21.1 An Introduction to Carbohydrates
|
|
|
- Jeffrey Norris
- 7 years ago
- Views:
Transcription
1 21.1 An Introduction to Carbohydrates Carbohydrates are a large class of naturally occurring polyhydroxy aldehydes and ketones. Monosaccharides, or simple sugars, have from three to seven carbon atoms, and one aldehyde or one ketone group. If the sugar has an aldehyde group, it is an aldose. If it has a ketone group, the sugar is classified as a ketose.
2 21.1 An Introduction to Carbohydrates
3 21.1 An Introduction to Carbohydrates Monosaccharides undergo a variety of structural changes and chemical reactions. They form disaccharides and polysaccharides (complex carbohydrates), which are polymers of monosaccharides. Functional groups are involved in reactions with alcohols, lipids, or proteins to form biomolecules.
4 21.2 Handedness of Carbohydrates Chiral compounds lack a plane of symmetry and exist as a pair of enantiomers in either a right-handed D- form or a left-handed L- form.
5 21.2 Handedness of Carbohydrates
6 21.2 Handedness of Carbohydrates Aldotetroses have two chiral carbon atoms and can exist in four isomeric forms.
7 21.2 Handedness of Carbohydrates By convention the carbonyl group and the terminal CH 2 OH are drawn pointing to the right. In general, a compound with n chiral carbon atoms has a maximum of 2 n possible stereoisomers and half that many pairs of enantiomers. In some cases, fewer than the maximum predicted number of stereoisomers exist because some of the molecules have symmetry planes that make them identical to their mirror images.
8 21.3 The D and L Families of Sugars: Drawing Sugar Molecules In a Fischer projection, the aldehyde or ketone group is always placed at the top. The result is that H and OH groups projecting above the page are on the left and right of the chiral carbons, and groups projecting behind the page are above and below the chiral carbons.
9 21.3 The D and L Families of Sugars: Drawing Sugar Molecules Monosaccharides are divided into D sugars and L sugars based on their structural relationships to glyceraldehyde.
10 21.3 The D and L Families of Sugars: Drawing Sugar Molecules In the D form, the OH group on carbon 2 comes out of the plane of the paper and points to the right; in the L form, the OH group at carbon 2 comes out of the plane of the paper and points to the left.
11 21.3 The D and L Families of Sugars: Drawing Sugar Molecules D and L derive from the Latin dextro for right and levo for left. In Fischer projections, the D form of a monosaccharide has the hydroxyl group on the chiral carbon atom farthest from the carbonyl group pointing toward the right, whereas the L form has the hydroxyl group on this carbon pointing toward the left.
12 21.3 The D and L Families of Sugars: Drawing Sugar Molecules Chirality and Drugs The goal of modern drug development is creation of a drug molecule that binds with a specific hormone, enzyme, or cellular receptor. Because most biomolecules are chiral, a chiral drug molecule (single isomer) is likely to meet the need most effectively. The route to a chiral drug molecule begins with chiral reactants and an enzyme, or with chemical synthesis of a pair of enantiomers that are then separated from each other. Enantiomers can have different effects. the active ingredient in the pain killer and anti-inflammatory Aleve, is sold as a single enantiomer. The other enantiomer causes liver damage.
13 21.4 Structure of Glucose and Other Monosaccharides Monosaccharides with five or six carbon atoms exist primarily in cyclic forms when in solution. Aldehydes and ketones react reversibly with alcohols to yield hemiacetals.
14 21.4 Structure of Glucose and Other Monosaccharides
15 21.4 Structure of Glucose and Other Monosaccharides D-Glucose can exist as an open-chain hydroxy aldehyde or as a pair of cyclic hemiacetals. The cyclic forms differ only at C1, where the OH group is either on the opposite side of the sixmembered ring from the CH 2 OH (a) or on the same side (b). The CH 2 OH group in D sugars is always above the plane of the ring. Cyclic monosaccharides that differ only in the positions of substituents at carbon 1 are known as anomers, and carbon 1 is said to be an anomeric carbon atom.
16 21.4 Structure of Glucose and Other Monosaccharides Although the structural difference between anomers appears small, it has enormous biological consequences. Crystalline glucose is entirely in the cyclic a form. In water, equilibrium is established among the open-chain form and the two anomers through mutarotation.
17 21.4 Structure of Glucose and Other Monosaccharides Monosaccharide Structures Summary Monosaccharides are polyhydroxy aldehydes or ketones. Monosaccharides have three to seven carbon atoms, and a maximum of 2 n possible stereoisomers, where n is the number of chiral carbon atoms. D and L enantiomers differ in the orientation of the OH group on the chiral carbon atom farthest from the carbonyl. In Fischer projections, D sugars have the OH on the right and L sugars have the OH on the left. D-Glucose (and other 6-carbon aldoses) forms cyclic hemiacetals conventionally represented so that OH groups on chiral carbons on the left in Fischer projections point up and those on the right in Fischer projections point down. In glucose, the hemiacetal carbon (the anomeric carbon) is chiral, and a and b anomers differ in the orientation of the OH groups on this carbon. The a anomer has the OH on the opposite side from the CH 2 OH and the b anomer has the OH on the same side as the CH 2 OH.
18 21.5 Some Important Monosaccharides Monosaccharides can form multiple hydrogen bonds, and are generally high-melting, white, crystalline solids that are soluble in water and insoluble in nonpolar solvents. Most monosaccharides and disaccharides are sweet-tasting, digestible, and nontoxic. Except for glyceraldehyde (an aldotriose) and fructose (a ketohexose), the carbohydrates of interest in human biochemistry are all aldohexoses or aldopentoses. Most are in the D family.
19 21.5 Some Important Monosaccharides Glucose Glucose is the most important simple carbohydrate in human metabolism. It is the final product of complex carbohydrate digestion and provides acetyl groups for entry into the citric acid cycle as acetyl-coa. Maintenance of an appropriate blood glucose level is essential to human health.
20 21.5 Some Important Monosaccharides Galactose D-Galactose is widely distributed in plant gums and pectins, and is a component of the disaccharide lactose. Galactose is an aldohexose; it differs from glucose only in the spatial orientation of the OH group at carbon 4. In the body, galactose is converted to glucose to provide energy and is synthesized from glucose as needed.
21 21.5 Some Important Monosaccharides Fructose D-Fructose, often called levulose or fruit sugar, occurs in honey and many fruits. It is one of the two monosaccharides combined in the disaccharide sucrose. It is a ketohexose rather than an aldohexose. In solution, fructose forms five-membered rings:
22 21.5 Some Important Monosaccharides Ribose and 2-Deoxyribose Ribose and its relative 2-deoxyribose are both 5-carbon aldehyde sugars. These two sugars are most important as parts of larger biomolecules. Ribose is a constituent of coenzyme A, ATP, oxidizing and reducing agent coenzymes and cyclic AMP. 2-deoxyribose differs from ribose by the absence of one oxygen atom, that in the OH group at C2.
23 21.5 Some Important Monosaccharides CELL-SURFACE CARBOHYDRATES AND BLOOD TYPE Human blood can be classified into four blood group types, called A, B, AB, and O, based on the presence of three different oligosaccharide units, designated A, B, and O. Among the targets of antibodies are cell-surface molecules that not present on the individual s own cells, thus foreign blood cells. Type O cell-surface oligosaccharides are similar in composition to those of types A and B. People with blood types A, B, and AB all lack antibodies to type O cells making individuals with type O blood universal donors. People with type AB blood have both A and B molecules on their red cells. Their blood contains no antibodies to A, B, or O, and they can, if necessary, receive blood of all types.
24 21.6 Reactions of Monosaccharides Reaction with Oxidizing Agents: Reducing Sugars Aldehydes can be oxidized to carboxylic acids (RCHO RCOOH). As the open-chain aldehyde is oxidized, its equilibrium with the cyclic form is displaced, so that the open-chain form continues to be produced. Carbohydrates that react with mild oxidizing agents are classified as reducing sugars.
25 21.6 Reactions of Monosaccharides Reaction with Oxidizing Agents: Reducing Sugars In basic solution, ketones can undergo rearrangement and will also act as reducing sugars. Keto enol tautomerism is an equilibrium that results from a shift in position of a hydrogen atom and a double bond. It is possible whenever there is a hydrogen atom on a carbon adjacent to a carbonyl carbon.
26 21.6 Reactions of Monosaccharides Reaction with Alcohols: Glycoside and Disaccharide Formation Hemiacetals react with alcohols with the loss of water to yield acetals, compounds with two OR groups bonded to the same carbon. Because glucose and other monosaccharides are cyclic hemiacetals, they also react with alcohols to form acetals, which are called glycosides.
27 21.6 Reactions of Monosaccharides In larger molecules monosaccharides are connected to each other by glycosidic bonds. The reverse of this reaction is a hydrolysis.
28 21.6 Reactions of Monosaccharides Formation of Phosphate Esters of Alcohols Phosphate esters of alcohols contain a PO 3 2 group bonded to the oxygen atom of an OH group. The OH groups of sugars can add PO 3 2 groups to form phosphate esters in the same manner. Phosphate esters of monosaccharides appear as reactants and products throughout the metabolism of carbohydrates.
29 21.7 Disaccharides Disaccharide Structure The two monosaccharides in a disaccharide are connected by a glycosidic bond. The bond may be a or b as in cyclic monosaccharides. The structures include glycosidic bonds that create a 1,4 link between C1 of one monosaccharide and C4 of the second monosaccharide.
30 21.7 Disaccharides Maltose Maltose can be prepared by enzyme-catalyzed degradation of starch. Two a-d-glucose molecules are joined in maltose by an a-1,4 link. It is both an acetal (at C1 in the glucose on the left) and a hemiacetal (at C1 in the glucose on the right), making maltose a reducing sugar.
31 21.7 Disaccharides Lactose Lactose, or milk sugar, is the major carbohydrate in mammalian milk. Human milk, for example, is about 7% lactose. Structurally, lactose is composed of D-galactose and D-glucose. The two monosaccharides are connected by a b-1,4 link. Like maltose, lactose is a reducing sugar because the glucose ring (on the right in the following structure) is a hemiacetal at C1.
32 21.7 Disaccharides Sucrose Hydrolysis of sucrose yields one molecule of D-glucose and one molecule of D-fructose. The 50:50 mixture of glucose and fructose that results, invert sugar, is sweeter than sucrose. Sucrose has no hemiacetal group because a 1,2 link joins both anomeric carbon atoms (C1 on glucose, C2 on fructose). The absence of a hemiacetal group means that sucrose is not a reducing sugar.
33 21.7 Disaccharides Carbohydrates and Fiber in the Diet The major monosaccharides in our diets are fructose and glucose from fruits and honey. The major disaccharides are sucrose and lactose. Our diets contain large amounts of the complex carbohydrate starch and the indigestible polysaccharide cellulose. Cellulose and all other indigestible carbohydrates are collectively known as dietary fiber. Consumption of the more easily digested carbohydrates results in rapid elevation of blood glucose levels followed by lower-than-desired levels a few hours later. Carbohydrates that are digested and absorbed more slowly are associated with healthier blood sugar responses. The Nutrition Facts labels on packaged foods give percentages based on a recommended 300 g per day of total carbohydrate and 25 g per day of dietary fiber. Pectins and vegetable gums comprise the soluble portion of dietary fiber. Hemicellulose and lignin are the major components of insoluble fiber.
34 21.8 Variations on the Carbohydrate Theme Variations on carbohydrates often incorporate modified glucose molecules.
35 21.8 Variations on the Carbohydrate Theme Chitin The shells of lobsters, beetles, and spiders are made of chitin, the second most abundant polysaccharide in the natural world. (Cellulose is the most abundant.) Chitin is a hard, structural polymer. It is composed of N-acetyl-D-glucosamine rather than glucose but is otherwise identical to cellulose.
36 21.8 Variations on the Carbohydrate Theme Connective Tissue and Polysaccharides Connective tissues are composed of protein fibers in a matrix that contains unbranched mucopolysaccharides. The gel-like mixtures of these polysaccharides with water serve as lubricants and shock absorbers. Hyaluronate molecules contain up to 25,000 disaccharide units. It forms the synovial fluid that lubricates joints and is present within the eye. Chondroitin 6-sulfate (also the 4-sulfate) is present in tendons and cartilage, where it is linked to proteins.
37 21.8 Variations on the Carbohydrate Theme Heparin Heparin is valuable medically as an anticoagulant (an agent that prevents or retards the clotting of blood). Heparin is composed of a variety of different monosaccharides, many of them containing sulfate groups.
38 21.8 Variations on the Carbohydrate Theme Glycoproteins Proteins that contain short carbohydrate chains (oligosaccharide chains) are glycoproteins. The carbohydrate is connected to the protein by a glycosidic bond between an anomeric carbon and a side chain of the protein. The oligosaccharide chains function as receptors or, in one case, antifreeze.
39 21.9 Some Important Polysaccharides Polysaccharides are polymers of tens, hundreds, or even many thousands of monosaccharides linked together through glycosidic bonds.
40 21.9 Some Important Polysaccharides Cellulose Cellulose provides structure in plants. Each molecule consists of several thousand D-glucose units joined in a long, straight chain by b-1,4 links. Because of the tetrahedral bonding at each carbon atom, the carbohydrate rings bent up at one end and down at the other in what is known as the chair conformation.
41 21.9 Some Important Polysaccharides The hydrogen bonds within chains and between chains (shown in red) contribute to the rigidity and toughness of cellulose fibers. Grazing animals, termites, and moths are able to digest cellulose because microorganisms colonizing their digestive tracts produce enzymes that hydrolyze b-glycosidic bonds. Humans neither produce such enzymes nor harbor such organisms, and therefore cannot hydrolyze cellulose.
42 21.9 Some Important Polysaccharides Starch In starch, individual glucose units are joined by a-1,4 links rather than by the b-1,4 links of cellulose. Starch is fully digestible and is an essential part of the human diet. It is present only in plant material; our major sources are beans, wheat and rice, and potatoes.
43 21.9 Some Important Polysaccharides Starch Amylose, accounts for about 20% of starch. It is somewhat soluble in hot water and consists of several hundred to a thousand D-glucose units linked in long chains. Amylose tends to coil into helices.
44 21.9 Some Important Polysaccharides Starch Amylopectin accounts for about 80% of starch. It has up to 100,000 glucose units per molecule and a-1,6 branches approximately every 25 units along its chain. It is not soluble.
45 21.9 Some Important Polysaccharides Glycogen Glycogen stores energy in animals in the liver and muscles. Structurally, glycogen is similar to amylopectin in being a long polymer of D-glucose with the same type of branch points in its chain. Glycogen has many more branches and contains up to one million glucose units per molecule.
46 21.9 Some Important Polysaccharides Cell Walls: Rigid Defense Systems Bacteria and higher plants surround the plasma membrane with a rigid cell wall. The rigidity of the wall prevents the cell from bursting due to osmotic pressure, gives shape to the cell, and protects it from pathogens. Plant cell walls are composed of fibrils of cellulose in a polymer matrix of pectins, lignin, and hemicellulose. Bacterial cell walls provide a rigid platform for the attachment of flagella and pilli. They do not contain cellulose. A majority of bacterial cell walls are composed of a polymer of peptidoglycan. Animals have developed natural defenses that can control many bacteria. For example, lysozyme an enzyme found naturally in tears, saliva, and egg white hydrolyzes the cell wall of pathogenic bacteria. Penicillin contains a beta-lactam ring that acts as a suicide inhibitor of the enzymes that synthesize the peptidoglycan cross-linking peptide chain. Unfortunately, many bacteria have developed enzymes that destroy the ring, granting resistance to penicillin.
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Solid Phase Peptide Synthesis
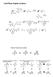 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Structure of proteins
 Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Lipids. There are 2 types of lipids; those that contain the structural component of a fatty acid; and
 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted from cells using
Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted from cells using
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline
 AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Qualitative Testing for Carbohydrates
 R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Fatty Acids carboxylic acids
 Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Reactions of Fats and Fatty Acids
 Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Digestive System Functions
 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
(Woods) Chem-131 Lec-19 09-4 Lipids 1. Lipids:
 (Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Classifying Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, p sphingolipids, p and cholesterol
(Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Classifying Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, p sphingolipids, p and cholesterol
The correct answer is d C. Answer c is incorrect. Reliance on the energy produced by others is a characteristic of heterotrophs.
 1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
CHEM 121. Chapter 19, Name: Date:
 CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
CHAPTER 6 AN INTRODUCTION TO METABOLISM. Section B: Enzymes
 CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
Lipids. Classes of Lipids. Types of Lipids. Saturated and Unsaturated Fatty Acids. Fatty Acids. 15.1 Lipids 15.2 Fatty Acids
 hapter 15 15.1 15.2 Fatty Acids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted
hapter 15 15.1 15.2 Fatty Acids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted
McMush. Testing for the Presence of Biomolecules
 Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Nucleotides and Nucleic Acids
 Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Name Date Period. Keystone Review Enzymes
 Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Summary of Metabolism. Mechanism of Enzyme Action
 Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Organic Functional Groups Chapter 7. Alcohols, Ethers and More
 Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because:
 Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
Methods of Grading S/N Style of grading Percentage Score 1 Attendance, class work and assignment 10 2 Test 20 3 Examination 70 Total 100
 COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
PHOTOSYNTHESIS AND CELLULAR RESPIRATION
 reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
Copyright 2010 Pearson Education, Inc. Chapter Twenty Three 1
 23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d.
 1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
CHEM 2353 Fundamentals of Organic Chemistry. Carbohydrates. Organic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R.
 hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
