Glucose Fructose Galactose
|
|
|
- Bathsheba Merritt
- 7 years ago
- Views:
Transcription
1 Name Date Per preap Biology Nutrient Molecules: Chemistry and Identification of Carbohydrates Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding of some chemistry of living things is necessary. In this activity, you will construct models that represent three major molecules of biology. Remember: Models do not represent the actual three-dimensional shapes of the molecules. Models serve to help you learn how smaller molecules can be grouped into larger, more complex molecules. Procedure Part A. Water Model 1. What is the molecular formula for water? 2. What elements make up water? 3. How many Hydrogen atoms are present in a molecule of water? 4. How many Oxygen atoms are present in a molecule of water? Part B. Carbohydrate Models Group 1. Monosaccharide (single molecule sugars) A single molecule sugar is called a monosaccharide. The prefix mono means one. However, the one molecule can have different shapes due to a different arrangement of atoms. Three monosaccharides are glucose, fructose, and galactose. Glucose Fructose Galactose Examine the structural formulas of these three sugars SHOWN ABOVE and answer questions 1 to What three chemical elements are present in all three monosaccharides shown? 2. How many atoms of carbon are present in a molecule of Glucose Fructose Galactose 3. Add subscripts to the following to indicate the proper molecular formula. Fill in the blanks by counting the total number of carbon, hydrogen, and oxygen atoms in each molecule. Glucose: C H O Fructose: C H O Galactose: C H O 1
2 4. What is the ratio of hydrogen atoms to oxygen atoms in a monosaccharide? 5. What is the ratio of hydrogen atoms to oxygen atoms in a molecule of water? 6. Compare the structural formula of glucose and fructose a. Are they exactly the same in shape? b. Are they both monosaccharides? Group 2. Disaccharides (double molecule sugars) Two monosaccharide sugar molecules can join chemically to form a larger carbohydrate molecule called a double sugar, or disaccharide. The prefix di- means two. By chemically joining a glucose molecule with a fructose molecule, a double sugar called sucrose is produced. Use the page of paper models given to you by your teacher to complete this section. Sucrose Cut out a model of ONE glucose and ONE fructose molecule. Cut along SOLID LINES ONLY. In order to join the molecules, remove an OH end from ONE molecule and an H end from THE OTHER molecule. Cut along the dotted lines. Fit the fructose and glucose together. Glue on the space below and label the molecule sucrose. 7. The H and OH ends that were removed can also fit together with each other to form a molecule. This new molecule has a molecular formula of and is called. Fit together and glue the H and OH next to the sucrose and label it. 2
3 8. Write the molecular formula for sucrose by adding together the molecular formulas of glucose and fructose FROM QUESTION #3 and then subtracting water, H 2 O Molecular Formula for Glucose + Molecular Formula for Fructose = - H 2 O Molecular Formula for Sucrose = Maltose Different disaccharide molecules can be made by joining other monosaccharides in different combinations. By chemically joining a glucose molecule to another glucose molecule, a double sugar called maltose is formed. Cut out two molecules of glucose. Cut along SOLID LINES ONLY. 9. What must be removed from the glucose models so that they easily fit together? Remove the necessary pieces for the two glucose models to fit together. Fit together the two glucose molecules and glue on the space below. Label the molecule maltose. Fit together and glue the H and OH next to the maltose and label it. 3
4 10. Write the molecular formula for maltose using the molecular formulas FROM QUESTION #3. Molecular Formula for Glucose + Molecular Formula for Glucose = - H 2 O Molecular Formula for Maltose = 11. How does the molecular formula for sucrose compare to maltose? 12. What is the ratio of hydrogen atoms to oxygen atoms in a disaccharide? 13. How many monosaccharide molecules are needed to form one sucrose molecule? 14. How many monosaccharide molecules are needed to form one maltose molecule? Group 3. Polysaccharides (many molecule sugars) Just as double sugars were formed from two single sugar molecules, polysaccharides are formed when many single sugars are joined chemically. The prefix poly- means many. Starch, glycogen, and cellulose are the three most common polysaccharides in biology. They consist of long chains of glucose molecules joined together. Construct a starch molecule by joining three glucose molecules. This model will represent only a small part of a starch molecule because starch consists of hundreds of glucose molecules. 15. What must be removed from the glucose models in order to have them easily fit together? Fit together and glue the glucose molecules in the space below. Label the molecule starch. Fit together and glue each of the two water molecules next to the starch molecule and label them. 4
5 5
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
19.2 Chemical Formulas
 In the previous section, you learned how and why atoms form chemical bonds with one another. You also know that atoms combine in certain ratios with other atoms. These ratios determine the chemical formula
In the previous section, you learned how and why atoms form chemical bonds with one another. You also know that atoms combine in certain ratios with other atoms. These ratios determine the chemical formula
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
The molecules of life. The molecules that make up living things are really big They are called macromolecules
 Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Lesson Plan: The Building Blocks of Photosynthesis
 Lesson Plan: The Building Blocks of Photosynthesis Summary In this lesson, students will use colored blocks to represent the elements in photosynthesis and illustrate how they are broken down and reassembled
Lesson Plan: The Building Blocks of Photosynthesis Summary In this lesson, students will use colored blocks to represent the elements in photosynthesis and illustrate how they are broken down and reassembled
We know from the information given that we have an equal mass of each compound, but no real numbers to plug in and find moles. So what can we do?
 How do we figure this out? We know that: 1) the number of oxygen atoms can be found by using Avogadro s number, if we know the moles of oxygen atoms; 2) the number of moles of oxygen atoms can be found
How do we figure this out? We know that: 1) the number of oxygen atoms can be found by using Avogadro s number, if we know the moles of oxygen atoms; 2) the number of moles of oxygen atoms can be found
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Digestive System Functions
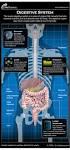 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
Metabolism: Cellular Respiration, Fermentation and Photosynthesis
 Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
2. PHOTOSYNTHESIS. The general equation describing photosynthesis is light + 6 H 2 O + 6 CO 2 C 6 H 12 O 6 + 6 O 2
 2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Photo Cell Resp Practice. A. ATP B. oxygen C. DNA D. water. The following equation represents the process of photosynthesis in green plants.
 Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Biology 13A Lab #13: Nutrition and Digestion
 Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Modelling Compounds. 242 MHR Unit 2 Atoms, Elements, and Compounds
 6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
MOLECULAR MASS AND FORMULA MASS
 1 MOLECULAR MASS AND FORMULA MASS Molecular mass = sum of the atomic weights of all atoms in the molecule. Formula mass = sum of the atomic weights of all atoms in the formula unit. 2 MOLECULAR MASS AND
1 MOLECULAR MASS AND FORMULA MASS Molecular mass = sum of the atomic weights of all atoms in the molecule. Formula mass = sum of the atomic weights of all atoms in the formula unit. 2 MOLECULAR MASS AND
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
What are the similarities between this equation for burning glucose and the equation for cellular respiration of glucose when oxygen is available?
 Cellular Respiration in Yeast Adapted from Alcoholic Fermentation in Yeast Investigation in the School District of Philadelphia Biology Core Curriculum 2009 by Dr. Jennifer Doherty and Dr. Ingrid Waldron,
Cellular Respiration in Yeast Adapted from Alcoholic Fermentation in Yeast Investigation in the School District of Philadelphia Biology Core Curriculum 2009 by Dr. Jennifer Doherty and Dr. Ingrid Waldron,
Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test
 Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test NAME Section 7.1 The Mole: A Measurement of Matter A. What is a mole? 1. Chemistry is a quantitative science. What does this term mean?
Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test NAME Section 7.1 The Mole: A Measurement of Matter A. What is a mole? 1. Chemistry is a quantitative science. What does this term mean?
Plants: The Ultimate Green Machines Science, Grade Level 7
 DRAFT 1 Lesson Title: Plants: The Ultimate Green Machines Grade Level: 7 Subject Area: Science Setting: Garden, Classroom, Laboratory Instructional Time: 1-2 class periods Plants: The Ultimate Green Machines
DRAFT 1 Lesson Title: Plants: The Ultimate Green Machines Grade Level: 7 Subject Area: Science Setting: Garden, Classroom, Laboratory Instructional Time: 1-2 class periods Plants: The Ultimate Green Machines
McMush. Testing for the Presence of Biomolecules
 Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Chapter 3. Chemical Reactions and Reaction Stoichiometry. Lecture Presentation. James F. Kirby Quinnipiac University Hamden, CT
 Lecture Presentation Chapter 3 Chemical Reactions and Reaction James F. Kirby Quinnipiac University Hamden, CT The study of the mass relationships in chemistry Based on the Law of Conservation of Mass
Lecture Presentation Chapter 3 Chemical Reactions and Reaction James F. Kirby Quinnipiac University Hamden, CT The study of the mass relationships in chemistry Based on the Law of Conservation of Mass
Simple vs. True. Simple vs. True. Calculating Empirical and Molecular Formulas
 Calculating Empirical and Molecular Formulas Formula writing is a key component for success in chemistry. How do scientists really know what the true formula for a compound might be? In this lesson we
Calculating Empirical and Molecular Formulas Formula writing is a key component for success in chemistry. How do scientists really know what the true formula for a compound might be? In this lesson we
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
green B 1 ) into a single unit to model the substrate in this reaction. enzyme
 Teacher Key Objectives You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating enzymatic activity.
Teacher Key Objectives You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating enzymatic activity.
Chapter 6 Notes. Chemical Composition
 Chapter 6 Notes Chemical Composition Section 6.1: Counting By Weighing We can weigh a large number of the objects and find the average mass. Once we know the average mass we can equate that to any number
Chapter 6 Notes Chemical Composition Section 6.1: Counting By Weighing We can weigh a large number of the objects and find the average mass. Once we know the average mass we can equate that to any number
Chemical Calculations: Formula Masses, Moles, and Chemical Equations
 Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
CHAPTER 8: CHEMICAL COMPOSITION
 CHAPTER 8: CHEMICAL COMPOSITION Active Learning: 1-4, 6-8, 12, 18-25; End-of-Chapter Problems: 3-4, 9-82, 84-85, 87-92, 94-104, 107-109, 111, 113, 119, 125-126 8.2 ATOMIC MASSES: COUNTING ATOMS BY WEIGHING
CHAPTER 8: CHEMICAL COMPOSITION Active Learning: 1-4, 6-8, 12, 18-25; End-of-Chapter Problems: 3-4, 9-82, 84-85, 87-92, 94-104, 107-109, 111, 113, 119, 125-126 8.2 ATOMIC MASSES: COUNTING ATOMS BY WEIGHING
Enzymes. Chapter 3. 3.1 Enzymes and catalysts. Vital mistake. What is an enzyme?
 Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Solid Phase Peptide Synthesis
 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Chapter 3, Lesson 4: Density: Sink and Float for Solids
 Chapter 3, Lesson 4: Density: Sink and Float for Solids Key Concepts The density of an object determines whether it will float or sink in another substance. An object will float if it is less dense than
Chapter 3, Lesson 4: Density: Sink and Float for Solids Key Concepts The density of an object determines whether it will float or sink in another substance. An object will float if it is less dense than
The Mole Concept. The Mole. Masses of molecules
 The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
Chemical Calculations: The Mole Concept and Chemical Formulas. AW Atomic weight (mass of the atom of an element) was determined by relative weights.
 1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
Chapter 2 Atoms and Molecules
 Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Chapter 4: Structure and Properties of Ionic and Covalent Compounds
 Chapter 4: Structure and Properties of Ionic and Covalent Compounds 4.1 Chemical Bonding o Chemical Bond - the force of attraction between any two atoms in a compound. o Interactions involving valence
Chapter 4: Structure and Properties of Ionic and Covalent Compounds 4.1 Chemical Bonding o Chemical Bond - the force of attraction between any two atoms in a compound. o Interactions involving valence
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
MOLAR MASS AND MOLECULAR WEIGHT Themolar mass of a molecule is the sum of the atomic weights of all atoms in the molecule. Molar Mass.
 Counting Atoms Mg burns in air (O 2 ) to produce white magnesium oxide, MgO. How can we figure out how much oxide is produced from a given mass of Mg? PROBLEM: If If 0.200 g of Mg is is burned, how much
Counting Atoms Mg burns in air (O 2 ) to produce white magnesium oxide, MgO. How can we figure out how much oxide is produced from a given mass of Mg? PROBLEM: If If 0.200 g of Mg is is burned, how much
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Elements and Compounds. Chemical Bonds compounds are made of atoms held together by chemical bonds bonds are forces of attraction between atoms
 Elements and Compounds elements combine together to make an almost limitless number of compounds the properties of the compound are totally different from the constituent elements Tro, Chemistry: A Molecular
Elements and Compounds elements combine together to make an almost limitless number of compounds the properties of the compound are totally different from the constituent elements Tro, Chemistry: A Molecular
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Overview... 1 What is the Outreach Program?... 1 Concepts... 2 Objectives... 3 Arizona Science Standards... 3 College and Career Ready ELA
 Overview... 1 What is the Outreach Program?... 1 Concepts... 2 Objectives... 3 Arizona Science Standards... 3 College and Career Ready ELA Standards... 4 Next Generation Science Standards... 4 Learning
Overview... 1 What is the Outreach Program?... 1 Concepts... 2 Objectives... 3 Arizona Science Standards... 3 College and Career Ready ELA Standards... 4 Next Generation Science Standards... 4 Learning
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
DNA is found in all organisms from the smallest bacteria to humans. DNA has the same composition and structure in all organisms!
 Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
= 16.00 amu. = 39.10 amu
 Using Chemical Formulas Objective 1: Calculate the formula mass or molar mass of any given compound. The Formula Mass of any molecule, formula unit, or ion is the sum of the average atomic masses of all
Using Chemical Formulas Objective 1: Calculate the formula mass or molar mass of any given compound. The Formula Mass of any molecule, formula unit, or ion is the sum of the average atomic masses of all
Carbohydrate Analysis: Column Chemistries and Detection
 Carbohydrate Analysis: Column Chemistries and Detection Joe Romano Waters Corporation Carbohydrates in Feeds Methodology Forum AOAC 2007 Annual Meeting Anaheim, CA September 18, 2007 2007 Waters Corporation
Carbohydrate Analysis: Column Chemistries and Detection Joe Romano Waters Corporation Carbohydrates in Feeds Methodology Forum AOAC 2007 Annual Meeting Anaheim, CA September 18, 2007 2007 Waters Corporation
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
DNA Worksheet BIOL 1107L DNA
 Worksheet BIOL 1107L Name Day/Time Refer to Chapter 5 and Chapter 16 (Figs. 16.5, 16.7, 16.8 and figure embedded in text on p. 310) in your textbook, Biology, 9th Ed, for information on and its structure
Worksheet BIOL 1107L Name Day/Time Refer to Chapter 5 and Chapter 16 (Figs. 16.5, 16.7, 16.8 and figure embedded in text on p. 310) in your textbook, Biology, 9th Ed, for information on and its structure
Chapter 4. Chemical Energy
 hapter 4 hemical Energy Perhaps the most convenient form in which to store energy is chemical energy. The foods we eat, combined with the oxygen we breathe, store energy that our bodies extract and convert
hapter 4 hemical Energy Perhaps the most convenient form in which to store energy is chemical energy. The foods we eat, combined with the oxygen we breathe, store energy that our bodies extract and convert
Activity Sheets Enzymes and Their Functions
 Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
CHAPTER 3 Calculations with Chemical Formulas and Equations. atoms in a FORMULA UNIT
 CHAPTER 3 Calculations with Chemical Formulas and Equations MOLECULAR WEIGHT (M. W.) Sum of the Atomic Weights of all atoms in a MOLECULE of a substance. FORMULA WEIGHT (F. W.) Sum of the atomic Weights
CHAPTER 3 Calculations with Chemical Formulas and Equations MOLECULAR WEIGHT (M. W.) Sum of the Atomic Weights of all atoms in a MOLECULE of a substance. FORMULA WEIGHT (F. W.) Sum of the atomic Weights
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Molecular Formula Determination
 Molecular Formula Determination Classical Approach Qualitative elemental analysis Quantitative elemental analysis Determination of empirical formula Molecular weight determination Molecular formula determination
Molecular Formula Determination Classical Approach Qualitative elemental analysis Quantitative elemental analysis Determination of empirical formula Molecular weight determination Molecular formula determination
