Larval organogenesis of Pagrus pagrus L., 1758 with special attention to the digestive system development
|
|
|
- Brenda Hampton
- 8 years ago
- Views:
Transcription
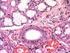
1 Histol Histopathol (2007) 22: Histology and Histopathology Cellular and Molecular Biology Larval organogenesis of Pagrus pagrus L., 1758 with special attention to the digestive system development M.J. Darias, J.B. Ortiz-Delgado, C. Sarasquete, G. Martínez-Rodríguez and M. Yúfera Andalusian Institute for Marine Sciences (CSIC), Puerto Real, Cadiz, Spain Summary. Organogenesis of the red porgy (Pagrus pagrus L., 1758) was examined from hatching until 63 days post-hatching (dph) using histological and histochemical techniques. At hatching, the heart appeared as a tubular structure which progressively developed into four differentiated regions at 2 dph: bulbus arteriosus, atrium, ventricle and sinus venosus. First ventricle and atrium trabeculae were appreciated at 6 and 26 dph, respectively. Primordial gill arches were evident at 2 dph. Primordial filaments and first lamellae were observed at 6 and 15 dph, respectively. At mouth opening (3dph), larvae exhausted their yolk-sac reserves. The pancreatic zymogen granules appeared at 6 dph. Glycogen granules, proteins and neutral lipids (vacuoles in paraffin sections) were detected in the cytoplasm of the hepatocytes from 4-6 dph. Hepatic sinusoids could be observed from 9 dph. Pharyngeal and buccal teeth were observed at 9 and 15 dph, respectively. Oesophageal goblet cells appeared around 6 dph, containing neutral and acid mucosubstances. An incipient stomach could be distinguished at 2 dph. The first signs of gastric gland development were detected at 26 dph, increasing in number and size by dph. Gastric glands were concentrated in the cardiac stomach region and presented a high content of protein rich in tyrosine, arginine and tryptophan. The intestinal mucous cells appeared at 15 dph and contained neutral and acid glycoconjugates, the carboxylated mucins being more abundant than the sulphated ones. Acidophilic supranuclear inclusions in the intestinal cells of the posterior intestine, related to pynocitosis of proteins, were observed at 4-6 dph. Key words: Gut development, Pagrus pagrus, Red porgy, Histology, Histochemistry, Fish larvae Offprint requests to: Dr. María J. Darias, Instituto de Ciencias Marinas de Andalucía (CSIC), Apartado Oficial 11510, Puerto Real, Cádiz, Spain. mariajose.darias@icman.csic.es Introduction Generally, teleosts present at hatching an undifferentiated digestive tract without any aperture to the exterior which develops in the following days to be ready for digestion at the beginning of the exogenous feeding. Meanwhile, energy needed for the metabolism of maintenance, swimming activity and growth is obtained from the yolk-sac reserves. In carnivorous species, proteins are the main dietary component that is firstly digested by alkaline proteases secreted by the exocrine pancreas (Kurokawa and Suzuki, 1996) and by intracellular proteases of the enterocytes (Zambonino- Infante and Cahu, 2001). A more efficient acidic protein digestion is carried out in the stomach when gastric glands are fully developed and synthesize pepsin, which is the main proteolytic enzyme acting at the end of the larval period and supposes the switch to the adult mode of digestion (Moyano et al., 1996; Douglas et al., 2000; Gawlicka et al., 2001; Zambonino-Infante and Cahu, 2001; Yúfera et al., 2004). It is assumed that fish larvae digestive efficiency is related to the presence or lack of gastric glands and to the proteolytic action of pancreatic juice but, interestingly, it also depends on goblet cell secretion (Kapoor et al., 1975; Sarasquete et al., 2001). It is well known that acid and/or neutral mucosubstances play an important role in several food-processing activities, such as lubricant or protective function, in osmoregulation, and possess a crucial role in regulating the interactions between cells and their microenvironment that are of great importance for the maintenance of differentiated cellular function. Even, for instance, some sulphated mucins have been identified as an important constituent of the mitogenic stimulus controlling cell growth (Gallagher et al., 1986; Parillo et al., 2004). The high mortality percentage obtained during the larval and fry stages of some fish species when they are intensively reared in aquaculture may be due to an incomplete secretory activity of the gut (Domeneghini et al., 1998). In spite of all this knowledge, there are inter-specific
2 754 Organogenesis of the red porgy larvae variations in the timing of organ formation and maturation. Therefore, it becomes necessary to carry out specific organogenesis studies for each species to optimise fish larval rearing techniques. Red porgy (Pagrus pagrus) is a marine teleost, belonging to the Sparidae family, with tropical and subtropical amphiatlantic distribution, specially located in the Mediterranean coasts, where it presents a high commercial value. This species is considered a good candidate to contribute to fish farming expansion. However, few studies have been made on its larval development (Kolios et al., 1997; Hernández-Cruz et al., 1999; Roo et al., 1999; Mihelakakis et al., 2001; Darias et al., 2005, 2006), which is the base of the rearing process to obtain a stable production. The present work aimed to analyse larval organogenesis of the red porgy using histological and histochemical techniques in order to determine the degree of morphological development and functionality of the digestive organs, which is correlated with the digestive enzyme ontogeny (Oozeki and Bailey, 1995; Hidalgo et al., 1999; Martínez et al., 1999; Ribeiro et al., 1999a,b; Gawlicka et al., 2000; Kim et al., 2001; Rojas-García et al., 2001). Thus, an adequate food supply according to their digestive capacity can be established based on this knowledge in order to optimise survival and growth during the larval period. Materials and methods Rearing conditions and larval growth Eggs of red porgy were obtained by natural spawning from a captive brood stock maintained at INIAP/IPIMAR (Olhão, Portugal). Newly hatched larvae were transferred to three 300 L tanks with flowthrough water supplied at a constant temperature of 19.5±0.5 C and salinity of 33. Constant illumination was provided during the first two weeks switching to a photoperiod of 12L:12D afterwards. Initial larval density ranged from 30 to 50 larvae L -1. The first 24 h post hatching was considered as day 0. Larvae were initially fed (3 dph) with rotifers (Brachionus plicatilis) at 10 ml -1 (fed with Nannochloropsis gaditana) and then gradually replaced by Artemia sp. nauplii, metanauplii and adults at 2 ml -1 from 23 dph onwards. A daily dose of microalgae (N. gaditana, at 3x10 5 cells ml-1 initial concentration) was added to the rearing tanks during the first month. Groups of 30 larvae (0-60 dph) were sampled from each tank, euthanized with 200 mg L -1 of ethyl-4-amino-benzoate and total length (TL) was measured under stereomicroscope. dehydrated through ethanol series, infiltrated and embedded in paraffin blocks. Each block contained a pool of larvae, the number of which decreased according to the larval size (from 10 larvae at 0 dph to 1 larva at 63 dph). Serial saggital and/or transversal 7 µm thick sections from whole specimens were placed on uncoated glass slides and baked overnight at 40 C. Subsequently, sections were stained with Haematoxylin-Eosin (H-E). Histochemical techniques were performed for identification of carbohydrates (glycogen, mucosubstances and/or glycoconjugates) (Table 1) and proteins rich in different aminoacids (Table 2). All methods and techniques used in this work were taken from Pearse (1985) and Bancroft and Stevens (1990) monographs. Slides were examined and photographed with light microscope (Leitz Etzlar 307). Results Larval growth of the red porgy, in terms of total length, during the experimental period is represented in Fig. 1. The main ontogenetic landmarks of the red porgy digestive system are shown in Fig. 2. Yolk-sac larvae After hatching, yolk-sac is formed by a squamous epithelium surrounding a homogeneous and acidophilic yolk-matrix (Fig. 3A) which contains neutral glycoconjugates, glycogen and proteins rich in tyrosine, arginine, lysine, cysteine and cystine (Table 3). At 2 dph, a pronounced decrease of the yolk-sac volume was observed, showing a fragmentation of the strongly acidophilic yolk-matrix (Fig. 3B). Yolk-sac reserves Histological and histochemical procedures Larvae and post-larvae were sampled in triplicate (three tanks) from hatching until 63 dph (0, 1, 2, 3, 5, 6, 9, 12, 15, 16, 19, 22, 23, 26, 30, 40, 50, 63 dph), fixed at 4 C in buffered formaldehyde (ph 7.2) overnight, Fig. 1. Red porgy larval growth expressed in terms of total length. The sequence of food supply (Brachionus plicatilis and Artemia sp.) is indicated by horizontal arrows under the curve. Data were adjusted to an exponential regression (TL = 3.91 e 0.02 T ; TL = total length, T = days post hatching; r = 0.96).
3 Organogenesis of the red porgy larvae 755 Table 1. Histochemical reactions of carbohydrates analysed in red porgy larvae. Reactions Periodic acid-schiff/pas Diastase-PAS Alcian Blue ph 2.5 Alcian Blue ph 1 Alcian Blue ph 0.5 Esterification / Alcian Blue ph 2.5 Esterification / Saponification / Alcian Blue ph 2.5 Esterification / Alcian Blue ph 0.5 Esterification / Saponification / Alcian Blue ph 0.5 Acid hydrolysis-alcian Blue ph 2.5 Functions and/or demonstrated compound Aldehydes from the oxidation of glycol or aminool contiguous groups Glycogen or neutral glycoconjugates Carboxylated mucosubstances or glycoconjugates (sulphated or not) Carboxylated glycoconjugates sulphated slightly ionized Glycoconjugates sulphated strongly ionized Carboxylated glycoconjugates blockage Carboxylated glycoconjugates reactivation Glycoconjugates sulphated loss (sulphatolysis) Not reactivation of sulphated glycoconjugates (lack of alcianophilia) Sialic acid extraction (breakdown of glycosides linkages) Table 2. Histochemical reactions of proteins analysed in red porgy larvae. Reactions Functions and/or demonstrated compound Bromophenol Blue general proteins Ninhydrin-Schiff proteins rich in lysine (-NH2) Ferric ferricyanide-fe III -SH/cysteine protein rich Thioglycolate reduction -S-S-/cystine protein rich 1,2 napthoquinone-4-sulphonic acid salt sodium proteins rich in arginine Hg-sulphate-sulfuric acid-sodium nitrate proteins rich in tyrosine P-dimethylaminobenzaldehyde proteins rich in tryptophan Fig. 2. Diagram showing the main histological ontogenetic landmarks during the larval development of the red porgy. Dotted lines indicate the age at which the corresponding structure is completely developed (comp. dev.) at histological level and only exhibited an increase in number and size of cells and tissues, respectively.
4 756 Organogenesis of the red porgy larvae were completely exhausted at 3 dph. Newly hatched larvae presented a rectilinear duct that curved in the caudal portion, localized dorsally to the yolk-sac, the lumen of which distended in both extremities without aperture to the exterior. The epithelium of the digestive tract was constituted of squamous cells of different height with a basal nucleus. From 2 dph three intestinal portions could be observed: anterior, medium and posterior (Fig. 3B). The enterocytes showed basal nucleus and cytoplasmic projections to the lumen. At 3 dph mouth and anus opening occurred in synchrony with the total absorption of the maternal reserves and the beginning of the exogenous feeding. At the same time, the growing intestine formed a loop to accommodate in the visceral cavity. Fig. 3. Light micrographs of sections stained with H-E of red porgy larvae from 0 to 6 dph. A. Recently hatched larvae showing a homogeneous yolk-sac matrix and a unique oil globule. The digestive tract is straight and undifferentiated and the mouth is closed. B. A 2 day-old larva showing the digestive system differentiated in buccal cavity, oesophagus and intestine in three portions (anterior, medium and posterior). Yolk-sac volume visibly reduced. C-D. A 6 day-old larva showing a primordial stomach, the first mucous cells in the anterior intestine and a detail of the posterior intestine with supranuclear inclusions. The first pharyngeal teeth, primordial filaments of the gill arches and pronephros are also evident. a, auricle, ai, anterior intestine; ba, bulbus arteriosus; bc, buccal cavity; cc, chloride cell; dt, digestive tract; e, eye; en, encephalon; ga, gill arch; gb, gall bladder; gc, goblet cell; h, heart; l, liver; m, muscle; mc, mucous cell; mi, medium intestine; n, notochord; o, operculum; oe, oesophagus; og, oil globule; p, pancreas; pf, primordial filament; pga, primordial gill arch; pht, pharyngeal tooth; pi, posterior intestine; pt, pronephric tubule; s, stomach; si, supranuclear inclusions; sv, sinus venosus; ub, urinary bladder; v, ventricle; ys, yolk-sac; z, zymogen granules.
5 Organogenesis of the red porgy larvae 757 Buccopharyngeal cavity, gills and pseudobranches Pharyngeal teeth were evident from 9 dph and increased in number and size according to larval development. Maxillary teeth and labial pulps were first observed at 15 dph. Abundant buccal mucous cells were observed at 40 dph. Primordial gill arches were appreciated at 2 dph (Fig. 3B) and four pairs of gill arches were observed at 6 dph, from which cartilaginous tissue (Alcian Blue positive) developed to form primordial filaments (Fig. 3C) in second and third arches firstly. Chloride cells, pillar cells and gill vascular structures were evident at 12 dph (Fig. 4E). Gills were completely formed at 15 dph (Fig. 4H), showing filaments that increased in length and primordial lamellae that increased in number and size throughout larval development (Fig. 4H, 7A, B). Pseudobranches began to differentiate from 2 dph being located in the posterior zone of the gill cavity and behind the eye. Two pairs of filaments were observed at 12 dph. At 22 dph, pseudobranches presented three pairs of filaments that progressively increased in number and length with larval development (Fig. 4D). First lamellae were observed at 26 dph. Oesophagus The oesophagus was located caudal to the pharynx and extended from the last gill-arch to the anterior intestine opening (Fig. 3B). Long longitudinal folds and a loose connective tissue were evidenced in larvae from 3-4 dph. The lumen, constituted by an epithelium of squamous cells, was relatively narrow and short. The proliferation of these cells forms a stratified epithelium of cubic cells at 2-3 dph. Goblet cells appeared from 6 dph (Fig. 3C; 4F, I). Around 12 dph, goblet cells contained neutral glycoconjugates and/or acid glycoconjugates, carboxylated and sulphated being equally abundant (Table 4). Some goblet cells were only stained with PAS (neutral glycoconjugates), while others were stained in purple with Alcian Blue ph PAS (neutral and carboxylated mucosubstances). Goblet cells also presented proteins rich in lysine, cysteine, tyrosine, arginine and tryptophan from 12 dph (Table 5). At 12 dph, the oesophageal wall was surrounded by an inner longitudinal thin muscle layer, a circular striated muscle layer and a thin tunica serosa. Abundant goblet cells were detected at 40 dph (Fig. 7E). Stomach At 6 dph, the future stomach appeared as a little pocket with a primordial pyloric sphincter (Fig. 3C). Its mucosa was composed of a simple cubic cell epithelium without any signal of secretion and a connective subepithelial tissue layer. The epithelial cells presented a Table 3. Histochemical distribution of carbohydrates and proteins in red porgy from hatching until 6 dph. Neutral mucosubstances/ Carboxylated mucosubstances/ Sulphated mucosubstances/ Glycogen Proteins glycoconjugates glycoconjugates glycoconjugates Yolk-sac/oilglobule 3/0 0/0 0/0 1/0 3/3 Liver/hepatocytes 2/2 0-1/0-1 0/0 2-3/2-3 2/2 Exocrine pancreas/ 2-3/ / /0-1 0/0 3/3 acidophilic zymogen granules Reaction intensity: 0, negative; 1, slight; 2, moderate; 3, strong. Table 4. Histochemical distribution of mucosubstances/glycoconjugates in oesophagus, stomach and intestine of the red porgy at 15 dph. Neutral glycoconjugates Carboxylated glycoconjugates Sulphated glycoconjugates Glycogen Oesophagus Epithelium/Enterocytes 0-1/ / /0-1 0/0 Goblet cells Stomach Epithelium/Enterocytes 0-1/0-1 1/1 0/0 1/1 Gastric glands (35 dph) Intestine Epithelium/Enterocytes 0-1/0-1 1/1 0-1/0-1 1/1 Mucous cells Supranuclear inclusions (posterior intestine) Reaction intensity: 0, negative; 1, slight; 2, moderate; 3, strong.
6 758 Organogenesis of the red porgy larvae Fig. 4. Light micrographs of sections stained with H-E of red porgy larvae from 9 to 15 dph. A. A 9 day-old larva showing mucous cells in the anterior intestine which is surrounded by a diffuse pancreas. B, C, E. A 12 dayold larvae showing the pyloric sphincter which connects the stomach with the anterior intestine, the kidney with pronephric tubules and gill arches with filaments. G-I. A 15 day-old larva showing a completely developed swim bladder, kidney, and gills with filament and lamella. D. A 22 day-old larva showing a pseudobranch with three filaments. a, atrium; ai, anterior intestine; ba, bulbus arteriosus; cc, chloride cell; f, filament; ga, gill arch; gb, gall bladder; gc, goblet cell; gg, gas gland; gvs, gill vascular structures; ht, haematopoietic tissue; hs, hepatic sinusoid; l, liver; lm, lamella; mc, mucous cell; mi, medium intestine; mn, mesonephros; p, pancreas; pc, pillar cell; pi, posterior intestine; pn, pronephros; ppg, primordial pronephros glomerulus; ps, pyloric sphincter; psb, pseudobranch; pt, pronephric tubules; rm, rete mirabile; s, stomach; sb, swim bladder; sv, sinus venosus; ub, urinary bladder; v, ventricle.
7 Organogenesis of the red porgy larvae 759 granular and narrow cytoplasm with an oval nucleus in basal or central position and short microvilli in their apical border. At 12 dph, the pyloric sphincter connected the stomach with the antero-median intestine (Fig. 4B). The first signs of gastric gland development were observed around 19 dph, being formed by dph and the number of which increased significantly at 35 dph (Fig. 6B-D). Gastric glands were composed of a unique type of secretory cell devoid of microvilli in its apical border and forming aggregated cells connected to the lumen and surrounded by a delicate connective tissue layer (Figs. 7F, 8C, D). The stomach wall consisted of mucosa, lamina propria-submucosa, muscularis and serosa layers. Three gastric regions could be distinguished: fundic, cardiac and pyloric. The muscular layer of the cardiac region was thin, and thickened in the pyloric region. Gastric glands were localized in the cardiac region. The stomach presented two layers of smooth muscle: inner circular and external longitudinal ones. Both the stomach epithelium and gastric glands contained glycogen and neutral and acid glycoconjugates, mainly the carboxylated ones. Proteins rich in arginine, tyrosine and tryptophan were especially abundant inside the gastric glands (Table 5). Intestine The epithelial intestine of red porgy larvae was formed by a single columnar enterocyte layer with nucleus in basal or medium position and microvilli in their apical border and by an inner circular and outer longitudinal thin muscular tissue layer, separated by a delicate connective tissue layer (Fig. 5A, C). At 2 dph, the intestine became curved to accommodate inside the visceral cavity and three regions could be observed: antero-median, median and posterior (Fig. 3B). The first region presented mucous cells with the same histochemical characteristics as the oesophageal goblet cells: neutral and/or carboxylated and/or sulphated glycoconjugates (Table 4). Such mucous cells first Fig. 5. Light micrographs of sections stained with H-E of 19 day-old red porgy larvae. A. Anterior intestine well developed in close contact with the pancreas which secretes enzymes for digestion. B. Nutrient absorption by pinocytosis in the posterior intestine is evidenced by the presence of supranuclear inclusions. C. Detail of the medium intestine showing numerous intestinal folds that increases the digestive surface. D. Detail of the pancreas showing both endocrine and exocrine pancreas and zymogen granules containing digestive enzymes precursors. ai, anterior intestine; ep, endocrine pancreas; exp, exocrine pancreas; hs, hepatic sinusoid; p, pancreas; si, supranuclear inclusion; z, zymogen granules.
8 760 Organogenesis of the red porgy larvae Table 5. Histochemical distribution of proteins in oesophagus, stomach and intestine of the red porgy between 6 and 15 dph. General proteins Lysine Tyrosine Arginine Cystine Cysteine Tryptophan Oesophagus Epithelium/Enterocytes 2/2 1-2/1-2 2/2 2/2 2/2 2/2 0/0 Goblet cells Stomach Epithelium/Enterocytes 2/2 1/1 2/2 2/2 3/3 0-1/0-1 2/2 Gastric glands (35 dph) Intestine Epithelium/Enterocytes 3/3 1/1 1/1 2/2 1/1 1/1 1/1 Mucous cells Supranuclear inclusions (posterior intestine) Reaction intensity: 0, negative; 1, slight; 2, moderate; 3, strong. Fig. 6. Light micrographs of sections stained with H-E of the red porgy larvae digestive system. A-C. 26 day-old larvae presenting a morphologically developed digestive system showing the first gastric glands in the cardiac region of the stomach. D. A 30 day-old larva showing high number of gastric glands responsible for the adult mode of digestion (extracellular acid digestion). ai, anterior intestine; bc, branchial cavity; gb, gall bladder; gc, goblet cell; gg, gastric glands; h, heart; l, liver; nt, nephric tubules; oe, oesophagus; p, pancreas; ps, pyloric sphincter; r, rectum; s, stomach; si, supranuclear inclusions; sb, swim bladder.
9 Fig. 7. Light micrographs of sections stained with H-E of 40 day-old red porgy larvae. A. General view of the mature digestive system. B. Detail of the gill arch showing filaments with numerous lamellas that improve the oxygen uptake, among other functions. C. Detail of the buccal cavity showing the mucous cells that lubricate the food to protect the mucosa. D. Detail of the heart showing the bulbus arteriosus, ventricle with trabeculae, atrium and sinus venosus directly connected to the liver. E. Detail of the oesophagus showing numerous goblet cells that secrete mucous to lubricate the food. F. Detail of the cardiac region of the stomach showing gastric glands. a, atrium; ai, anterior intestine; ba, bulbus arteriosus; bc, buccal cavity; e, eye; ga, gill arch; gc, goblet cell; l, liver; lm, lamella; m, muscle; mc, mucous cell; oe, oesophagus; r, rectum; s, stomach; sb, swim bladder; sv, sinus venosus; ub, urinary bladder; v, ventricle. 761
10 762 Organogenesis of the red porgy larvae appeared at 6 dph although they increased in number from 15 dph onwards (Figs. 3C, 4A,I, 8C). In the medium intestine, the intestinal folds were deeper and more abundant (Figs. 5C, 8A,B). Finally, acidophilic supranuclear inclusions in the posterior region of the intestine appeared at 6 dph (Fig. 3C,D), containing abundant proteins, especially rich in arginine, tryptophan, tyrosine, cysteine and cystine (Table 5). Only Alcian Blue ph 2.5 showed a positive staining in such inclusions. Active pinocytosis was observed in the base of the microvilli of the enterocytes, except for those placed near the anus. The rectum was short and formed by a simple cubic epithelium. The enterocytes of this region presented supranuclear inclusions during the studied period (Fig. 5B). Swim bladder The swim bladder differentiated from the gut, being initially composed of columnar cells identical to the enterocytes. Subsequently, the swim bladder developed into a simple cubic cell epithelium, surrounded by fibroblast, which inflated from dph (Figs. 4F,I, 6A,B, 7A). The rete mirabile, placed on the right side of the swim bladder, was well defined at this time (Fig. 4F). Liver After hatching, the liver was situated dorsally to the yolk-sac and ventrally to the developing digestive tract. Around 2 dph, the liver started to elongate and to adapt to the body cavity (Fig. 3B). Approximately at 4-6 dph, the hepatocytes organised in cords and showed an evident granular cytoplasm, eccentric nucleus and prominent nucleolus. Glycogen granules (PAS positive and diastase-pas negative), proteins (Table 3), as well as neutral lipids (vacuoles in paraffin) were easily detected in the cytoplasm of the hepatocytes from 4-6 dph. Hepatic sinusoids containing blood cells could be observed at 9 dph (Fig. 4A), which ended into the sinus Fig. 8. Light micrographs of sections stained with H-E of the red porgy larvae digestive system. A-C. 50 day-old larva. A. General view of the digestive system showing a large stomach. B. Detail of the medium intestine showing numerous intestinal folds which help in breaking down the food in the intestinal lumen and increase the digestive surface. C. Mucous cells in the anterior intestine, liver, pancreas and stomach with gastric glands. D. Detail of the gastric glands in the stomach of 63 day-old larvae. ai, anterior intestine; gg, gastric glands; if, intestinal folds; l, liver; mc, mucous cells; mi, medium intestine; oe; oesophagus; p, pancreas.
11 Organogenesis of the red porgy larvae 763 Table 6. Gastric gland appearance in the stomach of several fish species under different culture conditions. Species Age(dph) Rearing temperature (ºC) Authors Acipenser baeri Gisbert et al., 1998 Dentex dentex Santamaría et al., 2004 Dicentrarchus labrax García-Hernández et al., 2001 Diplodus sargas Ortiz-Delgado et al., 2003 Gadus morhua Pérez-Casanova et al., 2006 Hippoglossus hippoglossus Luizi et al., 1999 Melanogrammus aeglefinus Hamlin et al., 2000 Pagrus auriga Sánchez-Amaya et al., 2006 Pagrus pagrus Darias et al., 2005 and present study Paralichthys californicus Gisbert et al., 2003 Pleuronectes dentatus Bisbal & Bengtson, 1995 Pleuronectes ferruginea Baglole et al., 1997 Psetta maxima Cousin & Baudin Laurencin, 1985; Sala, 1993; Segner, 1994 Seriola lalandi Chen et al., 2006 Solea senegalensis Sarasquete et al., 1996, 2001; Ribeiro et al., 1999; Vieira, 2000 Sparus aurata Elbal et al., 2004 venosus (Fig. 4F, I). The gall bladder was evident from 2 dph composed of a simple epithelium surrounded by a delicate connective tissue layer (Figs. 3B, 4A,F,I, 6A-C). Pancreas The exocrine pancreas initially appeared placed along the right side of the stomach to subsequently extend to the liver and to the dorsally and ventrally mesenteries of the gastrointestinal region (Figs. 4I, 6A,D). Between 2-3 dph, the basophilic cytoplasm of the exocrine pancreas was homogeneous and its cells were similar to the hepatocytes in shape and possessed spherical nucleus (Fig. 3B). After yolk-sac exhaustion, the acinar pancreatic cells were grouped to form ducts. Around 6 dph, acidophilic zymogen granules containing proteins rich in tryptophan, tyrosine, cysteine, cystine, lysine and arginine were observed in the exocrine pancreas (Table 3; Figs. 3C, 5D). The morphology of liver and pancreas remained invariable from 15 dph onwards, only showing a progressive increase in size. Heart The heart was present at hatching as a tubular structure and subsequently divided into four regions (bulbus arteriosus, ventricle, atrium and sinus venosus) at 2 dph, which connected directly to the perivitelline space (Fig. 3B). First ventricle trabeculae appeared at 6 dph, increasing progressively with larval development, while first atrium trabeculae were observed from 26 dph. Valves between bulbus arteriosus and ventricle and between atrium and sinus venosus were also detected at that time (Figs. 6B, 7D). Kidney The kidney was already present from hatching as a straight duct located just behind the notochord. At 2 dph, pronephric tubules as well as pronephric ducts were observed. The urinary bladder connected with the posterior intestine close to the anus that was not yet opened (Fig. 3B). At 6 dph pronephros developed, showing tubules more convoluted, and the haematopoietic tissue increased (Fig. 3C). At 15 dph the urinary bladder opened directly to the exterior and two regions could be differentiated: haematopoietic tissue with pronephric glomeruli zone and mesonephric zone (Fig. 4C,G). Discussion Overall, red porgy presented a similar pattern of histo-physiological digestive system ontogeny to other teleosts, either belonging to the same family (Sarasquete et al., 1995, 2001; Roo et al., 1999; Ortiz-Delgado et al., 2003, Elbal et al., 2004; Santamaría et al., 2004; Sánchez-Amaya et al., 2006) or not (Blaxter et al., 1983; Cousin and Baudin-Laurencin, 1985; Kjørsvik et al., 1991; Boulhic and Gabaudan, 1992; Segner et al., 1994; Tanaka et al., 1996; Luizi et al., 1999; Ribeiro et al., 1999b; Hamlin et al., 2000). They presented a common organ location, as well as similar cellular characteristics. The main inter-specific variability encountered resides in the timing of tissue and organ development. The quality of spawners determines in the first place the quality and amount of larval energetic reserves and the environmental conditions directly affect the rate of larval development. Thus, it is possible to find differences in larval development when species inhabiting temperate water of the same geographic area are compared. For example, Senegal sole (Solea senegalensis), white sea bream (Diplodus sargus), common dentex (Dentex dentex), redbanded seabream (Pagrus auriga) and red porgy exhibited higher speed of tissue development than gilthead sea bream (Sparus aurata). Indeed, gastric glands were not observed in the last species before two months of life (Domeneghini et al., 1998; Sarasquete et al., 2001; Elbal et al., 2004), while these glands were
12 764 Organogenesis of the red porgy larvae completely developed within the first month in the other above mentioned species (Ribeiro et al., 1999; Roo et al., 1999; Ortiz-Delgado et al., 2003; Santamaría et al., 2004; Sánchez-Amaya et al., 2006 and red porgy present study). It is worth paying attention to the variations found in tissue development between red porgy and white sea bream (Ortiz-Delgado et al., 2003) since they were reared in the same installations under the same conditions of water temperature and salinity. Such differences could be attributed to feeding habits of each species as well as in ecological strategies. As in many other teleosts, red porgy larvae presented at hatching an undifferentiated straight gut without mouth and anus opening (Govoni et al., 1986; Segner et al., 1994; Sarasquete et al., 1996; Ribeiro et al., 1999b; García-Hernández et al., 2001; Ortiz-Delgado et al., 2003; Elbal et al., 2004; Sánchez-Amaya et al., 2006) which developed during the following days into an anatomically and functionally formed digestive tract at the beginning of the exogenous feeding period, with the exception of the stomach. During the first days of larval life, gills and pseudobranches are not developed yet and, therefore, osmorregulation and respiration functions are carried out by chloride cells of the buccopharyngeal epithelium and trough skin, respectively, as was pointed out in other fish species (Sarasquete et al., 2001; Santamaria et al., 2004). The primordial gill arches could be observed in 2 days-old red porgy larvae. Cartilaginous structure of gill arches was detected on 6 dph and primordial filaments of the second and third arches began to develop. Primordial lamellae were first observed at 15 dph, indicating the beginning of gills functionality. An incipient pseudobranch was detected in 2 day-old larvae that developed into a paired structure with two filaments at 12 dph and three filaments at 22 dph. At 26 dph, first lamellae could be appreciated, as well as a notable increase of filament size. Similar results were reported by Santamaría et al. (2004) and Sánchez-Amaya et al. (2006) for common dentex and redbanded seabream, respectively. The function of this structure is not yet well known, although several roles have been attributed, such as osmoregulation, regulation of hydrostatic pressure and sensorial, respiratory and endocrine functions, among others. According to Tanaka (1971), pharyngeal teeth appeared before mandible and maxillary teeth. However, the opposite has been observed in other fish species such as summer flounder (Paralichthys dentatus) (Bisbal and Bengtson, 1995), common pandora (Pagellus erythrinus) (Micale et al., 2006) and redbanded seabream (Sánchez- Amaya et al., 2006). The oesophageal goblet cells of red porgy, white sea bream (Ortiz-Delgado et al., 2003) and redbanded seabream (Sánchez-Amaya et al., 2006) appeared a few days after first feeding (5-6 dph), as well as in turbot (Psetta maxima) (Cousin and Baudin-Laurencin, 1985), gilthead sea bream (Sarasquete et al., 1995) and sea bass (Dicentrarchus labrax) (García-Hernández et al., 2001). In haddock (Melanogrammus aeglefinus), goblet cells were observed at 10 dph at 8 C (Hamlin et al., 2000). However, the mucin secretion was detected earlier in other species, coinciding with the mouth opening, such as in Dover sole (Solea solea) (Boulhic and Gabaudan, 1992) and Senegal sole (Sarasquete et al., 1996; Vieira, 2000). Goblet cells are components of the postgastric mucose layer of fish larvae and adults (Sarasquete et al., 1995, 2001; Ribeiro et al., 1999a; Arellano et al., 2001), being implied in absorption of easily digested substances (disaccharides and short-chain fatty acids) and transport processes, as well as in digestive tract protection (Rhodes et al., 1985; Anderson, 1986; Pajak and Danguy, 1993; Baglole et al., 1997; Ribeiro et al., 1999b). Grau et al. (1992) attributed the microelongations of the oesophageal epithelium to a protective function against mechanical impacts of the ingested food as well as to facilitate the anchorage of the mucous produced by the goblet cells. García Hernández et al. (2001) found oesophageal cells with similar ultrastructural features of chloride cells of gill epithelium in sea bass larvae, suggesting that oesophagus plays an osmoregulatory role in this species. Acidic mucosubstances were only found in goblet cells of Dover sole (Boulhic and Gabaudan, 1992), Senegal sole (Ribeiro et al., 1999b) and sea bass (García Hernández et al., 2001). In red porgy and white sea bream larvae (Ortiz-Delgado et al., 2003), as well as in other species (Sarasquete et al., 1995, 2001; Sánchez- Amaya et al., 2006) some goblet cells reacted exclusively to PAS (neutral mucosubstances). However, other cells were stained blue or purple when a double Alcian Blue 2.5-PAS staining was carried out, which indicates either the presence of a mixture of neutral (magenta) to acidic mucins (purple) or a secretion of an unique type of acidic glycoproteins (blue), especially the carboxylated ones (Ortiz-Delgado et al., 2003; Sánchez- Amaya et al. 2006). Harrison et al. (1987) considered this variability of staining of a determined goblet cell as a result of a temporal sequence of glycoprotein mucous synthesis and secretion. According to Elbal and Agulleiro (1986) and Sarasquete et al. (2001), the PASpositive reaction in the goblet cells of different fish species could represent an early stage of development, when cells are mainly producing neutral glycoproteins. Goblet cells are stained with Alcian blue (ph 2.5) when glycoproteins are being carboxylated. The presence of sulphated glycoproteins (Alcian blue, ph 0.5) coincides with the stage in which sulphated groups are conjugated with glycoproteins. Contrary to this, the acid type of mucins appeared before the neutral ones in the goblet cells of the oesophagus of sea bass larvae (Hernández- Cruz et al., 2001). As observed in other fish species (Boulhic and Gabaudan, 1992; Grau et al., 1992; Murray et al., 1994a,b; Bisbal and Bengtson, 1995; Arellano et al., 1999; Hamlin et al., 2000; Ortiz-Delgado et al., 2003; Elbal et al., 2004), in red porgy, the transition from oesophagus to stomach is evidenced by goblet cell
13 Organogenesis of the red porgy larvae 765 disappearance and by the replacement of a stratified epithelium by a columnar epithelium in the stomach. The incipient stomach could be observed in red porgy larvae by 6 dph, as well as in gilthead sea bream (Sarasquete et al., 1995), while in Senegal sole, white sea bream and redbanded seabass it was distinguished at 2-3 dph (Ribeiro et al., 1999; Ortiz-Delgado et al., 2003; Sánchez-Amaya et al., 2006). The columnar epithelium of the stomach could be the precursor of gastric glands (Douglas et al., 1999). Once again, red porgy showed a slight delay in tissue development with respect to the white sea bream, the first signs of gastric glands being detected around 19 dph, while in the white sea bream this occurred by dph (Ortiz-Delgado et al., 2003) and by 60 dph in gilthead sea bream (Elbal et al., 2004). An inter-specific variability of gastric gland formation was observed in reared species (Table 6). The complete maturation of gastric glands takes place when these are able to secret pepsin and hydrochloric acid, which supposes the adult mode of acid digestion and is considered by many authors as an indicator of the larval to juvenile transition (Baglole et al., 1997; Douglas et al., 1999; Hamlin et al., 2000; Gawlicka et al., 2001; Elbal et al., 2004). Gastric glands were morphologically developed in red porgy at 26 dph and pepsinogen expression was detected at 30 dph (Darias et al., 2005), while gastric glands were histologically developed in the white sea bream 10 days earlier (Ortiz-Delgado et al., 2003). Neutral glycoconjugates, cysteine and especially cystine residues were detected in the columnar epithelium of the stomach of the red porgy. It is interesting to note that proteins rich in tyrosine, arginine and tryptophan were very abundant in red porgy at 35 dph, while in white sea bream at 23 dph (Ortiz-Delgado et al., 2003) and in Senegal sole between dph (Vieira, 2000; Sarasquete et al., 2001). These substances could be related to the synthesis and secretion of enzymatic precursors such as pepsinogen (Gutiérrez et al., 1986; Grau et al., 1992; Gisbert et al., 1999; Vieira, 2000). Neutral glycoconjugates detected in the surface of epithelial gastric cells of red porgy larvae could indicate the existence of nutrient absorption processes in the stomach (Reifel and Travill, 1977; Elbal and Agulleiro, 1986; Grau et al., 1992). In addition, neutral mucins could serve as a protection against auto-digestion processes caused by HCl and enzymes secreted by gastric glands (Ferraris et al., 1987). Sulphated glycoconjugates were not present in red porgy larvae or in other species either (Sarasquete et al., 2001; Ortiz- Delgado et al., 2003). However, such mucosubstances have been found in other fish species and are considered to be pepsin stabilizers by forming complexes for buffering or neutralizing the enzymatic activity (Spicer and Schulte, 1992, Arellano et al., 2001). This could indicate that red porgy gastric glands do not secrete pepsin at this time. Gastric glands of red porgy were specifically located in the cardiac region of the stomach, the same as were observed in gilthead sea bream (Elbal and Agulleiro, 1986), European eel (Anguilla anguilla) (Ostos-Garrido et al., 1996) and white sea bream (Ortiz-Delgado et al., 2003). However, in other fish species gastric glands were found in the fundic region of the stomach, such as in yellowtail flounder (Pleuronectes ferruginea) (Baglole et al., 1997), summer flounder (Bisbal and Bengtson, 1995) and turbot (Segner et al., 1994). In redbanded seabream (Sánchez-Amaya et al., 2006) gastric glands were localized in the cardiac and fundic regions of the stomach, and in Senegal sole juveniles and adults those were concentrated in the fundic and pyloric zones (Vieira, 2000; Arellano et al., 2001), while the stomach of Atlantic halibut (Hipposglossus hippoglossus) adults was completely glandular, probably because this species consumes large preys (Murray et al., 1994a). Such inter-specific differences in the gastric glands localization in the stomach suggest different strategies for feeding and digestion. The intestinal mucosa of red porgy larvae presents primary and secondary folds that increases the digestive surface and helps in breaking down food and mixture with the aid of enzymes secreted by the exocrine pancreas. Intestinal mucous cells of red porgy larvae were rich in acidic mucosubstances, although they also contained neutral mucins. This result agrees with those obtained in gilthead sea bream, white sea bream and redbanded seabream (Sarasquete et al., 1995; Ortiz- Delgado et al., 2003; Sánchez-Amaya et al., 2006). However, Hernández-Cruz et al. (2001) and Ribeiro et al. (1999b) only observed neutral mucosubstances in sea bass and Senegal sole, respectively. Such differences in the content of the intestinal mucous cells could be due to variations in feeding conditions (Sarasquete et al., 1995, 2001). The final portion of the intestine is actively implied in the absorption of the resultant products of digestion during the larval stage. The existence of acidophilic supranuclear inclusions in the posterior intestine of fish larvae has frequently been observed (Govoni et al., 1986; Kjørsvik et al., 1991; Sarasquete et al., 1993, 1995; Gisbert et al., 1999; Ribeiro et al., 1999b; Hamlin et al., 2000, Ortiz-Delgado et al., 2003; Sánchez-Amaya et al., 2006) which normally disappear when the stomach is completely functional. While gastric glands are still developing, pynocitosis of proteins in the posterior intestine is responsible for the intracellular digestion of proteins (Govoni et al., 1986). Waldford and Lam (1993) suggested that, in absence of stomach, the anterior intestine takes charge of the food digestion through alkaline trypsin activity (Moyano et al., 1996). In white sea bream (Ortiz-Delgado et al., 2003) and Atlantic halibut (Luizi et al., 1999), pinocytotic supranuclear vesicles disappeared when gastric glands were developed and an elevated level of protease activity was measured (Cara et al., 2003). These inclusions were absent in starved larvae of other sparids (Yúfera et al., 1993; Crespo et al., 2001). However, in red porgy they did not disappear during the studied period, as was observed in sea bass (García Hernández et
14 766 Organogenesis of the red porgy larvae al., 2001). This result suggests that intracellular protein digestion occurs independently of the degree of stomach development. Acidophilic zymogen granules were detected at 6 dph in the exocrine pancreas of red porgy larvae, revealing the presence of enzymatic precursors (basophilic cytoplasm pancreocytes or RNA-proteins synthesis), as suggested by Sarasquete and Gutiérrez (2005). Yolk-sac fish larvae confer priority to the synthesis and accumulation of pancreatic digestive enzymes to be ready for food digestion at the beginning of the exogenous feeding. In summary, red porgy larval organogenesis was concentrated within the first three weeks of development and essentially exhibited an increase in tissue structure number and size from that date onwards. Generally, red porgy displayed a similar pattern of digestive system ontogeny to other teleosts, although showing some differences in the timing of organ development which should be taken into account for designing diets adequate to the degree of maturation of their digestive tract. In this sense, this study showed that red porgy larvae were able to digest food from first feeding as demonstrated by the good larval growth rate obtained (Fig. 1). However, the progressive development, both morphologic and functional, of the digestive tract and associated organs, implies a replacement of the less efficient intracellular and alkaline digestion by a more efficient acidic digestion inside the gastric glands, which occurred at 30 dph, allowing larvae to improve food assimilation. Considering this, red porgy larvae probably would grow better if Artemia sp. was supplied from 30 dph onwards. For the aquaculture potential of this species it would be interesting to test the effects of an easily digestible proteins-based inert diet until such critical date on larval assimilation efficiency. Studies based on the recently known digestive capacity of red porgy larvae to improve their nutritional needs will optimise survival and growth during the larval period. Acknowledgements. We thank Dr. P. Pousaõ from INIAP/IPIMAR (Olhão, Portugal) for supplying the biological material used in this work, and I. Viaña, E. García-Ramos Neto and J.A. Miquel for their helpful technical assistance. This work was supported by the Comisión Interministerial de Ciencia y Tecnología, Spain (CICYT Projects AGL C02-01 and AGL ). M.J. Darias was supported by a FPI fellowship from MCYT, Spain (FP ). J.B. Ortiz-Delgado was supported by a Juan de la Cierva Postdoctoral Contract from MCYT, Spain. References Anderson T.A. (1986). Histological and cytological study of the gastrointestinal tract of the luderick, Girella tricuspidata (Pisces, Kyphosidae), in relation to diet. J. Morphol. 190, Arellano J., Dinis M.T. and Sarasquete C. (1999). Histomorphological and histochemical characteristics of the intestine of the Senegal Sole, Solea senegalensis. Eur. J. Histochem. 43, Arellano J.M., Storch V. and Sarasquete C. (2001). Histological and histochemical observations in the stomach of the Senegal sole, Solea senegalensis. Histol. Histopathol. 16, Baglole C.J., Murray H.M., Goff G.P. and Wright G.M. (1997). Ontogeny of the digestive tract during larval development of yellowtail flounder: a light microscopic and mucous histochemical study. J. Fish Biol. 51, Bancroft J.D. and Stevens A. (1990). Theory and practice of histological techniques. 3rd ed.churchill Livingstone, Edinburgh, London, Melbourne and New York. pp 726. Bisbal G.A. and Bengtson D.A. (1995). Development of digestive tract in larval summer flounder. J. Fish Biol. 47, Blaxter J.H.S., Danielssen D., Moksness E. and Øiestad V. (1983). Description of the early development of the halibut Hippoglossus hippoglossus and attempts to rear the larvae past first feeding. Mar. Biol. 73, Boulhic M. and Gabaudan J. (1992). Histological study of the organogenesis of the digestive system and swim bladder of the Dover sole, Solea solea (Linnaeus 1758). Aquaculture 102, Cara J.B., Moyano F.J., Cárdenas S., Fernández-Díaz C. and Yúfera M. (2003). Assessment of digestive enzyme activities during larval development of white seabream (Diplodus sargus). J. Fish Biol. 63, Chen B.N., Qin J.G., Kumar M.S., Hutchinson W. and Clarke S. (2006). Ontogenetic development of the digestive system in yellowtail kingfish Seriola lalandi larvae. Aquaculture 256, Cousin J.C.B. and Baudin-Laurencin F. (1985). Morphogénêse de l appareil digestif de la vessie gazeuse du turbot, Scophthalmus maximus L. Aquaculture 47, Crespo S., Marín de Mateo M., Santamaría C.A., Sala R., Grau A. and Pastor E. (2001). Histopathological observations during larval rearing of common dentex Dentex dentex L. (Sparidae), Aquaculture 192, Darias M.J., Murray H.M., Martínez-Rodríguez G., Cárdenas S. and Yúfera M. (2005). Gene expression of pepsinogen during the larval development of red porgy (Pagrus pagrus). Aquaculture 248, Darias M.J., Murray H.M., Gallant J.W., Astola A., Douglas S.E., Yúfera M. and Martínez-Rodríguez G. (2006). Characterization of a partial α-amylase clone from red porgy (Pagrus pagrus): Expression during larval development. Comp. Biochem. Physiol. Part B 143, Domeneghini C., Pannelle S.R. and Veggetti A. (1998). Gut glycoconjugates in Sparus aurata L. (Pisces, Teleostei). A comparative histochemical study in larval and adult ages. Histol. Histopathol. 13, Douglas S.E., Gawlicka A., Mandla S. and Galland J.W. (1999). Ontogeny of the stomach in winter flounder: characterization and expression of the pepsinogen and proton pump genes and determination of pepsin activity. J. Fish Biol. 55, Douglas S.E., Mandla S. and Gallant J.W. (2000). Molecular analysis of the amylase gene and its expression during development in the winter flounder, Pleuronectes americanus. Aquaculture 190, Elbal M.T. and Agulleiro B. (1986). A histochemical and ultrastructural study of the gut of Sparus aurata (Teleostei). J. Submicrosc. Cytol. 18, Elbal M.T., García-Hernández M.P., Lozano M.T. and Agulleiro B. (2004). Development of the digestive tract of gilthead sea bream
15 Organogenesis of the red porgy larvae 767 (Sparus aurata L.). Light and electron microscopic studies. Aquaculture 234, Ferraris R.P., Tan J.D. and De la Cruz M.C. (1987). Development of the digestive tract of milkfish, Chanos chanos: Histology and histochemistry. Aquaculture 61, Gallagher J.T., Lyon M. and Steward W.P. (1986). Structure and function of heparin sulphate proteoglycans. Biochem. J. 236, García-Hernández M.P., Lozano M.T., Elbal M.T. and Agulleiro B. (2001). Development of the digestive tract of sea bass (Dicentrarchus labrax L). Light and electron microscopic studies. Anat embryol. 204, Gawlicka A., Parent B., Horn M.H., Ross N., Opstad I. and Torrissen O.J. (2000). Activity of digestive anzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): indication of readiness for first feeding. Aquaculture 184, Gawlicka A., Leggiadro C.T., Gallant J.W. and Douglas S.E. (2001). Cellular expression of the pepsinogen and gastric proton pump genes in the stomach of winter flounder as determined by in situ hybridization. J. Fish Biol. 58, Gisbert E., Sarasquete C., Williot P. and Castelló-Orvay F. (1999). Histochemistry of the development of the digestive system of Siberian sturgeon during early ontogeny. J. Fish Biol. 55, Govoni J.J., Boehlert G.W. and Watanabe Y. (1986). The physiology of digestion in fish larvae. Env. Biol. Fish. 16, Grau A., Crespo S., Sarasquete M.C. and González de Canales M.L. (1992). The digestive tract of the amberjack, Seriola dumerili Risso: a light and scanning microscope study. J. Fish. Biol. 41, Gutiérrez M., Sarasquete C. and González de Canales M.L. (1986). Distribución histoquímica de carbohidratos y proteínas en estómago e intestino de Angilla anguilla L., 1758 de las salinas de Cádiz. Inv. Pesq. 50, Hamlin H.J., Hunt von Herbing I. and Kling L.J. (2000). Histological and morphological evaluations of the digestive tract and associated organs of haddock throughout post-hatching ontogeny. J. Fish. Biol. 57, Harrison J.D., Auger D.W., Paterson K.L. and Rowley P.S.A. (1987). Mucin histochemistry of submandibular and parotid salivary glands of man: light and electron microscopy. Histochem. J. 19, Hernández-Cruz C.M., Salhi M., Bessonart M., Izquierdo M.S., González M.M. and Fernández-Palacios H. (1999). Rearing techniques for red porgy (Pagrus pagrus) during larval development. Aquaculture 179, Hidalgo M.C., Urea E. and Sanz A. (1999). Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 170, Kapoor B.G., Smith H. and Verighina I.A. (1975). The alimentary canal and digestion in teleosts. Adv. Marine Biol. 63, Kim B.G., Divakaran S., Brown C.L. and Ostrowski A.C. (2001). Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific threadfin (Polyactylus sexfilis) and bluefin trevally (Caranx melampygus). Fish. Physiol. Biochem. 24, Kjørsvik E., van der Meeren T., Kryvi H., Arnfinnson J. and Kvenseth P.G. (1991). Early development of the digestive tract of cod larvae, Gadus morhua L., during start-feeding and starvation. J. Fish Biol. 38, Kolios P., Kiritsis S. and Natribusas N. (1997). Larval-rearing and growout of the red porgy (Pagrus pagrus) in the Riopesca hatchery (Greece). Hydrobiologia 358, Kurokawa T. and Suzuki T. (1996). Formation of the diffuse pancreas and the development of digestive enzyme synthesis in larvae of the Japanese flounder Paralichthys olivaceus. Aquaculture 114, Luizi F.S., Gara B., Shields R.J. and Bromage N.R. (1999). Further description of the development of the digestive organs in Atlantic halibut (Hippoglossus hippoglossus) larvae, with notes on differential absorption of copepod and Artemia prey. Aquaculture 176, Martínez I., Moyano F.J., Fernández-Díaz C. and Yúfera M. (1999). Digestive enzyme activity during larval development of the Senegal sole (Solea senegalensis). Fish. Physiol. Biochem. 21, Micale V., Garaffo M., Genovese L., Spedicato M.T. and Muglia U. (2006). The ontogeny of the alimentary tract during larval development in common pandora Pagellus erythrinus L. Aquaculture 251, Mihelakakis A., Yoshimatsu T. and Tsolkas C. (2001). Spawning in captivity and early life history of cultured red porgy, Pagrus pagrus, Aquaculture 199, Moyano F.J., Díaz M., Alarcón F.J. and Sarasquete C. (1996). Characterization of the digestive enzyme activity during larval development of gilthead seabream Sparus aurata L. Fish Physiol. Biochem. 15, Murray H.M., Wright G.M. and Goff G.P. (1994a). A comparative histological and histochemical study of the stomach from three species of pleuronectid, the Atlantic halibut, Hippoglossus hippoglossus, the yellowtail flounder, Pleuronectes ferruginea, and the winter flounder, Pleuronectes americanus. Can. J. Zool. 72, Murray H.M., Wright G.M. and Goff G.P. (1994b). A study of the posterior oesophagus in the winter flounder, Pleuronectes americanus, and the yellowtail flounder, Pleuronectes ferruginea: a morphological evidence of pregastric digestion?. Can. J. Zool. 72, Oozeki Y. and Bailey K.M. (1995). Ontogenetic development of digestive enyme activities in larval walleye pollock, Theragra chalcogramma. Mar. Biol. 122, Ortiz-Delgado J.B., Darias M.J., Cañavate J.P., Yúfera M. and Sarasquete C. (2003). Organogenesis of the digestive tract in the white seabream, Diplodus sargus. Histological and histochemical approaches. Histol. Histopathol. 18, Ostos-Garrido M.V., González Oller C. and Abaurrea Equisoain A. (1996). Effect of diet on gastric mucosa cells in the European eel (Anguilla anguilla L.). Histochemical and ultrastructural study. Micron 27, Pajak B. and Danguy A. (1993). Characterization of sugar moieties and oligosaccharide sequences in the digital intestinal epithelium of the rainbow trout by means of lectin histochemistry. J. Fish Biol. 43, Parillo F., Gargiulo A.M. and Fagioli O. (2004). Complex carbohydrates occurring in the digestive apparatus of Umbrina cirrosa (L.) fry. Vet. Res. Commun. 28, Pearse A.G.E. (1985). Histochemistry. Theoretical and applied. Vol. 2 Analytical Technology. 4 th ed. Churchill Livingstone. New York. NY. Pérez-Casanova J.C., Murray H.M., Gallant J.W., Ross N.W., Douglas S.E. and Jonhson S.C. (2006). Development of the digestive capacity in larvae of haddock (Melanogrammus aeglefinus) and Atlantic cod (Gadus morhua). Aquaculture 251, Reifel C.W. and Travill A.A. (1977). Structure and carbohydrate histochemistry of the esophagus in ten teleostean species. J.
16 768 Organogenesis of the red porgy larvae Morphol. 152, Rhodes J.M., Black R.R., Gallimore R. and Savage A. (1985). Histochemical demostration of desialitation and desulphation of normal and inflammatory bowel disease rectal mucus by faecal extracts. Gut 26, Ribeiro L., Zambonino-Infante J.L., Cahu C. and Dinis M.T. (1999a). Development of digestive enzymes in larvae of Solea senegalensis, Kaup Aquaculture 179, Ribeiro L., Sarasquete C. and Dinis M.T. (1999b). Histological and histochemical development of the digestive system of Solea senegalensis (Kaup, 1858) larvae. Aquaculture, 171, Rojas-García C.R., Rønnestad I. and Ueberschär B. (2001). Combined sensitive analytical methods for cholecystokinin levels and tryptic activity in individual fish larvae. J. Exp. Mar. Biol. Ecol. 256, Roo F.J., Socorro J., Izquierdo M.S., Caballero M.J., Hernández-Cruz C.M., Fernández A. and Fernández-Palacios H. (1999). Development of red porgy Pagrus pagrus visual system in relation with changes in the digestive tract and larval feeding habits. Aquaculture, 179, Sánchez-Amaya M.I., Ortiz-Delgado J.B., García-Lopez A., Cárdenas S. and Sarasquete C. (2006). Ontogeny of the redbanded seabream, Pagrus auriga larvae: An Histological Study. Aquaculture (in press). Santamaría C.A., Marín de Mateo M., Traveset R., Sala R., Grau A., Pastor E., Sarasquete C. and Crespo S. (2004). Larval organogenesis in common dentex Dentex dentex L. (Sparidae): histological and histochemical aspects. Aquaculture 237, Sarasquete C. and Gutiérrez M. (2005). New tetrachromic VOF stain (Type III-G.S) for normal and pathological fish tissues. Eur. J. Histochem. 49, Sarasquete C., Polo A. and Gonzalez de Canales M.L. (1993). A histochemical and immunohistochemical study of digestive enzymes and hormones during the larval development of the seabream, Sparus aurata. Histochem. J., 25, Sarasquete C., Polo A. and Yúfera M. (1995). Histology and histochemistry of the development of the digestive system of larval gilthead seabream Sparus aurata L.. Aquaculture 130, Sarasquete M.C., Gónzalez de Canales M.L., Arellano J.M., Munoz- Cueto J.A., Ribeiro L. and Dinis M.T. (1996). Histochemical aspects of the yolk-sac and digestive tract of larvae of the Senegal sole, Solea senegalensis (Kaup, 1858). Histol. Histophatol. 11, Sarasquete C., Gisbert E., Ribeiro L., Vieira L. and Dinis M.T. (2001). Glyconjugates in epidermal, branchial and digestive mucous cells and gastric glands of gilthead sea bream, Sparus aurata, Senegal sole, Solea senegalensis and Siberian sturgeon, Acipenser baeri development. Eur. J. Histochem.45, Segner H., Storch V., Reinecke M., Kloas W. and Hanke W. (1994). The development of functional digestive and metabolic organs in turbot Scophthalmus maximus. Mar. Biol. 119, Spicer S.S. and Schulte B.A. (1992). Diversity of cell glycoconjugates shown histochemically: a perspective. J. Histochem. Cytochem. 40, Tanaka M. (1971). Studies on the structure and function of the digestive system in teleost larvae - III. Development of the digestive system during postlarval stage. -DS- FKWK\RO 18, Tanaka M., Kawai S., Seika T. and Burke J.S. (1996). Development of the digestive organ system in Japanese flounder in relation to metamorphosis and settlement. Mar. Fresh. Behav. Physiol. 28, Vieira L. (2000). Estudio histológico e histoquímico de juveniles de lenguado, Solea senegalensis, alimentados con dietas inertes. Tesis de licenciatura. Universidade do Algarve (Unidad de Ciencias y Tecnología de Recursos Acuáticos). Faro. (Portugal). Walford J. and Lam T.J. (1993). Development of digestive tract proteolytic enzyme activity in seabass (Lates calcarifer) larvae and juveniles. Aquaculture 109, Yúfera M., Pascual E., Polo A. and Sarasquete M.C. (1993). Effect of starvation on the feeding ability of gilthead seabream (Sparus aurata L.) larvae at first feeding. J. Exp. Mar. Biol. Ecol. 169, Yúfera M., Fernández-Díaz C., Vidaurreta A., Cara J.B. and Moyano F.J. (2004). Gastrointestinal ph and development of the acid digestion in larvae and early juveniles of Sparus aurata (Pisces: Teleostei). Mar. Biol. 144, Zambonino-Infante J.L. and Cahu C.L. (2001). Ontogeny of the gastrointestinal tract of marine fish larvae. Comp. Biochem. Physiol. Part C 130, Accepted January 26, 2007
Organogenesis of the digestive tract in the white seabream, Diplodus sargus. Histological and histochemical approaches
 Histol Histopathol (2003) 18: 1141-1154 http://www.hh.um.es Histology and Histopathology Cellular and Molecular Biology Organogenesis of the digestive tract in the white seabream, Diplodus sargus. Histological
Histol Histopathol (2003) 18: 1141-1154 http://www.hh.um.es Histology and Histopathology Cellular and Molecular Biology Organogenesis of the digestive tract in the white seabream, Diplodus sargus. Histological
The Digestive System. Chapter 16. Introduction. Histological Organization. Overview of Digestive System. Movement and Mixing of Digestive Materials
 The Digestive System Chapter 16 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
The Digestive System Chapter 16 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
5. Secretion: release of water, acids. Enzymes, buffers by digestive tract.
 Digestive System CH-16 Lecture topics Functions of the digestive system: p. 488. 1. Ingestion: Taking food in 2. Propulsion: movement of food thru alimentary canal p.490. voluntary: swalloing : skeletal
Digestive System CH-16 Lecture topics Functions of the digestive system: p. 488. 1. Ingestion: Taking food in 2. Propulsion: movement of food thru alimentary canal p.490. voluntary: swalloing : skeletal
Each gland has at least one duct that takes saliva to the oral cavity.
 kufa university Physiology College of Nursing first year student Ass. Lect :- Hisham Qassem M. Lecture No :-3 The Digestive System Digestive system consists of: 1. Gastrointestinal Tract (GIT). 2. Accessory
kufa university Physiology College of Nursing first year student Ass. Lect :- Hisham Qassem M. Lecture No :-3 The Digestive System Digestive system consists of: 1. Gastrointestinal Tract (GIT). 2. Accessory
Digestive System Digestive Tract
 Digestive System Digestive Tract Dept. of Histology and Embryology 周 莉 教 授 Introduction of digestive system * a long tube extending from the mouth to the anus, and associated with glands. * its main function:
Digestive System Digestive Tract Dept. of Histology and Embryology 周 莉 教 授 Introduction of digestive system * a long tube extending from the mouth to the anus, and associated with glands. * its main function:
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Digestive System AKA. GI System. Overview. GI Process Process Includes. G-I Tract Alimentary Canal
 Digestive System AKA G-I Tract Alimentary Canal Overview GI System Consists of Mouth, pharynx, esophagus, stomach, small intestine, large intestine, anus About 30 in length Accessory Organs Teeth, tongue,
Digestive System AKA G-I Tract Alimentary Canal Overview GI System Consists of Mouth, pharynx, esophagus, stomach, small intestine, large intestine, anus About 30 in length Accessory Organs Teeth, tongue,
I. The basic function of the digestive system is
 Chapter 15, Digestive System - ANATOMY OF THE DIGESTIVE SYSTEM I. The basic function of the digestive system is. This process is called. II. List 2 other names for the digestive tract: A. B. III. The digestive
Chapter 15, Digestive System - ANATOMY OF THE DIGESTIVE SYSTEM I. The basic function of the digestive system is. This process is called. II. List 2 other names for the digestive tract: A. B. III. The digestive
The Vertebrate (mostly human) Digestive System
 The Vertebrate (mostly human) Digestive System Mouth - mastication, lubrication, digestion Pharynx and Esophagus - swallowing Stomach - some digestion Small intestine - most digestion and absorption Large
The Vertebrate (mostly human) Digestive System Mouth - mastication, lubrication, digestion Pharynx and Esophagus - swallowing Stomach - some digestion Small intestine - most digestion and absorption Large
THE GI TRACT IS A CONTINUOUS MULTILAYERED TUBE EXTENDING FROM THE MOUTH TO THE ANUS THAT IS SUPPORTED AND PARTIALLY COVERED BY THE PERITONEUM.
 THE GI TRACT IS A CONTINUOUS MULTILAYERED TUBE EXTENDING FROM THE MOUTH TO THE ANUS THAT IS SUPPORTED AND PARTIALLY COVERED BY THE PERITONEUM. OVERVIEW OF THE DIGESTIVE SYSTEM Two groups of organs compose
THE GI TRACT IS A CONTINUOUS MULTILAYERED TUBE EXTENDING FROM THE MOUTH TO THE ANUS THAT IS SUPPORTED AND PARTIALLY COVERED BY THE PERITONEUM. OVERVIEW OF THE DIGESTIVE SYSTEM Two groups of organs compose
The Digestive System. You are what you eat!
 The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
DIGESTIVE SYSTEM Liver, pancreas, esophagus, stomach fundus, small intestine, large intestine, appendix; preparations B9, 10, 12, 15, 16, 17, 18, (19)
 DIGESTIVE SYSTEM Liver, pancreas, esophagus, stomach fundus, small intestine, large intestine, appendix; preparations B9, 10, 12, 15, 16, 17, 18, (19) Institute of Histology and Embryology RNDr. Lucie
DIGESTIVE SYSTEM Liver, pancreas, esophagus, stomach fundus, small intestine, large intestine, appendix; preparations B9, 10, 12, 15, 16, 17, 18, (19) Institute of Histology and Embryology RNDr. Lucie
Eating, pooping, and peeing THE DIGESTIVE SYSTEM
 THE DIGESTIVE SYSTEM Ingested food is not technically in the body until it is absorbed so it needs to be: Mechanically and chemically reduced Transported by the blood to the cells Large portions are not
THE DIGESTIVE SYSTEM Ingested food is not technically in the body until it is absorbed so it needs to be: Mechanically and chemically reduced Transported by the blood to the cells Large portions are not
Digestive System Notes
 Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
Divisions of Digestive System. Organs of the Alimentary Canal. Anatomy of the Digestive System: Organs of the Alimentary Canal. CHAPTER 14 p.
 Divisions of Digestive System Anatomy of the Digestive System: Organs of the Alimentary Canal CHAPTER 14 p. 412-423 1. Alimentary Canal or Gastrointestinal Tract (GI)-digests and absorbs food coiled hollow
Divisions of Digestive System Anatomy of the Digestive System: Organs of the Alimentary Canal CHAPTER 14 p. 412-423 1. Alimentary Canal or Gastrointestinal Tract (GI)-digests and absorbs food coiled hollow
Outline Digestive System
 Outline Digestive System The Digestive System Digestive System Lecture Packet 19 Chapter 15 I. Function II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine,
Outline Digestive System The Digestive System Digestive System Lecture Packet 19 Chapter 15 I. Function II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine,
Digestive system Review
 Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Small & Large Intestines
 Small & Large Intestines Small Intestine: principal site for digestion of food and absorption of the products of digestion Large Intestine: reabsorption of water and elimination of undigested food and
Small & Large Intestines Small Intestine: principal site for digestion of food and absorption of the products of digestion Large Intestine: reabsorption of water and elimination of undigested food and
Medical Physiology Z.H.Al-Zubaydi
 Lec.13 Medical Physiology Z.H.Al-Zubaydi Functions of the Digestive System The major functions of the digestive tract include the following six processes, summarized in Figure 1: 1. Ingestion Food must
Lec.13 Medical Physiology Z.H.Al-Zubaydi Functions of the Digestive System The major functions of the digestive tract include the following six processes, summarized in Figure 1: 1. Ingestion Food must
The 6 th International Junior Science Olympiad Training Programme
 The 6 th International Junior Science Olympiad Training Programme KEEPING HEALTHY SECTION 2: DIGESTIVE SYSTEM Notes to Teachers Learning Objectives: Processes of nutrition in human (0.25 hr) General plan
The 6 th International Junior Science Olympiad Training Programme KEEPING HEALTHY SECTION 2: DIGESTIVE SYSTEM Notes to Teachers Learning Objectives: Processes of nutrition in human (0.25 hr) General plan
The Digestive System. Chapter 14. The Digestive System and Body Metabolism. Metabolism. Organs of the Digestive System. Digestion.
 Chapter 14 The Digestive System The Digestive System and Body Metabolism Digestion of ingested food of nutrients into the blood Metabolism Production of Constructive and degradative cellular activities
Chapter 14 The Digestive System The Digestive System and Body Metabolism Digestion of ingested food of nutrients into the blood Metabolism Production of Constructive and degradative cellular activities
Functions of the GI Tract. Chapter 18. Functions of the GI Tract (continued)
 Functions of the GI Tract Chapter 18 The Digestive System Motility: Movement of of food through the GI tract. Ingestion: Taking food into the mouth. Mastication: Chewing the food and mixing it with saliva.
Functions of the GI Tract Chapter 18 The Digestive System Motility: Movement of of food through the GI tract. Ingestion: Taking food into the mouth. Mastication: Chewing the food and mixing it with saliva.
THE DIGESTIVE SYSTEM
 THE DIGESTIVE SYSTEM What is digestion? Digestion is the process of breaking down food so that it's small enough to be absorbed and used by the body for energy or in other bodily functions. Digestion involves
THE DIGESTIVE SYSTEM What is digestion? Digestion is the process of breaking down food so that it's small enough to be absorbed and used by the body for energy or in other bodily functions. Digestion involves
Biology 2402 Anatomy &Physiology II - Digestive system notes - Ch. 15
 Biology 2402 Anatomy &Physiology II - Digestive system notes - Ch. 15 Digestive system processes the food used as fuel and nutrients for the body. Composed of a tube through the body (digestive tract,
Biology 2402 Anatomy &Physiology II - Digestive system notes - Ch. 15 Digestive system processes the food used as fuel and nutrients for the body. Composed of a tube through the body (digestive tract,
Topic 4: Digestion and Nutrition
 Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
The Digestive System. Chapter 15
 The Digestive System Chapter 15 Introduction Digestion refers to the mechanical and chemical breakdown of food so the nutrients can be absorbed by cells Carried out by the digestive system Consists of
The Digestive System Chapter 15 Introduction Digestion refers to the mechanical and chemical breakdown of food so the nutrients can be absorbed by cells Carried out by the digestive system Consists of
Mechanical digestion: physical breaking of food chewing by teeth churning by stomach segmentation by intestines (= mixing food) p.611/ Fig. 22.
 The Digestive System 1. Describe the general functions of the digestive system Ingestion: Taking food in Propulsion: movement of food thru alimentary canal voluntary: swalloing involuntary: peristalsis
The Digestive System 1. Describe the general functions of the digestive system Ingestion: Taking food in Propulsion: movement of food thru alimentary canal voluntary: swalloing involuntary: peristalsis
26. Digestive System
 26. Digestive System Your body needs food for two primary purposes: growth and maintenance. Molecules and atoms in the food you eat are generally used to either build new molecules in your body or to provide
26. Digestive System Your body needs food for two primary purposes: growth and maintenance. Molecules and atoms in the food you eat are generally used to either build new molecules in your body or to provide
Chapter 15 Digestion and Nutrition
 Chapter 15 Digestion and Nutrition Digestive System: Digestion refers to the mechanical and chemical breakdown of foods so that nutrients can be absorbed by cells. Consists of the canal which is all of
Chapter 15 Digestion and Nutrition Digestive System: Digestion refers to the mechanical and chemical breakdown of foods so that nutrients can be absorbed by cells. Consists of the canal which is all of
Digestive System Functions
 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
THE DIGESTIVE SYSTEM Secretion Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 THE DIGESTIVE SYSTEM Secretion Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1: Title Page Digestive system secretion involves
THE DIGESTIVE SYSTEM Secretion Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1: Title Page Digestive system secretion involves
Chapter 48. Nutrients in Food. Carbohydrates, Proteins, and Lipids. Carbohydrates, Proteins, and Lipids, continued
 Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Digestive system. Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College
 Digestive system Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Oral cavity Alimentary Canal Accessory organs teeth salivary glands liver gall bladder pancreas appendix Components Functions Motility
Digestive system Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Oral cavity Alimentary Canal Accessory organs teeth salivary glands liver gall bladder pancreas appendix Components Functions Motility
Integumentary System Digestive System. Outline. Integumentary System 11/4/2008. Week 11 BA & BP November 4, 2008 Nadia Arora, ND
 Integumentary System Digestive System Week 11 BA & BP November 4, 2008 Nadia Arora, ND Outline Integumentary system and body membranes Types of body membranes and their function General structure and main
Integumentary System Digestive System Week 11 BA & BP November 4, 2008 Nadia Arora, ND Outline Integumentary system and body membranes Types of body membranes and their function General structure and main
h. Large intestine 3
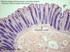 (1) General features (a) Large intestine is last organ of digestive tract proper divided into 3 or 4 regions cecum appendix in humans colon rectum 1 b) No villi lumenal epithelium has microvilli This brush
(1) General features (a) Large intestine is last organ of digestive tract proper divided into 3 or 4 regions cecum appendix in humans colon rectum 1 b) No villi lumenal epithelium has microvilli This brush
The Gastrointestinal System It consists of: The digestive tract Mouth Pharynx Oesophagus Stomach Small intestine Large intestine
 The Gastrointestinal System It consists of: The digestive tract Mouth Pharynx Oesophagus Stomach Small intestine Large intestine The digestive organs Teeth Tongue Salivary glands Liver Gall bladder Pancreas
The Gastrointestinal System It consists of: The digestive tract Mouth Pharynx Oesophagus Stomach Small intestine Large intestine The digestive organs Teeth Tongue Salivary glands Liver Gall bladder Pancreas
Digestive System. Gross Anatomy and Physiology
 Digestive System Gross Anatomy and Physiology I. Introduction A. Base Function: Working with the circulatory system the digestive system provides the body with fuel. B. Main players: 1. Digestive tract:
Digestive System Gross Anatomy and Physiology I. Introduction A. Base Function: Working with the circulatory system the digestive system provides the body with fuel. B. Main players: 1. Digestive tract:
The Human Digestive System
 The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
2) Digestion the breakdown of. There are two types of digestion: Mechanical and Chemical. 3) Absorption when the nutrients enter into the blood.
 The Digestive System Video on the digestive system (5 min) The digestive system is responsible for the breakdown of the we eat so that it can be absorbed into the. There are four main stages of the digestive
The Digestive System Video on the digestive system (5 min) The digestive system is responsible for the breakdown of the we eat so that it can be absorbed into the. There are four main stages of the digestive
1. Which substances in the small intestine of humans serve to increase the surface area for absorption?
 Digestion Review 1. Which substances in the small intestine of humans serve to increase the surface area for absorption? (a.) intestinal glands (b.) villi (c.) pseudopodia (d.) cilia (e.) flagella 2. The
Digestion Review 1. Which substances in the small intestine of humans serve to increase the surface area for absorption? (a.) intestinal glands (b.) villi (c.) pseudopodia (d.) cilia (e.) flagella 2. The
BIO 137: CHAPTER 1 OBJECTIVES
 BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
Chapter 15 Digestive System.
 Chapter 15 Digestive System. I. The Gastrointestinal Tract. a. The digestive system mechanically and chemically breaks down food into molecules that can be absorbed into the bloodstream or lymph. Residues
Chapter 15 Digestive System. I. The Gastrointestinal Tract. a. The digestive system mechanically and chemically breaks down food into molecules that can be absorbed into the bloodstream or lymph. Residues
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Chapter 17 Digestive System. Alimentary Canal. Movements of the Tube
 Chapter 17 Digestive System Functions of Digestive System ingestion mechanical digestion chemical digestion propulsion absorption defecation Consists of the alimentary canal and accessory organs 1 Alimentary
Chapter 17 Digestive System Functions of Digestive System ingestion mechanical digestion chemical digestion propulsion absorption defecation Consists of the alimentary canal and accessory organs 1 Alimentary
Classifications of animals: ruminant vs non-ruminant carnivore: meat-eating herbivore: plant-eating omnivore: both meat and plant-eating
 Digestion and Metabolism Digestive tract one long, continuous tube starting at the mouth and ending at the anus Functions Ingestion Grinding Digestion/absorption of food Elimination of solid wastes Classifications
Digestion and Metabolism Digestive tract one long, continuous tube starting at the mouth and ending at the anus Functions Ingestion Grinding Digestion/absorption of food Elimination of solid wastes Classifications
Lab 18 The Digestive System
 Lab 18 The Digestive System Laboratory Objectives Identify on a diagram, model or cadaver the parts of the digestive system and accessory organs. Describe the general histology of the digestive system.
Lab 18 The Digestive System Laboratory Objectives Identify on a diagram, model or cadaver the parts of the digestive system and accessory organs. Describe the general histology of the digestive system.
Researcher 2013;5(12) http://www.sciencepub.net/researcher
 The Mouth and Gastro-Intestinal Tract of Pomadasys Jubelini (Cuvier, 1830) In the New Calabar-Bonny River, Rivers State, Nigeria Agbugui, M. O. Department of Biological Sciences, Ahmadu Bello University
The Mouth and Gastro-Intestinal Tract of Pomadasys Jubelini (Cuvier, 1830) In the New Calabar-Bonny River, Rivers State, Nigeria Agbugui, M. O. Department of Biological Sciences, Ahmadu Bello University
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
The Respiratory System
 Human Anatomy III: Respiratory, Urinary & Digestive Systems The Respiratory System Major functions include: Obtaining oxygen Removing carbon dioxide Maintenance of ph balance Respiration may be accomplished
Human Anatomy III: Respiratory, Urinary & Digestive Systems The Respiratory System Major functions include: Obtaining oxygen Removing carbon dioxide Maintenance of ph balance Respiration may be accomplished
SMALL AND LARGE INTESTINE SECRETIONS
 SMALL AND LARGE INTESTINE SECRETIONS Objectives At the end of lecture student should be able to know, Digestive system Digestive system secretions Small intestine Component of small intestine Intestinal
SMALL AND LARGE INTESTINE SECRETIONS Objectives At the end of lecture student should be able to know, Digestive system Digestive system secretions Small intestine Component of small intestine Intestinal
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems
 Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Animal Tissues. I. Epithelial Tissue
 Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
The Excretory and Digestive Systems
 The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
Human Anatomy & Physiology II with Dr. Hubley
 Human Anatomy & Physiology II with Dr. Hubley Practice Exam III Name: Instructions This exam consists of 50 questions. You may write on the exam itself, but be sure to answer all your questions on a Scantron
Human Anatomy & Physiology II with Dr. Hubley Practice Exam III Name: Instructions This exam consists of 50 questions. You may write on the exam itself, but be sure to answer all your questions on a Scantron
Histology and Glycoconjugates Histochemistry in the Small and Large Intestinal Epithelium of the Malayan Pangolin, Manis javanica
 Kasetsart J. (Nat. Sci.) 41 : 186-191 (2007) Histology and Glycoconjugates Histochemistry in the Small and Large Intestinal Epithelium of the Malayan Pangolin, Manis javanica Apinun Suprasert*, Maleewan
Kasetsart J. (Nat. Sci.) 41 : 186-191 (2007) Histology and Glycoconjugates Histochemistry in the Small and Large Intestinal Epithelium of the Malayan Pangolin, Manis javanica Apinun Suprasert*, Maleewan
CHAPTER 9 BODY ORGANIZATION
 CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
The Tissue Level of Organization
 The Tissue Level of Organization Tissues A groups of similar cells, usually having similar embryonic origin and specialized function Histology: the study of tissues Four general types Epithelial Muscle
The Tissue Level of Organization Tissues A groups of similar cells, usually having similar embryonic origin and specialized function Histology: the study of tissues Four general types Epithelial Muscle
CHAPTER 23 DIGESTIVE
 CHAPTER 23 DIGESTIVE nutrition requires : getting nutrients digesting nutrients transporting nutrients Digestive System musculo-skeletal digestive circulatory Digestive System alimentary canal ~ gastrointestinal
CHAPTER 23 DIGESTIVE nutrition requires : getting nutrients digesting nutrients transporting nutrients Digestive System musculo-skeletal digestive circulatory Digestive System alimentary canal ~ gastrointestinal
The Digestive System
 The Digestive System What do you know?? quiz-digestive-health Digestion Videos The Digestive System Inside-Dr-Ozs-Digestive-System-Video Now it is your turn to recreate the digestive system. How is food
The Digestive System What do you know?? quiz-digestive-health Digestion Videos The Digestive System Inside-Dr-Ozs-Digestive-System-Video Now it is your turn to recreate the digestive system. How is food
AP Biology. What do animals need to live? Animal Nutrition. Nutritional requirements. How do animals get their food? Different diets; different lives
 Animal Nutrition What do animals need to live? Animals make energy using: food food oxygen Animals build bodies using: food for raw materials amino acids, sugars, fats, nucleotides O 2 ATP energy for synthesis
Animal Nutrition What do animals need to live? Animals make energy using: food food oxygen Animals build bodies using: food for raw materials amino acids, sugars, fats, nucleotides O 2 ATP energy for synthesis
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Digestion. What we ll cover. Main stages of digestion. Digestion: A Closer Look. A Tour of the Human Digestive System. Mechanical digestion
 Digestion What we ll cover What are the digestive system structures and their functions? Where does carbohydrate, protein and fat digestion and absorption occur? What are the 3 accessory organs of digestion?
Digestion What we ll cover What are the digestive system structures and their functions? Where does carbohydrate, protein and fat digestion and absorption occur? What are the 3 accessory organs of digestion?
Archimer http://archimer.ifremer.fr
 In Feeding and Digestive Functions of Fishes 2008, p281-348 Archimer http://archimer.ifremer.fr Ontogeny and physiology of the digestive system of marine fish larvae J.L. Zambonino Infante 1*, Gisbert
In Feeding and Digestive Functions of Fishes 2008, p281-348 Archimer http://archimer.ifremer.fr Ontogeny and physiology of the digestive system of marine fish larvae J.L. Zambonino Infante 1*, Gisbert
The Digestive System
 The Digestive System Digestive Structures Mouth including teeth and tongue Esophagus Stomach Small intestine Large intestine Accessory structures - salivary glands, liver, gallbladder, & pancreas Digestive
The Digestive System Digestive Structures Mouth including teeth and tongue Esophagus Stomach Small intestine Large intestine Accessory structures - salivary glands, liver, gallbladder, & pancreas Digestive
North Bergen School District Benchmarks
 Grade: 10,11, and 12 Subject: Anatomy and Physiology First Marking Period Define anatomy and physiology, and describe various subspecialties of each discipline. Describe the five basic functions of living
Grade: 10,11, and 12 Subject: Anatomy and Physiology First Marking Period Define anatomy and physiology, and describe various subspecialties of each discipline. Describe the five basic functions of living
Session 20. The Digestive Organs. Session Outline. Introduction. Unit 6 Digestive System
 Unit 6 Digestive System Session 20 The Digestive Organs Session Outline Introduction 20.1Alimentary tract 20.2 Mouth 20.3Tongue 20.4Teeth 20.5Oesophagus 20.6 Stomach 20.7Small intestine 20.8 large intestine
Unit 6 Digestive System Session 20 The Digestive Organs Session Outline Introduction 20.1Alimentary tract 20.2 Mouth 20.3Tongue 20.4Teeth 20.5Oesophagus 20.6 Stomach 20.7Small intestine 20.8 large intestine
SEER Training Modules
 http://training.seer.cancer.gov/anatomy/digestive/ WiRED International wishes to thank the National Cancer Institute for use of this information. SEER Training Modules Introduction to the Digestive System
http://training.seer.cancer.gov/anatomy/digestive/ WiRED International wishes to thank the National Cancer Institute for use of this information. SEER Training Modules Introduction to the Digestive System
Alimentary canal (gastrointestinal or GI tract) continuous coiled hollow tube
 The Digestive System and Body Metabolism Gross Anatomy Function The Digestive System Functions Ingestion taking in food Digestion breaking food down both physically and chemically Absorption movement of
The Digestive System and Body Metabolism Gross Anatomy Function The Digestive System Functions Ingestion taking in food Digestion breaking food down both physically and chemically Absorption movement of
Note Taking Guide. Topic # 3024 Comparative Digestive Systems
 Note Taking Guide Topic # 3024 Comparative Digestive Systems Digestive Systems Overview 1. Digestion Digestion: Food enters the mouth and goes through mechanical and chemical changes as it passes through
Note Taking Guide Topic # 3024 Comparative Digestive Systems Digestive Systems Overview 1. Digestion Digestion: Food enters the mouth and goes through mechanical and chemical changes as it passes through
Common features: - longitudinal tube through body - regional specializations along length - basic wall plan common to all vertebrate groups
 VERTEBRATE DIGESTIVE SYSTEM Functions: - mechanical breakdown - big lumps of food to small - chemical breakdown - digestion monomers - absorption of monomers - compact waste feces, extract water eliminate
VERTEBRATE DIGESTIVE SYSTEM Functions: - mechanical breakdown - big lumps of food to small - chemical breakdown - digestion monomers - absorption of monomers - compact waste feces, extract water eliminate
Histology. Epithelial Tissue
 Histology Epithelial Tissue Epithelial Tissue Lines internal and external body surfaces Forms glands Epithelial Tissue Little extracellular matrix Attached on one side Avascular Basement membrane Apical
Histology Epithelial Tissue Epithelial Tissue Lines internal and external body surfaces Forms glands Epithelial Tissue Little extracellular matrix Attached on one side Avascular Basement membrane Apical
RAD 223. Radiography physiology. Lecture Notes. First lecture: Cell and Tissue
 RAD 223 Radiography physiology Lecture Notes First lecture: Cell and Tissue Physiology: the word physiology derived from a Greek word for study of nature. It is the study of how the body and its part work
RAD 223 Radiography physiology Lecture Notes First lecture: Cell and Tissue Physiology: the word physiology derived from a Greek word for study of nature. It is the study of how the body and its part work
Functions of the digestive system
 Digestive system Functions of the digestive system Digestion-mechanical and chemical breakdown of material Motility-movement of material from the oral cavity to the anus-swallowing / peristalsis Secretion-exocrine
Digestive system Functions of the digestive system Digestion-mechanical and chemical breakdown of material Motility-movement of material from the oral cavity to the anus-swallowing / peristalsis Secretion-exocrine
Compartmentalization of the Cell. Objectives. Recommended Reading. Professor Alfred Cuschieri. Department of Anatomy University of Malta
 Compartmentalization of the Cell Professor Alfred Cuschieri Department of Anatomy University of Malta Objectives By the end of this session the student should be able to: 1. Identify the different organelles
Compartmentalization of the Cell Professor Alfred Cuschieri Department of Anatomy University of Malta Objectives By the end of this session the student should be able to: 1. Identify the different organelles
GI TRACT ORGANS ACCESSORY ORGANS
 Digestive System GI TRACT ORGANS Oral cavity Oropharynx Esophagus Stomach Small intestine Large Intestine Anus ACCESSORY ORGANS Salivary glands Pancreas Liver Gall bladder GI TRACT LAYERS Mucosa Submucosa
Digestive System GI TRACT ORGANS Oral cavity Oropharynx Esophagus Stomach Small intestine Large Intestine Anus ACCESSORY ORGANS Salivary glands Pancreas Liver Gall bladder GI TRACT LAYERS Mucosa Submucosa
Absorption and Transport of Nutrients
 Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Human Anatomy & Physiology I with Dr. Hubley. Practice Exam 1
 Human Anatomy & Physiology I with Dr. Hubley Practice Exam 1 1. Which definition is the best definition of the term gross anatomy? a. The study of cells. b. The study of tissues. c. The study of structures
Human Anatomy & Physiology I with Dr. Hubley Practice Exam 1 1. Which definition is the best definition of the term gross anatomy? a. The study of cells. b. The study of tissues. c. The study of structures
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
DIGESTIVE SYSTEM Five Basic Processes The Gastrointestinal tract (alimentary canal)
 DIGESTIVE SYSTEM Five Basic Processes 1. Ingestion - eating 2. Movement of the food along the G.I. tract. 3. Digestion- chemical and mechanical breakdown of food. 4. Absorption of the breakdown products
DIGESTIVE SYSTEM Five Basic Processes 1. Ingestion - eating 2. Movement of the food along the G.I. tract. 3. Digestion- chemical and mechanical breakdown of food. 4. Absorption of the breakdown products
Anatomy PHL 212. By Dr Tajdar Husain Khan
 Anatomy PHL 212 By Dr Tajdar Husain Khan Overview of Anatomy Anatomy(from the Greek word anatome,"dissection") is a branch of natural science dealing with the structural organization of living things The
Anatomy PHL 212 By Dr Tajdar Husain Khan Overview of Anatomy Anatomy(from the Greek word anatome,"dissection") is a branch of natural science dealing with the structural organization of living things The
Digestion in the small and Large Intestines
 9.5 Digestion in the small and Large Intestines Do some foods keep you feeling full for a long time? Do you ever feel that after eating certain foods, you are hungry again in a short time? Some foods stay
9.5 Digestion in the small and Large Intestines Do some foods keep you feeling full for a long time? Do you ever feel that after eating certain foods, you are hungry again in a short time? Some foods stay
Special organ structures and functions conduct these tasks through the successive parts of the overall system.
 Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Vertebrate Body Organization
 Vertebrate Body Organization Digestive tube suspended in coelom from mouth to anus Body supported by internal skeleton of jointed bones Vertebrae and Cranium protects nervous system Diaphragm divides coelom
Vertebrate Body Organization Digestive tube suspended in coelom from mouth to anus Body supported by internal skeleton of jointed bones Vertebrae and Cranium protects nervous system Diaphragm divides coelom
Continuing Education Independent Study Series
 Continuing Education Independent Study Series Professional Development Manager Association of Surgical Technologists Englewood, Colorado Association of Surgical Technologists Publication made possible
Continuing Education Independent Study Series Professional Development Manager Association of Surgical Technologists Englewood, Colorado Association of Surgical Technologists Publication made possible
Section B: Epithelial Tissue 1. Where are epithelial tissues found within the body? 2. What are the functions of the epithelial tissues?
 Tissue worksheet Name Section A: Intro to Histology Cells are the smallest units of life. In complex organisms, cells group together with one another based on similar structure and function to form tissues.
Tissue worksheet Name Section A: Intro to Histology Cells are the smallest units of life. In complex organisms, cells group together with one another based on similar structure and function to form tissues.
2012 Anatomy & Physiology C Division. National Competition-Science
 2012 Anatomy & Physiology C Division National Competition-Science Olympiad 1. The alimentary canal includes 8 different parts and 4 accessory organs. Name 5 parts from the alimentary canal and 3 accessory
2012 Anatomy & Physiology C Division National Competition-Science Olympiad 1. The alimentary canal includes 8 different parts and 4 accessory organs. Name 5 parts from the alimentary canal and 3 accessory
Weds 5/20/15. Membranes - finish last lecture outline. Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system
 Membranes - finish last lecture outline Weds 5/20/15 Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system structures and functions // accessory organs mechanism
Membranes - finish last lecture outline Weds 5/20/15 Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system structures and functions // accessory organs mechanism
Chapter 24: DIGESTIVE SYSTEM
 Chapter 24: DIGESTIVE SYSTEM I. OVERVIEW A. Gross anatomy (Fig. 24.1) and functions (Table 24.1) B. "You are what you eat." 1. The problem: Ingestion (eating) is not the same as absorption. 2. The solution:
Chapter 24: DIGESTIVE SYSTEM I. OVERVIEW A. Gross anatomy (Fig. 24.1) and functions (Table 24.1) B. "You are what you eat." 1. The problem: Ingestion (eating) is not the same as absorption. 2. The solution:
Digestion. Processing of food Types. Mechanical (physical) Chemical. Chew Tear Grind Mash Mix. Catabolic reactions Enzymatic hydrolysis
 Digestive System Digestion Processing of food Types Mechanical (physical) Chew Tear Grind Mash Mix Chemical Catabolic reactions Enzymatic hydrolysis Carbohydrate Protein Lipid 2 Digestion Phases Ingestion
Digestive System Digestion Processing of food Types Mechanical (physical) Chew Tear Grind Mash Mix Chemical Catabolic reactions Enzymatic hydrolysis Carbohydrate Protein Lipid 2 Digestion Phases Ingestion
By Casey Schmidt and Wendy Ford
 By Casey Schmidt and Wendy Ford Body systems Digestive System Circulatory System Respiratory System Excretory System Immune System Reproductive System Nervous System Muscular System Skeletal System Endocrine
By Casey Schmidt and Wendy Ford Body systems Digestive System Circulatory System Respiratory System Excretory System Immune System Reproductive System Nervous System Muscular System Skeletal System Endocrine
PCB 4023 Cell Biology. Lab 8: Organology II Digestive tract and accessory organs
 PCB 4023 Cell Biology Lab 8: Organology II Digestive tract and accessory organs Name: Name: SSN: SSN: N.B. Since this document is in pdf format, the URLs (web addresses) cannot be linked. To use them,
PCB 4023 Cell Biology Lab 8: Organology II Digestive tract and accessory organs Name: Name: SSN: SSN: N.B. Since this document is in pdf format, the URLs (web addresses) cannot be linked. To use them,
DIGESTIVE SYSTEM II Esophagus and Stomach. Dr. Paul W. L. Kwan Tufts University School of Medicine
 DIGESTIVE SYSTEM II Esophagus and Stomach Dr. Paul W. L. Kwan Tufts University School of Medicine Learning Objectives 1. The esophagus is a typical hollow organ. 2. Know the histology of each of the layers
DIGESTIVE SYSTEM II Esophagus and Stomach Dr. Paul W. L. Kwan Tufts University School of Medicine Learning Objectives 1. The esophagus is a typical hollow organ. 2. Know the histology of each of the layers
Environmental influences on the development of lordosis and musculoskeletal tissues in Sea bass (Dicentrarchus labrax): the ORCIS project
 Environmental influences on the development of lordosis and musculoskeletal tissues in Sea bass (Dicentrarchus labrax): the ORCIS project The ORCIS Partnership Prof Neil Stickland & Dr Clare Ashton, The
Environmental influences on the development of lordosis and musculoskeletal tissues in Sea bass (Dicentrarchus labrax): the ORCIS project The ORCIS Partnership Prof Neil Stickland & Dr Clare Ashton, The
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Magic School Bus Digestive System Brainpop Digestive System
 The Digestive System Magic School Bus Digestive System Brainpop Digestive System 1 Functions of the Digestive System: 1. Break up food into smaller pieces 2. Absorbing nutrients into the blood 3. Excreting
The Digestive System Magic School Bus Digestive System Brainpop Digestive System 1 Functions of the Digestive System: 1. Break up food into smaller pieces 2. Absorbing nutrients into the blood 3. Excreting
CHAPTER 20: URINARY SYSTEM
 OBJECTIVES: 1. Name the major function of the urinary system, and name and locate (on a diagram) the organs that compose the system. 2. Explain what the term renal refers to. 3. Define the term retroperitoneal.
OBJECTIVES: 1. Name the major function of the urinary system, and name and locate (on a diagram) the organs that compose the system. 2. Explain what the term renal refers to. 3. Define the term retroperitoneal.
Introduction to Anatomy and Physiology: Tissues and Integumentary System. Biology 105 Lecture 7 Chapter 4
 Introduction to Anatomy and Physiology: Tissues and Integumentary System Biology 105 Lecture 7 Chapter 4 Outline I. Tissues A. Epithelial B. Connective C. Muscle D. Nervous tissues II. Cell-to-cell contact
Introduction to Anatomy and Physiology: Tissues and Integumentary System Biology 105 Lecture 7 Chapter 4 Outline I. Tissues A. Epithelial B. Connective C. Muscle D. Nervous tissues II. Cell-to-cell contact
