/21014327.jpg) Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Determination of Specific Nutrients in Various Foods. Abstract. Humans need to consume food compounds such as carbohydrates, proteins, fats,
 Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Get It Right. Answers. Chapter 1: The Science of Life. A biologist studies all living things.
 Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Copyright 2010 Pearson Education, Inc. Chapter Twenty Three 1
 23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
H.W. 1 Bio 101 Prof. Fournier
 H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
Biological Molecules
 Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
AP Biology. What do animals need to live? Animal Nutrition. Nutritional requirements. How do animals get their food? Different diets; different lives
 Animal Nutrition What do animals need to live? Animals make energy using: food food oxygen Animals build bodies using: food for raw materials amino acids, sugars, fats, nucleotides O 2 ATP energy for synthesis
Animal Nutrition What do animals need to live? Animals make energy using: food food oxygen Animals build bodies using: food for raw materials amino acids, sugars, fats, nucleotides O 2 ATP energy for synthesis
T F T F in childr T F
 T F T F in childr T F 108 Plant-derived energy nutrients 3 mon. 123 124. 110 Glucose is the preferred source of energy for the brain. What As noted earlier (in Chapter 1), carbohydrates are one of the
T F T F in childr T F 108 Plant-derived energy nutrients 3 mon. 123 124. 110 Glucose is the preferred source of energy for the brain. What As noted earlier (in Chapter 1), carbohydrates are one of the
The Excretory and Digestive Systems
 The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
Chapter 2: Cell Structure and Function pg. 70-107
 UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
Cells & Cell Organelles
 Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Working With Enzymes. a world of learning. Introduction. How Enzymes Work. Types and Sources of Enzymes
 Working With Enzymes a world of learning Presented by Peter J Ball, Southern Biological. For further information, please contact the author by phone (03) 9877-4597 or by email peterjball@southernbiological.com.
Working With Enzymes a world of learning Presented by Peter J Ball, Southern Biological. For further information, please contact the author by phone (03) 9877-4597 or by email peterjball@southernbiological.com.
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Helices From Readily in Biological Structures
 The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Digestive system Review
 Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
