PHYSICAL AND CHEMICAL CHANGES
|
|
|
- Alexina Atkins
- 7 years ago
- Views:
Transcription
1 reflect A student conducted an experiment with two identical candles. She measured the mass of each candle first. Then she lit one candle with a match, causing the candle to burn. She placed the other candle in a pan and heated it on the stove, causing it to melt. Later, she measured the masses of the two candles again. Study her data below. Why did she fi nd that the candle s mass decreased in one case but not in the other? What type of changes occurred in each case? Beginning mass Ending mass Burned candle 5.00 g 3.50 g Melted candle 5.00 g 5.00 g Matter changes in two ways: physically and chemically. The two candles experienced different types of change. The candle in the pan on the stove underwent a physical change only. The burned candle also underwent a physical change some of its wax melted. However, the burned candle also underwent a chemical change. How do these changes differ? Let s analyze the chemical change first. When wax burns, it reacts with oxygen in the air to produce two new substances. The new substances are carbon dioxide and water vapor. Both of these products are gases that diffuse, or scatter, into the air. The candle s mass decreases because some of the wax is converted to these gases. wax + oxygen chemical change carbon dioxide + water vapor Next, analyze the physical change. When wax is heated on a stove, it melts. A solid is transformed into a liquid. Because the wax keeps its original identity, the candle s mass does not change. Only the physical state of the candle changes. (Another term for state is phase.) physical change solid wax + heat liquid wax Whether a substance undergoes a chemical change or a physical change depends on whether the chemical identity of the substance changes. A chemical change involves a change in the identity or chemical composition of a substance. A physical change involves changes to the form of a substance, but not to its chemical identity. 1
2 When a substance changes physically, its physical properties change. Physical changes involve changes to the physical properties of a substance. You have already seen that a change in the state of a substance (gas, liquid, and solid) is a physical change. Another kind of physical change occurs when a solid substance is broken into smaller pieces. For example, when you crush a sugar cube, you change its size and shape. When you tear a piece of paper, you change its size and shape. These are physical changes because they do not cause the chemical identity of the substance to change. Only the substance s size and shape change. When a substance changes chemically, its chemical properties change. Chemical changes involve changes to the chemical identity of a substance. During a chemical change, the atoms in one or more substances rearrange. Once they rearrange, these atoms bond to one another to form new substances. Because we cannot see how atoms rearrange, we have to rely on changes that we can see to help us recognize a chemical change. What types of changes indicate chemical change? Gas production: The formation of a gas is often an indication of chemical change. When you mix vinegar with baking soda, for example, carbon dioxide gas is produced as the result of a chemical reaction taking place. You can see this gas as air bubbles in the vinegar. atom: the smallest unit of an element that has all the properties of that element Solid formation: Like the formation of a gaseous product, a solid product can provide evidence of a chemical change. The rust that forms on metal is an example of a solid forming from a chemical reaction. Color change: A change in color can be a sign that a chemical change has occurred. When silver tarnishes, it changes from shiny silver to dull gray. This color change is due to a chemical reaction between silver metal and sulfur-containing compounds present in air. 2
3 Temperature change: Chemical reactions involve changes in energy. Some reactions release energy. Others absorb energy from the surroundings. If the energy change is great enough, you may be able to feel it. A reaction that releases energy feels warm to the touch. A reaction that absorbs energy feels cool. look out! You cannot conclude that a chemical change accompanies every change in color or temperature. Color and temperature changes sometimes accompany physical changes, too. For example, when you mix two colors of paint together, a new color often results. No chemical change occurred in this case. You see a physical change as two substances mix together. In another example, a can of compressed air feels cool to the touch when you activate the nozzle to dispense a stream of air. This change in temperature does not result from a chemical reaction. Instead the temperature drop is the result of physical changes in the number of gas particles inside the can. Both physical and chemical changes happen in the human digestive system. We can learn more about physical and chemical changes by studying your body s digestive system. When you eat food, you take a variety of organic compounds into your body. Organic compounds contain carbon and other elements that you need to stay healthy. In the digestive process, your body breaks down these compounds by both physical and chemical processes. The digestive system involves both kinds of changes because food molecules are large and complex. Food must be broken down into much smaller pieces before our bodies can absorb it. Once broken down, food molecules are absorbed into the blood stream. The blood then transports food molecules to all the cells in the body. These molecules provide nourishment for cells to keep them functioning well. D igestion can be divided into two broad types: mechanical digestion and chemical digestion. Mechanical digestion involves breaking large pieces of food into smaller pieces of food. Physical actions of biting, chewing, mixing, and churning all contribute to mechanical digestion. These processes cause physical changes in food. Foods undergoing mechanical digestion include changes in size and shape. 3
4 Following mechanical digestion, chemical digestion can begin. Chemical digestion involves breaking large molecules into smaller molecules. This type of digestion is often carried out by large protein molecules called enzymes. Each enzyme breaks down a specifi c type of molecule. For example, pepsin is an enzyme that breaks down proteins. Amylase is an enzyme that breaks down starch. The end result of chemical digestion is a Food is made up of many kinds of molecules. mixture of small molecules that can pass Different enzymes are necessary to break through cell membranes into cells. Once down different molecules. inside the cells, these molecules supply a source of chemical energy and chemical building blocks for the cell to carry out life processes. what do you think? Like other living things, bacteria must obtain food to live. Because it is so small, a bacterial cell absorbs chemical compounds from its environment. The cell uses these compounds as food. Do you think that a single-celled organism like a bacterium carries out mechanical digestion? Chemical digestion? In terms of food digestion, how is a bacterial cell like a human muscle cell? What kinds of organisms have digestive systems that perform mechanical and chemical digestion? The human digestive system contains many specialized parts. Your body causes both physical and chemical change in the food you eat. We can follow food through the digestive process. At each stage, we can analyze the types of changes taking place in the food. Mouth: Once you take a bite of food, you begin chewing it. Chewing reduces food size. At the same time, your mouth brings about a change in state in some foods. For example, a chocolate bar changes from a solid to a liquid as it melts in your mouth. Both changes in size and state are physical changes that happen to food in the mouth. Chemical changes occur in the mouth, too. Your saliva contains enzymes that break bonds in large molecules to form smaller molecules. Large starch molecules in bread undergo this type of change in the mouth. Starch is chemically digested into molecules of glucose (C 6 H 12 O 6 ), a simple sugar. 4
5 Stomach: More physical and chemical changes take place as food enters your stomach. Muscular movement in the stomach causes food particles to become even smaller. This is a physical change. Enzymes and digestive juices in the stomach work to chemically break down proteins into amino acids. This is a chemical change. Small intestine: By the time food has reached the small intestine, its size has been reduced considerably. Now in the form of large and small molecules, the food undergoes mostly chemical changes. Many enzymes produced by the small intestine work to reduce remaining large molecules into smaller compounds. Small molecules pass through the walls of the small intestine to enter blood vessels. Most of the absorption of molecules takes place in the small intestine. Large intestine: Matter not absorbed in the small intestine enters the large intestine. Digestion is over by this stage. However, the body still needs to rid itself of the leftover matter. The large intestine works to pass this material along. It also works to absorb water from this matter. The remaining waste passes out of the body as fecal matter. Everyday Life: Why do some people have difficulty digesting milk? Some people cannot digest milk. They have a condition known as lactose intolerance. Lactose is a sugar found naturally in milk. People with lactose intolerance do not produce enzymes in their small intestines that break down lactose into smaller molecules. When this enzyme is absent, lactose builds up and causes discomfort. This condition is not dangerous, and people with lactose intolerance are fi ne if they avoid foods containing lactose. There are also enzyme solutions for sale in pharmacies and grocery stores that can help. When taken with milk or dairy products, these enzyme solutions can help a person with lactose intolerance digest the lactose. What do you know? The human digestive system can be described using a flowchart like the one shown on the next page. The four boxes at the left of the fl owchart represent the four main parts of the digestive system: Large intestine, Mouth, Small intestine, and Stomach. 5
6 Place each part in the correct box; the top box represents the first part that food passes through and the bottom box represents the final part that food passes through. Then, complete the fl ow chart by writing descriptions of any mechanical or chemical digestive processes in the spaces next to each part. 6
7 connecting with your child Observing Digestion at Work To help students learn more about the process of digestion, have them research the work of physician William Beaumont. Beaumont was an army physician stationed at a fort in 1822 when an accident led to an unusual research opportunity. A gunshot wound in a soldier s abdomen created a hole in the man s stomach. Even when the man healed, the hole remained, which made it possible for Beaumont to observe changes in the contents of the man s stomach during digestion. Encourage your child to do enough research to discover what scientists thought about digestion at the time, and how Beaumont was able to contribute to the scientifi c understanding of the digestive process as a result of the experiments he performed on the man. Here are some questions to discuss with your child: How did scientists understanding of the human digestive process in the early 1800s differ from our understanding today? Why was the soldier s wound so unusual? What scientific opportunity did it give Beaumont? What kinds of experiments did Beaumont do that helped increase our understanding of chemical digestion? 7
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems
 Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Chemical Changes. Measuring a Chemical Reaction. Name(s)
 Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES
 PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
Physical and Chemical Changes Pre Test Questions
 Pre Test Questions Name: Period: Date: 1. Which of the following is an example of physical change? a. Mixing baking soda and vinegar together, and this causes bubbles and foam. b. A glass cup falls from
Pre Test Questions Name: Period: Date: 1. Which of the following is an example of physical change? a. Mixing baking soda and vinegar together, and this causes bubbles and foam. b. A glass cup falls from
The Excretory and Digestive Systems
 The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
ANSWER KEY. Acids, Bases, and Solutions. Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries,
 Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
The Human Digestive System
 The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
Getting Energy from Food Your Digestive System
 9 Getting Energy from Food Your Digestive System The Digestive System You know how your body gets the oxygen it needs. But how do your body s cells get the nutrients they need? Nutrients come from the
9 Getting Energy from Food Your Digestive System The Digestive System You know how your body gets the oxygen it needs. But how do your body s cells get the nutrients they need? Nutrients come from the
Chemistry Worksheet: Matter #1
 Chemistry Worksheet: Matter #1 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up
Chemistry Worksheet: Matter #1 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up
Review and apply Investigation 5. Let s review Pages 311-312
 Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
What happens to the food we eat? It gets broken down!
 Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
Physical and Chemical Properties of Matter
 Physical and Chemical Properties of Matter What is matter? Anything that has mass and takes up space Chemical or Physical Property? Physical properties of matter: characteristics that can be observed or
Physical and Chemical Properties of Matter What is matter? Anything that has mass and takes up space Chemical or Physical Property? Physical properties of matter: characteristics that can be observed or
Warm-Up 9/9. 1. Define the term matter. 2. Name something in this room that is not matter.
 Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Unit B Understanding Animal Body Systems. Lesson 1 Understanding Animal Digestion
 Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
20.2 Chemical Equations
 All of the chemical changes you observed in the last Investigation were the result of chemical reactions. A chemical reaction involves a rearrangement of atoms in one or more reactants to form one or more
All of the chemical changes you observed in the last Investigation were the result of chemical reactions. A chemical reaction involves a rearrangement of atoms in one or more reactants to form one or more
Topic 4: Digestion and Nutrition
 Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name Date Class CHAPTER 1 REVIEW. Answer the following questions in the space provided.
 CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
Lesson 2: Digestion of fats, proteins, and carbohydrates
 Lesson 2: Digestion of fats, proteins, and carbohydrates Inquiry Focus: How does the body get energy from food? Student Learning Objectives: By the end of the lesson, students will be able to do the following:
Lesson 2: Digestion of fats, proteins, and carbohydrates Inquiry Focus: How does the body get energy from food? Student Learning Objectives: By the end of the lesson, students will be able to do the following:
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
2) Digestion the breakdown of. There are two types of digestion: Mechanical and Chemical. 3) Absorption when the nutrients enter into the blood.
 The Digestive System Video on the digestive system (5 min) The digestive system is responsible for the breakdown of the we eat so that it can be absorbed into the. There are four main stages of the digestive
The Digestive System Video on the digestive system (5 min) The digestive system is responsible for the breakdown of the we eat so that it can be absorbed into the. There are four main stages of the digestive
Name Date Period. Keystone Review Enzymes
 Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document.
 Name: Period: Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document. 1. Which of the following is a NOT a physical property of hydrogen? A. It is gas C. It is
Name: Period: Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document. 1. Which of the following is a NOT a physical property of hydrogen? A. It is gas C. It is
Chapter 48. Nutrients in Food. Carbohydrates, Proteins, and Lipids. Carbohydrates, Proteins, and Lipids, continued
 Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Magic School Bus Digestive System Brainpop Digestive System
 The Digestive System Magic School Bus Digestive System Brainpop Digestive System 1 Functions of the Digestive System: 1. Break up food into smaller pieces 2. Absorbing nutrients into the blood 3. Excreting
The Digestive System Magic School Bus Digestive System Brainpop Digestive System 1 Functions of the Digestive System: 1. Break up food into smaller pieces 2. Absorbing nutrients into the blood 3. Excreting
The Digestive System: Where does food go? Teacher Version
 The Digestive System: Where does food go? Teacher Version In this lab you will learn about your digestive system. We will use everyday objects like yarn and a ziplock bag to understand how long our digestive
The Digestive System: Where does food go? Teacher Version In this lab you will learn about your digestive system. We will use everyday objects like yarn and a ziplock bag to understand how long our digestive
EXAMPLE EXERCISE 4.1 Change of Physical State
 EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
Digestive System Notes
 Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
Name: Class: Date: ID: A
 Name: Class: Date: ID: A Chapter 2 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. Complete short answer questions on a separate sheet of paper.
Name: Class: Date: ID: A Chapter 2 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. Complete short answer questions on a separate sheet of paper.
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Enzymes. Chapter 3. 3.1 Enzymes and catalysts. Vital mistake. What is an enzyme?
 Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Chemical reactions allow living things to grow, develop, reproduce, and adapt.
 Section 2: Chemical reactions allow living things to grow, develop, reproduce, and adapt. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the parts of a chemical reaction?
Section 2: Chemical reactions allow living things to grow, develop, reproduce, and adapt. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the parts of a chemical reaction?
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
THE DIGESTIVE SYSTEM
 THE DIGESTIVE SYSTEM What is digestion? Digestion is the process of breaking down food so that it's small enough to be absorbed and used by the body for energy or in other bodily functions. Digestion involves
THE DIGESTIVE SYSTEM What is digestion? Digestion is the process of breaking down food so that it's small enough to be absorbed and used by the body for energy or in other bodily functions. Digestion involves
PHOTOSYNTHESIS AND CELLULAR RESPIRATION
 reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter Test A. Elements, Compounds, and Mixtures MULTIPLE CHOICE. chemically combined? MIXs2 a. element b. compound c. mixture d.
 Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Name: Unit 2- Elements, Compounds and Mixtures and Physical/Chemical Properties and Changes. Elements, Compounds and Mixtures
 Name: Unit 2- Elements, Compounds and Mixtures and Physical/Chemical Properties and Changes Day Page # Description IC/HW All 2 Warm-up IC 1 3 5 Matter Notes IC 1 6 Nuts & Bolts IC 1 7 Elements, Compounds
Name: Unit 2- Elements, Compounds and Mixtures and Physical/Chemical Properties and Changes Day Page # Description IC/HW All 2 Warm-up IC 1 3 5 Matter Notes IC 1 6 Nuts & Bolts IC 1 7 Elements, Compounds
Heterogeneous Homogenous. Mixtures; Solutions. Phases of matter: Solid. Phases of Matter: Liquid. Phases of Matter: Gas. Solid, Liquid, Gas
 Phases of matter: Solid Heterogeneous Homogenous Mixtures Solutions Phases of Matter: Liquid Atoms and molecules are more spaced out and now can move. The material can be slightly compressed into a smaller
Phases of matter: Solid Heterogeneous Homogenous Mixtures Solutions Phases of Matter: Liquid Atoms and molecules are more spaced out and now can move. The material can be slightly compressed into a smaller
By Casey Schmidt and Wendy Ford
 By Casey Schmidt and Wendy Ford Body systems Digestive System Circulatory System Respiratory System Excretory System Immune System Reproductive System Nervous System Muscular System Skeletal System Endocrine
By Casey Schmidt and Wendy Ford Body systems Digestive System Circulatory System Respiratory System Excretory System Immune System Reproductive System Nervous System Muscular System Skeletal System Endocrine
2 MATTER. 2.1 Physical and Chemical Properties and Changes
 2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
The Digestive System. You are what you eat!
 The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
Chemical Reactions Practice Test
 Chemical Reactions Practice Test Chapter 2 Name Date Hour _ Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The only sure evidence for a chemical reaction
Chemical Reactions Practice Test Chapter 2 Name Date Hour _ Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The only sure evidence for a chemical reaction
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Properties and Classifications of Matter
 PS-3.1 Distinguish chemical properties of matter (including reactivity) from physical properties of matter (including boiling point, freezing/melting point, density [with density calculations], solubility,
PS-3.1 Distinguish chemical properties of matter (including reactivity) from physical properties of matter (including boiling point, freezing/melting point, density [with density calculations], solubility,
DIGESTION is the physical and
 Digestion DIGESTION is the physical and chemical breakdown of feeds as they pass through the gastrointestinal tract. The structures of the gastrointestinal tract include the mouth, the esophagus, the stomach,
Digestion DIGESTION is the physical and chemical breakdown of feeds as they pass through the gastrointestinal tract. The structures of the gastrointestinal tract include the mouth, the esophagus, the stomach,
A Study of Matter. Video Notes
 A Study of Matter Video Notes In this lesson you will: Define physical property, chemical property and chemical change. Describe the phases of matter. Label properties as physical or chemical. Label changes
A Study of Matter Video Notes In this lesson you will: Define physical property, chemical property and chemical change. Describe the phases of matter. Label properties as physical or chemical. Label changes
Chemical versus Physical Changes
 Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
reflect look out! organisms: living things
 reflect Imagine that a student in your school fell down and is having difficulty breathing. Sirens wail as an ambulance pulls into the school parking lot. The emergency workers rush over to help the student.
reflect Imagine that a student in your school fell down and is having difficulty breathing. Sirens wail as an ambulance pulls into the school parking lot. The emergency workers rush over to help the student.
Absorption and Transport of Nutrients
 Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Digestive System Functions
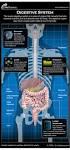 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
The Digestive System Grade 5
 TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
Biology 13A Lab #13: Nutrition and Digestion
 Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
The Digestive System
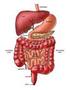 The Digestive System What do you know?? quiz-digestive-health Digestion Videos The Digestive System Inside-Dr-Ozs-Digestive-System-Video Now it is your turn to recreate the digestive system. How is food
The Digestive System What do you know?? quiz-digestive-health Digestion Videos The Digestive System Inside-Dr-Ozs-Digestive-System-Video Now it is your turn to recreate the digestive system. How is food
Oxygen Give and Take. Correlation to National Science Education Standards
 Chemistry and Environmental Sciences Oxygen Give and Take Summary This is a series of three activities followed by a worksheet. The concepts taught include gas production (O 2 and CO 2 ), chemical reactions,
Chemistry and Environmental Sciences Oxygen Give and Take Summary This is a series of three activities followed by a worksheet. The concepts taught include gas production (O 2 and CO 2 ), chemical reactions,
tissues are made of cells that work together, organs are )
 Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
6023-1 - Page 1. Name: 4) The diagram below represents a beaker containing a solution of various molecules involved in digestion.
 Name: 6023-1 - Page 1 1) Which one of the following situations indicates a serious organ system malfunction? A) Mitochondria stop functioning in a unicellular organism exposed to pollutants. B) White blood
Name: 6023-1 - Page 1 1) Which one of the following situations indicates a serious organ system malfunction? A) Mitochondria stop functioning in a unicellular organism exposed to pollutants. B) White blood
FIRST GRADE CHEMISTRY
 FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Acids and Bases. AND a widemouth container of the following solids:
 Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
O o. Thomas Jefferson National Accelerator Facility - Office of Science Education http://education.jlab.org/
 O o b l ekk c What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for
O o b l ekk c What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for
PLANT AND ANIMAL CELL ORGANELLES
 reflect The heart is an example of an organ. Think for a minute about your body. It s organized into parts that perform specific functions. For example, your heart functions to help transport materials
reflect The heart is an example of an organ. Think for a minute about your body. It s organized into parts that perform specific functions. For example, your heart functions to help transport materials
Pre-requisites: Successful completion of 4th grade science and the 4th grade science assessment.
 Throughout each unit, assessments are incorporated into lessons. These assessments are activities that occur within the context of each lesson providing the guidelines for assessing students' progress.
Throughout each unit, assessments are incorporated into lessons. These assessments are activities that occur within the context of each lesson providing the guidelines for assessing students' progress.
Activity Sheets Enzymes and Their Functions
 Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
PHOTOSYNTHESIS. reflect. what do you think?
 reflect Suppose you place a plant on a sunny windowsill and water it regularly. At the same time you place a similar plant in a dark closet and keep it watered, too. The only difference between the two
reflect Suppose you place a plant on a sunny windowsill and water it regularly. At the same time you place a similar plant in a dark closet and keep it watered, too. The only difference between the two
Medical Physiology Z.H.Al-Zubaydi
 Lec.13 Medical Physiology Z.H.Al-Zubaydi Functions of the Digestive System The major functions of the digestive tract include the following six processes, summarized in Figure 1: 1. Ingestion Food must
Lec.13 Medical Physiology Z.H.Al-Zubaydi Functions of the Digestive System The major functions of the digestive tract include the following six processes, summarized in Figure 1: 1. Ingestion Food must
Thermodynamics and Equilibrium
 Chapter 19 Thermodynamics and Equilibrium Concept Check 19.1 You have a sample of 1.0 mg of solid iodine at room temperature. Later, you notice that the iodine has sublimed (passed into the vapor state).
Chapter 19 Thermodynamics and Equilibrium Concept Check 19.1 You have a sample of 1.0 mg of solid iodine at room temperature. Later, you notice that the iodine has sublimed (passed into the vapor state).
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
NAME: DATE: Home Economics: The parts of the digestive system and their functions. Home Economics
 Home Economics The parts of the digestive system and their functions It is not necessary to carry out all the activities contained in this unit. Please see Teachers Notes for explanations, additional activities,
Home Economics The parts of the digestive system and their functions It is not necessary to carry out all the activities contained in this unit. Please see Teachers Notes for explanations, additional activities,
I. The basic function of the digestive system is
 Chapter 15, Digestive System - ANATOMY OF THE DIGESTIVE SYSTEM I. The basic function of the digestive system is. This process is called. II. List 2 other names for the digestive tract: A. B. III. The digestive
Chapter 15, Digestive System - ANATOMY OF THE DIGESTIVE SYSTEM I. The basic function of the digestive system is. This process is called. II. List 2 other names for the digestive tract: A. B. III. The digestive
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Get It Right. Answers. Chapter 1: The Science of Life. A biologist studies all living things.
 Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
TEACHER BACKGROUND INFORMATION THERMAL ENERGY
 TEACHER BACKGROUND INFORMATION THERMAL ENERGY In general, when an object performs work on another object, it does not transfer all of its energy to that object. Some of the energy is lost as heat due to
TEACHER BACKGROUND INFORMATION THERMAL ENERGY In general, when an object performs work on another object, it does not transfer all of its energy to that object. Some of the energy is lost as heat due to
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Determination of Specific Nutrients in Various Foods. Abstract. Humans need to consume food compounds such as carbohydrates, proteins, fats,
 Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
CHEMICAL FORMULAS AND EQUATIONS
 reflect Imagine that you and three other classmates had enough supplies and the recipe to make one pepperoni pizza. The recipe might include a ball of dough, a cup of pizza sauce, a cup of cheese, and
reflect Imagine that you and three other classmates had enough supplies and the recipe to make one pepperoni pizza. The recipe might include a ball of dough, a cup of pizza sauce, a cup of cheese, and
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
THE ACTIVITY OF LACTASE
 THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Ecology Pre-Test (High School)
 Ecology Pre-Test (High School) Science is easier to understand if you can make connections between what you know now and the new ideas that you are studying. This is a test that will help us to understand
Ecology Pre-Test (High School) Science is easier to understand if you can make connections between what you know now and the new ideas that you are studying. This is a test that will help us to understand
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction
 Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
B2 1 Cells, Tissues and Organs
 B2 Cells, Tissues and Organs 5 minutes 5 marks Page of 7 Q. The diagram shows a bacterium. On the drawing, name the structures labelled A, B, C and D. (Total 4 marks) Q2. (a) The diagrams show cells containing
B2 Cells, Tissues and Organs 5 minutes 5 marks Page of 7 Q. The diagram shows a bacterium. On the drawing, name the structures labelled A, B, C and D. (Total 4 marks) Q2. (a) The diagrams show cells containing
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
The Huntington Library, Art Collections, and Botanical Gardens. How Sweet It Is: Enzyme Action in Seed Germination
 The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
Sample Liver Enzyme Lab
 Sample Liver Enzyme Lab Design Aspect 1: Research Question This lab will be driven by the research question, Do changes in temperature have an effect on the activity of the enzyme catalase? Pearson Baccalaureate:
Sample Liver Enzyme Lab Design Aspect 1: Research Question This lab will be driven by the research question, Do changes in temperature have an effect on the activity of the enzyme catalase? Pearson Baccalaureate:
Classifying Matter. reflect. look out!
 reflect Do you know what air, water, and an apple all have in common? They are all examples of matter. Matter is a word we use a lot in science. It means stuff. All of the stuff in the world that has mass
reflect Do you know what air, water, and an apple all have in common? They are all examples of matter. Matter is a word we use a lot in science. It means stuff. All of the stuff in the world that has mass
Letter to the Student... 5 Test-Taking Checklist... 6 Next Generation Sunshine State Standards Correlation Chart... 7
 Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
ACIDS AND BASES SAFETY PRECAUTIONS
 ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
Chapter 4 Practice Quiz
 Chapter 4 Practice Quiz 1. Label each box with the appropriate state of matter. A) I: Gas II: Liquid III: Solid B) I: Liquid II: Solid III: Gas C) I: Solid II: Liquid III: Gas D) I: Gas II: Solid III:
Chapter 4 Practice Quiz 1. Label each box with the appropriate state of matter. A) I: Gas II: Liquid III: Solid B) I: Liquid II: Solid III: Gas C) I: Solid II: Liquid III: Gas D) I: Gas II: Solid III:
Biopharmaceuticals and Biotechnology Unit 2 Student Handout. DNA Biotechnology and Enzymes
 DNA Biotechnology and Enzymes 35 Background Unit 2~ Lesson 1 The Biotechnology Industry Biotechnology is a process (or a technology) that is used to create products like medicines by using micro-organisms,
DNA Biotechnology and Enzymes 35 Background Unit 2~ Lesson 1 The Biotechnology Industry Biotechnology is a process (or a technology) that is used to create products like medicines by using micro-organisms,
