LAB 5 - Enzymes BACKGROUND INFORMATION
|
|
|
- Hugo Welch
- 7 years ago
- Views:
Transcription
1 LAB 5 - Enzymes Chemical Reactions BACKGROUND INFORMATION The cells of organisms, from bacteria to plants to animals, carry out hundreds to thousands of chemical reactions that must be properly coordinated and controlled. We call the molecules at the start of a chemical reaction the reactants, and the resulting molecules are called the products. Chemical reactions can be represented as shown below: Reactants Products 1) A + B C 2) X Y + Z In the first example, molecules A and B undergo a chemical reaction to form a larger product C. In the second example molecule X undergoes a chemical reaction to form two smaller molecules Y and Z. This is exactly what occurs in two common biological reactions: 3) Glucose + Fructose Sucrose 4) Sucrose Glucose + Fructose In many plants, the monosaccharides glucose and fructose are combined in a chemical reaction to form the disaccharide sucrose as shown for reaction 3. Organisms that consume sucrose from plants (such as you!) carry out reaction 4 to digest the disaccharide sucrose to the monosaccharides glucose and fructose, which can then be effectively absorbed into the bloodstream and used by cells. Biochemical reactions that build larger molecules from smaller ones (such as reactions 1 and 3) are generally referred to as anabolic. Reactions that break down larger molecules into smaller ones (such as reactions 2 and 4) are referred to as catabolic. The sum of all biochemical reactions in a living organism, both anabolic and catabolic, is referred to as metabolism. Energy Another way of looking at chemical reactions is in terms of energy. All chemical reactions involve changes in the energy state of the reactants relative to the products. If the stored or potential energy of the products of a reaction are greater than that of the reactants, then the reaction requires a net input of energy. Such reactions are called endergonic (endo- into and - ergonic energy ) and absorb energy. Other chemical reactions have a net release of energy since the products contain less potential energy than the reactants. Such reactions are called exergonic (exo- off or out and -ergonic energy ) and release energy.
2 ENDERGONIC: amino acid-1 + amino acid-2 + ENERGY polypeptide EXERGONIC: gasoline + O 2 CO 2 + H 2 O + ENERGY As indicated above, the synthesis of a polypeptide from free amino acids is a complex series of chemical reactions that requires energy and thus is endergonic. There is more stored energy in a polypeptide than in the free amino acids from which it is made. The burning of gasoline, on the other hand, is a chemical reaction that releases energy and thus is exergonic. There is less stored energy in the CO 2 and water products than in the reactants gasoline and oxygen. As a general rule, anabolic reactions such as polypeptide synthesis are endergonic and catabolic reactions are exergonic, with exergonic reactions providing the needed energy for endergonic ones. Activation Energy and Catalysts Another important aspect of chemical reactions and energy is the concept of activation energy (E a ). Regardless of whether a chemical reaction is endergonic or exergonic, every chemical reaction requires a certain amount of energy to get the reaction started. It is clear that the burning of gasoline is an exergonic reaction, however gasoline doesn t just burn spontaneously, it requires some sort of energy input to spark the reaction. This is why car engines have spark plugs, to supply the necessary activation energy to burn gasoline in a very controlled manner. When you strike a match on a rough surface and then light a candle you are doing the same thing, providing the necessary activation energy to get each of these exergonic reactions started. Chemical reactions in biological systems generally occur at a negligible rate by themselves. Without changing the amount of reactants, the only ways to increase the rate of a chemical reaction are to 1) increase the temperature (which is what you do when you light a match, burn a candle, or burn gasoline), or 2) introduce a catalyst. Catalysts are substances that interact directly with chemical reactants and position them so that they react more easily. No increase in temperature is necessary. Catalysts actually lower the activation energy requirement for a reaction, allowing it to occur much more readily as illustrated in this graph: 2
3 Enzymes Since it would be impossible for living cells to control and coordinate their many biochemical reactions by adjusting the temperature, cells rely on biological catalysts. The biological catalysts in cells are proteins called enzymes, and just about every biochemical reaction in a cell has its own specifically shaped enzyme catalyst. By controlling the production and activity of enzymes (which are encoded by genes), cells control and coordinate their biochemical activity, i.e., their metabolism. As with any catalyst, an enzyme works by binding and positioning the reactant(s) for a specific reaction in a way that lowers the activation energy. The biochemical reactant(s) that a given enzyme binds to is referred to as its substrate, and the part of the enzyme that binds the substrate is called the active site. The diagram below illustrates this for the enzyme sucrase and its substrate sucrose (the suffix ase denotes an enzyme, whereas ose denotes a carbohydrate): As you can see, the enzyme sucrase, a protein, binds directly to its substrate sucrose and positions it so the covalent bond between the monosaccharides glucose and fructose is strained in a way that lowers the activation energy enough to break the bond. This yields the products glucose and fructose, which are then released. The enzyme is free to repeat this process, catalyzing the reaction over and over again until it is no longer active. Like any protein, the action of an enzyme is dependent upon its unique three-dimensional shape. Anything that causes an enzyme to adopt a non-functional shape is said to denature the enzyme. Factors that can denature an enzyme and cause it to become non-functional include changes in temperature, ph and salt concentration. For example, most human enzymes have evolved to function best at normal cellular conditions: 37 o C, ph 7.4 and 0.9% NaCl. If the temperature, ph or salt concentration deviates significantly from the normal state, enzymes and other proteins will begin to denature and lose their function. This is largely why high fevers and deviations in ph (acidosis, alkalosis), for example, can be so dangerous. In today s lab you will examine the functions of three digestive enzymes and test the effect of denaturing conditions on one of these enzymes 3
4 DIGESTIVE ENZYME FUNCTION In the first three exercises you will observe how three different digestive enzymes catalyze biochemical reactions that break down their substrates into smaller molecules. Below is a list of each enzyme with its substrate and resulting product(s): Enzyme Substrate Product lipase triglycerides fatty acids, glycerol trypsin proteins smaller polypeptides amylase starch glucose Each of these digestive enzymes is produced in the pancreas as part of a cocktail of digestive enzymes in what we call pancreatic juice. Pancreatic juice is released into the first section of the small intestine, the duodenum, when it receives partially digested food called chyme from the stomach. The enzymes contained in pancreatic juice will complete the chemical digestion of a meal so that the monomeric nutrients it contains (e.g., amino acids, monosaccharide sugars, fatty acids) can be absorbed. Digestion of Triglycerides by the Enzyme Lipase The first enzyme you will examine is pancreatic lipase. Lipase is produced by the pancreas to catalyze the break down of lipids such as triglycerides into free fatty acids and glycerol: Triglycerides are the main form of lipid found in animal fats and vegetable oils, however they must be digested to fatty acids and glycerol via pancreatic lipase to be absorbed. Since lipids are not soluble in water they will form large droplets to minimize contact with the surrounding aqueous environment. This limits their interaction with lipase thus making their digestion very slow and inefficient. To avoid this problem, your liver produces bile, a greenish fluid with properties similar to soap that helps to emulsify lipids, i.e., break them up into smaller droplets. Bile is stored in the gall bladder until your partially digested meal reaches the duodenum. This triggers the release of bile into the duodenum to help emulsify the lipids. In the laboratory, digestion of triglycerides to fatty acids and glycerol can be detected by a decrease in ph. As their name implies, fatty acids are acidic (they release H + into solution) due to their carboxyl groups. The release of fatty acids from neutral triglycerides will thus result in an increase in the H + concentration (i.e., lowering of the ph value). In the following exercise, you will detect such changes in ph by using a ph indicator that changes color in response to ph. 4
5 *Exercise 1 Digestion of cream by lipase In this exercise you will test the ability of pancreatic lipase to digest triglycerides in cream, both with and without bile. The cream contains litmus, a ph indicator that turns red under acidic conditions, blue under basic conditions, and is purple at neutral ph. The litmus cream you will use is a neutral lavender color and will gradually turn reddish-pink if free fatty acids are released due to the digestion of triglycerides. Your reactions should be performed as follows: 1. label four test tubes 1, 2, 3 & 4 and add 2 ml of litmus cream to each tube 2. add a pinch (~0.02 g or 20 mg) of bile powder to tubes 2 & 4 only 3. add 0.5 ml of water to tubes 1 & 2 and 0.5 ml of 1% lipase solution to tubes 3 & 4 4. mix each tube well and incubate them in a 37 o C water bath for 1 hour 5. record the results on your worksheet and answer the associated questions NOTE: For exercises 1 & 2, begin the next exercise while the current one is incubating. Digestion of Proteins by the Enzyme Trypsin The next enzyme you will examine is trypsin, one of many enzymes your body produces to digest or break down proteins. Trypsin will catalyze the breakage of peptide bonds in proteins at lysine and arginine amino acid residues. This results in larger polypeptides being broken down into smaller polypeptides (commonly referred to as peptides ). The protein source you will subject to trypsin digestion is gelatin. Gelatin consists primarily of the protein collagen extracted from animal bones and other connective tissues. At room temperature and below, gelatin is a semisolid gel due to interactions between the collagen fibers that form a fishnet-like structure. Trypsin will partially digest the collagen fibers, disrupting their interaction and causing the gelatin to liquefy and remain liquid, even at cool temperatures. *Exercise 2 Digestion of gelatin by trypsin To demonstrate the ability of trypsin to catalyze the partial digestion of gelatin, you will carry out two reactions as described below. If digestion of the gelatin has occurred, you will see that the gelatin remains liquid even on ice: 1. obtain two tubes of molten gelatin from the 37 o C water bath and label them 1 & 2 2. add 0.5 ml of water to tube 1 and 0.5 ml of 1% trypsin solution to tube 2 3. mix well and place both tubes in the 37 o C water bath for 30 minutes 4. place both tubes on ice for 15 minutes (or long enough for tube 1 to solidify) 5. invert each tube to see if the gelatin is liquid or solid and record on your worksheet 5
6 Digestion of Starch by the Enzyme Amylase Starch is a large polymer of the monosaccharide glucose. In order for your body to obtain glucose from the starch you eat, it must be digested by the enzyme amylase: Amylase is present in human saliva as well as pancreatic juice. As you learned in the previous lab, starch can be detected by the addition of a small amount of iodine solution. If starch is present the sample will turn dark blue or black when iodine solution is added, if there is no starch then the sample should be a clear light brown color. The complete digestion of starch to free glucose should result in no dark blue or black color when iodine solution is added. In the next exercise you will use iodine solution to determine if starch is digested by amylase. *Exercise 3 Digestion of starch by amylase In this exercise you will set up three reaction tubes. Two reactions will serve as controls, one lacking the enzyme and the other lacking starch. The third reaction will contain both the enzyme amylase and its substrate, starch. Your three reactions should be performed as follows: 1. label three test tubes 1, 2 & 3 2. add 0.5 ml of water to tube 1 and 2.5 ml of water to tube 2 3. add 2.5 ml of starch solution to tubes 1 & 3 4. add 0.5 ml of 1% amylase solution to tubes 2 & 3 5. mix well and incubate each tube at room temperature for 10 minutes 6. add 2 drops of iodine to each tube, mix and record the results on your worksheet NOTE: Save your control tubes from Exercise 3 for use in Exercise 4. Effect of ph on Enzyme Function As you learned earlier, for an enzyme to function properly it must be in its native conformation or shape. If anything causes the enzyme to become misshapen or denatured, it will no longer function as a catalyst since it can no longer bind properly to its substrate. Changes in temperature, ph and salt concentration all can denature an enzyme and inhibit its activity. To illustrate this, we will focus on the effects of changes in ph. 6
7 Pancreatic enzymes such as lipase, trypsin and amylase normally carry out their catalytic activities at a relatively neutral ph 7 to ph 8. To test the effect of ph on the activity of such enzymes, you will test the activity of the enzyme amylase at a variety of ph values *Exercise 4 Effect of ph on amylase activity In this exercise you will carry out five amylase reactions much like you did in Exercise 3, however each reaction will occur at a different ph. The control tubes from Exercise 3 can be used as controls for this experiment. Perform this experiment as described below: 1. label five test tubes 2, 4, 7, 10 & 12 for the ph values you will be testing 2. add 2.5 ml of starch solution to each tube 3. add 1 ml of the corresponding ph buffer to each tube (e.g., add ph 7 buffer to tube 7) 4. add 0.5 ml of 1% amylase solution to each tube 5. mix well and incubate each tube at room temperature for 10 minutes 6. add 2 drops of iodine to each tube and mix 7. record the color of each reaction on your worksheet, and score* each reaction for amylase activity as indicated below: 0 no amylase activity (completely dark blue/black) 1 a little amylase activity (dark but not completely dark blue/black) 2 significant amylase activity (slightly darkened) 3 high amylase activity (clear light brown) 8. graph your results (ph vs amylase activity) * Use the control tubes from Exercise 3 to help you score each reaction. The tube with no enzyme represents a score of 0, and the tube with no starch represents a score of 3. 7
8 8
9 LABORATORY 5 WORKSHEET Ex. 1 Digestion of cream by lipase Your control tubes are the tubes without lipase. Why is it important to include the second control (tube #2)? Which of your reactions was most acidic? What ph indicator allowed you to observe changes in ph? Name Section tube # color What product caused the ph to drop in the acidic reactions? Did bile alone (without lipase) result in triglyceride digestion? Why or why not? Did bile aid the digestion of triglycerides in the cream? If so, what is its role in this reaction? Ex. 2 Digestion of gelatin by trypsin State your hypothesis for this experiment and identify the control. Which of your reactions, if any, remained liquid after incubating on ice? Explain your results below: Human beings can make only 12 of the 20 amino acids needed to build proteins, the other 8 are referred to as essential amino acids since they can only be obtained from food. Gelatin is mostly collagen extracted from animal connective tissues and contains little to none of the essential amino acids methionine and tryptophan. Is gelatin by itself a nutritious protein source? Why or why not?
10 Ex. 3 Digestion of starch by amylase Tubes 1 and 2 served as controls with or without starch. Explain your result for tube 3 with starch and amylase: tube # color starch? When starch is digested, what product is produced? Blood sugar is actually blood glucose. Why does eating starch raise your blood sugar level? Would a person with diabetes be better off eating a scoop of mashed potatoes or ice cream? Why? Ex. 4 Effect of ph on amylase activity State your hypothesis for this experiment: ph color amylase activity At what ph value does amylase work best? worst? Did these results support or refute your hypothesis? A person with a blood ph below 7.35 will be diagnosed with acidosis. What effect might an abnormal ph such as this have on proteins in the blood?
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Chemical Processes of Digestion
 Chemical Processes of Digestion Objective: To explain in short essays or diagrams how carbohydrates, proteins, and fats are digested into end products that can be absorbed into the blood, at the level
Chemical Processes of Digestion Objective: To explain in short essays or diagrams how carbohydrates, proteins, and fats are digested into end products that can be absorbed into the blood, at the level
Absorption and Transport of Nutrients
 Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
CHAPTER 6 AN INTRODUCTION TO METABOLISM. Section B: Enzymes
 CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
The Digestive System. You are what you eat!
 The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
Name Date Period. Keystone Review Enzymes
 Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Do not put any wastes down the sink! All materials will be collected as-is at the end of class.
 Chemical and Physical Processes of Digestion Exercise 39A / 39 (begins page 597 in 9 th &10 th eds, page 595 in 11 th edition, page 599 in 12 th edition) Lab 7 Objectives Read lab Exercise 39A / 39 Do
Chemical and Physical Processes of Digestion Exercise 39A / 39 (begins page 597 in 9 th &10 th eds, page 595 in 11 th edition, page 599 in 12 th edition) Lab 7 Objectives Read lab Exercise 39A / 39 Do
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Cellular Energy: ATP & Enzymes. What is it? Where do organism s get it? How do they use it?
 Cellular Energy: ATP & Enzymes What is it? Where do organism s get it? How do they use it? Where does Energy come from? Ultimately, from the sun. It is transferred between organisms in the earth s lithosphere,
Cellular Energy: ATP & Enzymes What is it? Where do organism s get it? How do they use it? Where does Energy come from? Ultimately, from the sun. It is transferred between organisms in the earth s lithosphere,
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Working With Enzymes. a world of learning. Introduction. How Enzymes Work. Types and Sources of Enzymes
 Working With Enzymes a world of learning Presented by Peter J Ball, Southern Biological. For further information, please contact the author by phone (03) 9877-4597 or by email peterjball@southernbiological.com.
Working With Enzymes a world of learning Presented by Peter J Ball, Southern Biological. For further information, please contact the author by phone (03) 9877-4597 or by email peterjball@southernbiological.com.
What happens to the food we eat? It gets broken down!
 Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Experiment 10 Enzymes
 Experiment 10 Enzymes Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without being used up themselves. They do this by lowering the
Experiment 10 Enzymes Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without being used up themselves. They do this by lowering the
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
The digestive system, also called the gastrointestinal
 exercise 8 Chemical and Physical Processes of Digestion Objectives 1. To define digestive tract, accessory glands, digestion, hydrolases, salivary amylase, carbohydrates, proteins, lipids, bile salts,
exercise 8 Chemical and Physical Processes of Digestion Objectives 1. To define digestive tract, accessory glands, digestion, hydrolases, salivary amylase, carbohydrates, proteins, lipids, bile salts,
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
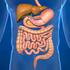 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Biology 3A Laboratory: Enzyme Function
 Biology 3A Laboratory: Enzyme Function Objectives To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate the effect
Biology 3A Laboratory: Enzyme Function Objectives To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate the effect
Energy & Enzymes. Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy.
 Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Enzymes: Practice Questions #1
 Enzymes: Practice Questions #1 1. Compound X increases the rate of the reaction below. Compound X is most likely A. an enzyme B. a lipid molecule C. an indicator D. an ADP molecule 2. The equation below
Enzymes: Practice Questions #1 1. Compound X increases the rate of the reaction below. Compound X is most likely A. an enzyme B. a lipid molecule C. an indicator D. an ADP molecule 2. The equation below
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Digestive System Notes
 Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
Digestive System Notes Structure Function Relation Mouth cavity Mechanical digestion by teeth; chemical digestion of starch by saliva. Salivary glands Three pairs of glands which secrete saliva containing
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Topic 4: Digestion and Nutrition
 Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Digestion, Absorption. How & where?
 Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Digestion, Absorption How & where? What happens to food? Three processes Digestion Absorption Elimination Where do they occur? GI tract Overview of Digestion GI tract Gastrointestinal (GI) tract: series
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Enzymes and Metabolism
 Enzymes and Metabolism Enzymes and Metabolism Metabolism: Exergonic and Endergonic Reactions Chemical Reactions: Activation Every chemical reaction involves bond breaking and bond forming A chemical reaction
Enzymes and Metabolism Enzymes and Metabolism Metabolism: Exergonic and Endergonic Reactions Chemical Reactions: Activation Every chemical reaction involves bond breaking and bond forming A chemical reaction
General Properties Protein Nature of Enzymes Folded Shape of Enzymes H-bonds complementary
 Proteins that function as biological catalysts are called enzymes. Enzymes speed up specific metabolic reactions. Low contamination, low temperature and fast metabolism are only possible with enzymes.
Proteins that function as biological catalysts are called enzymes. Enzymes speed up specific metabolic reactions. Low contamination, low temperature and fast metabolism are only possible with enzymes.
The Huntington Library, Art Collections, and Botanical Gardens. How Sweet It Is: Enzyme Action in Seed Germination
 The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Lesson 2: Digestion of fats, proteins, and carbohydrates
 Lesson 2: Digestion of fats, proteins, and carbohydrates Inquiry Focus: How does the body get energy from food? Student Learning Objectives: By the end of the lesson, students will be able to do the following:
Lesson 2: Digestion of fats, proteins, and carbohydrates Inquiry Focus: How does the body get energy from food? Student Learning Objectives: By the end of the lesson, students will be able to do the following:
Digestive system Review
 Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Digestive system Review 1. Distinguish between chemical digestion and mechanical digestion. The physical breakdown of food begins in the mouth with two types of processes. The mouth is a complex structure
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
2) Digestion the breakdown of. There are two types of digestion: Mechanical and Chemical. 3) Absorption when the nutrients enter into the blood.
 The Digestive System Video on the digestive system (5 min) The digestive system is responsible for the breakdown of the we eat so that it can be absorbed into the. There are four main stages of the digestive
The Digestive System Video on the digestive system (5 min) The digestive system is responsible for the breakdown of the we eat so that it can be absorbed into the. There are four main stages of the digestive
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Figure 5. Energy of activation with and without an enzyme.
 Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
The Human Digestive System
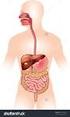 The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
Digestive System Functions
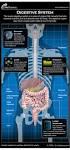 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d.
 1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
8/20/2012 H C OH H R. Proteins
 Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Unit B Understanding Animal Body Systems. Lesson 1 Understanding Animal Digestion
 Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
10-ml Graduated cylinder 40 ml 3% Hydrogen peroxide solution (found in stores) Straight-edged razor blade Scissors and Forceps (tweezers)
 Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
1. Enzymes. Biochemical Reactions. Chapter 5: Microbial Metabolism. 1. Enzymes. 2. ATP Production. 3. Autotrophic Processes
 Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
6023-1 - Page 1. Name: 4) The diagram below represents a beaker containing a solution of various molecules involved in digestion.
 Name: 6023-1 - Page 1 1) Which one of the following situations indicates a serious organ system malfunction? A) Mitochondria stop functioning in a unicellular organism exposed to pollutants. B) White blood
Name: 6023-1 - Page 1 1) Which one of the following situations indicates a serious organ system malfunction? A) Mitochondria stop functioning in a unicellular organism exposed to pollutants. B) White blood
Biology 13A Lab #13: Nutrition and Digestion
 Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
green B 1 ) into a single unit to model the substrate in this reaction. enzyme
 Teacher Key Objectives You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating enzymatic activity.
Teacher Key Objectives You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating enzymatic activity.
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Determination of Specific Nutrients in Various Foods. Abstract. Humans need to consume food compounds such as carbohydrates, proteins, fats,
 Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Biopharmaceuticals and Biotechnology Unit 2 Student Handout. DNA Biotechnology and Enzymes
 DNA Biotechnology and Enzymes 35 Background Unit 2~ Lesson 1 The Biotechnology Industry Biotechnology is a process (or a technology) that is used to create products like medicines by using micro-organisms,
DNA Biotechnology and Enzymes 35 Background Unit 2~ Lesson 1 The Biotechnology Industry Biotechnology is a process (or a technology) that is used to create products like medicines by using micro-organisms,
Sample Liver Enzyme Lab
 Sample Liver Enzyme Lab Design Aspect 1: Research Question This lab will be driven by the research question, Do changes in temperature have an effect on the activity of the enzyme catalase? Pearson Baccalaureate:
Sample Liver Enzyme Lab Design Aspect 1: Research Question This lab will be driven by the research question, Do changes in temperature have an effect on the activity of the enzyme catalase? Pearson Baccalaureate:
pencil. Vocabulary: 1. Reactant 2. Product 3. Activation energy 4. Catalyst 5. substrate 6. Chemical reaction Keep your textbooks when you are done
 Objectives Students will explore the importance of chemical reactions in biology Students will discuss the role of enzymes as catalysts in biological reactions. Students will analyze graphs showing how
Objectives Students will explore the importance of chemical reactions in biology Students will discuss the role of enzymes as catalysts in biological reactions. Students will analyze graphs showing how
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
The Excretory and Digestive Systems
 The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
The Excretory and Digestive Systems 38.2 The Process of Digestion Organs of the Digestive System The digestive system includes the: Mouth Pharynx Esophagus Stomach Small and large intestine. Other structures
Activity Sheets Enzymes and Their Functions
 Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
Investigation 2- ENZYME ACTIVITY BACKGROUND catalase Learning Objectives
 Investigation 2-13 ENZYME ACTIVITY How do abiotic or biotic factors influence the rates of enzymatic reactions? BACKGROUND Enzymes are the catalysts of biological systems. They speed up chemical reactions
Investigation 2-13 ENZYME ACTIVITY How do abiotic or biotic factors influence the rates of enzymatic reactions? BACKGROUND Enzymes are the catalysts of biological systems. They speed up chemical reactions
Special organ structures and functions conduct these tasks through the successive parts of the overall system.
 Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
The purpose of this lab is to investigate the impact of temperature, substrate concentration,
 Lee 1 Jessica Lee AP Biology Mrs. Kingston 23 October 2013 Abstract: The purpose of this lab is to investigate the impact of temperature, substrate concentration, enzyme concentration, and the presence
Lee 1 Jessica Lee AP Biology Mrs. Kingston 23 October 2013 Abstract: The purpose of this lab is to investigate the impact of temperature, substrate concentration, enzyme concentration, and the presence
LAB TOPIC 4: ENZYMES. Enzyme catalyzed reactions can be expressed in the following way:
 LAB TOPIC 4: ENZYMES Objectives Define enzyme and describe the activity of enzymes in cells. Discuss the effects of varying enzyme concentrations on the rate of enzyme activity. Discuss the effects of
LAB TOPIC 4: ENZYMES Objectives Define enzyme and describe the activity of enzymes in cells. Discuss the effects of varying enzyme concentrations on the rate of enzyme activity. Discuss the effects of
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
Effect of temperature and ph on the enzymatic activity of salivary amylase
 Effect of temperature and ph on the enzymatic activity of salivary amylase Gae Khalil Rodillas, Nonia Carla Ysabel Samson, Raphael Jaime Santos* and Brylle Tabora Department of Biological Sciences, College
Effect of temperature and ph on the enzymatic activity of salivary amylase Gae Khalil Rodillas, Nonia Carla Ysabel Samson, Raphael Jaime Santos* and Brylle Tabora Department of Biological Sciences, College
Enzymes. Chapter 3. 3.1 Enzymes and catalysts. Vital mistake. What is an enzyme?
 Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
The Digestive System
 The Digestive System What do you know?? quiz-digestive-health Digestion Videos The Digestive System Inside-Dr-Ozs-Digestive-System-Video Now it is your turn to recreate the digestive system. How is food
The Digestive System What do you know?? quiz-digestive-health Digestion Videos The Digestive System Inside-Dr-Ozs-Digestive-System-Video Now it is your turn to recreate the digestive system. How is food
Metabolism: Cellular Respiration, Fermentation and Photosynthesis
 Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems
 Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Topic 3.0 Healthy human function depends on a variety of interacting and reacting systems Organ Systems Organ systems must have the ability to to changes within and outside of your body to maintain life
Name. Lab 3: ENZYMES. In this lab, you ll investigate some of the properties of enzymes.
 Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
Overview... 1 What is the Outreach Program?... 1 Concepts... 2 Objectives... 3 Arizona Science Standards... 3 College and Career Ready ELA
 Overview... 1 What is the Outreach Program?... 1 Concepts... 2 Objectives... 3 Arizona Science Standards... 3 College and Career Ready ELA Standards... 4 Next Generation Science Standards... 4 Learning
Overview... 1 What is the Outreach Program?... 1 Concepts... 2 Objectives... 3 Arizona Science Standards... 3 College and Career Ready ELA Standards... 4 Next Generation Science Standards... 4 Learning
Enzymes: Amylase Activity in Starch-degrading Soil Isolates
 Enzymes: Amylase Activity in Starch-degrading Soil Isolates Introduction This week you will continue our theme of industrial microbiologist by characterizing the enzyme activity we selected for (starch
Enzymes: Amylase Activity in Starch-degrading Soil Isolates Introduction This week you will continue our theme of industrial microbiologist by characterizing the enzyme activity we selected for (starch
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
CHAPTER 4: Enzyme Structure ENZYMES
 CHAPTER 4: ENZYMES Enzymes are biological catalysts. There are about 40,000 different enzymes in human cells, each controlling a different chemical reaction. They increase the rate of reactions by a factor
CHAPTER 4: ENZYMES Enzymes are biological catalysts. There are about 40,000 different enzymes in human cells, each controlling a different chemical reaction. They increase the rate of reactions by a factor
Enzymes. OpenStax College
 OpenStax-CNX module: m44429 1 Enzymes OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able
OpenStax-CNX module: m44429 1 Enzymes OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able
Enzymes and Metabolic Pathways
 Enzymes and Metabolic Pathways Enzyme characteristics Made of protein Catalysts: reactions occur 1,000,000 times faster with enzymes Not part of reaction Not changed or affected by reaction Used over and
Enzymes and Metabolic Pathways Enzyme characteristics Made of protein Catalysts: reactions occur 1,000,000 times faster with enzymes Not part of reaction Not changed or affected by reaction Used over and
ANSWER KEY. Acids, Bases, and Solutions. Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries,
 Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
Table of Content. Enzymes and Their Functions Teacher Version 1
 Enzymes and Their Functions Jeisa Pelet, Cornell University Carolyn Wilczynski, Binghamton High School Cornell Learning Initiative in Medicine and Bioengineering (CLIMB) Table of Content Title Page Abstract..
Enzymes and Their Functions Jeisa Pelet, Cornell University Carolyn Wilczynski, Binghamton High School Cornell Learning Initiative in Medicine and Bioengineering (CLIMB) Table of Content Title Page Abstract..
Digestion. What we ll cover. Main stages of digestion. Digestion: A Closer Look. A Tour of the Human Digestive System. Mechanical digestion
 Digestion What we ll cover What are the digestive system structures and their functions? Where does carbohydrate, protein and fat digestion and absorption occur? What are the 3 accessory organs of digestion?
Digestion What we ll cover What are the digestive system structures and their functions? Where does carbohydrate, protein and fat digestion and absorption occur? What are the 3 accessory organs of digestion?
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Two Forms of Energy
 Module 2D - Energy and Metabolism Objective # 19 All living organisms require energy for survival. In this module we will examine some general principles about chemical reactions and energy usage within
Module 2D - Energy and Metabolism Objective # 19 All living organisms require energy for survival. In this module we will examine some general principles about chemical reactions and energy usage within
