Absorption properties of β-sn nanocrystals in SiO 2
|
|
|
- Darren Chase
- 7 years ago
- Views:
Transcription
1 Absorption properties of β-sn nanocrystals in SiO 2 Jonas Günsel Winter 2010 i
2 ii This thesis has been submitted to the faculty of science at Aarhus University to fulfill the requirements for obtaining a PhD degree. The work has been carried out under supervision of professor Brian Bech Nielsen at the department of Physics and Astronomy and the Interdisciplinary Nanoscience Center (inano).
3 List of publications Jonas Günsel, Jacques Chevallier and Brian Bech Nielsen. Absorption enhancement by a layered structure compared to randomly distributed β-sn nanocrystals in SiO 2, in preparation for Physical Review B iii
4 List of Figures 1.1 β-sn unit cell Schematic representation of a RBS experiment Energy loss in RBS Depth resolution of RBS Principle of TEM operation As grown random Sn sample Schematic overview of the spectrophotometer Overview of the sputtering process Quartz wafer transmittance Multilayered and random Sn samples Formation of nanocrystals Homogeneous nucleation barrier TEM picture of Sn nanocrystals randomly distributed in SiO RBS spectrum of Sn randomly distributed in SiO TEM pictures of multilayered nanocrystals Diffraction from Sn nanocrystals Full and reduced Mie expression Absorption from bulk vs nanocrystals Garcia model for composite dielectric function Effect of the nanocrystal distribution on the refractive index Lorentz model for ɛ Dielectric function of a free electron metal Sn banddiagram Bulk vs nanocrystal iv
5 List of Figures 4.9 Sn dielectric functions Reflection from a surface Schematic overview of the matrix method Reflection from Quartz wafer and thin film Minimization procedure point by point simulation Refractive index of a quartz wafer Sn peak in RBS for RSn TEM and size distribution of RSn RBS spectrum of multi layered sample Size distribution of ML with different PV-TEM preparation techniques TEM of two multi layered samples Simulation structure for RSn samples BaSO 4 reflectance measurement BaSO 4 reflectance measurement RSn1 measurement and simulation n and κ for RSn σ nc for RSn1 compared to MG Mie vs MG for the RSn1 sample Comparison between absorption from RSn samples Comparison between transmittance from as grown and annealed samples Simulation structure for multi layered samples Refractive index of β-sn nanocrystals MLSn4 reflection and transmission MLSn4 reflection and transmission Comparison of σ a for RSn and ML samples MLSn2 compared to MG theory Absorption from multi layered samples Enhancement of absorption from nanocrystals in a nearby layer Enhancement of absorption from nanocrystals within a single layer Absorption enhancement relative to MG theory Model vs experimental absorption enhancement Absorption from single slab v
6 List of Figures 5.27 Comparison of σ a for MLSn samples from direct measurement and the thin film modeling procedure Multi layered samples considered as effective media Interlayer reflections Absorption comparison with previous work Absorption of SnO 2 vs Sn nanocrystals in SiO Absorption comparison with previous work A.1 Field from a layer of nanocrystals a distance z 0 away A.2 Field from a layer of nanocrystals A.3 Field from randomly distributed nanocrystals vi
7 Contents List of Figures Contents Acknowledgements List of abbreviations iv vii ix xi 1 Introduction 1 2 Characterization techniques Rutherford Backscattering Spectrometry RBS theory Extracting information from experiments Experimental details Transmission Electron Microscopy (TEM) Principle of operation Bright field imaging Diffraction mode High Resolution TEM Sample preparation Optical measurements Synthesis of Sn nanocrystals RF magnetron sputtering The sputtering chamber Wafer wedging Thin films composed of Sn and SiO vii
8 Contents Sn nanocrystals randomly distributed in SiO Sn nanocrystals in a layered structure Interactions between light and matter The Maxwell equations and electromagnetic waves EM waves in unbounded media Nanocrystals embedded in a host material Origin of the dielectric function The β-sn dielectric function The matrix method for determining reflection and transmission Simulation based determination of absorption Sn nanocrystals in SiO Introduction Experimental details Random Sn samples Sn in a multi layered structure Simulation based determination of absorption The correction factor for reflection measurements Refractive indices of the quartz wafer and SiO 2 layers β-sn absorption cross sections Comparison with previous studies Conclusion A A simple model for the impact on nanocrystal absorption from the surrounding nanocrystals 101 A.1 Nanocrystals in a different layer A.1.1 Electric field from a layer of nanocrystals A.2 Nanocrystals in the same layer A.3 Randomly distributed nanocrystals Bibliography 115 viii
9 Acknowledgements For more than 5 years I have been working in the semiconductor group at Aarhus University starting out doing a bachelor project before launching into the PhD study to be described in this thesis. I have met many challenges and obstacles during the process, which undoubtedly would have been very hard to overcome without the help and support from a lot of people. First and foremost I would like to thank my supervisor Brian Bech Nielsen for his help and guidance throughout this project. His great enthusiasm towards physics and his ability to focus on the interesting physical aspects of a given problem has many a time send me smiling from his office eager to investigate the matter further. Also his readiness to always take time to discuss the recent results despite of his very busy schedule has been much appreciated. Jacques Chevalier has prepared all the samples used in this project and has been very helpful in discussing their structural properties. His divine powers operating the TEM has been a big support and I am very grateful for his help throughout my time here. Also John Lundsgaard Hansen has provided invaluable help with the X-ray and RBS equipment which sometimes could seem to have a mind of its own. Pia Bomholt has prepared all the TEM samples during my study and furthermore served as a wonderful guide in all kinds of different tasks carried out in the chemistry lab. Her help with all this is much appreciated, just as her ability to create a pleasant working environment where conversations do not have to be related to physics. I also wish to thank Jesper Skov Jensen, Christian Uhrenfeldt and Amélie Têtu for introduction to and guidance in the experimental work in the lab. Christians detailed knowledge of obstacles and pitfalls in optical measurements has been very helpful and Amélie has helped me on numerous occasions with TEM, PL lab and where not. Also I appreciate her kindness to volunteer to proof read this thesis. Duncan Sutherland is much appreciated for letting me use his ix
10 Acknowledgements spectrophotometer for optical characterization and for always being helpful when I had questions regarding its use. I would like to thank all my friends and fellow students for creating an inspiring and pleasant environment and for making the past 8 years behind the yellow walls be about more than just physics. Finally I am grateful to my family for their support and interest and especially to Caroline Arnfeldt for her love and support that has kept me going during the long hours of this project. x
11 List of abbreviations Here is an alphabetically ordered list of abbreviations that will be used in the thesis. amu : Atomic mass unit BF : Bright Field CCD : Charge Coupled Device DF : Dark Field DFT : Density Functional Theory EDX : Energy Dispersive X-ray EM : Electromagnetic HR-TEM: High Resolution Transmission Electron Microscopy MBE : Molecular Beam Epitaxy MG : Maxwell-Garnett ML : Multi Layered NC : Nanocrystal PVD : Physical Vapor Deposition R : Reflectance RBS : Rutherford Backscattering Spectrometry RF : Radio Frequency SCCM : Standard Cubic Centimeter pr Minute Si : Silicon Sn : Tin T : Transmittance TEM : Transmission Electron Microscopy UV : Ultra Violet xi
12
13 Chapter 1 Introduction One of the greatest challenges of the 21st century is to secure the worlds energy need and preferably do it in an environmentally sustainable way. As the coal and oil reserves on the planet will eventually be exhausted the incentive to find renewable energy sources is right at hand, and with the current debate about global warming and greenhouse gases directly related to the fossil fuels, the interest in clean energy is bigger than ever. There are a number of different areas with the potential to provide clean and renewable energy such as wind power, waves in the sea, geothermal, and solar energy. Of these the solar energy has by far the greatest potential, as the world combined energy need is an insignificant fraction of the sunlight hitting the earth. For instance, it has been calculated that covering only 4% of the global desert area with solar panels is sufficient to account for the worlds electrical energy need [1]. On the bottom line the important parameter is the price to pay for the energy, and solar cells have yet to become competitive to the fossil fuels. Therefore either the efficiency of the solar cells has to be improved, the production cost reduced or a combination of the two strategies. This process is already ongoing for the silicon (Si) based solar cells where the first generation was based on thick wafers of crystalline Si. The second generation cells use thin films to reduce the fabrication price [2]. When using sufficiently thin films however the absorption efficiency is reduced due to the smaller distance travelled by the sunlight in the film. Methods, such as texturing of the backside of the thin Si layer [3, 4, 5, 6], that has been used to enhance the light path and thus the chance of absorption, has been shown to improve the efficiency to cost ratio of the solar cells. There is still a long way to go to reach efficiencies around 43%, 1
14 1. Introduction a a c Figure 1.1: β-sn unit cell. It is a tetragonal structure with a 4 atoms basis. which has been predicted as an upper limit for single junction solar cells [7]. One of the major issues to improve the efficiency is to use some of the energy lost to thermalization of hot electrons and holes. This has been adressed recently as multi exciton generation in silicon nanocrystals has been observed [8], but there are still some scepticism about whether this will in fact improve the solar cell efficiency [9]. Another method which has proven to enhance the efficiency of thin film Si solar cells exploit the surface plasmon resonance effect of metallic nanoparticles [10, 11, 12, 13, 14]. In this approach the excitation of a surface plasmon on a metal nanoparticle help to scatter the light into the Si layer and can thereby increase the efficiency of the solar cell significantly. The efficiency increase depends on the nanocrystal material, size, shape, and environment, and a lot of effort is being put into such studies in recent years, with the vast majority focusing on the noble metals silver or gold. Usually the nanocrystals are placed on top of the Si film in order to avoid the creation of metallic defect states in the band gap often seen for metals in Si [15]. As tin (Sn) belongs to the same group in the periodic table as Si a Sn atom can substitute for a Si atom without introducing unwanted electron states in the Si band gap which makes Sn a possible candidate for device fabrication. Therefore knowledge of the optical properties of Sn nanostructures is expected to be important for future photo-voltaic devices. Another area where metal nanocrystals are gaining a footing is in the shading area such as sunglasses, where a transparency in the visible spectrum along with UV absorptivity are desired [16] [17]. 2
15 Basically Sn comes in two allotropes at atmospheric conditions termed α- Sn and β-sn. The α form is a diamond structured semiconductor with at very small band gap of around 0.1 ev which is only stable at temperatures below 13.2 C [18]. The metallic β form has a body-centered tetragonal structure (figure 1.1) and is the preferred form at room temperature and above. As the transition temperature lies within the range of temperatures experienced in the earth s climate effects of the α β transition has been known for many years. The transition from metallic to semiconducting Sn has been termed the tin plague as it causes the Sn to become powdery and fall apart, as has been seen for church organ pipes and for Napoleons soldiers as they marched into the Russian winter in 1812 [19]. Even though the α β transition is heavily favored from a kinetic point of view [18, 20, 21] nanostructures of alpha Sn can be kept stable at temperatures significantly above the transition temperature by incorporating them into a diamond structured matrix of for instance Ge [22], Si [23] or CdTe [24, 25]. These structures are very interesting for optoelectronic devices due to their direct and tunable band gap. As β-sn is thermodynamically stable at room temperature nanocrystals in that form can be studied in a wide variety of materials, though for photovoltaic devices those based on silicon are of greatest interest. The aim of this project has been to investigate the optical properties of β-sn nanocrystals in a SiO 2 matrix. The oxide has been chosen as a host material because of its very high band gap which makes optical measurements across a wide range of wavelengths feasible. Furthermore SiO 2 is fully compatible with silicon based photo-voltaic device fabrication. It would be ideal to study the nanocrystals without a surrounding matrix, but if Sn gets in contact with oxygen it is immediately oxidized so it is necessary to keep the nanocrystals inside a host material. The thesis has been split into 5 chapters and an appendix. First a brief introduction to the most important experimental techniques used for structural and compositional characterization of the samples will be given. This is followed by a chapter devoted to the synthesis of nanocrystals which describes the technique used for thin film growth and some general features of the samples studied. After that there is a chapter dedicated to some of the basic theory on interaction between light and matter and the geometrical effects of the nanocrystals. Furthermore it gives a description of thin film interference effects and how to model such effects in order not to be mislead when 3
16 1. Introduction interpreting absorption spectra. In the fifth chapter the main findings of this work will be presented and a detailed description of a model used to describe absorption enhancement is given in the appendix. 4
17 Chapter 2 Characterization techniques In order to understand and model the optical effects of the composite systems investigated in this thesis it is of utmost importance to know the detailed structure of the samples under study. The Rutherford Backscattering Spectrometry (RBS) technique provides a depth resolved chemical composition of the sample and such quantitative information is very important in order to compare measured data with theoretical modeling. Another important sample parameter is the nanocrystal size which can be obtained using Transmission Electron Microscopy (TEM), a very powerful technique for visualizing various nanostructures. A brief discussion of these important techniques will be given in the following paragraphs followed by a description of the setup used for optical measurements. The RBS theory can be found in various textbooks and the following section is mainly based on [26]. 2.1 Rutherford Backscattering Spectrometry The RBS technique is based on the famous Geiger-Marsden experiment from the beginning of the 20th century, where the backwards scattering of He ions from a gold foil led scientist to abandon the plum-pudding model for the atom. Basically positive He ions are accelerated to a kinetic energy of a few MeV and focused onto the sample surface. The ions penetrate into the sample and some of them will be scattered by the atomic nuclei. A multichannel detector collect the backscattered ions in a certain angle θ and determine their energy distribution. One very favorable aspect of RBS is the lack of 5
18 2. Characterization techniques Ion accelerator Detector Sample Θ He + ion Figure 2.1: Sketch of the experimental setup for RBS measurements. sample preparation needed to perform the experiment. The overall geometry of the RBS experiment is sketched in figure RBS theory As mentioned above, the theory behind RBS is based on elastic scattering of alpha particles by heavier atoms, the basis of which being the Coulomb repulsion between the nuclei. Therefore the energy lost in the scattering event can be derived classically by considering conservation of energy and momentum. For an ion of mass M i and initial energy E 0 scattered into an angle θ from an atom with mass M t the energy of the ion after collision is E 1 = E 0 M icosθ + Mt 2 M i 2sin2 θ M i + M t 2 KE 0, (2.1) where K is called the kinematic factor which, for an experiment where M i and θ are fixed, is seen solely to depend on the mass of the target nuclei. 6
19 2.1. Rutherford Backscattering Spectrometry x E in E scat E in E out E out t Figure 2.2: The different contributions to the energy loss of a He ion during a RBS experiment. Placing the detector in an angle close to 180 will give the maximum energy transfer between ion and target resulting in the optimum mass resolution of the experiment. For scattering by heavy nuclei, that is M i << M t, the scattering cross section can be approximated by ( Zi Z t e 2 ) 2 1 σ (θ, E i ) = 4E i sin 4 (θ/2), (2.2) where Z i (Z t ) is the atomic number of the ion (target) and e is the elementary charge. As implied by the Z 2 t behavior RBS has a much higher sensitivity for heavy atoms than light ones. So far only the scattering event in itself has been discussed, but there are other equally important energy losses in the experiment to be considered, as sketched in figure 2.2. Both before and after scattering by a sample nuclei the He ions travel through the sample where they lose energy from inelastic scattering by electrons and small angle scattering by nuclei. Since the energy lost from the lat- 7
20 2. Characterization techniques 8 ter type is orders of magnitude much smaller than the first one, it will not be taken into account. Although the inelastic ion-electron collisions are discrete in nature, the energy lost is sufficiently small that the ions energy loss passing through the sample can be considered as a continuous process as a function of distance. The energy loss pr unit distance is termed stopping power and is given by de i dx = 2πZ2 i e4 NZ t E i ( Mi m e ) ln 2m ev i, (2.3) I where m e is the electron mass, v i is the velocity of the He ion and N and I are the atomic density and ionization energy of the sample respectively. Thus on the inward trip of length t into the sample the He ion will lose E in = t 0 de i dx dx tde i, (2.4) dx in where the last part is an approximation where the stopping power is evaluated at an average between E in and the energy just before backscattering. This is a standard approximation used when studying thin films, where the low penetration depth makes it rather good. Since the energy loss depends on the sample electron density it is convenient to introduce the stopping cross section ɛ = 1 de N dx. (2.5) For a composite material the stopping cross section is a sum of the individual atomic stopping cross sections weighted by their relative amount in the sample, which is known as Bragg s rule. With this, and the fact that the same story goes for the outward path, the He ion emerges at the detector with an energy of E out (t) = K (E in tnɛ in ) t cosθ Nɛ out, (2.6) where the first part E scat = K(E in tnɛ in ) is the energy of the ion just after scattering, and the final part is the energy loss on its way back out. The energy difference of an ion scattered on the surface and one scattered in a depth t (by the same type of atom) is then ( E = Nt Kɛ in + 1 ) cosθ Nɛ out [S]Nt, (2.7)
21 2.1. Rutherford Backscattering Spectrometry where the stopping cross section factor [S] is introduced. The position of the peaks in the spectrum identifies the element and the width of the peak is now seen to be directly proportional to the depth of penetration t Extracting information from experiments The output of an RBS measurement is the yield of backscattered ions at an angle θ as a function of energy and in order to extract the composition and depth profile some computer modeling is necessary. One option is the RUMP [27] software package where a structure containing different layers with given compositions and thicknesses is entered and simulated to obtain a RBS spectrum. By comparing the simulated spectrum to the measurement one can adjust the composition and thickness of the layers in the modeled structure until it matches the measurement. RUMP has a database of atomic stopping cross sections and atomic densities it uses to calculate stopping powers of the user proposed layers exploiting Bragg s rule for composite layers. The accuracy of the final output is influenced both by measurement and simulation. In the measurement a certain amount of total charge is collected by the detector. The more charge the better signal to noise ratio is obtained but it comes at the expense of prolonged measurement time. On the other hand the simulations is based on tabulated atomic densities which may not be exactly the same as for the sputtered films investigated. With the values used in this study the accuracy of the chemical composition is expected to be around 10% [28] Experimental details All RBS measurements have been performed with a 5 MeV van de Graff accelerator supplying 2 MeV 4 He + ions incident on the sample at normal angle (the sample was tilted up to 2 during measurement to avoid channeling). A silicon solid state detector, with an energy resolution of about 40 kev placed at an angle of θ = 161 with respect to the incoming beam, as shown in figure 2.1, collected the backscattered alpha particles. The penetration depth of the alpha particles was significantly higher than the film thickness, so compositional information throughout the sample was available. Due to the high penetration depth the silicon substrate signal can be used to normalize the measurement to the simulation, as the substrate has a well described density. A 400 V 9
22 2. Characterization techniques a) b) Recoil energy [MeV] Figure 2.3: Sn peak in a RBS spectrum of a multi layered structure with 5 Sn layers separated by SiO 2 layers of 15nm (a) and 75nm (b) respectively. The layered structure is not resolved for the smallest distance between the layers. electron suppressor ensured reliable counting of the 20 µc charge directed at the sample. With the experimental settings used the depth resolution in RBS is about 40 nm. In order to resolve smaller features or to obtain a higher precision in the thickness estimate other techniques such as TEM are used. An example of the depth resolution in RBS measurements is shown in figure 2.3. Here the part of the RBS spectrum showing the Sn peak of two samples with alternating layers of Sn and SiO 2 is shown where the difference between the samples is the separation of the Sn layers. As the Sn layers come closer together they become unresolvable by RBS. 2.2 Transmission Electron Microscopy (TEM) As noted in the introduction TEM is an indispensable tool for size determination when working with nanocrystals both embedded in a solid host or in a solution. The huge advantage of the technique is the ability to actually see the structures in the sample without having to extract the information from elaborate simulation procedures. On the other hand the sample preparation necessary is time consuming and destructive in addition to potentially influencing the sample structure. A description of TEM can be found in a number of textbooks since the technique has been used for decades, but the following 10
23 2.2. Transmission Electron Microscopy (TEM) LaB 6 cathode High voltage acceleration Condenser lenses Condenser aperture Sample Objective aperture Objective lenses Projector lenses CCD camera / fluorescent screen Figure 2.4: Schematic overview of a Transmission Electron Microscope. part is mainly based on [29] Principle of operation In a transmission electron microscope electrons are emitted at a cathode and accelerated through a voltage difference of a few hundred kev. The electrons are focused through a set of magnetic lenses and apertures both before and after hitting a sample as sketched in figure 2.4. Finally the electrons are collected on a fluorescent screen or by a CCD camera. In general the technique does not differ much in concept from conventional optical microscopes, but the superiority of the electron microscope is the low electron de Broglie wavelength compared to visible light, which results in a significant enhancement in resolution. 11
24 2. Characterization techniques In this study a V = 200keV acceleration voltage was applied which corresponds to a wavelength of λ = 2m e ev h ( ) = nm. (2.8) 1 + ev 2m ec 2 Here h is Plank s constant, m e is the electron mass, c is the speed of light and e is the electron charge. The wavelength is seen to be orders of magnitude less than visible light, but the limiting factor in resolution turns out to be aberration in the lenses [30]. The point resolution of the Philips CM20 system used in this study is 2.7 Å which is sufficient for the systems studied. A pressure of 10 7 mbar is sustained in the column to avoid electron scattering and sample contamination. The microscope can be operated in a number of different modes which offer different kinds of information and some of those will be reviewed in the following paragraphs Bright field imaging The bright field (BF) imaging mode is the one in closest resemblance with an optical microscope. The image is made from the central spot of the electron beam, as all electrons scattered on their way through the sample have been removed by the objective aperture. In that way the picture will consist of bright and dark regions corresponding to areas of the sample where few or many electrons are scattered respectively. As the dominant mechanism for scattering is interactions with core electrons which increases with atomic number, higher atomic numbers will look increasingly dark in BF mode. In order to get the optimal contrast the apertures are set to remove as much of the scattered light as possible, both for amorphous and crystalline structures present in the sample Diffraction mode In diffraction mode the screen shows the diffraction pattern formed in the back focal plane of the objective lens and this mode is used to determine the crystallinity and crystal structure of the sample. When crystalline nanostructures are present in an amorphous environment the electron scattering will be most intense whenever the Bragg conditions in the nanocrystals are met. 12
25 2.2. Transmission Electron Microscopy (TEM) This happens when a lattice plane in the nanocrystal happens to align with the electron beam in such a way that elastically scattered electrons have a change in wave vector equal to the reciprocal lattice vector. For a single nanocrystal a pattern of bright spots will appear on the fluorescent screen and their distance from the center spot determine the plane spacing and thus the crystal structure. When looking at a large number of randomly oriented nanocrystals, as is present in the samples studied in this thesis, the diffraction pattern will instead become concentric circles but the plane distance is measured in the same way. In order to get rid of diffraction from the silicon substrate a selected-area aperture can be inserted and the beam is then focused only on a small spot. The diffraction rings are rarely extremely well defined when looking at nanocrystals due to the small size that limits the number of electron scattered and thereby the brightness of the diffraction spots. This would result in an inaccurate determination of the inter planar distance but since the atomic nature of the nanocrystals is often already known, the precision only needs to be good enough to discriminate between different crystal structures High Resolution TEM In High Resolution TEM (HR-TEM) the aperture used to block out the diffracted beam in BF mode is widened enough to include some of the diffracted electrons as well. This is done on a spot where no diffraction from the substrate is included. The interference between the direct and diffracted beam will produce a picture of the periodic charge distribution seen by the electrons (the lattice planes) superimposed on the BF image. The experimental conditions for doing HR-TEM is very demanding. The focus has to be perfect and the lenses have to be corrected for astigmatism for the interference effects to become visible, and in general these conditions are fairly hard to meet Sample preparation The samples used for TEM measurements has to be very thin in order for the majority of the electrons to pass through the specimen, that is 100 nm. The first step is a rough polishing of a small piece of sample until it is about 10 µm thick followed by ion milling with 5 kev Ar + ions, which sputters away material 1. Two different types of samples have been prepared; cross sectional 1 The sputtering process is explained in chapter 3 13
26 2. Characterization techniques SiO 2 SiO 2 Figure 2.5: Cross sectional TEM picture of an as grown sample of randomly distributed Sn in SiO 2. and planar view samples. When studying thin films a planar view sample is thinned in a direction perpendicular to the film as opposed to cross sectional where the thinning occurs parallel to the film. Thus cross sectional view provides depth resolution of the thin film whereas the planar view provides information about a thin layer of the film. The samples were coated with carbon after ion milling in order to prevent charging of the SiO 2 layers. The ion milling step potentially raises the temperature in the TEM sample enough for the Sn to form nano-clusters, as seen in figure 2.5. The formed clusters however, did not show any diffraction pattern, so they are considered to be amorphous. Whether the formation is in fact a result of the sample preparation or originate from the sputtering process is impossible to determine from the TEM pictures, but it is a thing to keep in mind when interpreting the pictures. 2.3 Optical measurements Transmission and reflection measurements on the samples prepared on quartz substrates were performed using a Shimadzu UV-3600 double beam spec- 14
27 2.3. Optical measurements trophotometer, which is sketched in figure 2.6. The measurements were performed in the wavelength range from nm ( ev). A deuterium lamp is used for wavelengths from nm whereas a halogen lamp covers the rest of the spectrum. From the lamp compartment the light is led into the main body of the spectrophotometer through the entrance window. After hitting the first grating a slit limits the beam divergence and after another grating the following slit is also equipped with a filter to remove higher order diffracted light. The (ideally) monochromatic beam is led through a chopper mirror which is alternating between reflecting the beam and letting it pass, which gives rise to two beams termed the sample beam and the reference beam. These are let through the exit windows into the sample compartment where an integrating sphere is installed. The walls of the sphere are coated with BaSO 4 white paint which is essentially 100% reflecting across a wide range of wavelengths. The detectors used are a photomultiplier tube and a PbS solid state detector placed in the top and bottom of the integrating sphere respectively. The reference beam enters the integrating sphere at an 8 angle such that the specular reflection from a sample placed on the opposite side of the sphere can be measured. In order to calculate the 100% reflection or transmission line compressed BaSO 4 white powder was used as a reference. Reflection data for such powders has been measured previously [31, 32], but as they are somewhat dependent on the exact nature and thickness of the paint, a measurement of its reflectance has been performed. The sensitivity of the spectrophotometer is ±0.003 absorbance and it can measure up to 6 absorbances. At high absorbances the measurement is very sensitive towards microscopic holes in the sample as the absorbance would level off at some value depending on how big the hole is compared to the beam profile. In this work the measured samples never have an absorbance much above 1 so microscopic holes will not have a big influence. In any case, each sample was measured at different spots and the spectra were seen to be in accordance for all samples. Besides the lamp change at 325 nm a grating and detector change occur at 900 nm, which often give rise to fluctuations in the spectra at these wavelengths. 15
28 2. Characterization techniques Slit Gratings Gratings Slit Halogen lamp = Mirror Slit with filter Entrance window D2 lamp Sample compartment Reference beam Integrating sphere Exit windows Sample beam Chopper mirror Figure 2.6: Schematic overview of the Shimadzu UV-3600 spectrophotometer used to perform transmission and reflection measurements. 16
29 Chapter 3 Synthesis of Sn nanocrystals In this work Radio Frequency (RF) magnetron sputtering has been the preferred technique for nanocrystal synthesis. This physical vapor deposition (PVD) technique was chosen due to its ability to grow thin amorphous layers with good uniformity and thickness controllability in a relatively short time [33]. The technique is furthermore better suited for large scale production compared to other thin film growth techniques such as molecular beam epitaxy [34]. The magnetron sputtering technique will be presented here and the description is mainly based on [29]. This will be followed by a description of the different types of samples produced in this work. 3.1 RF magnetron sputtering The basis of sputtering is the ability for high energy ions to knock off atoms or molecules from a target upon impact. The released atoms with the appropriate direction will condense on the substrate and form a film. An overview of the process is given in figure 3.1. Basically a vacuum chamber is flooded with an inert gas such as argon and the target is placed at a negative potential compared to the substrate. This will accelerate the positive Ar ions towards the target, ionizing additional Ar atoms on their way. Upon collision with the target the Ar atoms will knock out target atoms in different directions as well as secondary electrons. The electrons are accelerated towards the substrate and ionize more Ar atoms. This will result in a self sustaining ion plasma and due to the magnetic field generated by the magnet below the target, the 17
30 3. Synthesis of Sn nanocrystals Substrate Target atom Ar + Target material Target Magnet Figure 3.1: Schematic representation of the sputtering process used to grow thin films in this work. plasma will be located right above the target. The magnetic field causes the electrons to move in a helical trajectory which increases their path length towards the substrate and thus their probability of ionizing Ar atoms. In that way a lower Ar pressure is sufficient to sustain a plasma which facilitates higher sputtering rates, as both target atoms on their way to the substrate and Ar + ions moving in the opposite direction undergo less collisions with Ar atoms. Sputtering of insulating materials, such as SiO 2 used in this work, requires an alternating potential between substrate and target, otherwise the target surface will accumulate positive charge which eventually terminates the sputtering process. The alternating potential ensures that the target is hit by alternating periods of Ar + ions and electrons, and as the electrons are much easier to set in motion due to their lower mass, more electrons than Ar + ions will hit the target during a full cycle keeping the target at a negative potential The sputtering chamber The sputtering equipment used in this work was a homebuilt system with four separate targets, so each sample can consist of up to four different materials.
31 3.1. RF magnetron sputtering The targets were placed 70 mm from the substrate which was water cooled to a temperature of about 15 C during sputtering. To ensure the quality of the sputtered films the chamber was initially pumped down to a base pressure of 10 7 mbar. A 30 sccm flow of % pure Ar gas was let into the chamber and the Ar pressure during sputtering was kept fixed at mbar. By tuning the sputtering power and the deposition time the thickness of a given layer can be controlled. 20x20 mm pieces of both silicon and quartz were used as substrates. Those on silicon were used for RBS measurements and to prepare TEM samples whereas those on quartz were used for optical measurements. In order to deposit mixed layers of Sn and SiO 2 small pieces of Sn were put on top of a SiO 2 target covering a carefully calculated area in order to produce a desired atomic ratio in the film. The substrate is rotated at a speed of 2 rounds per minute during sputtering to ensure a homogeneous film growth Wafer wedging As will be discussed in a later chapter multiple reflections give rise to interference fringes in the transmission and reflection spectra in thin films, but such effects may also occur in thick non-absorbing samples, such as a quartz wafer. In figure 3.2 the transmittance of a 10 µm quartz wafer has been calculated (blue line) by the matrix method revealing a high frequency oscillation on top of the smooth transmission (red dashed line). Such oscillations would obscure the measurement, but can be removed by either measuring at a sufficiently low resolution or by polishing the wafers in a wedge shaped profile, as pointed out in [35]. In order for the oscillations to be removed the difference in height δh across the quartz wafer must satisfy δh >> λ 4n q (3.1) where n q is the quartz refractive index. After the quartz wafers were mechanically ground into a wedged shape they were thoroughly polished in order to get a smooth and clean surface. All samples for optical measurements were grown on wedge-shaped quartz wafers in order to prevent substrate oscillations from contaminating the optical measurements. 19
32 3. Synthesis of Sn nanocrystals 110 Transmittance [%] Wavelength [nm] Figure 3.2: Transmittance spectrum of a 10 µm thick quartz wafer calculated by the matrix method described in the following chapter (blue line). This is compared to a calculation on a wedge-shaped wafer of the same thickness (red dashed line). The quartz dielectric function is taken from [36]. 3.2 Thin films composed of Sn and SiO 2 Two different types of samples have been investigated in this work: one with Sn nanocrystals randomly distributed in SiO 2 and one with the Sn nanocrystals arranged in layers. The two types are sketched in figure 3.3, and will be explained in more details in the remainder of this chapter Sn nanocrystals randomly distributed in SiO 2 First of all the basic theory covering nanocrystal formation will be presented. Reference [37] has a very thorough description of the thermodynamics involved in cluster formation and serves as the basis for the following section. After that the heat treatment will be described and in the end some of the structural parameters of the samples will be discussed. Thermodynamically driven nucleation When Sn atoms are initially randomly distributed in SiO 2 a driving force is needed in order for them to aggregate and form nanocrystals. This driving force originates from the decrease in Gibbs free energy involved in crystallization process, which is sketched in figure 3.4. There are three different 20
33 3.2. Thin films composed of Sn and SiO 2 SiO 2 Sn nanocrystal (a) (b) Figure 3.3: Cross sectional schematic slice through a) Sn layers sandwiched between SiO 2 in a multilayered structure and b) randomly distributed Sn nanocrystals in SiO 2 Sn atom Sn nanocrystal G 0 G 0 + G Figure 3.4: Homogeneous nucleation of Sn nanocrystals in SiO 2. The nanocrystal will form if there is an overall decrease in energy, meaning G must be negative. contributions to the Gibbs free energy change in the formation of a cluster of volume V and surface area A as seen in equation 3.2. G = V G v + Aγ + V G s (3.2) The first term describes the decrease in free energy from atoms joining to form a cluster, the second term is the interface energy between the cluster and the matrix and the last term is the induced strain energy if the newly formed 21
34 3. Synthesis of Sn nanocrystals Surface term G 0 G * R * Volume and strain term R Figure 3.5: Barrier in homogeneous nucleation and the different thermodynamic factors causing it. The cluster has to pass the barrier in order to get the critical size necessary to start growing to become a nanocrystal. cluster does not fit perfectly into the host matrix. It should be noted that with the form of the surface free energy term γ introduced in the second term in equation 3.2 it is assumed to be isotropic, which is a valid assumption if the nanocrystals are spherical. In addition the concentration of Sn atoms in the neighborhood of the clusters is assumed to be unchanged by their formation. For spherical clusters this can be rewritten in terms of the nanocrystal radius R to G = 4π 3 R3 ( G v G s ) + 4πR 2 γ. (3.3) If the misfit strain energy i small ( G s < G v ) which is often the case in an amorphous matrix such as SiO 2, the behavior of G will look as sketched in figure 3.5. From this it is evident that a critical radius R exist, which defines the number of Sn atoms needed to cluster together in order to overcome the barrier where growth is thermodynamically favorable. By differentiation of eq. 3.3 the critical radius R and the barrier height G becomes 22
35 3.2. Thin films composed of Sn and SiO 2 R = 2γ ( G v G s ) (3.4) G = 16πγ 3 3 ( G v G s ) 2. (3.5) The energy needed to surmount the barrier can be supplied in form of a post growth heat treatment, thus nanocrystal formation is often said to be thermally activated. If the concentration of Sn atoms is C Sn the concentration of clusters reaching the critical size C at a given temperature T can be shown to be [37] C = C Sn e G k b T (3.6) where k b is the Boltzmann constant. Thus depending on the hight of the barrier heat treatment may be a necessity or just a means of speeding up the nanocrystal formation. This section has described how homogeneous nucleation occurs, but if there are impurities or other irregularities present in the SiO 2 the cluster formation may begin at specific sites. This would result in a lowering of the energy barrier G which would encourage cluster formation, without influencing the growth kinetics. Annealing procedure In the literature annealing temperatures between 400 C and 1100 C [38, 39, 40] have been used to form Sn nanocrystals in SiO 2 depending on the deposition method and annealing atmosphere. It was found in [40] that increasing the annealing temperature led to formation of larger nanocrystals, which was also seen in this work. This could seem in contradiction to the conclusions of the previous section, where higher temperature lowers the barrier for cluster formation which would indicate the formation of many small nanocrystals. The phenomenon which can be accredited for this is the Ostwald Ripening effect [41] which describes how larger nanocrystals grow at the expense of smaller ones. In this work an annealing temperature of 400 C in vacuum for 1 hour was chosen, as it turned out to be sufficient to produce nanocrystals. Furthermore Huang et al. [40] discovered that annealing at 400 C resulted in a more uniform distribution of nanocrystals than at higher annealing temperatures. Annealing was performed in a vacuum furnace at a pressure of P 23
36 3. Synthesis of Sn nanocrystals 20 nm Figure 3.6: BF-TEM picture of randomly distributed Sn nanocrystals in SiO 2. The crystallinity was determined by looking at the diffraction pattern. 24 < 10 4 mbar in order to avoid oxidation of the nanocrystals, which can occur when annealing in a N 2 atmosphere [42, 43, 44, 45]. In figure 3.6 a TEM picture of Sn nanocrystals randomly distributed in SiO 2 is shown. TEM diffraction analysis showed that the nanocrystals were in the β-sn phase as would be expected [40]. The Sn content in the films was measured by RBS before annealing and such a spectrum together with the RUMP simulation is shown in figure 3.7. The RBS spectrum has been normalized to the RUMP simulation in the part originating from the silicon wafer in order to be able to extract the areal density Ω for the thin film constituents. In that way the amount of Sn in the sample can be determined by integration of the area under the Sn peak. From the figure it is also clear that some Ar is present in the sample. This is an unavoidable side effect from the sputtering process that some of the Ar atoms get incorporated into the thin film. Most of the Ar is released from the samples during annealing, but due to the low annealing temperature a very small amount is still present. Annealing at higher temperatures would probably remove all of the Ar [46, 47] but in order to avoid complications with the film quality (to be described in the following section) the temperature was kept at 400 C. The remaining Ar was found to be less than 0.2 at% for samples annealed for 1 hour at 400 C. This low concentration is expected to
37 3.3. Sn nanocrystals in a layered structure O Measured Simulation Normalized Yield Si from wafer Si from film Ar Sn 5 0 Blow up of the Sn peak Recoil Energy [MeV] Figure 3.7: RBS spectrum of Sn randomly distributed in SiO 2 before annealing. The inset to the right is a blow up of the Sn peak in the spectrum. have minimal influence on the film parameters. By comparing the Sn peak in the spectrum with the RUMP simulation (the blow up in the right part of figure 3.7) one can see that the experimental curve is slightly higher than the simulation at the left part of the peak. This indicates that the Sn distribution is not completely homogeneous across the layer, as it is assumed in the simulation. It does not affect the fact that the nanocrystals are randomly distributed, but it will cause the filling fraction to vary in depth and it should be kept in mind when modeling such structures. 3.3 Sn nanocrystals in a layered structure Samples with multiple layers of Sn sandwiched between SiO 2 (see figure 3.3(a)) were produced and annealed under the same conditions as the random samples described above. This was done in order to investigate how the distribution of the nanocrystal affected their absorption. Ge nanocrystals have shown an enhanced absorption for an equivalent amount of material when placed in lay- 25
38 3. Synthesis of Sn nanocrystals 50 nm a) b) Number of observations Diameter [nm] c) Figure 3.8: Cross sectional (a) and plane view (b) TEM pictures of a sample with layers of nanocrystals. The size corresponding size distribution is shown in (c). ers compared to random distributions [48], and the effect of the layer distance was also an element of interest. The layers were produced by sputtering of individual targets of Sn and SiO 2 and the number of Sn layers were kept at 5 in order to limit the total film thickness to approximately 500 nm. Just as the randomly distributed Sn samples the multilayered structures were annealed in vacuum at 400 C for 1 hour in order to form nanocrystals. At this temperature the nanocrystals stay in the layered structure, and their mean diameter is 4-5 times the thickness of the sputtered Sn layer. Annealing at 700 C showed loss of the layer structure as nanocrystals migrated into the SiO 2 layers leaving voids in the structure and earlier heat treatments performed in an N 2 atmosphere showed cracked films when annealed at C. Therefore the annealing temperature was kept at 400 C which proved sufficient to form nanocrystals. TEM was used to find the nanocrystal size, crystal structure and the distance between neighboring layers whereas the total amount of Sn was measured by RBS. BF-TEM pictures of a multilayered structure is presented in figure 3.8 along with the corresponding size distribution and the diffraction from such a structure is seen in figure 3.9. The distance from the center spot to the diffraction rings is connected to 26
39 3.3. Sn nanocrystals in a layered structure (200) (101) (211) Figure 3.9: Diffraction pattern from a sample with layers of Sn nanocrystals. The ring like structure is a result of the random orientation of the nanocrystals and by looking carefully the innermost ring is actually two rings close together. The 3 most intense rings have been identified and labeled on the figure, as they are the ones most easily identified. the plane distance in the nanocrystals, so from pictures like this the crystal structure can be verified. All samples showed the nanocrystals to be in the β form. The physical process driving nanocrystal formation in the case of the layered structure can be considered to be the reduction of surface area between Sn an SiO 2 by converting a Sn layer into nanocrystals. If a layer of area A and thickness t is converted into n nanocrystals with a radius R the relative change in surface area is given by A = 2A 4πR2 n A total 2A (3.7) If all the Sn from the layer is converted into nanocrystals then 4πR 3 n/3 = ta (assuming the same Sn density in layer and nanocrystals) which will turn equation 3.7 into A = 1 3t A total 2R. (3.8) Thus if R > 1.5t one gets a positive number signifying a surface area reduction and ss mentioned above the diameter observed in TEM was 4-5 times the layer thickness. In this calculation strain energies have not been considered at all even though the increase in nanocrystal size compared to the Sn layer 27
40 3. Synthesis of Sn nanocrystals thickness could very well introduce a strain in the SiO 2 layers. Films with thicker Sn layers compared to the separating SiO 2 layers resulted in cracked and partially peeled off films when annealed at 400 C and higher, so strain effects may certainly play a role in such structures. The films presented in this work, however, all looked smooth and showed no sign of such strain related effects. 28
41 Chapter 4 Interactions between light and matter Before venturing into the area of optical interactions in composite systems some of the basic theory of the interaction between light and matter will be discussed. At first the Maxwell equations and the resulting fields in bulk media will be introduced followed by a discussion of the geometrical effects of nanocrystals embedded in a host material. After an overview of the dielectric function and its connection to the electronic states in the material, the matrix model for determining reflection and transmission through a multi layered structure will be presented. Finally there will be given a description of how the matrix method can be applied to extract information about the individual layers in such multi layered structures. 4.1 The Maxwell equations and electromagnetic waves The physical laws obeyed by an electromagnetic (EM) wave traveling in any medium are summarized in the Maxwell equations. These equations describe, in macroscopic terms, which electric and magnetic fields that are allowed to propagate given the electronic structure of the material, and are found in almost any textbook related to light-matter interactions. Assuming a nonmagnetic and current free material the Maxwell equations are given by [35] 29
42 4. Interactions between light and matter ( ɛ E) = ρ ɛ 0 (4.1) B = 0 (4.2) E = B t B = µ 0 ɛ 0 ( ( ɛ E) ) t (4.3) (4.4) where E is the electric field, B is the magnetic induction, ρ is the free charge density and ɛ 0 is the vacuum permittivity. These equations must be satisfied at any point and time in the material. The dielectric function ɛ, which is connected to the electronic structure of the material, describes the materials response to an electric field. For linear isotropic materials the polarization P caused by an electric field 1 is given by P = ɛ 0 ( ɛ 1) E (4.5) and the dielectric function can be written as ɛ = ɛ r + iɛ i. The form of the dielectric function depends on the crystal structure of the material in question. A cubic crystal lattice would result in an isotropic dielectric function and it can be represented by a scalar whereas for anisotropic materials ɛ has to be represented by a tensor. For materials with a tetragonal structure such as β-sn there are two crystallographic axes along which the dielectric function will differ. However when studying the optical responses of nanocrystals with a random crystallographic orientation the dielectric function can be described as an average of the components along each crystallographic axis EM waves in unbounded media Among the solutions to the Maxwell equations in a homogeneous unbounded medium the plane waves are probably those most commonly encountered due 1 For very large electric fields the polarization is no longer linearly related to the field, but these fields are mostly encountered when working with very intense beams such as lasers. For normal optical measurements equation 4.5 is obeyed. 30
43 4.1. The Maxwell equations and electromagnetic waves to their simple form and the fact that they form a complete set of functions 2. The E and B fields for a plane wave can be described by the following equations E( r, t) = E 0 e i( k r ωt) (4.6) B( r, t) = B 0 e i( k r ωt) (4.7) where E 0 and B 0 are mutual perpendicular amplitude vectors, each also perpendicular to the wave vector k. In order to satisfy the Maxwell equations k and the frequency ω must be related by k 2 = µ 0 ɛ 0 ɛω 2. (4.8) Introducing the speed of light in vacuum c = 1 ɛ0 µ 0 this reduces to k = ω ɛ. (4.9) c For bulk materials it is often convenient to introduce the refractive index Ñ = n + iκ = ɛ. The electric field of a homogeneous plane wave traveling in the z direction is then given by ( ) E(z, t) = E 0 e 2π κz i 2π nz ωt λ 0 e λ 0 (4.10) where the free space wavelength λ 0 = 2πc ω has been introduced. As shown in equation 4.10 the complex part of the refractive index can be associated with the field attenuation during propagation through a material. Since light intensity is what typically is measured experimentally the attenuation can be described in terms of the initial intensity I 0 and the intensity after traversing a length z through a given material I(z) through I(z) = I 0 e αz (4.11) where the attenuation is described by α. In bulk materials the dominant mechanism for attenuation is absorption in the material and for that reason α is known as the absorption coefficient. From equation 4.10 and 4.11, using the waves. 2 Such that all possible fields satisfying the Maxwell equations can be expanded in plane 31
44 4. Interactions between light and matter fact that intensity is proportional to the electric field squared, the absorption coefficient can be described by α = 4π λ 0 κ. (4.12) It is evident from equation 4.12 that the absorption coefficient is a pure material property related to its electronic structure since it depends only on κ. To get a quantity related to the individual atoms instead of a whole ensemble the atomic absorption cross section σ a is often used σ a = α ρ. (4.13) Here ρ is the material density, and the introduction of σ a provides a way to compare measurements on samples where the amount of absorbing material is important Nanocrystals embedded in a host material So far bulk materials have been considered and the endless repetition of unit cells have imposed little boundary conditions on the Maxwell equations. When dealing with composite materials there are interfaces between the constituents where the material symmetry is broken and boundary conditions must be applied in order to determine the electromagnetic fields. This applies to a nanocrystal embedded in a host medium and for spherical nanocrystals the problem is described in a theory developed in the early 1900 s and accredited to Gustav Mie [49]. The Mie theory, which is treated thoroughly in [35], describes how a plane wave is scattered and absorbed by a single spherical particle in a host material by expanding the wave in spherical Bessel functions and impose continuity of the electric and magnetic fields across the material interfaces. Although the complete analytical solution can be found, an approximation valid for small particles such as nanocrystals is often used. This approximation is imposed by expanding the Bessel functions in the parameter x, which is the nanocrystal circumference divided by the wavelength in the surrounding medium x = 2πRn h λ 0, (4.14)
45 4.2. Nanocrystals embedded in a host material where n h is the real part of the host refractive index 3 and R is the nanocrystal radius. Keeping only terms up to x 4 in the expansion the extinction cross section becomes [35] { m σ ext = πr 2 2 ( 1 4xIm [1 m 2 + x2 m 2 ) 1 m m 2 ]} m m { (m + πr ) 2 } 1 3 x4 Re m where the nanocrystal dielectric function relative to the host dielectric function has been replaced by m 2 = ɛnc ɛ h for clarity. If m x << 1 this can be further reduced and the scattering and absorption cross sections can be identified as σ scat = 128π5 R 6 n 4 h ɛ nc ɛ h 2 3λ 4 0 ɛ nc + 2 ɛ h (4.15) σ abs = 8π2 R 3 { } n h ɛnc ɛ h Im. λ 0 ɛ nc + 2 ɛ h (4.16) Reformulated in words the assumption that m x << 1 can be phrased like the dimension of the nanocrystals is much smaller than the wavelength inside it. Thus the field across the nanocrystal is homogeneous at a given time which is called the electrostatic approximation. Equations 4.15 and 4.16 can be shown to be identical to the scattering and absorption cross sections obtained from an oscillating dipole [35] so the nanocrystals can be viewed as small dipoles in a host material. In figure 4.1 the Mie absorption cross section from the full Mie expansion is compared to equation 4.16 for two different sizes of Sn nanocrystals in SiO 2, and it is clear that for sufficiently large nanocrystals equation 4.16 is no longer valid. It has been verified that m x < 0.2 for the sizes and wavelengths use in this work so the formulas for σ scat and σ abs given above can be used with sufficient accuracy. It can be noted how the scattering cross section in equation 4.15 is proportional to x 4 whereas the absorption cross section is proportional to x. Thus for small nanocrystals the absorption will dominate the extinction. From figure 4.2 it is clear that the Sn absorption depends heavily on the geometry and dielectric surroundings. Here the atomic absorption cross section 3 As the host material used in this work is SiO 2 the refractive index is purely real in the wavelength range studied. 33
46 4. Interactions between light and matter Full Mie Reduced Mie σ nc [arb. unit] Full Mie 800 Reduced Mie Wavelength [nm] Figure 4.1: Nanocrystal absorption cross section for Sn nanocrystals in SiO 2 with a diameter of 5 nm (top) and 50 nm (bottom). The red curve labeled Reduced Mie is calculated from equation 4.16 wheres the black Full Mie curve is calculated using the MiePlot software available at and based on the computer code presented in [35]. Dielectric functions for Sn and SiO 2 respectively are taken from [50] and [36]. of bulk Sn found from equation 4.13 is compared to Sn nanocrystals embedded in a SiO 2 host or in a vacuum where the dielectric function from Sn and SiO 2 respectively has been taken from [50] and [36]. As the absorption cross section from the Mie expression in equation 4.16 is for a nanocrystal it has been converted to an atomic cross section by dividing with the number of atoms pr nanocrystal 4 ρvnc, where Vnc is the nanocrystal volume. The shape of the bulk absorption differ substantially from the spherical nanocrystals both in SiO 2 and in vacuum which in turn are mostly distinct regarding the absorp- 4 The density of Sn in nanocrystals is assumed to be the bulk density, which is reasonable since they share the bulk crystal structure. On another note, the comparison in figure 4.2 is only possible for arbitrarily sized nanocrystals because the Mie absorption cross section is directly proportional to the nanocrystal volume, which cancels out from the equation when converting to atomic absorption cross section. 34
47 4.2. Nanocrystals embedded in a host material σ a [cm 2 ] 1 x Bulk Sn in vacuum Sn in SiO Wavelength [nm] Figure 4.2: Atomic absorption cross sections from bulk Sn compared to Sn nanocrystals surrounded by either vacuum or SiO 2. The origin of the dielectric functions used is explained in the text. tion onset. The most pronounced effect going from bulk to nanocrystal is the appearance of a peak in the spectrum. This is best seen for the Sn in SiO 2 around 230 nm and is a result of the ( ɛ nc + 2 ɛ host ) denominator in equation When this approaches 0 the absorption increases rapidly, a phenomenon known as a Mie plasmon [51]. The position of the absorption peak can be varied by choosing a host material where ( ɛ nc + 2 ɛ host ) 0 is satisfied at a different wavelength. The Mie theory accurately describe a single particle embedded in an infinite host matrix, but in practice measurements will often be conducted on a large number of nanocrystals, so interactions between those has to be taken into account. This has been done in the Maxwell-Garnet (MG) theory [52], which describe a random distribution of spherical particles in a host medium using an average dielectric function for the composite medium given by ( 3f ɛnc ɛ h ɛ ave = ɛ h f ɛnc ɛ. (4.17) h ɛ nc+2 ɛ h ɛ nc+2 ɛ h ) Here ɛ h is the host dielectric function and f = V nc ρ nc is the filling factor or volume fraction occupied by the nanocrystals. From equation 4.13 and 35
48 4. Interactions between light and matter 4.12, which can be reformulated in terms of ɛ i in place of κ to α = 2π λ 0 ɛ i n, the nanocrystal absorption cross section in MG theory is given by 36 σ nc = α ρ nc = 2πV nc λ 0 n ave f Im{ ɛ ave} (4.18) where n ave = Re{ ɛ ave } is the real part of the refractive index of the composite layer. Assuming that the host dielectric function has no complex part in the wavelength range of interest (which is true for SiO 2 used in this work) this can be reduced to σ nc = 6πV nc λ 0 n ave Im { ɛ h ɛ nc ɛ h ɛ nc+2 ɛ h 1 f ɛnc ɛ h ɛ nc+2 ɛ h }. (4.19) In the MG theory the spherical inclusions are, as in Mie theory, assumed to be dipoles and the interaction between them arises from mutual polarization fields. Thus the MG theory will only be accurate up to a certain filling factor above which initially neglected multipole effects will become important. For a filling factor below f = the simple expression in equation 4.17 is sufficiently accurate [53, 54, 55]. The MG formula assumes the nanocrystal size to be much smaller than the wavelength in the same way as described above for the Mie theory, and by expanding equation 4.17 in the volume fraction f keeping only the leading term, the Mie result from equation 4.16 emerges. For a low filling factor the nanocrystals will be far apart and interactions between them will be negligible which support that the two theories should be related. For nanocrystals arranged in a layered structure the ordering of the nanocrystals need to be taken into consideration. This has been done for a single layer by Toudert et al. [56] who used ellipsometric measurements in conjunction with thin film modeling to extract the dielectric function from a layer containing nanocrystals and relate it to their morphology. For more layers, a method is described in [57] to account for the spatial arrangement of nanocrystals. This method, originally proposed by Garcia et al. [58, 59], is essentially an extension of the MG theory to account for the non-random distribution of the nanocrystals. Here the local electric field experienced by a nanocrystal in
49 4.2. Nanocrystals embedded in a host material R L i r ij j Figure 4.3: Imaginary sphere in a plane of nanocrystals used for calculation of the electric field exerted on particle i in the center of the sphere. the point i, E loc i, is the sum of the external field and the field from all other nanocrystals (treated as dipoles) E i loc = E ext + E i nc. (4.20) The field from the nanocrystals can be calculated by setting up an imaginary sphere, as seen in figure 4.3, (it is usually termed the Lorentz sphere) and adding the contributions from the nanocrystals inside (E in i ) and outside (E out i ) the sphere E i nc = E i in + E i out. (4.21) The size of the sphere has to be large enough to be representative of the nanocrystal distribution in a layer. By considering an external electric field directed along the x-axis the author in [57] derive an expression for the effective dielectric function describing the composite medium given by ɛ ave = ɛ m (1 + ) f( ɛ nc ɛ m ). (4.22) ɛ m + S( ɛ nc ɛ m )) Here f is the filling fraction previously introduced and S = L f 3 fk 4π is a factor dependent on the structural and geometrical arrangement of the nanocrystals. It has been assumed that the nanocrystals are similar in respect to dielectric function, volume, shape and surroundings. L is the depolarization factor which equals 1/3 for spherical clusters and K is given by 37
50 4. Interactions between light and matter MG Positive K Negative K 0.3 κ ave Wavelength [nm] Figure 4.4: The average refractive index κ ave of spherical Sn nanocrystals in SiO 2 calculated by the MG formula (equation 4.17) compared to the modified MG expression (equation 4.22) with a positive and negative value for K respectively. As the absorption cross section is directly proportional to κ this demonstrates how a distribution giving rise to a positive value of K will shift the absorption towards higher wavelengths compared to the MG case. K = j [ 3x 2 ij r 5 ij ] 1 p xj rij 3 P. (4.23) and describes the dependence on the spatial arrangement of the nanocrystals. The summation is over the nanocrystals with x ij being the x component of the position vector r ij between them. p xj is the x component of the dipole moment and P is the polarization density. If K=0 equation 4.22 reduces to the MG expression in equation 4.17, so K is related to how much the distribution deviates from random. Figure 4.4 shows how the imaginary part of the refractive index for Sn nanocrystals in SiO 2 changes with the sign of K. Although the derivation above was made for randomly distributed nanocrystals it was shown in [57] that this approach could adequately describe the response from samples with silver nanocrystals placed in parallel layers Origin of the dielectric function In the beginning of this chapter when the Maxwell equations were introduced, it was noted that the influence of the medium was contained solely in the dielectric function (assuming non-magnetic media). It seems now only appro- 38
51 4.2. Nanocrystals embedded in a host material priate to introduce the dielectric function in a more thorough way. As already mentioned the refractive index and the dielectric function are related quantities linked to the same physical properties and since these relations are widely used throughout this thesis they will be summarized here. ɛ = Ñ 2 = (n + iκ) 2 ɛ r = n 2 κ 2 ɛ i = 2nκ (4.24) For historical reasons n and κ are often called optical constants, which can look rather puzzling since they are connected to the dielectric function. As this thesis is probably not going to change the terminology on this matter it will just be noted that both the dielectric function and refractive index are (often strongly) dependent on the wavelength. In the following sections the classical and quantum mechanical origins of the dielectric function are described. As the classical formulation contains some intuitive physics this approach is still useful as an introduction to the quantum mechanical description. Classical formulation of the dielectric function The contributions to the classical description of the dielectric function were largely made by Lorentz and Drude who addressed different aspects of the electronic structure of a solid. Lorentz considered the force attaching the electrons in an atom to the nucleus to be like small springs, which would then describe the bound electrons in a metal. The free electrons on the other hand can be described by the Drude model, which is basically a special case of the Lorentz spring model. If we assume that an electron is attached to the nucleus with a spring like force and is acted upon by a periodically varying field such as a plane wave described in equation 4.10 the equation of motion for a small displacement r is [60] m d2 r dt 2 + mγd r dt + K r = e E (4.25) where m and e are the mass and charge of the electron, Γ is the damping coefficient necessary for dissipating energy from the system and K = mω 2 0 is the restoring force. A number of assumptions have been done here. First 39
52 4. Interactions between light and matter of all it is assumed that the electron interacts negligibly with the magnetic field of the incident light wave which is justifiable as the interaction is given by e v B/c and the speed of the electron is much smaller than the speed of light c. Secondly the mass of the nucleus is assumed to be infinite compared to the electron. This could be sorted out by using the reduced mass instead, but as the purpose of this section is mainly to give some qualitative insight the use of the electron mass will suffice. For the same reason the electric field responsible for the displacement is taken to be the incident plane wave even though it should be the local field experienced by the electron. Finally interactions between the electron under consideration and others are neglected too. As the time variation of the electric field is e iωt (see equation 4.6) the solution to equation (4.25) becomes r = e E m(ω 2 0 ω2 iγω) (4.26) where the natural frequency of the oscillator ω 0 has been substituted for the restoring force K. The movement of the electron with respect to the nucleus will induce a dipole moment p proportional to the displacement r given by p = e r = e 2 E m(ω 2 0 ω2 iγω) = α(ω) E (4.27) where α(ω) is the polarizability of the one electron atom. The total contribution for N of such oscillators pr unit volume becomes P = Nα(ω) E. (4.28) Using equation (4.5) the resulting dielectric function becomes ɛ = 1 + Nα(ω) ɛ 0 Ne 2 = 1 + mɛ 0 (ω0 2 ω2 iγω) (4.29) which can be separated into a real and a complex part. Before doing so the theory is usually extended to bulk structure by allowing for more than one spring constant since electrons are bound with different strength. If the 40
53 4.2. Nanocrystals embedded in a host material 10 8 ε r ε i ω Figure 4.5: Real and imaginary part of the dielectric function from the Lorentz oscillator model. The imaginary part which is connected to absorption in the material is seen to peak at the natural frequency of the oscillators ω 0 ω density of oscillators with the natural frequency ω j is N j the dielectric function becomes [60] ɛ r = 1 + e2 N j (ωj 2 ω2 ) mɛ 0 (ωj 2 ω2 ) 2 + Γ 2 ω 2 (4.30) ɛ i = j e2 N j Γω mɛ 0 (ωj 2 ω2 ) 2 + Γ 2 ω 2 (4.31) j Equations 4.30 and 4.31 are then the classical contribution to the dielectric function from electrons bound the the nucleus. The behavior of the ɛ r and ɛ i are sketched for a single oscillator with natural frequenzy ω 0 in figure 4.5. In the beginning of this section the damping coefficient Γ was introduced as a mean for energy to dissipate from the system. For bulk materials this happens primarily by absorption and from equation (4.31) this is seen to be associated with the imaginary part of the dielectric function. The absorption is strongest at the natural frequency of the oscillator, ω 0, as seen in figure 4.5. The contribution to ɛ from free electrons in a metal follow directly from the Lorentz model. As the electrons are not attached to any nucleus ω 0 = 0 and equations 4.30 and 4.31 turn into ɛ r = 1 Ne2 mɛ 0 1 ω 2 + Γ 2 (4.32) 41
54 4. Interactions between light and matter 5 ω p 0 ε r, ε i -5 ε r ε i Figure 4.6: Real and imaginary contribution to the dielectric function from free electrons in the classical model. ω ɛ i = Ne2 Γ mɛ 0 ω(ω 2 + Γ 2 ). (4.33) It should be noted that Γ here is still a damping coefficient, but it is related to the free electron scattering responsible for classical resistivity, and is as such related to another mechanism than for the bound electrons. Therefore in its place it makes sense to introduce the lifetime τ describing the mean time between an electron undergoes a scattering event. As τ = 1/Γ [60] the above equations turn into ɛ r = 1 ω2 pτ ω 2 τ 2 (4.34) ɛ i = ω 2 pτ ω(1 + ω 2 τ 2 ) (4.35) where the plasma frequency ω p = Ne2 mɛ 0 has been introduced. These equations are known as the Drude model for free electron metals and the behavior of the real and complex part of the dielectric function is sketched in figure 4.6. The total dielectric function is the sum of the free and bound electron contributions, but it is not always possible to distinguish the two. By comparing figure 4.5 and 4.6 it can be noted that for low frequencies ɛ r for the Lorentz 42
55 4.2. Nanocrystals embedded in a host material 10 5 ev K Z Figure 4.7: β-sn band structure taken from [50]. Interband and intraband transitions corresponding to bound and free electrons respectively are sketched with a red and blue arrow. The dashed line is the Fermi energy which is placed at 0 ev in the figure. model approaches a constant, whereas the free electron Drude contribution approaches so the behavior in that range can be expected to be governed by the free electrons. This can be understood by looking at the band diagram 5 for Sn in figure 4.7. The free electron contribution to the dielectric function describe intraband transitions, meaning that the electron is excited into a vacant state within the same energy band, as shown with a blue arrow in the figure, whereas the interband transitions shown in red are contained in the bound electron oscillator model. For low energies bound electrons cannot jump to another band so the behavior is expected to be free electron like. Quantum theory of the dielectric function The quantum mechanical approach rely on the interaction between the applied field and the multi electron wave function describing an atom. To perform a 5 The concept of energy bands is borrowed from quantum mechanics. 43
56 4. Interactions between light and matter complete quantum mechanical treatment the electromagnetic waves should be quantized into photons, but as this approach become unnecessarily complicated for the purpose here (see for instance [61]), a simpler semi-classical approach is given. Here the field is treated classically while the electrons are described by their quantum mechanical wave functions. By treating the interaction between the field and an electron as a small perturbation on the electron states, the quantum mechanical analog to the Lorentz model describing interband transitions can be derived [62]. For convenience the electromagnetic field is described by a vector potential A given by A = 1 ( ) 2 A 0ˆr e i( k r ωt) + e i( k r ωt) (4.36) where ˆr is a unit polarization vector and the constant A 0 can be chosen such that A 0 = E(ω) ω. This way the vector potential is related to the electric field by E = A t. (4.37) The two exponentials in equation 4.36 describe absorption and stimulated emission respectively, and since the absorption is the interesting part here 6 only the first part is considered in the following. The perturbation to the electronic Hamiltonian describing an electron in an external field in the weak field regime becomes [62] H pert = e m A p (4.38) where p is the momentum operator for the electron. Applying first order perturbation theory results in the Fermi golden rule for the transition probability W if for an electron between an initial state ψ i ( r) and a final state ψ f ( r) via interaction with the field [60] 44 W if = 2π ψ f H pert ψ i 2 δ(e f E i ω). (4.39) Here the delta function is the condition for energy conservation, and the matrix element 6 It could be assumed that the atom is initially in its ground state, making stimulated emission impossible.
57 4.2. Nanocrystals embedded in a host material ψ f H pert ψ i 2 = e2 m 2 ψ f A p ψi 2 (4.40) describes the transition amplitude between states. If the electron states are described by Bloch functions with u k being a lattice periodic function ψ k ( r) = u k ( r)e i k r (4.41) and the dipole approximation is invoked, the transition probability becomes [62] W if = 2π ( e ) 2 E(ω) 2 P if 2 δ(e f ( 2mω k) E i ( k) ω) (4.42) k where P if 2 = u f ˆr p u i 2 has been inserted. This is the probability for a vertical band to band transition in a crystal as the summation is over all the filled electron states in the valence band. By considering the continuity equation for energy lost from absorption in a unit volume of the crystal the imaginary part of the dielectric function is readily obtained 7 [62] ɛ i (ω) = πe2 m 2 ω 2 ɛ 0 k P if 2 δ(e f ( k) E i ( k) ω). (4.43) The real and imaginary parts of ɛ are related via the Kramers-Kronig relations given by [60] ɛ r 1 = 2 π P V 0 ɛ i = 2ω π P V 0 ω ɛ i (ω ) (ω ) 2 ω 2 dω (4.44) ɛ r (ω ) 1 (ω ) 2 ω 2 dω (4.45) where P V denotes the principal value of the following integral. Using equation 4.44 the real part of the dielectric function turn out to be ɛ r (ω) = 1 + e2 2 2 P if 1 mɛ 0 m ω if ω if 2 (4.46) ω2 k 7 The summation is now over allowed k vectors per unit volume of the crystal. 45
58 4. Interactions between light and matter 46 where ω if = E f ( k) E i ( k) has been inserted. The current form of equation 4.46 has been chosen to emphasize the resemblance with the classical Lorentz oscillator model. If Γ is assumed to be zero, equation 4.30 reduces to ɛ r = 1 + e2 mɛ 0 j ω 2 j N j ω2. (4.47) The quantity f if = 2 P if 2 m ω if, which is referred to as the oscillator strength of an optical transition, can be interpreted as the number of oscillators with the frequency ω if and is seen to be the quantum mechanical analog to the density of oscillators in the Lorentz model. The contribution from the free electrons to the dielectric function via intraband transitions can also be calculated quantum mechanically. This is done for instance in [60] where the results for a free electron gas is generalized to a solid by the use of Bloch wave functions and in the dipole approximation the real part of the dielectric function is given by ɛ intra r = 1 e2 2 ω 2 ɛ 0 k,l F (E k,l ) 2 E k,l k 2 (4.48) where l is the band index and F is the Fermi function. This is seen to be identical to the Drude result in equation 4.32 when a monovalent metal is considered 8. Introducing the reduced mass of the electron 1 m = 1 2 E and 2 k 2 the average value of the Fermi function across a Brillouin zone (= 1 2 ) and carrying out the summation over k points in a Brillouin zone results in ɛ intra r = 1 Ne2 m ɛ 0 ω 2 (4.49) which, besides from the damping factor, is identical to equation Equations for the imaginary part of ɛ can of course be derived as well, but the comparison to the classical counterparts is not easily done. Often a single expression encompassing both the real and imaginary parts are used, such as for instance in [50] where the total dielectric function is written as absent. ɛ(ω) = e2 2 ɛ 0 m 2 i,f [f(e i ) f(e f )] P if 2 E if (E 2 if 2 ω 2 ) (4.50) 8 In this discussion lifetime broadening has been ignored so the damping coefficient Γ is
59 4.2. Nanocrystals embedded in a host material Unit cell Bulk Nanocrystal Figure 4.8: Difference between bulk matter and nanocrystals in terms of the number of unit cells. The small number of unit cells present in a nanocrystal change the electronic structure compared to bulk. for each crystallographic axis. By introducing the Bloch functions the dielectric function becomes a bulk parameter, as the Bloch functions describe the symmetry across a large number of unit cells. Therefore using the bulk dielectric function can be misleading when studying sufficiently small nanocrystals, as the translational symmetry described by the Bloch functions is not present. The difference between bulk and a nanocrystal in terms of the number of unit cells is sketched in figure 4.8. Using bulk dielectric functions, either measured or calculated, to describe optical properties for structures on the nanometer scale could thus very well be misleading and the results should at the very least be given proper consideration The β-sn dielectric function As the following section will explain how modeling based on layers with a known dielectric function can be used to extract the absorption coefficient from a thin film sample, a brief description of the β-sn dielectric function will be given here. There have been several papers aimed at finding the Sn dielectric function [63] [64] [65] [66] including more recent experimental [67] and theoretical studies [50]. The data for the Sn dielectric function from Takeuchi [67] and Pedersen [50] are compared in figure 4.9. As Takeuchi only measures the component along the c axis (see figuer 1.1) of the β-sn unit cell, this is the one shown in figure The two are seen to be in fair agreement only for wavelengths 9 In the theoretical calculations by Pedersen the components along both axis of the unit 47
60 4. Interactions between light and matter ε r 60 ε i Pedersen Takeuchi Wavelength [nm] 20 Pedersen Takeuchi Figure 4.9: Comparison between real (left) and imaginary (right) part of the β-sn dielectric function from two recent studies by Pedersen [50] and Takeuchi [67]. The intraband contribution in [50] has been added by using the values for plasma frequencies and damping coefficient given in the article. It should be noted that it is only the part of the dielectric function parallel to the c axis of the β-sn unit cell that is shown here. below 400 nm, which may reflect their different origin. Takeuchi uses spectroscopic ellipsometry on a 25 nm β-sn film at room temperature together with some thin film modeling to extract a bulk dielectric function. Pedersen on the other hand uses DFT band structure calculations to get the real and imaginary parts of the dielectric function for both crystallographic directions. The calculated data are then found to agree well with previous measurements at -200 C performed in [65] and the components does not show significant differences between the two crystallographic directions. Therefore the dielectric function found by Takeuchi [67] is considered to be representative for the total Sn dielectric function, which for randomly oriented nanocrystals will be given by an average of the crystallographic directions. As Takeuchi extract his dielectric function from room-temperature measurements they can be expected to be somewhat different from the low temperature calculations of Pedersen, such as seen in [65]. As optical measurements in this work have been conducted at room temperature the dielectric function given by Takeuchi can be expected to best fit the conditions. cell are given. 48
61 4.3. The matrix method for determining reflection and transmission n 1 n 2 n 1 n 2 Θ 1 Θ 2 Θ 1 Θ 2 d a) b) Figure 4.10: Reflection contributions from a) a thick absorbing slab and b) thin weakly absorbing film. Multiple reflections contribute to the total reflection in the latter. 4.3 The matrix method for determining reflection and transmission Whenever light is incident on an interface a part of it will be reflected and a part of it will be transmitted and continue to travel through the material. This situation is sketched in figure 4.10a. For a thick absorbing slab all the transmitted part will be absorbed and the reflection from such a slab would simply be given by the Fresnel equation for the front interface, which for a plane wave incident on a plane interface looks like R = ( ) ±n1 cos(θ 1 ) n 2 cos(θ 2 ) 2 (4.51) n 1 cos(θ 1 ) + n 2 cos(θ 2 ) where the ± depends on the polarization. For light incident normal to the surface θ 1 = θ 2 the cosines and the distinction between polarizations disappear, and equation 4.51 reduces to ( ) n1 n 2 2 R =. (4.52) n 1 + n 2 The reflection from such a surface is easily deduced from the refractive indexes of the involved materials and similar equations exist for the transmission. If the slab is not completely absorbing a part the transmitted light 49
62 4. Interactions between light and matter will be reflected from the back side of the slab and contribute to the total reflection. If the slab is sufficiently thin or weakly absorbing the light may be reflected multiple times, as shown in figure 4.10b. When looking at thin films this will very often be the case and the different reflection contributions may interact and create interference effects in the reflection and transmission measurements. For thin films containing nanocrystals it is thus important to be able to separate the effects of such interferences from those related to the nanocrystal properties as pointed out in [68]. One way to address the effect of multiple reflections is simply by adding all the contributions for each layer. The simplest example is a single slab of material as in figure 4.10b, where the successive contributions to the final reflection 10 have traversed the slab an increasing number of times before attenuated enough to be negligible. Assuming normal incidence and that the slab has an absorption coefficient α the sum of these contributions can be written as a geometric series [69] R slab = R 12 + T 12R 21 T 21 e 2αd 1 R 12 R 21 e 2αd (4.53) where T is the transmittance across an interface. As R 12 = R 21 from equation 4.52 and the same applies to the transmission equation 4.53 is rather simple. For more than one layer the summation approach becomes increasingly inconvenient as reflections across layers need to be taken into account and it gets difficult to keep track of all the different contributions. Another way to treat the multiple reflections is by the matrix formalism described in [48] and [70] 11. This method keeps track of the different transmission and reflection contributions across all boundaries by itself and is thus much easier to work with for a large number of layers. The matrix method can be shown to be equivalent to the summation method described above [71] and is chosen for the simulations performed in this work. Figure 4.11 shows a schematic example of a sample consisting of N layers where the i th layer is described by a refractive index Ñi = n i + iκ i. In each layer the electric field can be divided into parts traveling left and right and a prime will be used to distinguish between the field in each end of the layers. 10 Transmission could be considered equivalently. 11 The two citations create the basis for the following part. 50
63 4.3. The matrix method for determining reflection and transmission 1... i j... N E li E' li E lj E' lj d i d j E ri E' ri E rj E' rj L i H ij L j Figure 4.11: Overview of the notation used in the matrix method. The different operators are explained in the text. The notation for the field at the left and right side of layer i, ( E i ) and ( E i ) are ( ) E E i = li E ri ) E (E i = li E ri If we limit the discussion to incident light normal to the interfaces the Fresnel coefficients for transmission and reflection from the ij th interface is given by τ ij = 2Ñi Ñ i + Ñj (4.54) r ij = Ñi Ñj. (4.55) Ñ i + Ñj Each of the fields in figure 4.11 can be considered as a sum of two contributions. For instance E li is the transmitted part of E lj across the ij interface plus the reflected part of E ri from the same interface. In that way all the fields are connected and the exercise is to describe the link between the initial field incident on the first interface and the field exiting in the N th layer. This can be accomplished by applying the symmetry relations of the Fresnel coefficients 51
64 4. Interactions between light and matter which follow directly from equations 4.54 and 4.55 to the connected fields just described. r ij = r ji τ ij = 1 + r ij τ ij τ ji + (r ij ) 2 = 1 In that way the correlation between fields across a layer can be found. Introducing the interface transition matrix [ H ij = 1 τ ij 1 r ij r ij 1 the relation between fields across a boundary can be described by ] E i = H ij E j. (4.56) In the same way a propagation matrix relating the fields across a single layer can be defined as resulting in the field relation [ L i = 1 τ ij e iβ i 0 0 e iβ i ] (4.57) β i is given by E i = L i E i. (4.58) 52 β i = 2πÑid i λ 0 where d i is the layer thickness and λ 0 is the vacuum wavelength. From equations 4.56 and 4.58 it is evident that the field in one layer can be related to the field in another layer. The relation between layer 1 and the N th layer is given by E 1 = H 12L 2 H 23 L 3...H N 2,N 1 L N 1 H N 1,N EN,
65 4.3. The matrix method for determining reflection and transmission which is more conveniently written as [ E 1 = S 11 S 12 S 21 S 22 ] E N = S E N. (4.59) S is the stack matrix and contains all the contributions to the total reflectance and transmittance for all the layers (often called a stack). The boundary conditions for layer 1 and layer N ( E 1 = E r E i ) ( ) 0 E N = can be inserted in equation 4.59 to isolate the reflection and transmission coefficient for the entire stack. These state that in layer N there is only a field component moving away from the stack, which is the transmitted portion E t, in contrary to layer 1 where there are both the incident E i and reflected field E r. This results in the following equations for the transmission and reflection coefficient respectively E t r stack = E r E i = S 12 S 22 τ stack = E t E i = 1 S 22. By definition the reflectance (R) and transmittance (T ) then become R = r stack 2 (4.60) T = n N n 1 τ stack 2. (4.61) In most cases the sample is surrounded by air, which means n N = n 1 = 1 and the refractive indexes in equation 4.61 can be disregarded. With the procedure outlined here it is possible to calculate the reflectance and transmittance for an arbitrary number of layers where multiple reflections in and between the layers are fully accounted for. It can be done analytically, but 53
66 4. Interactions between light and matter for more than a few layers the equations become terribly cumbersome making computer assistance crucial. In order to calculate the reflectance (R) and transmittance (T ) at a given wavelength the complex refractive index Ñ = n + iκ along with the thickness of each layer comprising the stack need to be known. On the other hand, if R and T can be measured the matrix method can be used to find the refractive index of a given layer. Since the thickness of any given layer can be determined from BF-TEM pictures the only two unknown parameters are the real and imaginary part of its refractive index. With independent measurements of R and T it should be possible to deduce n and κ for a layer by comparing measurement with simulation, for instance, by varying the refractive index input to the simulation until measurement and simulation are in agreement. This procedure works better on paper than in practice, since not all possible combinations of n and κ can be examined. In order to find values that are likely to be close to the actual ones, an iterative process with a carefully chosen start point has been applied. The process is described in more detail in the following section Simulation based determination of absorption Extracting the absorption coefficient for a given sample from a measurement of the transmitted part of a beam of light has been performed for many years. If the transmission can be measured without any reflection taking place across a certain range of wavelengths experimentalists have often used the absorbance A defined by A = log(t ) = log ( It I i ) (4.62) where I i and I t are the incident and transmitted intensity. If the thickness of the absorbing material is d and using equation 4.11 equation 4.62 becomes A = αd log(e) (4.63) and the absorption coefficient is readily acquired by measuring the transmission. Since a reflection free measurement of the transmission is not always possible this method has its limitations. If the reflection is sufficiently constant across the measured wavelength range the correct spectral dependence
67 4.4. Simulation based determination of absorption 15 Quartz wafer Quartz wafer + thin film Reflectance [%] Wavelength [nm] Figure 4.12: Measured reflectance spectra of a 0.4 mm thick wedge shaped quartz wafer and a similar wafer with a 500 nm thick SiO 2 thin film on top. Whereas the pure quartz wafer has an almost constant reflection the thin film shows substantial interference effects. The feature at 900 nm is a result of a grating change in the instrument. for the absorption coefficient is found. The reason these simple equations have found wide applications throughout the scientific world is the simplicity of the experiment required. Simply take a beam of light and measure how much the intensity is reduced upon introducing the sample in the light path. For many purposes the reflection is not sufficiently constant (see figure 4.12) for equation 4.62 to be useful and other means of extracting the absorption coefficient must be used. Especially for thin films the interference effects become important and if they are not properly taken into account it can result in misinterpretation of absorption properties, as pointed out in [72]. Therefore a multitude of ways to remove interference and retain the correct absorption coefficient have been developed. In [73], a method for determining n and α as a function of wavelength for an amorphous silicon film using the interference fringes in the transmission spectrum, is developed. This procedure however, seem useful only for a homogeneous film with a very uniform thickness. Others [68] [74] use both T and R to compute 1 R T, which for certain sufficiently absorbing films removes the interference and allows determination of the absorption coefficient. Often computer simulation based methods aid in extracting the parameters of interest. One method is to remove the interference fringes from the transmission measurement as in [48] or alternatively 55
68 4. Interactions between light and matter 56 do as in [68] where the authors simulate R and compare it to the experimental curve to check their values for α. As the methods described failed to produce an interference free absorption coefficient for the samples used in this work an iterative process based on the matrix method was used instead. In the previous section it was pointed out that the only unknown parameters for determining the reflection and transmission of a multi layered film is the real and imaginary part of the refractive index (or equivalently the dielectric function) for the layers 12. By constructing a stack of layers with composition and distances determined from RBS and TEM measurements the resulting reflectance and transmittance can be calculated by the matrix method for a suitable input of the refractive indexes. The calculated values are then compared to the measured ones and the refractive index values are optimized to ensure a good agreement. This is done separately for the real and imaginary part by use of the fminsearch function in MATLAB. The fminsearch function is based on the Nelder-Mead algorithm [75] [76] which is a direct search method for minimizing a given function. The function to minimize is given by F = (R m R s ) 2 + (T m T s ) 2 (4.64) where m and s refer to measured and simulated respectively. Thus by minimizing the function F the simulated R and T will approach the measured ones. The minimization is performed with respect to n and κ for the layers containing Sn and for all layer thicknesses involved, d i. The function requires an initial parameter guess for the optimization algorithm to start from and some care must be taken concerning this choice. As the Nelder-Mead algorithm performs a direct search for the minimum it can potentially end up in local minima if such exist closer to the start point than the global minimum. This situation is sketched on figure Therefore the start point for the optimization should be chosen close to the expected value to increase the chance for the optimization algorithm to end up in the global minimum. The optimization of n, κ and d i were performed individually and as one parameter had been optimized it was held fixed during optimization of the other two. In this way the optimization iteratively approaches a set of values that are consistent with the experimentally measured R and T. 12 Their thicknesses can be determined by TEM, but as will be explained later this point will also be considered in the simulations.
69 4.4. Simulation based determination of absorption F x 2 x 1 x 0 x min x Figure 4.13: Schematic illustration of the importance of selecting a good starting point for the optimization process with a arbitrary parameter x. The function value F is described in equation 4.64, but the function showed here is just a random function. Starting out in the point x 0 will result in the Nelder-Mead algorithm finding the correct minimum x min, but on the other hand, starting in x 1 will lead to the local minimum in x 2 instead. It turned out that κ could be precisely determined on a wavelength to wavelength basis for all the samples studied resulting in nice smooth curves. For some of the samples this was the case for n as well, but for others the simulations jumped abruptly at some wavelength and did not return to the expected value immediately after. An example of this behavior is seen in figure 4.14(a). At wavelengths around 220 nm and 310 nm the otherwise nicely continuous values from the optimization procedure change abruptly. The reason behind this can be seen in the parts (b) and (c) of the same figure, showing simulations of the transmittance and reflectance from a stack of thin film layers where n has been varied with ±10%. At the wavelengths mentioned R and T are almost identical despite the large variation in n, which is why minimizing equation 4.64 with respect to n fails to produce a valid output at these wavelengths. This unsmooth behavior was deemed unphysical as the refractive index is expected to be smooth in the wavelength interval studied. To overcome this problem n was fit as a polynomial across the full wavelength range for those samples in question. In figure 4.15 the optimization of n for a quartz wafer performed both on a wavelength to wavelength basis and with a polynomial 57
70 4. Interactions between light and matter Optimized Input a T [%] R [%] n n n + 10% n - 10% 80 n n + 10% 60 n -10% Wavelength [nm] b c Figure 4.14: a) Real part of the refractive index resulting from the minimization of equation 4.64 for a stack of thin film layers (black line) compared to the input guess (red dashed line). The resulting reflectance (b) and transmittance (c) computed for the stack with n varied within 10%. 58
71 4.4. Simulation based determination of absorption Pointwise Polynomial n Wavelength [nm] Figure 4.15: Refractive index from a quartz wafer obtained from minimizing equation 4.64 from measured R and T spectra by the procedure outlined in this section. The comparison is made between the results of a minimization where n is found on a wavelength by wavelength basis and using the polynomial description from equation fit. The two are seen to be in excellent agreement. n(λ) = A + B(λ λ 0 ) + C(λ λ 0 ) 2 + D(λ λ 0 ) 3...O((λ λ 0 ) N ) (4.65) The polynomial is given by equation 4.65 with λ 0 chosen in the center of the wavelength interval and the minimization in equation 4.64 is then performed with respect to the coefficients A,B,C etc. The order of the polynomial has to be chosen high enough to retrieve the characteristics of n, but not too high as the computational time would be too long and the result would be dominated be the high order terms at high and low wavelength. Typically a polynomial of order 8-10 has been chosen as they fit the purpose well. 59
72
73 Chapter 5 Sn nanocrystals in SiO 2 This chapter presents the main results of the investigations of β-sn nanocrystals in SiO 2 in order to gain insight into their optical properties. The size of the nanocrystals was chosen small enough that scattering could be disregarded in the optical extinction leading to the absorption cross section as the main physical parameter of interest. The absorption cross section was determined from the measured reflectance (R) and transmittance (T ) spectra together with structural information from TEM and RBS characterization by the simulation based method described in chapter 4. The absorption cross sections are then compared to Mie and MG theory with the use of bulk dielectric functions and to previous experimental work. It is also discussed how care should be taken when applying effective medium theories to layered structures. 5.1 Introduction During the past 15 years the interest in structures on the nanometer scale has exploded [77] and today there are few research areas which are not in some way exploiting the opportunities of nanotechnology. With the current technology providing the opportunity to tailor materials to acquire various desirable properties there are a vast number of technological applications in sight. The computer industry has been interested in semiconductor nanocrystals for a long time due to their potential use in optoelectronic devices [78] [79]. The quantum confinement effect [80] relaxes the requirement of momentum conservation in optical transitions and nanocrystals of semiconductor materi- 61
74 5. Sn nanocrystals in SiO 2 62 als with an indirect band gap becomes optically active. Nanocrystals of the semiconducting form of Sn, α-sn, has been extensively studied in recent years due to their direct tunable band gap [22, 23, 81, 82, 83] but their usefulness is somewhat limited by the precautions needed to stabilize the α-sn. Metallic nano-structures have been attracting interest due to their potential use in single electron memory devices [84, 85] and the majority of studies into β-sn nanocrystals are related to that aspect [86, 87]. In recent years the potential for metallic nano particles to improve solar cell efficiency has led to a more pronounced focus on optical properties [10, 88]. A wide variety of techniques have been used to fabricate Sn nanocrystals in a host material including ion implantation [38, 39, 42, 86, 89], molecular beam epitaxy (MBE) [22, 23, 90], evaporation condensation [91, 92] and sputtering [40, 93, 94]. As explained earlier the sputtering technique has been chosen in this work since the grown films need not be crystalline and it is very suitable to produce amorphous layers with well defined thicknesses. Previous work investigating the optical absorption properties of β-sn nanocrystals in SiO 2 have been done by Huang et al. [40] and Kjeldsen et al. [94]. Huang et al. produce β-sn nanocrystals randomly distributed in SiO 2 by co-sputtering of individual Sn and SiO 2 targets and subsequent vacuum annealing. In the optical measurements they look for band to band absorption in order to identify quantum confinement effects for the Sn nanocrystals. As they see absorption in the UV range they ascribe that to oxidized Sn clusters that they identify in their samples as their β-sn nanocrystals are considered to big to show confinement effects and thus absorption in the UV range. They do not consider the absorption to be related to the composite medium, as described in section 4.2, but entirely as related to the individual tin oxide nanocrystals or Sn related defects. They report their absorption as the percentage of absorbed light instead of absorption cross section and they do not make a quantitative comparison of the absorption to Mie or MG theory. Kjeldsen et al. [94] produce a single layer of β-sn nanocrystals in SiO 2 by the sputtering technique and subsequent annealing in a N 2 atmosphere at 450 C for 30 minutes. The size of the nanocrystals is varied by increasing the thickness of the sputtered Sn layer and they report a slight redshift in the position of the extinction peak as the nanocrystal size is increased. Again there is no comparison to Mie or MG theory for the extinction properties. In this work the first experimental atomic absorption cross section for
75 5.2. Experimental details Sample Mixed layer thickness (nm) Surrounding SiO 2 layer thickness (nm) Sn content (at%) Sn Areal density Ω (1/cm 2 ) Mean diameter (nm) Filling fraction f RSn1 72±5 220± RSn2 295±15 105± Table 5.1: TEM and RBS parameters for the two RSn samples. Mixed layer refers to the layer containing both Sn and SiO 2. No nanocrystals were identified in BF-TEM for the RSn2 sample and no diffraction pattern was seen either, thus no nanocrystal size is given for this sample. β-sn nanocrystals in SiO 2 is reported. This is done both for nanocrystals randomly distributed throughout SiO 2 and for nanocrystals arranged in a layered structure and the absorption cross section is compared to Mie and MG theory. It is shown that the absorption increases when the nanocrystals are arranged in layers and a simple model is developed in order to understand this increase. 5.2 Experimental details As described in chapter 3 two types of samples have been investigated. The Sn nanocrystals have been formed either randomly distributed throughout an amorphous SiO 2 (a-sio 2 ) layer or in layers separated by different thickness of a-sio 2. The samples with randomly distributed nanocrystals will be labeled RSn whereas MLSn will be used to denote those with layers of nanocrystals Random Sn samples Two different samples with Sn randomly distributed in a-sio 2 were fabricated as sketched in figure 3.3b. Both had a total film thickness of 500 nm but the thickness of the Sn containing layer was varied and consequently also the thicknesses of the surrounding oxide layers. Various structural details for the random Sn samples are summarized in table 5.1. TEM measurements did not show any nanocrystals in the RSn2 63
76 5. Sn nanocrystals in SiO 2 Sn peak in RBS spectrum Yield Measured Simulated Recoil energy [MeV] Figure 5.1: Part of the RBS spectrum of the RSn1 sample showing the Sn peak. From the very good agreement between the measured curve and the one simulated by the RUMP software [27] the Sn atoms are expected to be evenly distributed throughout the layer. sample in BF mode and in diffraction mode no diffraction pattern was observed. A doubling of the annealing time compared to the RSn1 sample was attempted, but still no nanocrystals were identified. For the RSn1 sample the amount of Sn was determined from the RBS spectrum, a part of which is shown in figure 5.1. From the agreement between the measurement and simulation of the Sn peak it is concluded that the Sn is evenly distributed throughout the layer. The Sn content in the RSn2 sample was also determined by RBS and the areal density was very close to that of the RSn1 sample. Therefore it is concluded that the Sn content is present either in nanometer scale structures too small to be seen in TEM or possibly as randomly scattered Sn atoms throughout the sample. For the other RSn1 sample the individual nanocrystals were visible in TEM and a BF picture of this sample together with its size distribution is given in figure 5.2. The average diameter is 5.0±0.1 nm. The Sn nanocrystals was confirmed to by in the β phase by TEM diffraction. There was no signs 64
77 5.2. Experimental details 10 nm Number of observations Diameter [nm] a) b) Figure 5.2: a) BF-TEM image of randomly distributed Sn nanocrystals in SiO 2 for the RSn1 sample. b) The corresponding size distribution for RSn1 where the average diameter is 5.0±0.1 nm. of crystalline tin oxide diffraction seen in either TEM or X-ray diffraction measurements. Based on Energy Dispersive X-ray (EDX) studies in [86] it was concluded that the oxygen stay on the SiO 2 after heat treatment in a Sn implanted SiO 2 layer. As the Si=O double bond is stronger than the Sn=O bond thermodynamics also expect the oxygen to stay attached to the Si atoms [95]. It could be speculated that oxygen could diffuse from the air during storage and oxidize the Sn atoms but this has been shown in [94] to be unlikely. SiO 2 has also previously proved to be an efficient diffusion barrier [96, 97]. The relatively long annealing time of 1 hour should give all Sn atoms enough time to diffuse to a growing nanocrystal and therefore it is expected that the majority of the Sn is in the form of β-sn nanocrystals Sn in a multi layered structure A series of multi layered samples with approximately the same Sn content but with a different distance between the layers was also produced. The structural parameters of the multi layered samples based on TEM and RBS measurements are summarized in table 5.2. The RBS spectra were compared to RUMP [27] simulations, as explained in chapter 3, such that the Sn areal density could be extracted. For the samples with a Sn layer separation less than 45 nm the layer structure was 65
78 5. Sn nanocrystals in SiO 2 Sample Separating SiO 2 layer thickness (nm) Surrounding SiO 2 layer thickness (nm) Sn Areal density Ω (1/cm 2 ) Mean NC diameter (nm) Standard deviation (nm) MLSn1 3± ± MLSn2 11±2 224± MLSn3 24±4 181± MLSn4 42±3 158± MLSn5 54±4 135± MLSn6 68±5 90± Table 5.2: Structural data for the multi layered samples. The Sn areal density and atomic content are found from RBS whereas the nanocrystal diameter and layer thicknesses are measured by TEM. 66 not resolved by RBS and they could be fitted with a mixed Sn and SiO 2 layer as for the RSn samples. For the samples with a larger Sn layer separation the layers were resolved, as shown for sample MLSn6 in figure 5.3. In this the RUMP simulation is based on a structure of alternating layers of Sn and SiO 2 and whereas the layer structure in the Sn peak is clearly resolvable, the simulation does not agree fully with the measurement. This agreement can be improved considerably by expanding the thickness of the Sn layers a bit and include some SiO 2 in their composition. This is can be justified by comparison with TEM pictures, where the Sn is seen to form clusters already in the as grown samples. If this cluster formation occurs during sputtering the following SiO 2 layer will cover the grooves, and the layer can best be described as a mixed layer of Sn and SiO 2. In this way the TEM and RBS measurements are in accordance. The Sn clusters observed in as grown TEM pictures, as mentioned in section 2.2.5, thus appear to be a result of the sputtering process and not of the ion milling step in the TEM sample preparation. To ensure that the ion milling step is not responsible for nanocrystal formation plane view TEM samples for the MLSn2 sample were prepared both by the ion milling procedure previously described and by etching in a hydrofluoric acid solution. Comparison between the size distributions from the two approaches are seen in figure 5.4 and did not suggest that the ion milling step influence
79 5.2. Experimental details Measured Simulated Recoil energy [MeV] Figure 5.3: RBS spectrum of MLSn6 as grown. The correspondence between measurement and simulation can be increased by expanding the thickness of the Sn layers and letting them be composed of both Sn and SiO 2. the nanocrystal size 1. TEM characterization provides a more precise estimate of different layer thicknesses in the ML samples compared to RBS and additionally the size distribution of the nanocrystals can be determined. The layer thicknesses are directly extracted from cross sectional TEM samples, whereas the size distribution are most easily derived from plane view pictures. There will always be a slight ambiguity in this approach since nanocrystals from more than one layer can be taken into account where ideally only a single layer should be considered. This effect should be more pronounced when the layers are close, but as the average size of the nanocrystals turn out to be very consistent throughout all the samples, as seen from table 5.2, the size distributions are considered to satisfactorily determined. In figure 5.5 cross sectional TEM pictures of the MLSn2 and MLSn4 samples are presented. The layer thicknesses are measured at different places and between the different layers in order to get the most precise values and an 1 A standard test for difference in mean diameter was performed based on the size distributions in figure 5.4 and the result was within the 95% confidence interval for equal diameter. Although the test assumes the diameters so be normally distributed, which can be debated grounded on the figure, it is concluded that the sample preparation does not influence the nanocrystal size. 67
80 5. Sn nanocrystals in SiO 2 Number of observations Diameter [nm] Number of observations Diameter [nm] Figure 5.4: Size distributions from the MLSn2 sample from PV-TEM samples prepared by chemical etching in a hydrofluoric acid solution (left) and by ion milling (right). The average sizes are 5.9±0.11 and 6.1±0.09 respectively. 50 nm 50 nm Figure 5.5: Cross sectional TEM picture of MLSn2 (left) and MLSn4 (right) showing Sn nanocrystals surrounded by SiO 2 layers of different thickness. 68
Vacuum Evaporation Recap
 Sputtering Vacuum Evaporation Recap Use high temperatures at high vacuum to evaporate (eject) atoms or molecules off a material surface. Use ballistic flow to transport them to a substrate and deposit.
Sputtering Vacuum Evaporation Recap Use high temperatures at high vacuum to evaporate (eject) atoms or molecules off a material surface. Use ballistic flow to transport them to a substrate and deposit.
Coating Technology: Evaporation Vs Sputtering
 Satisloh Italy S.r.l. Coating Technology: Evaporation Vs Sputtering Gianni Monaco, PhD R&D project manager, Satisloh Italy 04.04.2016 V1 The aim of this document is to provide basic technical information
Satisloh Italy S.r.l. Coating Technology: Evaporation Vs Sputtering Gianni Monaco, PhD R&D project manager, Satisloh Italy 04.04.2016 V1 The aim of this document is to provide basic technical information
Physics 441/2: Transmission Electron Microscope
 Physics 441/2: Transmission Electron Microscope Introduction In this experiment we will explore the use of transmission electron microscopy (TEM) to take us into the world of ultrasmall structures. This
Physics 441/2: Transmission Electron Microscope Introduction In this experiment we will explore the use of transmission electron microscopy (TEM) to take us into the world of ultrasmall structures. This
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS
 PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light
 AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light Name: Period: Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Reflection,
AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light Name: Period: Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Reflection,
Laboratory #3 Guide: Optical and Electrical Properties of Transparent Conductors -- September 23, 2014
 Laboratory #3 Guide: Optical and Electrical Properties of Transparent Conductors -- September 23, 2014 Introduction Following our previous lab exercises, you now have the skills and understanding to control
Laboratory #3 Guide: Optical and Electrical Properties of Transparent Conductors -- September 23, 2014 Introduction Following our previous lab exercises, you now have the skills and understanding to control
How To Understand Light And Color
 PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
Ion Beam Sputtering: Practical Applications to Electron Microscopy
 Ion Beam Sputtering: Practical Applications to Electron Microscopy Applications Laboratory Report Introduction Electron microscope specimens, both scanning (SEM) and transmission (TEM), often require a
Ion Beam Sputtering: Practical Applications to Electron Microscopy Applications Laboratory Report Introduction Electron microscope specimens, both scanning (SEM) and transmission (TEM), often require a
Lectures about XRF (X-Ray Fluorescence)
 1 / 38 Lectures about XRF (X-Ray Fluorescence) Advanced Physics Laboratory Laurea Magistrale in Fisica year 2013 - Camerino 2 / 38 X-ray Fluorescence XRF is an acronym for X-Ray Fluorescence. The XRF technique
1 / 38 Lectures about XRF (X-Ray Fluorescence) Advanced Physics Laboratory Laurea Magistrale in Fisica year 2013 - Camerino 2 / 38 X-ray Fluorescence XRF is an acronym for X-Ray Fluorescence. The XRF technique
Preface Light Microscopy X-ray Diffraction Methods
 Preface xi 1 Light Microscopy 1 1.1 Optical Principles 1 1.1.1 Image Formation 1 1.1.2 Resolution 3 1.1.3 Depth of Field 5 1.1.4 Aberrations 6 1.2 Instrumentation 8 1.2.1 Illumination System 9 1.2.2 Objective
Preface xi 1 Light Microscopy 1 1.1 Optical Principles 1 1.1.1 Image Formation 1 1.1.2 Resolution 3 1.1.3 Depth of Field 5 1.1.4 Aberrations 6 1.2 Instrumentation 8 1.2.1 Illumination System 9 1.2.2 Objective
NANO SILICON DOTS EMBEDDED SIO 2 /SIO 2 MULTILAYERS FOR PV HIGH EFFICIENCY APPLICATION
 NANO SILICON DOTS EMBEDDED SIO 2 /SIO 2 MULTILAYERS FOR PV HIGH EFFICIENCY APPLICATION Olivier Palais, Damien Barakel, David Maestre, Fabrice Gourbilleau and Marcel Pasquinelli 1 Outline Photovoltaic today
NANO SILICON DOTS EMBEDDED SIO 2 /SIO 2 MULTILAYERS FOR PV HIGH EFFICIENCY APPLICATION Olivier Palais, Damien Barakel, David Maestre, Fabrice Gourbilleau and Marcel Pasquinelli 1 Outline Photovoltaic today
View of ΣIGMA TM (Ref. 1)
 Overview of the FESEM system 1. Electron optical column 2. Specimen chamber 3. EDS detector [Electron Dispersive Spectroscopy] 4. Monitors 5. BSD (Back scatter detector) 6. Personal Computer 7. ON/STANDBY/OFF
Overview of the FESEM system 1. Electron optical column 2. Specimen chamber 3. EDS detector [Electron Dispersive Spectroscopy] 4. Monitors 5. BSD (Back scatter detector) 6. Personal Computer 7. ON/STANDBY/OFF
X-ray thin-film measurement techniques
 Technical articles X-ray thin-film measurement techniques II. Out-of-plane diffraction measurements Toru Mitsunaga* 1. Introduction A thin-film sample is two-dimensionally formed on the surface of a substrate,
Technical articles X-ray thin-film measurement techniques II. Out-of-plane diffraction measurements Toru Mitsunaga* 1. Introduction A thin-film sample is two-dimensionally formed on the surface of a substrate,
8.1 Radio Emission from Solar System objects
 8.1 Radio Emission from Solar System objects 8.1.1 Moon and Terrestrial planets At visible wavelengths all the emission seen from these objects is due to light reflected from the sun. However at radio
8.1 Radio Emission from Solar System objects 8.1.1 Moon and Terrestrial planets At visible wavelengths all the emission seen from these objects is due to light reflected from the sun. However at radio
Electron Microscopy 3. SEM. Image formation, detection, resolution, signal to noise ratio, interaction volume, contrasts
 Electron Microscopy 3. SEM Image formation, detection, resolution, signal to noise ratio, interaction volume, contrasts 3-1 SEM is easy! Just focus and shoot "Photo"!!! Please comment this picture... Any
Electron Microscopy 3. SEM Image formation, detection, resolution, signal to noise ratio, interaction volume, contrasts 3-1 SEM is easy! Just focus and shoot "Photo"!!! Please comment this picture... Any
X-ray diffraction techniques for thin films
 X-ray diffraction techniques for thin films Rigaku Corporation Application Laboratory Takayuki Konya 1 Today s contents (PM) Introduction X-ray diffraction method Out-of-Plane In-Plane Pole figure Reciprocal
X-ray diffraction techniques for thin films Rigaku Corporation Application Laboratory Takayuki Konya 1 Today s contents (PM) Introduction X-ray diffraction method Out-of-Plane In-Plane Pole figure Reciprocal
Raman spectroscopy Lecture
 Raman spectroscopy Lecture Licentiate course in measurement science and technology Spring 2008 10.04.2008 Antti Kivioja Contents - Introduction - What is Raman spectroscopy? - The theory of Raman spectroscopy
Raman spectroscopy Lecture Licentiate course in measurement science and technology Spring 2008 10.04.2008 Antti Kivioja Contents - Introduction - What is Raman spectroscopy? - The theory of Raman spectroscopy
E/M Experiment: Electrons in a Magnetic Field.
 E/M Experiment: Electrons in a Magnetic Field. PRE-LAB You will be doing this experiment before we cover the relevant material in class. But there are only two fundamental concepts that you need to understand.
E/M Experiment: Electrons in a Magnetic Field. PRE-LAB You will be doing this experiment before we cover the relevant material in class. But there are only two fundamental concepts that you need to understand.
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
Lecture 12. Physical Vapor Deposition: Evaporation and Sputtering Reading: Chapter 12. ECE 6450 - Dr. Alan Doolittle
 Lecture 12 Physical Vapor Deposition: Evaporation and Sputtering Reading: Chapter 12 Evaporation and Sputtering (Metalization) Evaporation For all devices, there is a need to go from semiconductor to metal.
Lecture 12 Physical Vapor Deposition: Evaporation and Sputtering Reading: Chapter 12 Evaporation and Sputtering (Metalization) Evaporation For all devices, there is a need to go from semiconductor to metal.
Indiana's Academic Standards 2010 ICP Indiana's Academic Standards 2016 ICP. map) that describe the relationship acceleration, velocity and distance.
 .1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
.1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
MCQ - ENERGY and CLIMATE
 1 MCQ - ENERGY and CLIMATE 1. The volume of a given mass of water at a temperature of T 1 is V 1. The volume increases to V 2 at temperature T 2. The coefficient of volume expansion of water may be calculated
1 MCQ - ENERGY and CLIMATE 1. The volume of a given mass of water at a temperature of T 1 is V 1. The volume increases to V 2 at temperature T 2. The coefficient of volume expansion of water may be calculated
KINETIC MOLECULAR THEORY OF MATTER
 KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
UV/VIS/IR SPECTROSCOPY ANALYSIS OF NANOPARTICLES
 UV/VIS/IR SPECTROSCOPY ANALYSIS OF NANOPARTICLES SEPTEMBER 2012, V 1.1 4878 RONSON CT STE K SAN DIEGO, CA 92111 858-565 - 4227 NANOCOMPOSIX.COM Note to the Reader: We at nanocomposix have published this
UV/VIS/IR SPECTROSCOPY ANALYSIS OF NANOPARTICLES SEPTEMBER 2012, V 1.1 4878 RONSON CT STE K SAN DIEGO, CA 92111 858-565 - 4227 NANOCOMPOSIX.COM Note to the Reader: We at nanocomposix have published this
Light as a Wave. The Nature of Light. EM Radiation Spectrum. EM Radiation Spectrum. Electromagnetic Radiation
 The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
Experiment #5: Qualitative Absorption Spectroscopy
 Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
PHYS 222 Spring 2012 Final Exam. Closed books, notes, etc. No electronic device except a calculator.
 PHYS 222 Spring 2012 Final Exam Closed books, notes, etc. No electronic device except a calculator. NAME: (all questions with equal weight) 1. If the distance between two point charges is tripled, the
PHYS 222 Spring 2012 Final Exam Closed books, notes, etc. No electronic device except a calculator. NAME: (all questions with equal weight) 1. If the distance between two point charges is tripled, the
Chapter 8. Low energy ion scattering study of Fe 4 N on Cu(100)
 Low energy ion scattering study of 4 on Cu(1) Chapter 8. Low energy ion scattering study of 4 on Cu(1) 8.1. Introduction For a better understanding of the reconstructed 4 surfaces one would like to know
Low energy ion scattering study of 4 on Cu(1) Chapter 8. Low energy ion scattering study of 4 on Cu(1) 8.1. Introduction For a better understanding of the reconstructed 4 surfaces one would like to know
ATOMIC SPECTRA. Apparatus: Optical spectrometer, spectral tubes, power supply, incandescent lamp, bottles of dyed water, elevating jack or block.
 1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
Secondary Ion Mass Spectrometry
 Secondary Ion Mass Spectrometry A PRACTICAL HANDBOOK FOR DEPTH PROFILING AND BULK IMPURITY ANALYSIS R. G. Wilson Hughes Research Laboratories Malibu, California F. A. Stevie AT&T Bell Laboratories Allentown,
Secondary Ion Mass Spectrometry A PRACTICAL HANDBOOK FOR DEPTH PROFILING AND BULK IMPURITY ANALYSIS R. G. Wilson Hughes Research Laboratories Malibu, California F. A. Stevie AT&T Bell Laboratories Allentown,
Coating Thickness and Composition Analysis by Micro-EDXRF
 Application Note: XRF Coating Thickness and Composition Analysis by Micro-EDXRF www.edax.com Coating Thickness and Composition Analysis by Micro-EDXRF Introduction: The use of coatings in the modern manufacturing
Application Note: XRF Coating Thickness and Composition Analysis by Micro-EDXRF www.edax.com Coating Thickness and Composition Analysis by Micro-EDXRF Introduction: The use of coatings in the modern manufacturing
Crystal Optics of Visible Light
 Crystal Optics of Visible Light This can be a very helpful aspect of minerals in understanding the petrographic history of a rock. The manner by which light is transferred through a mineral is a means
Crystal Optics of Visible Light This can be a very helpful aspect of minerals in understanding the petrographic history of a rock. The manner by which light is transferred through a mineral is a means
Interference. Physics 102 Workshop #3. General Instructions
 Interference Physics 102 Workshop #3 Name: Lab Partner(s): Instructor: Time of Workshop: General Instructions Workshop exercises are to be carried out in groups of three. One report per group is due by
Interference Physics 102 Workshop #3 Name: Lab Partner(s): Instructor: Time of Workshop: General Instructions Workshop exercises are to be carried out in groups of three. One report per group is due by
Nanometer-scale imaging and metrology, nano-fabrication with the Orion Helium Ion Microscope
 andras@nist.gov Nanometer-scale imaging and metrology, nano-fabrication with the Orion Helium Ion Microscope Bin Ming, András E. Vladár and Michael T. Postek National Institute of Standards and Technology
andras@nist.gov Nanometer-scale imaging and metrology, nano-fabrication with the Orion Helium Ion Microscope Bin Ming, András E. Vladár and Michael T. Postek National Institute of Standards and Technology
P R E A M B L E. Facilitated workshop problems for class discussion (1.5 hours)
 INSURANCE SCAM OPTICS - LABORATORY INVESTIGATION P R E A M B L E The original form of the problem is an Experimental Group Research Project, undertaken by students organised into small groups working as
INSURANCE SCAM OPTICS - LABORATORY INVESTIGATION P R E A M B L E The original form of the problem is an Experimental Group Research Project, undertaken by students organised into small groups working as
Spectrophotometry and the Beer-Lambert Law: An Important Analytical Technique in Chemistry
 Spectrophotometry and the Beer-Lambert Law: An Important Analytical Technique in Chemistry Jon H. Hardesty, PhD and Bassam Attili, PhD Collin College Department of Chemistry Introduction: In the last lab
Spectrophotometry and the Beer-Lambert Law: An Important Analytical Technique in Chemistry Jon H. Hardesty, PhD and Bassam Attili, PhD Collin College Department of Chemistry Introduction: In the last lab
Introduction to microstructure
 Introduction to microstructure 1.1 What is microstructure? When describing the structure of a material, we make a clear distinction between its crystal structure and its microstructure. The term crystal
Introduction to microstructure 1.1 What is microstructure? When describing the structure of a material, we make a clear distinction between its crystal structure and its microstructure. The term crystal
GRID AND PRISM SPECTROMETERS
 FYSA230/2 GRID AND PRISM SPECTROMETERS 1. Introduction Electromagnetic radiation (e.g. visible light) experiences reflection, refraction, interference and diffraction phenomena when entering and passing
FYSA230/2 GRID AND PRISM SPECTROMETERS 1. Introduction Electromagnetic radiation (e.g. visible light) experiences reflection, refraction, interference and diffraction phenomena when entering and passing
Sputtered AlN Thin Films on Si and Electrodes for MEMS Resonators: Relationship Between Surface Quality Microstructure and Film Properties
 Sputtered AlN Thin Films on and Electrodes for MEMS Resonators: Relationship Between Surface Quality Microstructure and Film Properties S. Mishin, D. R. Marx and B. Sylvia, Advanced Modular Sputtering,
Sputtered AlN Thin Films on and Electrodes for MEMS Resonators: Relationship Between Surface Quality Microstructure and Film Properties S. Mishin, D. R. Marx and B. Sylvia, Advanced Modular Sputtering,
Electron Beam and Sputter Deposition Choosing Process Parameters
 Electron Beam and Sputter Deposition Choosing Process Parameters General Introduction The choice of process parameters for any process is determined not only by the physics and/or chemistry of the process,
Electron Beam and Sputter Deposition Choosing Process Parameters General Introduction The choice of process parameters for any process is determined not only by the physics and/or chemistry of the process,
6) How wide must a narrow slit be if the first diffraction minimum occurs at ±12 with laser light of 633 nm?
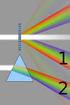 Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
2. Deposition process
 Properties of optical thin films produced by reactive low voltage ion plating (RLVIP) Antje Hallbauer Thin Film Technology Institute of Ion Physics & Applied Physics University of Innsbruck Investigations
Properties of optical thin films produced by reactive low voltage ion plating (RLVIP) Antje Hallbauer Thin Film Technology Institute of Ion Physics & Applied Physics University of Innsbruck Investigations
Name Date Class STATES OF MATTER. SECTION 13.1 THE NATURE OF GASES (pages 385 389)
 13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains how temperature
13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains how temperature
Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect
 Objectives: PS-7.1 Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect Illustrate ways that the energy of waves is transferred by interaction with
Objectives: PS-7.1 Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect Illustrate ways that the energy of waves is transferred by interaction with
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
 Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
h e l p s y o u C O N T R O L
 contamination analysis for compound semiconductors ANALYTICAL SERVICES B u r i e d d e f e c t s, E v a n s A n a l y t i c a l g r o u p h e l p s y o u C O N T R O L C O N T A M I N A T I O N Contamination
contamination analysis for compound semiconductors ANALYTICAL SERVICES B u r i e d d e f e c t s, E v a n s A n a l y t i c a l g r o u p h e l p s y o u C O N T R O L C O N T A M I N A T I O N Contamination
Raman Spectroscopy Basics
 Raman Spectroscopy Basics Introduction Raman spectroscopy is a spectroscopic technique based on inelastic scattering of monochromatic light, usually from a laser source. Inelastic scattering means that
Raman Spectroscopy Basics Introduction Raman spectroscopy is a spectroscopic technique based on inelastic scattering of monochromatic light, usually from a laser source. Inelastic scattering means that
Science In Action 8 Unit C - Light and Optical Systems. 1.1 The Challenge of light
 1.1 The Challenge of light 1. Pythagoras' thoughts about light were proven wrong because it was impossible to see A. the light beams B. dark objects C. in the dark D. shiny objects 2. Sir Isaac Newton
1.1 The Challenge of light 1. Pythagoras' thoughts about light were proven wrong because it was impossible to see A. the light beams B. dark objects C. in the dark D. shiny objects 2. Sir Isaac Newton
SOLAR ELECTRICITY: PROBLEM, CONSTRAINTS AND SOLUTIONS
 SOLAR ELECTRICITY: PROBLEM, CONSTRAINTS AND SOLUTIONS The United States generates over 4,110 TWh of electricity each year, costing $400 billion and emitting 2.5 billion metric tons of carbon dioxide (Yildiz,
SOLAR ELECTRICITY: PROBLEM, CONSTRAINTS AND SOLUTIONS The United States generates over 4,110 TWh of electricity each year, costing $400 billion and emitting 2.5 billion metric tons of carbon dioxide (Yildiz,
WAVELENGTH OF LIGHT - DIFFRACTION GRATING
 PURPOSE In this experiment we will use the diffraction grating and the spectrometer to measure wavelengths in the mercury spectrum. THEORY A diffraction grating is essentially a series of parallel equidistant
PURPOSE In this experiment we will use the diffraction grating and the spectrometer to measure wavelengths in the mercury spectrum. THEORY A diffraction grating is essentially a series of parallel equidistant
Geometric Optics Converging Lenses and Mirrors Physics Lab IV
 Objective Geometric Optics Converging Lenses and Mirrors Physics Lab IV In this set of lab exercises, the basic properties geometric optics concerning converging lenses and mirrors will be explored. The
Objective Geometric Optics Converging Lenses and Mirrors Physics Lab IV In this set of lab exercises, the basic properties geometric optics concerning converging lenses and mirrors will be explored. The
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* PHYSICS 0625/04 Paper 4 Theory (Extended) For Examination from 2016 SPECIMEN PAPER 1
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* PHYSICS 0625/04 Paper 4 Theory (Extended) For Examination from 2016 SPECIMEN PAPER 1
The Three Heat Transfer Modes in Reflow Soldering
 Section 5: Reflow Oven Heat Transfer The Three Heat Transfer Modes in Reflow Soldering There are three different heating modes involved with most SMT reflow processes: conduction, convection, and infrared
Section 5: Reflow Oven Heat Transfer The Three Heat Transfer Modes in Reflow Soldering There are three different heating modes involved with most SMT reflow processes: conduction, convection, and infrared
ILLUSTRATIVE EXAMPLE: Given: A = 3 and B = 4 if we now want the value of C=? C = 3 + 4 = 9 + 16 = 25 or 2
 Forensic Spectral Anaylysis: Warm up! The study of triangles has been done since ancient times. Many of the early discoveries about triangles are still used today. We will only be concerned with the "right
Forensic Spectral Anaylysis: Warm up! The study of triangles has been done since ancient times. Many of the early discoveries about triangles are still used today. We will only be concerned with the "right
13C NMR Spectroscopy
 13 C NMR Spectroscopy Introduction Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for structural determination. A nucleus with an odd number of protons, an odd number
13 C NMR Spectroscopy Introduction Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for structural determination. A nucleus with an odd number of protons, an odd number
Practice Test. 4) The planet Earth loses heat mainly by A) conduction. B) convection. C) radiation. D) all of these Answer: C
 Practice Test 1) Increase the pressure in a container of oxygen gas while keeping the temperature constant and you increase the A) molecular speed. B) molecular kinetic energy. C) Choice A and choice B
Practice Test 1) Increase the pressure in a container of oxygen gas while keeping the temperature constant and you increase the A) molecular speed. B) molecular kinetic energy. C) Choice A and choice B
Current Staff Course Unit/ Length. Basic Outline/ Structure. Unit Objectives/ Big Ideas. Properties of Waves A simple wave has a PH: Sound and Light
 Current Staff Course Unit/ Length August August September September October Unit Objectives/ Big Ideas Basic Outline/ Structure PS4- Types of Waves Because light can travel through space, it cannot be
Current Staff Course Unit/ Length August August September September October Unit Objectives/ Big Ideas Basic Outline/ Structure PS4- Types of Waves Because light can travel through space, it cannot be
A Remote Plasma Sputter Process for High Rate Web Coating of Low Temperature Plastic Film with High Quality Thin Film Metals and Insulators
 A Remote Plasma Sputter Process for High Rate Web Coating of Low Temperature Plastic Film with High Quality Thin Film Metals and Insulators Dr Peter Hockley and Professor Mike Thwaites, Plasma Quest Limited
A Remote Plasma Sputter Process for High Rate Web Coating of Low Temperature Plastic Film with High Quality Thin Film Metals and Insulators Dr Peter Hockley and Professor Mike Thwaites, Plasma Quest Limited
Physics 9e/Cutnell. correlated to the. College Board AP Physics 1 Course Objectives
 Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Solid State Detectors = Semi-Conductor based Detectors
 Solid State Detectors = Semi-Conductor based Detectors Materials and their properties Energy bands and electronic structure Charge transport and conductivity Boundaries: the p-n junction Charge collection
Solid State Detectors = Semi-Conductor based Detectors Materials and their properties Energy bands and electronic structure Charge transport and conductivity Boundaries: the p-n junction Charge collection
Overview. What is EMR? Electromagnetic Radiation (EMR) LA502 Special Studies Remote Sensing
 LA502 Special Studies Remote Sensing Electromagnetic Radiation (EMR) Dr. Ragab Khalil Department of Landscape Architecture Faculty of Environmental Design King AbdulAziz University Room 103 Overview What
LA502 Special Studies Remote Sensing Electromagnetic Radiation (EMR) Dr. Ragab Khalil Department of Landscape Architecture Faculty of Environmental Design King AbdulAziz University Room 103 Overview What
Waves Sound and Light
 Waves Sound and Light r2 c:\files\courses\1710\spr12\wavetrans.doc Ron Robertson The Nature of Waves Waves are a type of energy transmission that results from a periodic disturbance (vibration). They are
Waves Sound and Light r2 c:\files\courses\1710\spr12\wavetrans.doc Ron Robertson The Nature of Waves Waves are a type of energy transmission that results from a periodic disturbance (vibration). They are
Chemical Synthesis. Overview. Chemical Synthesis of Nanocrystals. Self-Assembly of Nanocrystals. Example: Cu 146 Se 73 (PPh 3 ) 30
 Chemical Synthesis Spontaneous organization of molecules into stable, structurally well-defined aggregates at the nanometer length scale. Overview The 1-100 nm nanoscale length is in between traditional
Chemical Synthesis Spontaneous organization of molecules into stable, structurally well-defined aggregates at the nanometer length scale. Overview The 1-100 nm nanoscale length is in between traditional
Types of Epitaxy. Homoepitaxy. Heteroepitaxy
 Epitaxy Epitaxial Growth Epitaxy means the growth of a single crystal film on top of a crystalline substrate. For most thin film applications (hard and soft coatings, optical coatings, protective coatings)
Epitaxy Epitaxial Growth Epitaxy means the growth of a single crystal film on top of a crystalline substrate. For most thin film applications (hard and soft coatings, optical coatings, protective coatings)
Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs
 Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs TracePro Opto-Mechanical Design Software s Fluorescence Property Utility TracePro s Fluorescence Property
Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs TracePro Opto-Mechanical Design Software s Fluorescence Property Utility TracePro s Fluorescence Property
Fundamentals of modern UV-visible spectroscopy. Presentation Materials
 Fundamentals of modern UV-visible spectroscopy Presentation Materials The Electromagnetic Spectrum E = hν ν = c / λ 1 Electronic Transitions in Formaldehyde 2 Electronic Transitions and Spectra of Atoms
Fundamentals of modern UV-visible spectroscopy Presentation Materials The Electromagnetic Spectrum E = hν ν = c / λ 1 Electronic Transitions in Formaldehyde 2 Electronic Transitions and Spectra of Atoms
Introduction to VLSI Fabrication Technologies. Emanuele Baravelli
 Introduction to VLSI Fabrication Technologies Emanuele Baravelli 27/09/2005 Organization Materials Used in VLSI Fabrication VLSI Fabrication Technologies Overview of Fabrication Methods Device simulation
Introduction to VLSI Fabrication Technologies Emanuele Baravelli 27/09/2005 Organization Materials Used in VLSI Fabrication VLSI Fabrication Technologies Overview of Fabrication Methods Device simulation
The photoionization detector (PID) utilizes ultraviolet
 Chapter 6 Photoionization Detectors The photoionization detector (PID) utilizes ultraviolet light to ionize gas molecules, and is commonly employed in the detection of volatile organic compounds (VOCs).
Chapter 6 Photoionization Detectors The photoionization detector (PID) utilizes ultraviolet light to ionize gas molecules, and is commonly employed in the detection of volatile organic compounds (VOCs).
Blackbody Radiation References INTRODUCTION
 Blackbody Radiation References 1) R.A. Serway, R.J. Beichner: Physics for Scientists and Engineers with Modern Physics, 5 th Edition, Vol. 2, Ch.40, Saunders College Publishing (A Division of Harcourt
Blackbody Radiation References 1) R.A. Serway, R.J. Beichner: Physics for Scientists and Engineers with Modern Physics, 5 th Edition, Vol. 2, Ch.40, Saunders College Publishing (A Division of Harcourt
Experiment: Crystal Structure Analysis in Engineering Materials
 Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Physics 30 Worksheet # 14: Michelson Experiment
 Physics 30 Worksheet # 14: Michelson Experiment 1. The speed of light found by a Michelson experiment was found to be 2.90 x 10 8 m/s. If the two hills were 20.0 km apart, what was the frequency of the
Physics 30 Worksheet # 14: Michelson Experiment 1. The speed of light found by a Michelson experiment was found to be 2.90 x 10 8 m/s. If the two hills were 20.0 km apart, what was the frequency of the
2 Absorbing Solar Energy
 2 Absorbing Solar Energy 2.1 Air Mass and the Solar Spectrum Now that we have introduced the solar cell, it is time to introduce the source of the energy the sun. The sun has many properties that could
2 Absorbing Solar Energy 2.1 Air Mass and the Solar Spectrum Now that we have introduced the solar cell, it is time to introduce the source of the energy the sun. The sun has many properties that could
13.1 The Nature of Gases. What is Kinetic Theory? Kinetic Theory and a Model for Gases. Chapter 13: States of Matter. Principles of Kinetic Theory
 Chapter 13: States of Matter The Nature of Gases The Nature of Gases kinetic molecular theory (KMT), gas pressure (pascal, atmosphere, mm Hg), kinetic energy The Nature of Liquids vaporization, evaporation,
Chapter 13: States of Matter The Nature of Gases The Nature of Gases kinetic molecular theory (KMT), gas pressure (pascal, atmosphere, mm Hg), kinetic energy The Nature of Liquids vaporization, evaporation,
Copyright 1999 2010 by Mark Brandt, Ph.D. 12
 Introduction to Absorbance Spectroscopy A single beam spectrophotometer is comprised of a light source, a monochromator, a sample holder, and a detector. An ideal instrument has a light source that emits
Introduction to Absorbance Spectroscopy A single beam spectrophotometer is comprised of a light source, a monochromator, a sample holder, and a detector. An ideal instrument has a light source that emits
Chemical Sputtering. von Kohlenstoff durch Wasserstoff. W. Jacob
 Chemical Sputtering von Kohlenstoff durch Wasserstoff W. Jacob Centre for Interdisciplinary Plasma Science Max-Planck-Institut für Plasmaphysik, 85748 Garching Content: Definitions: Chemical erosion, physical
Chemical Sputtering von Kohlenstoff durch Wasserstoff W. Jacob Centre for Interdisciplinary Plasma Science Max-Planck-Institut für Plasmaphysik, 85748 Garching Content: Definitions: Chemical erosion, physical
1. The Kinetic Theory of Matter states that all matter is composed of atoms and molecules that are in a constant state of constant random motion
 Physical Science Period: Name: ANSWER KEY Date: Practice Test for Unit 3: Ch. 3, and some of 15 and 16: Kinetic Theory of Matter, States of matter, and and thermodynamics, and gas laws. 1. The Kinetic
Physical Science Period: Name: ANSWER KEY Date: Practice Test for Unit 3: Ch. 3, and some of 15 and 16: Kinetic Theory of Matter, States of matter, and and thermodynamics, and gas laws. 1. The Kinetic
Lenses and Apertures of A TEM
 Instructor: Dr. C.Wang EMA 6518 Course Presentation Lenses and Apertures of A TEM Group Member: Anup Kr. Keshri Srikanth Korla Sushma Amruthaluri Venkata Pasumarthi Xudong Chen Outline Electron Optics
Instructor: Dr. C.Wang EMA 6518 Course Presentation Lenses and Apertures of A TEM Group Member: Anup Kr. Keshri Srikanth Korla Sushma Amruthaluri Venkata Pasumarthi Xudong Chen Outline Electron Optics
GREEN NANOTECHNOLOGY. Geoffrey. Energy in the Built Environment. Solutions for Sustainability and. B. Smith Claes G. Granqvist.
 GREEN NANOTECHNOLOGY Solutions for Sustainability and Energy in the Built Environment Geoffrey B. Smith Claes G. Granqvist CRC Press Taylor & Francis Group Boca Raton London NewYork CRC Press is an imprint
GREEN NANOTECHNOLOGY Solutions for Sustainability and Energy in the Built Environment Geoffrey B. Smith Claes G. Granqvist CRC Press Taylor & Francis Group Boca Raton London NewYork CRC Press is an imprint
Conductivity of silicon can be changed several orders of magnitude by introducing impurity atoms in silicon crystal lattice.
 CMOS Processing Technology Silicon: a semiconductor with resistance between that of conductor and an insulator. Conductivity of silicon can be changed several orders of magnitude by introducing impurity
CMOS Processing Technology Silicon: a semiconductor with resistance between that of conductor and an insulator. Conductivity of silicon can be changed several orders of magnitude by introducing impurity
Graduate Student Presentations
 Graduate Student Presentations Dang, Huong Chip packaging March 27 Call, Nathan Thin film transistors/ liquid crystal displays April 4 Feldman, Ari Optical computing April 11 Guerassio, Ian Self-assembly
Graduate Student Presentations Dang, Huong Chip packaging March 27 Call, Nathan Thin film transistors/ liquid crystal displays April 4 Feldman, Ari Optical computing April 11 Guerassio, Ian Self-assembly
Lab 9: The Acousto-Optic Effect
 Lab 9: The Acousto-Optic Effect Incoming Laser Beam Travelling Acoustic Wave (longitudinal wave) O A 1st order diffracted laser beam A 1 Introduction qb d O 2qb rarefractions compressions Refer to Appendix
Lab 9: The Acousto-Optic Effect Incoming Laser Beam Travelling Acoustic Wave (longitudinal wave) O A 1st order diffracted laser beam A 1 Introduction qb d O 2qb rarefractions compressions Refer to Appendix
Defects Introduction. Bonding + Structure + Defects. Properties
 Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
PHYSICS PAPER 1 (THEORY)
 PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
Using the Spectrophotometer
 Using the Spectrophotometer Introduction In this exercise, you will learn the basic principals of spectrophotometry and and serial dilution and their practical application. You will need these skills to
Using the Spectrophotometer Introduction In this exercise, you will learn the basic principals of spectrophotometry and and serial dilution and their practical application. You will need these skills to
Light and its effects
 Light and its effects Light and the speed of light Shadows Shadow films Pinhole camera (1) Pinhole camera (2) Reflection of light Image in a plane mirror An image in a plane mirror is: (i) the same size
Light and its effects Light and the speed of light Shadows Shadow films Pinhole camera (1) Pinhole camera (2) Reflection of light Image in a plane mirror An image in a plane mirror is: (i) the same size
Lecture 3: Optical Properties of Bulk and Nano. 5 nm
 Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
Laue lens for Nuclear Medicine
 Laue lens for Nuclear Medicine PhD in Physics Gianfranco Paternò Ferrara, 6-11-013 Supervisor: prof. Vincenzo Guidi Sensors and Semiconductors Lab, Department of Physics and Earth Science, University of
Laue lens for Nuclear Medicine PhD in Physics Gianfranco Paternò Ferrara, 6-11-013 Supervisor: prof. Vincenzo Guidi Sensors and Semiconductors Lab, Department of Physics and Earth Science, University of
Advancements in High Frequency, High Resolution Acoustic Micro Imaging for Thin Silicon Applications
 Advancements in High Frequency, High Resolution Acoustic Micro Imaging for Thin Silicon Applications Janet E. Semmens Sonoscan, Inc. 2149 E. Pratt Boulevard Elk Grove Village, IL 60007 USA Phone: (847)
Advancements in High Frequency, High Resolution Acoustic Micro Imaging for Thin Silicon Applications Janet E. Semmens Sonoscan, Inc. 2149 E. Pratt Boulevard Elk Grove Village, IL 60007 USA Phone: (847)
AS COMPETITION PAPER 2008
 AS COMPETITION PAPER 28 Name School Town & County Total Mark/5 Time Allowed: One hour Attempt as many questions as you can. Write your answers on this question paper. Marks allocated for each question
AS COMPETITION PAPER 28 Name School Town & County Total Mark/5 Time Allowed: One hour Attempt as many questions as you can. Write your answers on this question paper. Marks allocated for each question
. Tutorial #3 Building Complex Targets
 . Tutorial #3 Building Complex Targets. Mixed Gas/Solid Targets Gas Ionization Chamber Previous Tutorials have covered how to setup TRIM, determine which ion and energy to specify for a semiconductor n-well
. Tutorial #3 Building Complex Targets. Mixed Gas/Solid Targets Gas Ionization Chamber Previous Tutorials have covered how to setup TRIM, determine which ion and energy to specify for a semiconductor n-well
Name Date Class ELECTRONS IN ATOMS. Standard Curriculum Core content Extension topics
 13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
TOF FUNDAMENTALS TUTORIAL
 TOF FUNDAMENTALS TUTORIAL Presented By: JORDAN TOF PRODUCTS, INC. 990 Golden Gate Terrace Grass Valley, CA 95945 530-272-4580 / 530-272-2955 [fax] www.rmjordan.com [web] info@rmjordan.com [e-mail] This
TOF FUNDAMENTALS TUTORIAL Presented By: JORDAN TOF PRODUCTS, INC. 990 Golden Gate Terrace Grass Valley, CA 95945 530-272-4580 / 530-272-2955 [fax] www.rmjordan.com [web] info@rmjordan.com [e-mail] This
The Earth s Atmosphere
 THE SUN-EARTH SYSTEM III The Earth s Atmosphere Composition and Distribution of the Atmosphere The composition of the atmosphere and the way its gases interact with electromagnetic radiation determine
THE SUN-EARTH SYSTEM III The Earth s Atmosphere Composition and Distribution of the Atmosphere The composition of the atmosphere and the way its gases interact with electromagnetic radiation determine
Automatic and Objective Measurement of Residual Stress and Cord in Glass
 Automatic and Objective Measurement of Residual Stress and Cord in Glass GlassTrend - ICG TC15/21 Seminar SENSORS AND PROCESS CONTROL 13-14 October 2015, Eindhoven Henning Katte, ilis gmbh copyright ilis
Automatic and Objective Measurement of Residual Stress and Cord in Glass GlassTrend - ICG TC15/21 Seminar SENSORS AND PROCESS CONTROL 13-14 October 2015, Eindhoven Henning Katte, ilis gmbh copyright ilis
Dry Etching and Reactive Ion Etching (RIE)
 Dry Etching and Reactive Ion Etching (RIE) MEMS 5611 Feb 19 th 2013 Shengkui Gao Contents refer slides from UC Berkeley, Georgia Tech., KU, etc. (see reference) 1 Contents Etching and its terminologies
Dry Etching and Reactive Ion Etching (RIE) MEMS 5611 Feb 19 th 2013 Shengkui Gao Contents refer slides from UC Berkeley, Georgia Tech., KU, etc. (see reference) 1 Contents Etching and its terminologies
The Physics of Energy sources Renewable sources of energy. Solar Energy
 The Physics of Energy sources Renewable sources of energy Solar Energy B. Maffei Bruno.maffei@manchester.ac.uk Renewable sources 1 Solar power! There are basically two ways of using directly the radiative
The Physics of Energy sources Renewable sources of energy Solar Energy B. Maffei Bruno.maffei@manchester.ac.uk Renewable sources 1 Solar power! There are basically two ways of using directly the radiative
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
EDS system. CRF Oxford Instruments INCA CRF EDAX Genesis EVEX- NanoAnalysis Table top system
 EDS system Most common X-Ray measurement system in the SEM lab. Major elements (10 wt% or greater) identified in ~10 secs. Minor elements identifiable in ~100 secs. Rapid qualitative and accurate quantitative
EDS system Most common X-Ray measurement system in the SEM lab. Major elements (10 wt% or greater) identified in ~10 secs. Minor elements identifiable in ~100 secs. Rapid qualitative and accurate quantitative
Reflectance Measurements of Materials Used in the Solar Industry. Selecting the Appropriate Accessories for UV/Vis/NIR Measurements.
 T e c h n i c a l N o t e Reflectance Measurements of Materials Used in the Solar Industry UV/Vis/NIR Author: Dr. Jeffrey L. Taylor PerkinElmer, Inc. 710 Bridgeport Avenue Shelton, CT 06484 USA Selecting
T e c h n i c a l N o t e Reflectance Measurements of Materials Used in the Solar Industry UV/Vis/NIR Author: Dr. Jeffrey L. Taylor PerkinElmer, Inc. 710 Bridgeport Avenue Shelton, CT 06484 USA Selecting
1051-232 Imaging Systems Laboratory II. Laboratory 4: Basic Lens Design in OSLO April 2 & 4, 2002
 05-232 Imaging Systems Laboratory II Laboratory 4: Basic Lens Design in OSLO April 2 & 4, 2002 Abstract: For designing the optics of an imaging system, one of the main types of tools used today is optical
05-232 Imaging Systems Laboratory II Laboratory 4: Basic Lens Design in OSLO April 2 & 4, 2002 Abstract: For designing the optics of an imaging system, one of the main types of tools used today is optical
