GRID AND PRISM SPECTROMETERS
|
|
|
- Grant Cain
- 9 years ago
- Views:
Transcription
1 FYSA230/2 GRID AND PRISM SPECTROMETERS 1. Introduction Electromagnetic radiation (e.g. visible light) experiences reflection, refraction, interference and diffraction phenomena when entering and passing an (optically) active medium like a grid and prism. The phenomena can be explained partly with classical wave-mechanics but are basically quantum mechanical or better quantum electrodynamical in character. Grids and prisms belong to such media. Visible light (spectra) emitted by atoms of gas-discharge tubes are well suited to study properties of atomic excitations and functioning principles of optically active devices, simultaneously. 2. Devices 2.1 Spectrometer A spectrometer is an (optically) active medium (device) sensitive to the energy, polarisation and intensity of electromagnetic or particle radiation (beam) selecting it e.g. according energy into energy spectra. When the energy is 1 kev, an electromagnetic radiation is customary to classify with wavelength (E = h = hc/). The grid and prism spectrometers used here are designed to handle electromagnetic radiation in visible regime. A schematic principle is presented in figure 1 and in more detail in appendix 2. prism red light source slit collimator grid telescope violet Figure 1. Grid and prism spectrometers. Grid deflects and prism refracts the light into different angles according to the wavelength creating energy spectra that can be observed by looking at the transmitted light with a telescope in various angles. Knowing the geometry (number of slits per
2 - 2 - distance) of the grid or the refraction index of the prism the angle information can be translated to wavelength. In practise the energy or wavelength response of a spectrometer is calibrated using known spectra of standard sources and the resulting calibration function = f(θ) is used to analyse spectra of radiation studied. Various optical elements like slits, collimators and focusing devices are needed in experiments with some accuracy desired. A slit (S) narrows the beam from the light source. The collimator (C) parallels it before the grid or prism. The refracted rays with different wavelengths are focused with the objective of the telescope on the position of a hair grate located between the focus point and the ocular. The ocular magnifies the picture formed by the hair grate and the radiation to be observed by naked eye. Read appendix 2! 2.2. Diffraction grid Monochromatic light passing a narrow slit experiences diffraction i.e. deflects forming a diffraction pattern characteristic to the wavelength and the width b of the slit. The intensity minima of the diffraction pattern are located at angles sin(θ) = n/b, (n = 0, 1, 2, ). With several slits the diffracted light from different slits interfere and an interference pattern modulated by the diffraction one appears. The location (angle) of intensity minima and maxima depend now also on the density (location) of the diffraction slits. A regular, 2-dimensional diffraction grid with parallel, rectangular slits (width b, distance a, s.c. grid constant) forms principal intensity maxima at angles defined by n sin( ) (1) a If the light is non monochromatic, each wavelength yields its own diffraction pattern. Usually the main components of a light source are well separated in energy and a clean diffraction pattern of a grid for each wavelength will be observed. The grid and prism can be characterised with their ability to resolve different wavelengths, the dispersion D, which derived from eq. (1) is d n D (2) d acos( ) Irregular grids with 2- or 3-dimensional geometry's yield very complicated diffractioninterference patterns and are used as versatile, optically active devices Prism Light passing through a prism will be reflected and refracted by the entrance and exit surfaces. Monochromatic light (beam) will be refracted by an angle from its original direction. A minimal refraction ( = min ) follows, when the light passes the prism symmetrically (see fig. 2). Now it holds sin(½( min A)) n, (3) sin(½a)
3 - 3 - where A is the angle of the deflecting edge and n = c/c n is the refraction coefficient of the material of the prism (c and c n are the speed of light in air (or vacuum) and in the prism, correspondingly). Since the refraction coefficient n depends of the wavelength, each wavelength has a characteristic min. A 1 2 n min 1 A Figure 2. Minimal deflection. Non-monochromatic light will thus disperse in prism according to the wavelengths. The spectrum created differs in structure from the corresponding grid spectrum. The dispersion of a prism can be derived using eq. (3) and relation n = c/c n d min 2sin(½A) 2B D cos(½( 3, (4) d min A)) where B is a constant. 3. Radiation emitted by an excited atom Possible excitation of an atom are quantum mechanical states of (many) electron configurations. The lowest in energy (E 1 ) is the (stable) ground state and other (meta stable) configurations have higher energies E i (i = 2, 3,...), decaying to lower lying states either directly or via transition states (figure 3). The energy shift occurs emitting electromagnetic radiation, a photon (e.g. visible light) corresponding to the energy difference E i E j (i > j; i = 2, 3,...). The frequency of the photon is thus h Ei E 2 (5) where = c/ and h the Planck s constant. Each atom has a discrete, characteristic energy spectrum.
4 - 4 - E 4 transition E 3 excited E 2 E 1 ground Figure 3. States and transitions of an atom. Here we study electromagnetic radiation of excited atoms in a gas-discharge tube in the regime of visible light. The discrete spectra predicted by the theory presented before are analysed with the aid of grid and prism spectrometers. J. Balmer observed on 19th century that the wavelengths of visible spectral lines of a hydrogen atom obey the rule where RZ 2 '2 n n R = Rydberg s constant = 1, m -1 Z = 1 = atomic number of hydrogen n = 2 = the quantum number of the final state n' = the quantum number of the initial state = 3 for the hydrogen - line = 4 for the hydrogen - line = 5 for the hydrogen - line. (6) Eq. (6) is valid for all atoms with a single electron (like H, He +, Li 2+ ) and it can be derived from Bohr s model of the atom Lymanlines Balmerlines Figure 4. Partial decay scheme of a hydrogen atom.
5 Measurements 4.1. Focusing the spectrometer The spectrometer used is described in details in appendix 2. Fig. 5 shows a schematic diagram of the optics used. A B C D E F G Figure 5. Optical elements of the spectrometer: Slit A, objective of the collimator B, objective of the telescope C, hair grate D, ocular lenses of the telescope E and G, half-transparent mirror F which illuminates the hair grate. The distance between the ocular lenses E and G can be varied so that the image and the hair grate are seen sharp, simultaneously. Focusing the spectrometer is done as follows: If the lines with different colours and the hair grate are seen sharp using the grid, focusing is not necessary. Otherwise, set the lens G so that the hair grate is seen sharp. Then the entire set D, E, F, G is moved so that an object in distance is seen sharp and does not move with the position of the eye (elimination of s.c. parallax). Now the image of the hair grade and the object coincide. The position of the hair grate can be adjusted with the focusing ring M (see figure A1). The collimator B is focused by moving the slit A so that the slit is seen sharp independent of the position of the eye. The slit should be set vertical, exactly, and as narrow as possible depending of the intensity of the light source Grid Place the grid (about 900 slits per mm) on the turn table perpendicular to the optical axis. Optimise the height of the table. Use the helium lamp (He) and measure positions of the 6 brightest lines of the first order and the 3 corresponding of the second order on the both sides of the straight incoming light (angles 1 and 2 ). The deviation is =1/2( 1-2 ). Calculate the grid constant with eq. (1). By using the mercury-cadmium lamp (Hg/Cd), measure deflection angles of the 9 brightest lines of the first order. For hydrogen (H 2 ) observation of 3 brightest lines is sufficient. If you are not able to discern all the lines on both sides of the straight incoming light, record the angles from the the straight incoming light and the line on the other side. The deviation can then be calculated with help of these.
6 Prism With the Hg/Cd lamp optimise the set-up for the prism according to fig. 1 so that the spectrum is clearly visible. Search the minimal deflection angle. This can be done as follows: monitor the position of a selected spectral line and rotate the prism, until you find an angle, where the lines start to move in reverse direction when rotating the prism to same direction. In the limits of the accuracy of the spectrometer it is sufficient to use only one line (e.g. the green one) to define the minimal angle for the entire spectrum. For the other lines move now the position of the telescope, only, to measure the angles (θ 1 ). The minimal deflection for each line is now the difference between the angles measured and the angle (θ 0 ) for straight light when the prism is removed. The wavelengths of the lines are taken from the measurements with the grid. 5. Analysis of results Grid: By using your results for the He lamp and the wavelengths in appendix 1, determine the grid constant a. For H and Hg/Cd calculate the wavelengths using eq. (1) and the measured grid constant a. Verify and compare your values for Hg/Cd to those given in appendix 1 and in the literature to find the lines of mercury. Furthermore draw the curve () using measured results for the Hg/Cd lamp. Prism: Draw the minimal deflection min as a function of the wavelength. Furthermore, calculate the reflection coefficient n in eq. (3) (A = 60) and plot n as a function of. For several materials the so-called Cauchy s formula is valid B C n A, (7) 2 4 where A, B and C are constant parameters characteristic to the material, but independent of the wavelength. Define these parameters and study how well Cauchy s formula is valid. Both grid and prism: To determine the dispersions D = dθ/dλ and D = dδ min /dλ, draw a few tangents of curves θ(λ) and δ min (λ) with fit intervals and calculate their slopes. Plot in the same figure d/d and d min /d as a function of. Balmer s formula Calculate with eq. (6) the wavelengths of -, - and - lines of hydrogen. By comparing the calculated values with your experimental ones (obtained with the grid) define the colours of -, - and - lines.
7 - 7 - Answer to the following questions: What are most striking differences of the spectra obtained with the grid and the prism? Based on the curves D(), what can you tell about properties of the grid and the prism? What is dispersion? Why you don t see the Lyman-lines? Derive eq. (3) using fig. 2 and the Snell s law. Recall that a degree ( ) is not a SI unit. Appendix 1 Wavelengths given in literature (nm): He: Hg: red yellow yellow yellow green (strong) green green (weak) blue blue green violet blue
8 - 8 - Appendix 2 The Spencer-spectrometer In principle the spectrometer is simple (fig. L1), but has many components worth to study and try out their functions carefully before the measurements. Figure A1. Spencer-spectrometer. Slit A, objective of the collimator B, objective of the telescope C, prism D, hair grate E, fixing screw of the table I, fine-tuning screw of the table J, fine-tuning screw of the telescope K, ring of ocular L, focusing ring of telescope M, cover of the Nonie-ring N, height-setting screws of the table O, height-setting screws of the collimator and the telescope P, fixingscrew of the telescope Q, fixing-screws of the telescope R, fixing-screw of the collimator S. The telescope and the optical table rotate around common axis and they can be fixed with screws K and J. The height of the table is set by screw I. Do not over tighten them. When screws K and J are fixed, the position of the table and telescope can be tuned with fine tuning screws. To determine the angles precisely either the table (usually) or the telescope has to be kept fixed. The minimal deflection angle is best to find out with the fine tuning the table. The spectrometer is equipped with two 30' Nonie-scales. It is sufficient to use one of them, only. Learn to read the Nonie-scale correctly!
THE BOHR QUANTUM MODEL
 THE BOHR QUANTUM MODEL INTRODUCTION When light from a low-pressure gas is subject to an electric discharge, a discrete line spectrum is emitted. When light from such a low-pressure gas is examined with
THE BOHR QUANTUM MODEL INTRODUCTION When light from a low-pressure gas is subject to an electric discharge, a discrete line spectrum is emitted. When light from such a low-pressure gas is examined with
WAVELENGTH OF LIGHT - DIFFRACTION GRATING
 PURPOSE In this experiment we will use the diffraction grating and the spectrometer to measure wavelengths in the mercury spectrum. THEORY A diffraction grating is essentially a series of parallel equidistant
PURPOSE In this experiment we will use the diffraction grating and the spectrometer to measure wavelengths in the mercury spectrum. THEORY A diffraction grating is essentially a series of parallel equidistant
O6: The Diffraction Grating Spectrometer
 2B30: PRACTICAL ASTROPHYSICS FORMAL REPORT: O6: The Diffraction Grating Spectrometer Adam Hill Lab partner: G. Evans Tutor: Dr. Peter Storey 1 Abstract The calibration of a diffraction grating spectrometer
2B30: PRACTICAL ASTROPHYSICS FORMAL REPORT: O6: The Diffraction Grating Spectrometer Adam Hill Lab partner: G. Evans Tutor: Dr. Peter Storey 1 Abstract The calibration of a diffraction grating spectrometer
ATOMIC SPECTRA. Apparatus: Optical spectrometer, spectral tubes, power supply, incandescent lamp, bottles of dyed water, elevating jack or block.
 1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
Experiment #12: The Bohr Atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes, Holder, and Variac Flashlight
 Experiment #12: The Bohr Atom Purpose: To observe the visible spectrum of hydrogen and helium and verify the Bohr model of the hydrogen atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes,
Experiment #12: The Bohr Atom Purpose: To observe the visible spectrum of hydrogen and helium and verify the Bohr model of the hydrogen atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes,
AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light
 AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light Name: Period: Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Reflection,
AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light Name: Period: Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Reflection,
How To Understand Light And Color
 PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
Interference. Physics 102 Workshop #3. General Instructions
 Interference Physics 102 Workshop #3 Name: Lab Partner(s): Instructor: Time of Workshop: General Instructions Workshop exercises are to be carried out in groups of three. One report per group is due by
Interference Physics 102 Workshop #3 Name: Lab Partner(s): Instructor: Time of Workshop: General Instructions Workshop exercises are to be carried out in groups of three. One report per group is due by
Basic Optics System OS-8515C
 40 50 30 60 20 70 10 80 0 90 80 10 20 70 T 30 60 40 50 50 40 60 30 C 70 20 80 10 90 90 0 80 10 70 20 60 50 40 30 Instruction Manual with Experiment Guide and Teachers Notes 012-09900B Basic Optics System
40 50 30 60 20 70 10 80 0 90 80 10 20 70 T 30 60 40 50 50 40 60 30 C 70 20 80 10 90 90 0 80 10 70 20 60 50 40 30 Instruction Manual with Experiment Guide and Teachers Notes 012-09900B Basic Optics System
Reflection and Refraction
 Equipment Reflection and Refraction Acrylic block set, plane-concave-convex universal mirror, cork board, cork board stand, pins, flashlight, protractor, ruler, mirror worksheet, rectangular block worksheet,
Equipment Reflection and Refraction Acrylic block set, plane-concave-convex universal mirror, cork board, cork board stand, pins, flashlight, protractor, ruler, mirror worksheet, rectangular block worksheet,
Experiment IV: Atomic Spectra and the Bohr model
 P19: INTRODUCTORY PHYSICS III Experiment IV: Atomic Spectra and the Bohr model Department of Physics and Astronomy Dartmouth College 6127 Wilder Laboratory Hanover, NH 03755 USA Overview In this lab, we
P19: INTRODUCTORY PHYSICS III Experiment IV: Atomic Spectra and the Bohr model Department of Physics and Astronomy Dartmouth College 6127 Wilder Laboratory Hanover, NH 03755 USA Overview In this lab, we
Experiment #5: Qualitative Absorption Spectroscopy
 Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
P R E A M B L E. Facilitated workshop problems for class discussion (1.5 hours)
 INSURANCE SCAM OPTICS - LABORATORY INVESTIGATION P R E A M B L E The original form of the problem is an Experimental Group Research Project, undertaken by students organised into small groups working as
INSURANCE SCAM OPTICS - LABORATORY INVESTIGATION P R E A M B L E The original form of the problem is an Experimental Group Research Project, undertaken by students organised into small groups working as
Interferometers. OBJECTIVES To examine the operation of several kinds of interferometers. d sin = n (1)
 Interferometers The true worth of an experimenter consists in his pursuing not only what he seeks in his experiment, but also what he did not seek. Claude Bernard (1813-1878) OBJECTIVES To examine the
Interferometers The true worth of an experimenter consists in his pursuing not only what he seeks in his experiment, but also what he did not seek. Claude Bernard (1813-1878) OBJECTIVES To examine the
Fundamentals of modern UV-visible spectroscopy. Presentation Materials
 Fundamentals of modern UV-visible spectroscopy Presentation Materials The Electromagnetic Spectrum E = hν ν = c / λ 1 Electronic Transitions in Formaldehyde 2 Electronic Transitions and Spectra of Atoms
Fundamentals of modern UV-visible spectroscopy Presentation Materials The Electromagnetic Spectrum E = hν ν = c / λ 1 Electronic Transitions in Formaldehyde 2 Electronic Transitions and Spectra of Atoms
Theremino System Theremino Spectrometer Technology
 Theremino System Theremino Spectrometer Technology theremino System - Theremino Spectrometer Technology - August 15, 2014 - Page 1 Operation principles By placing a digital camera with a diffraction grating
Theremino System Theremino Spectrometer Technology theremino System - Theremino Spectrometer Technology - August 15, 2014 - Page 1 Operation principles By placing a digital camera with a diffraction grating
ILLUSTRATIVE EXAMPLE: Given: A = 3 and B = 4 if we now want the value of C=? C = 3 + 4 = 9 + 16 = 25 or 2
 Forensic Spectral Anaylysis: Warm up! The study of triangles has been done since ancient times. Many of the early discoveries about triangles are still used today. We will only be concerned with the "right
Forensic Spectral Anaylysis: Warm up! The study of triangles has been done since ancient times. Many of the early discoveries about triangles are still used today. We will only be concerned with the "right
Chemistry 111 Lab: Intro to Spectrophotometry Page E-1
 Chemistry 111 Lab: Intro to Spectrophotometry Page E-1 SPECTROPHOTOMETRY Absorption Measurements & their Application to Quantitative Analysis study of the interaction of light (or other electromagnetic
Chemistry 111 Lab: Intro to Spectrophotometry Page E-1 SPECTROPHOTOMETRY Absorption Measurements & their Application to Quantitative Analysis study of the interaction of light (or other electromagnetic
Physics 441/2: Transmission Electron Microscope
 Physics 441/2: Transmission Electron Microscope Introduction In this experiment we will explore the use of transmission electron microscopy (TEM) to take us into the world of ultrasmall structures. This
Physics 441/2: Transmission Electron Microscope Introduction In this experiment we will explore the use of transmission electron microscopy (TEM) to take us into the world of ultrasmall structures. This
Blackbody Radiation References INTRODUCTION
 Blackbody Radiation References 1) R.A. Serway, R.J. Beichner: Physics for Scientists and Engineers with Modern Physics, 5 th Edition, Vol. 2, Ch.40, Saunders College Publishing (A Division of Harcourt
Blackbody Radiation References 1) R.A. Serway, R.J. Beichner: Physics for Scientists and Engineers with Modern Physics, 5 th Edition, Vol. 2, Ch.40, Saunders College Publishing (A Division of Harcourt
Photons. ConcepTest 27.1. 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy. Which has more energy, a photon of:
 ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
Name Date Class ELECTRONS IN ATOMS. Standard Curriculum Core content Extension topics
 13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect
 Objectives: PS-7.1 Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect Illustrate ways that the energy of waves is transferred by interaction with
Objectives: PS-7.1 Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect Illustrate ways that the energy of waves is transferred by interaction with
What s in the Mix? Liquid Color Spectroscopy Lab (Randy Landsberg & Bill Fisher)
 What s in the Mix? Liquid Color Spectroscopy Lab (Randy Landsberg & Bill Fisher) Introduction: There is more to a color than a name. Color can tell us lots of information. In this lab you will use a spectrophotometer
What s in the Mix? Liquid Color Spectroscopy Lab (Randy Landsberg & Bill Fisher) Introduction: There is more to a color than a name. Color can tell us lots of information. In this lab you will use a spectrophotometer
Alignement of a ring cavity laser
 Alignement of a ring cavity laser 1 Introduction This manual describes a procedure to align the cavity of our Ti:Sapphire ring laser and its injection with an Argon-Ion pump laser beam. The setup is shown
Alignement of a ring cavity laser 1 Introduction This manual describes a procedure to align the cavity of our Ti:Sapphire ring laser and its injection with an Argon-Ion pump laser beam. The setup is shown
PHYSICS PAPER 1 (THEORY)
 PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
 Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
A Guide to Acousto-Optic Modulators
 A Guide to Acousto-Optic Modulators D. J. McCarron December 7, 2007 1 Introduction Acousto-optic modulators (AOMs) are useful devices which allow the frequency, intensity and direction of a laser beam
A Guide to Acousto-Optic Modulators D. J. McCarron December 7, 2007 1 Introduction Acousto-optic modulators (AOMs) are useful devices which allow the frequency, intensity and direction of a laser beam
WAVES AND ELECTROMAGNETIC RADIATION
 WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
PHYA2. General Certificate of Education Advanced Subsidiary Examination June 2010. Mechanics, Materials and Waves
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 2 For this paper you must have: a ruler a calculator a Data and Formulae Booklet.
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 2 For this paper you must have: a ruler a calculator a Data and Formulae Booklet.
Study Guide for Exam on Light
 Name: Class: Date: Study Guide for Exam on Light Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which portion of the electromagnetic spectrum is used
Name: Class: Date: Study Guide for Exam on Light Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which portion of the electromagnetic spectrum is used
Chapter 17: Light and Image Formation
 Chapter 17: Light and Image Formation 1. When light enters a medium with a higher index of refraction it is A. absorbed. B. bent away from the normal. C. bent towards from the normal. D. continues in the
Chapter 17: Light and Image Formation 1. When light enters a medium with a higher index of refraction it is A. absorbed. B. bent away from the normal. C. bent towards from the normal. D. continues in the
EXPERIMENT 11 UV/VIS Spectroscopy and Spectrophotometry: Spectrophotometric Analysis of Potassium Permanganate Solutions.
 EXPERIMENT 11 UV/VIS Spectroscopy and Spectrophotometry: Spectrophotometric Analysis of Potassium Permanganate Solutions. Outcomes After completing this experiment, the student should be able to: 1. Prepare
EXPERIMENT 11 UV/VIS Spectroscopy and Spectrophotometry: Spectrophotometric Analysis of Potassium Permanganate Solutions. Outcomes After completing this experiment, the student should be able to: 1. Prepare
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES. PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points
 TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
Review of the isotope effect in the hydrogen spectrum
 Review of the isotope effect in the hydrogen spectrum 1 Balmer and Rydberg Formulas By the middle of the 19th century it was well established that atoms emitted light at discrete wavelengths. This is in
Review of the isotope effect in the hydrogen spectrum 1 Balmer and Rydberg Formulas By the middle of the 19th century it was well established that atoms emitted light at discrete wavelengths. This is in
Light and its effects
 Light and its effects Light and the speed of light Shadows Shadow films Pinhole camera (1) Pinhole camera (2) Reflection of light Image in a plane mirror An image in a plane mirror is: (i) the same size
Light and its effects Light and the speed of light Shadows Shadow films Pinhole camera (1) Pinhole camera (2) Reflection of light Image in a plane mirror An image in a plane mirror is: (i) the same size
Light as a Wave. The Nature of Light. EM Radiation Spectrum. EM Radiation Spectrum. Electromagnetic Radiation
 The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation?
 From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation? From lowest energy to highest energy, which of the following correctly
From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation? From lowest energy to highest energy, which of the following correctly
Diffraction and Young s Single Slit Experiment
 Diffraction and Young s Single Slit Experiment Developers AB Overby Objectives Preparation Background The objectives of this experiment are to observe Fraunhofer, or far-field, diffraction through a single
Diffraction and Young s Single Slit Experiment Developers AB Overby Objectives Preparation Background The objectives of this experiment are to observe Fraunhofer, or far-field, diffraction through a single
PUMPED Nd:YAG LASER. Last Revision: August 21, 2007
 PUMPED Nd:YAG LASER Last Revision: August 21, 2007 QUESTION TO BE INVESTIGATED: How can an efficient atomic transition laser be constructed and characterized? INTRODUCTION: This lab exercise will allow
PUMPED Nd:YAG LASER Last Revision: August 21, 2007 QUESTION TO BE INVESTIGATED: How can an efficient atomic transition laser be constructed and characterized? INTRODUCTION: This lab exercise will allow
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Flame Tests & Electron Configuration
 Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
6) How wide must a narrow slit be if the first diffraction minimum occurs at ±12 with laser light of 633 nm?
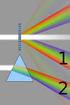 Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
LIGHT REFLECTION AND REFRACTION
 QUESTION BANK IN SCIENCE CLASS-X (TERM-II) 10 LIGHT REFLECTION AND REFRACTION CONCEPTS To revise the laws of reflection at plane surface and the characteristics of image formed as well as the uses of reflection
QUESTION BANK IN SCIENCE CLASS-X (TERM-II) 10 LIGHT REFLECTION AND REFRACTION CONCEPTS To revise the laws of reflection at plane surface and the characteristics of image formed as well as the uses of reflection
EXPERIMENT O-6. Michelson Interferometer. Abstract. References. Pre-Lab
 EXPERIMENT O-6 Michelson Interferometer Abstract A Michelson interferometer, constructed by the student, is used to measure the wavelength of He-Ne laser light and the index of refraction of a flat transparent
EXPERIMENT O-6 Michelson Interferometer Abstract A Michelson interferometer, constructed by the student, is used to measure the wavelength of He-Ne laser light and the index of refraction of a flat transparent
Infrared Spectroscopy: Theory
 u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
1051-232 Imaging Systems Laboratory II. Laboratory 4: Basic Lens Design in OSLO April 2 & 4, 2002
 05-232 Imaging Systems Laboratory II Laboratory 4: Basic Lens Design in OSLO April 2 & 4, 2002 Abstract: For designing the optics of an imaging system, one of the main types of tools used today is optical
05-232 Imaging Systems Laboratory II Laboratory 4: Basic Lens Design in OSLO April 2 & 4, 2002 Abstract: For designing the optics of an imaging system, one of the main types of tools used today is optical
Physics 30 Worksheet # 14: Michelson Experiment
 Physics 30 Worksheet # 14: Michelson Experiment 1. The speed of light found by a Michelson experiment was found to be 2.90 x 10 8 m/s. If the two hills were 20.0 km apart, what was the frequency of the
Physics 30 Worksheet # 14: Michelson Experiment 1. The speed of light found by a Michelson experiment was found to be 2.90 x 10 8 m/s. If the two hills were 20.0 km apart, what was the frequency of the
Atoms Absorb & Emit Light
 Atoms Absorb & Emit Light Spectra The wavelength of the light that an element emits or absorbs is its fingerprint. Atoms emit and absorb light First Test is Thurs, Feb 1 st About 30 multiple choice questions
Atoms Absorb & Emit Light Spectra The wavelength of the light that an element emits or absorbs is its fingerprint. Atoms emit and absorb light First Test is Thurs, Feb 1 st About 30 multiple choice questions
Physics 41 Chapter 38 HW Key
 Physics 41 Chapter 38 HW Key 1. Helium neon laser light (63..8 nm) is sent through a 0.300-mm-wide single slit. What is the width of the central imum on a screen 1.00 m from the slit? 7 6.38 10 sin θ.11
Physics 41 Chapter 38 HW Key 1. Helium neon laser light (63..8 nm) is sent through a 0.300-mm-wide single slit. What is the width of the central imum on a screen 1.00 m from the slit? 7 6.38 10 sin θ.11
Experiment 3 Lenses and Images
 Experiment 3 Lenses and Images Who shall teach thee, unless it be thine own eyes? Euripides (480?-406? BC) OBJECTIVES To examine the nature and location of images formed by es. THEORY Lenses are frequently
Experiment 3 Lenses and Images Who shall teach thee, unless it be thine own eyes? Euripides (480?-406? BC) OBJECTIVES To examine the nature and location of images formed by es. THEORY Lenses are frequently
Electron Orbits. Binding Energy. centrifugal force: electrostatic force: stability criterion: kinetic energy of the electron on its orbit:
 Electron Orbits In an atom model in which negatively charged electrons move around a small positively charged nucleus stable orbits are possible. Consider the simple example of an atom with a nucleus of
Electron Orbits In an atom model in which negatively charged electrons move around a small positively charged nucleus stable orbits are possible. Consider the simple example of an atom with a nucleus of
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
1 Laboratory #5: Grating Spectrometer
 SIMG-215-20061: LABORATORY #5 1 Laboratory #5: Grating Spectrometer 1.1 Objective: To observe and measure the spectra of different light sources. 1.2 Materials: 1. OSA optics kit. 2. Nikon digital camera
SIMG-215-20061: LABORATORY #5 1 Laboratory #5: Grating Spectrometer 1.1 Objective: To observe and measure the spectra of different light sources. 1.2 Materials: 1. OSA optics kit. 2. Nikon digital camera
Code number given on the right hand side of the question paper should be written on the title page of the answerbook by the candidate.
 Series ONS SET-1 Roll No. Candiates must write code on the title page of the answer book Please check that this question paper contains 16 printed pages. Code number given on the right hand side of the
Series ONS SET-1 Roll No. Candiates must write code on the title page of the answer book Please check that this question paper contains 16 printed pages. Code number given on the right hand side of the
Physics 41, Winter 1998 Lab 1 - The Current Balance. Theory
 Physics 41, Winter 1998 Lab 1 - The Current Balance Theory Consider a point at a perpendicular distance d from a long straight wire carrying a current I as shown in figure 1. If the wire is very long compared
Physics 41, Winter 1998 Lab 1 - The Current Balance Theory Consider a point at a perpendicular distance d from a long straight wire carrying a current I as shown in figure 1. If the wire is very long compared
Understanding astigmatism Spring 2003
 MAS450/854 Understanding astigmatism Spring 2003 March 9th 2003 Introduction Spherical lens with no astigmatism Crossed cylindrical lenses with astigmatism Horizontal focus Vertical focus Plane of sharpest
MAS450/854 Understanding astigmatism Spring 2003 March 9th 2003 Introduction Spherical lens with no astigmatism Crossed cylindrical lenses with astigmatism Horizontal focus Vertical focus Plane of sharpest
Procedure: Geometrical Optics. Theory Refer to your Lab Manual, pages 291 294. Equipment Needed
 Theory Refer to your Lab Manual, pages 291 294. Geometrical Optics Equipment Needed Light Source Ray Table and Base Three-surface Mirror Convex Lens Ruler Optics Bench Cylindrical Lens Concave Lens Rhombus
Theory Refer to your Lab Manual, pages 291 294. Geometrical Optics Equipment Needed Light Source Ray Table and Base Three-surface Mirror Convex Lens Ruler Optics Bench Cylindrical Lens Concave Lens Rhombus
Building your own Spectroscope
 Building your own Spectroscope 0-0.341-0.445-0.606-0.872-1.36 Lyman Balmer Paschen n=4 n=8 n=7 n=6 n=5 n=4 ENERGY/10-19 J -2.42-5.45 E 5 2 E 4 2 E 3 2 E E 5 3 4 3 n=3 n=2 (Many other transitions beyond
Building your own Spectroscope 0-0.341-0.445-0.606-0.872-1.36 Lyman Balmer Paschen n=4 n=8 n=7 n=6 n=5 n=4 ENERGY/10-19 J -2.42-5.45 E 5 2 E 4 2 E 3 2 E E 5 3 4 3 n=3 n=2 (Many other transitions beyond
Polarization of Light
 Polarization of Light References Halliday/Resnick/Walker Fundamentals of Physics, Chapter 33, 7 th ed. Wiley 005 PASCO EX997A and EX999 guide sheets (written by Ann Hanks) weight Exercises and weights
Polarization of Light References Halliday/Resnick/Walker Fundamentals of Physics, Chapter 33, 7 th ed. Wiley 005 PASCO EX997A and EX999 guide sheets (written by Ann Hanks) weight Exercises and weights
waves rays Consider rays of light from an object being reflected by a plane mirror (the rays are diverging): mirror object
 PHYS1000 Optics 1 Optics Light and its interaction with lenses and mirrors. We assume that we can ignore the wave properties of light. waves rays We represent the light as rays, and ignore diffraction.
PHYS1000 Optics 1 Optics Light and its interaction with lenses and mirrors. We assume that we can ignore the wave properties of light. waves rays We represent the light as rays, and ignore diffraction.
Review for Test 3. Polarized light. Action of a Polarizer. Polarized light. Light Intensity after a Polarizer. Review for Test 3.
 Review for Test 3 Polarized light No equation provided! Polarized light In linearly polarized light, the electric field vectors all lie in one single direction. Action of a Polarizer Transmission axis
Review for Test 3 Polarized light No equation provided! Polarized light In linearly polarized light, the electric field vectors all lie in one single direction. Action of a Polarizer Transmission axis
C) D) As object AB is moved from its present position toward the left, the size of the image produced A) decreases B) increases C) remains the same
 1. For a plane mirror, compared to the object distance, the image distance is always A) less B) greater C) the same 2. Which graph best represents the relationship between image distance (di) and object
1. For a plane mirror, compared to the object distance, the image distance is always A) less B) greater C) the same 2. Which graph best represents the relationship between image distance (di) and object
The Phenomenon of Photoelectric Emission:
 The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
v = fλ PROGRESSIVE WAVES 1 Candidates should be able to :
 PROGRESSIVE WAVES 1 Candidates should be able to : Describe and distinguish between progressive longitudinal and transverse waves. With the exception of electromagnetic waves, which do not need a material
PROGRESSIVE WAVES 1 Candidates should be able to : Describe and distinguish between progressive longitudinal and transverse waves. With the exception of electromagnetic waves, which do not need a material
Fraunhofer Diffraction
 Physics 334 Spring 1 Purpose Fraunhofer Diffraction The experiment will test the theory of Fraunhofer diffraction at a single slit by comparing a careful measurement of the angular dependence of intensity
Physics 334 Spring 1 Purpose Fraunhofer Diffraction The experiment will test the theory of Fraunhofer diffraction at a single slit by comparing a careful measurement of the angular dependence of intensity
Experiment 5. Lasers and laser mode structure
 Northeastern University, PHYS5318 Spring 2014, 1 1. Introduction Experiment 5. Lasers and laser mode structure The laser is a very important optical tool that has found widespread use in science and industry,
Northeastern University, PHYS5318 Spring 2014, 1 1. Introduction Experiment 5. Lasers and laser mode structure The laser is a very important optical tool that has found widespread use in science and industry,
Efficiency, Dispersion and Straylight Performance Tests of Immersed Gratings for High Resolution Spectroscopy in the Near Infra-red
 Changing the economics of space Efficiency, Dispersion and Straylight Performance Tests of Immersed Gratings for High Resolution Spectroscopy in the Near Infra-red J. Fernandez-Saldivar 1, F. Culfaz 1,
Changing the economics of space Efficiency, Dispersion and Straylight Performance Tests of Immersed Gratings for High Resolution Spectroscopy in the Near Infra-red J. Fernandez-Saldivar 1, F. Culfaz 1,
Cathode Ray Tube. Introduction. Functional principle
 Introduction The Cathode Ray Tube or Braun s Tube was invented by the German physicist Karl Ferdinand Braun in 897 and is today used in computer monitors, TV sets and oscilloscope tubes. The path of the
Introduction The Cathode Ray Tube or Braun s Tube was invented by the German physicist Karl Ferdinand Braun in 897 and is today used in computer monitors, TV sets and oscilloscope tubes. The path of the
Refractive Index Measurement Principle
 Refractive Index Measurement Principle Refractive index measurement principle Introduction Detection of liquid concentrations by optical means was already known in antiquity. The law of refraction was
Refractive Index Measurement Principle Refractive index measurement principle Introduction Detection of liquid concentrations by optical means was already known in antiquity. The law of refraction was
Care and Use of the Compound Microscope
 Revised Fall 2011 Care and Use of the Compound Microscope Objectives After completing this lab students should be able to 1. properly clean and carry a compound and dissecting microscope. 2. focus a specimen
Revised Fall 2011 Care and Use of the Compound Microscope Objectives After completing this lab students should be able to 1. properly clean and carry a compound and dissecting microscope. 2. focus a specimen
STAAR Science Tutorial 30 TEK 8.8C: Electromagnetic Waves
 Name: Teacher: Pd. Date: STAAR Science Tutorial 30 TEK 8.8C: Electromagnetic Waves TEK 8.8C: Explore how different wavelengths of the electromagnetic spectrum such as light and radio waves are used to
Name: Teacher: Pd. Date: STAAR Science Tutorial 30 TEK 8.8C: Electromagnetic Waves TEK 8.8C: Explore how different wavelengths of the electromagnetic spectrum such as light and radio waves are used to
Friday 18 January 2013 Morning
 Friday 18 January 2013 Morning AS GCE PHYSICS B (ADVANCING PHYSICS) G492/01 Understanding Processes / Experimentation and Data Handling *G411640113* Candidates answer on the Question Paper. OCR supplied
Friday 18 January 2013 Morning AS GCE PHYSICS B (ADVANCING PHYSICS) G492/01 Understanding Processes / Experimentation and Data Handling *G411640113* Candidates answer on the Question Paper. OCR supplied
DOING PHYSICS WITH MATLAB COMPUTATIONAL OPTICS RAYLEIGH-SOMMERFELD DIFFRACTION INTEGRAL OF THE FIRST KIND
 DOING PHYSICS WITH MATLAB COMPUTATIONAL OPTICS RAYLEIGH-SOMMERFELD DIFFRACTION INTEGRAL OF THE FIRST KIND THE THREE-DIMENSIONAL DISTRIBUTION OF THE RADIANT FLUX DENSITY AT THE FOCUS OF A CONVERGENCE BEAM
DOING PHYSICS WITH MATLAB COMPUTATIONAL OPTICS RAYLEIGH-SOMMERFELD DIFFRACTION INTEGRAL OF THE FIRST KIND THE THREE-DIMENSIONAL DISTRIBUTION OF THE RADIANT FLUX DENSITY AT THE FOCUS OF A CONVERGENCE BEAM
Efficiency of a Light Emitting Diode
 PHYSICS THROUGH TEACHING LABORATORY VII Efficiency of a Light Emitting Diode RAJESH B. KHAPARDE AND SMITHA PUTHIYADAN Homi Bhabha Centre for Science Education Tata Institute of Fundamental Research V.
PHYSICS THROUGH TEACHING LABORATORY VII Efficiency of a Light Emitting Diode RAJESH B. KHAPARDE AND SMITHA PUTHIYADAN Homi Bhabha Centre for Science Education Tata Institute of Fundamental Research V.
Diffraction of Laser Light
 Diffraction of Laser Light No Prelab Introduction The laser is a unique light source because its light is coherent and monochromatic. Coherent light is made up of waves, which are all in phase. Monochromatic
Diffraction of Laser Light No Prelab Introduction The laser is a unique light source because its light is coherent and monochromatic. Coherent light is made up of waves, which are all in phase. Monochromatic
2 Absorbing Solar Energy
 2 Absorbing Solar Energy 2.1 Air Mass and the Solar Spectrum Now that we have introduced the solar cell, it is time to introduce the source of the energy the sun. The sun has many properties that could
2 Absorbing Solar Energy 2.1 Air Mass and the Solar Spectrum Now that we have introduced the solar cell, it is time to introduce the source of the energy the sun. The sun has many properties that could
Measuring. User Manual
 0 1 2 3 4 5 6 7 8 9 10 11 Measuring User Manual Accessories for measuring tasks Stage micrometer (1) for calibration Graticules with various measuring pitches (2) in mm and inches Graticule with mesh (3)
0 1 2 3 4 5 6 7 8 9 10 11 Measuring User Manual Accessories for measuring tasks Stage micrometer (1) for calibration Graticules with various measuring pitches (2) in mm and inches Graticule with mesh (3)
1 of 9 2/9/2010 3:38 PM
 1 of 9 2/9/2010 3:38 PM Chapter 23 Homework Due: 8:00am on Monday, February 8, 2010 Note: To understand how points are awarded, read your instructor's Grading Policy. [Return to Standard Assignment View]
1 of 9 2/9/2010 3:38 PM Chapter 23 Homework Due: 8:00am on Monday, February 8, 2010 Note: To understand how points are awarded, read your instructor's Grading Policy. [Return to Standard Assignment View]
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS
 PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
Acousto-optic modulator
 1 of 3 Acousto-optic modulator F An acousto-optic modulator (AOM), also called a Bragg cell, uses the acousto-optic effect to diffract and shift the frequency of light using sound waves (usually at radio-frequency).
1 of 3 Acousto-optic modulator F An acousto-optic modulator (AOM), also called a Bragg cell, uses the acousto-optic effect to diffract and shift the frequency of light using sound waves (usually at radio-frequency).
ENGINEERING METROLOGY
 ENGINEERING METROLOGY ACADEMIC YEAR 92-93, SEMESTER ONE COORDINATE MEASURING MACHINES OPTICAL MEASUREMENT SYSTEMS; DEPARTMENT OF MECHANICAL ENGINEERING ISFAHAN UNIVERSITY OF TECHNOLOGY Coordinate Measuring
ENGINEERING METROLOGY ACADEMIC YEAR 92-93, SEMESTER ONE COORDINATE MEASURING MACHINES OPTICAL MEASUREMENT SYSTEMS; DEPARTMENT OF MECHANICAL ENGINEERING ISFAHAN UNIVERSITY OF TECHNOLOGY Coordinate Measuring
Fiber Optics: Fiber Basics
 Photonics Technical Note # 21 Fiber Optics Fiber Optics: Fiber Basics Optical fibers are circular dielectric wave-guides that can transport optical energy and information. They have a central core surrounded
Photonics Technical Note # 21 Fiber Optics Fiber Optics: Fiber Basics Optical fibers are circular dielectric wave-guides that can transport optical energy and information. They have a central core surrounded
5. The Nature of Light. Does Light Travel Infinitely Fast? EMR Travels At Finite Speed. EMR: Electric & Magnetic Waves
 5. The Nature of Light Light travels in vacuum at 3.0. 10 8 m/s Light is one form of electromagnetic radiation Continuous radiation: Based on temperature Wien s Law & the Stefan-Boltzmann Law Light has
5. The Nature of Light Light travels in vacuum at 3.0. 10 8 m/s Light is one form of electromagnetic radiation Continuous radiation: Based on temperature Wien s Law & the Stefan-Boltzmann Law Light has
Crystal Optics of Visible Light
 Crystal Optics of Visible Light This can be a very helpful aspect of minerals in understanding the petrographic history of a rock. The manner by which light is transferred through a mineral is a means
Crystal Optics of Visible Light This can be a very helpful aspect of minerals in understanding the petrographic history of a rock. The manner by which light is transferred through a mineral is a means
Holography 1 HOLOGRAPHY
 Holography 1 HOLOGRAPHY Introduction and Background The aesthetic appeal and commercial usefulness of holography are both related to the ability of a hologram to store a three-dimensional image. Unlike
Holography 1 HOLOGRAPHY Introduction and Background The aesthetic appeal and commercial usefulness of holography are both related to the ability of a hologram to store a three-dimensional image. Unlike
Experiment 2 - Grating Spectrometer
 Experiment 2 - Grating Spectrometer References: Optics by Eugene Hecht. Section 10.2.8 contains a good discussion of gratings and grating spectroscopy. Quantum Physics of Atoms, Molecules, Solids, Nuclei,
Experiment 2 - Grating Spectrometer References: Optics by Eugene Hecht. Section 10.2.8 contains a good discussion of gratings and grating spectroscopy. Quantum Physics of Atoms, Molecules, Solids, Nuclei,
Bohr's Theory of the Hydrogen Atom
 OpenStax-CNX module: m42596 1 Bohr's Theory of the Hydrogen Atom OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Describe
OpenStax-CNX module: m42596 1 Bohr's Theory of the Hydrogen Atom OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Describe
THE COMPOUND MICROSCOPE
 THE COMPOUND MICROSCOPE In microbiology, the microscope plays an important role in allowing us to see tiny objects that are normally invisible to the naked eye. It is essential for students to learn how
THE COMPOUND MICROSCOPE In microbiology, the microscope plays an important role in allowing us to see tiny objects that are normally invisible to the naked eye. It is essential for students to learn how
Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs
 Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs TracePro Opto-Mechanical Design Software s Fluorescence Property Utility TracePro s Fluorescence Property
Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs TracePro Opto-Mechanical Design Software s Fluorescence Property Utility TracePro s Fluorescence Property
3.5.4.2 One example: Michelson interferometer
 3.5.4.2 One example: Michelson interferometer mirror 1 mirror 2 light source 1 2 3 beam splitter 4 object (n object ) interference pattern we either observe fringes of same thickness (parallel light) or
3.5.4.2 One example: Michelson interferometer mirror 1 mirror 2 light source 1 2 3 beam splitter 4 object (n object ) interference pattern we either observe fringes of same thickness (parallel light) or
About Coffee and Refractometers 2008-2010 Voice Systems Technology, Inc. (VST)
 About Coffee and Refractometers 2008-200 Voice Systems Technology, Inc. (VST) www.mojotogo.us.0 Coffee and Refractive Index Refractive Index measurements have been used for process control in the food
About Coffee and Refractometers 2008-200 Voice Systems Technology, Inc. (VST) www.mojotogo.us.0 Coffee and Refractive Index Refractive Index measurements have been used for process control in the food
2 Spectrophotometry and the Analysis of Riboflavin
 2 Spectrophotometry and the Analysis of Riboflavin Objectives: A) To become familiar with operating the Platereader; B) to learn how to use the Platereader in determining the absorption spectrum of a compound
2 Spectrophotometry and the Analysis of Riboflavin Objectives: A) To become familiar with operating the Platereader; B) to learn how to use the Platereader in determining the absorption spectrum of a compound
Solution Derivations for Capa #14
 Solution Derivations for Capa #4 ) An image of the moon is focused onto a screen using a converging lens of focal length (f = 34.8 cm). The diameter of the moon is 3.48 0 6 m, and its mean distance from
Solution Derivations for Capa #4 ) An image of the moon is focused onto a screen using a converging lens of focal length (f = 34.8 cm). The diameter of the moon is 3.48 0 6 m, and its mean distance from
A NEW LOOK AT RISLEY PRISMS. By Craig Schwarze Senior Systems Engineer OPTRA Inc.
 As seen in Photonics Spectra June 2006: A NEW LOOK AT RISLEY PRISMS By Craig Schwarze Senior Systems Engineer OPTRA Inc. Introduction With laser beams finding more and more applications that require aiming
As seen in Photonics Spectra June 2006: A NEW LOOK AT RISLEY PRISMS By Craig Schwarze Senior Systems Engineer OPTRA Inc. Introduction With laser beams finding more and more applications that require aiming
RAY OPTICS II 7.1 INTRODUCTION
 7 RAY OPTICS II 7.1 INTRODUCTION This chapter presents a discussion of more complicated issues in ray optics that builds on and extends the ideas presented in the last chapter (which you must read first!)
7 RAY OPTICS II 7.1 INTRODUCTION This chapter presents a discussion of more complicated issues in ray optics that builds on and extends the ideas presented in the last chapter (which you must read first!)
19 - RAY OPTICS Page 1 ( Answers at the end of all questions )
 19 - RAY OPTICS Page 1 1 ) A ish looking up through the water sees the outside world contained in a circular horizon. I the reractive index o water is 4 / 3 and the ish is 1 cm below the surace, the radius
19 - RAY OPTICS Page 1 1 ) A ish looking up through the water sees the outside world contained in a circular horizon. I the reractive index o water is 4 / 3 and the ish is 1 cm below the surace, the radius
Sample Exercise 6.1 Concepts of Wavelength and Frequency
 Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Radiation Transfer in Environmental Science
 Radiation Transfer in Environmental Science with emphasis on aquatic and vegetation canopy media Autumn 2008 Prof. Emmanuel Boss, Dr. Eyal Rotenberg Introduction Radiation in Environmental sciences Most
Radiation Transfer in Environmental Science with emphasis on aquatic and vegetation canopy media Autumn 2008 Prof. Emmanuel Boss, Dr. Eyal Rotenberg Introduction Radiation in Environmental sciences Most
