Reduction of Monosaccharides
|
|
|
- Priscilla Casey
- 7 years ago
- Views:
Transcription
1 Reduction of Monosaccharides Monosaccharides can be reduced turning the carbonyl group into an alcohol group. producing sugar alcohols called alditols. and the products named by replacing the - ose ending with -itol. D-Glucose reduces to D-Glucitol, also called D-Sorbitol.
2 duction of Monosaccharides O O C O O + 2 Pt O C 2 O O O Other artificial sweeteners: xylitol mannitol O O C 2 O D-glucose C 2 O D-glucitol or D-sorbitol
3 Oxidation of D-Glucose, a Reducing Sugar Glucose is oxidized to a carboxylic acid. Benedict s reagent 2013 Pearson Education, Inc. Chapter 15, Section 2 3
4 Oxidation of Fructose Fructose, a ketohexose, is also a reducing sugar. In a basic solution such as Benedict's, the carbonyl group moves from carbon 2 to carbon 1, so it can be oxidized as glucose Pearson Education, Inc. Chapter 15, Section 2 4
5 Learning Check Write the products of the oxidation and reduction of D-Mannose. O C O O O O C 2 O D-Mannose 2013 Pearson Education, Inc. Chapter 15, Section 2 5
6 Solution Write the products of the oxidation and reduction of D-Mannose. O O O O C 2 O Reduction O O C 2 O O O C O O C 2 O Oxidation O O C O O O C 2 O D-Mannitol D-Mannose D-Mannonic acid 2013 Pearson Education, Inc. Chapter 15, Section 2 6
7 Important Disaccharides A disaccharide consists of two monosaccharides linked together. When two monosaccharides combine in a dehydration reaction, the product is an acetal and water.
8 Cyclic Acetals Cyclic acetals form when an alcohol adds to a cyclic hemiacetal. are very important in carbohydrate chemistry. It is the type of linkage that bonds glucose molecules to other glucose molecules in the formation of disaccharides and polysaccharides.
9 Maltose Maltose is a disaccharide also known as malt sugar. used in cereals, candies, and brewing. When maltose in the starches in barley is hydrolyzed by yeast with an enzyme, maltase, two molecules of glucose are obtained. The glucose can undergo fermentation and we have beer. Maltose + 2 O (in barley) maltase glucose + glucose fermentation ethanol (beer)
10 Formation of Maltose
11 Lactose Lactose Makes up 6-8% of human milk and 4-5% of cow s milk. People who are lactose intolerant, do not produce enough of the enzyme lactase, which is used to break down lactose. The lactose reaches the colon and is broken down there by bacteria. (this causes discomfort) 2013 Pearson Education, Inc. Chapter 15, Section 1 11
12 Lactose 2013 Pearson Education, Inc. Chapter 15, Section 1 12
13 Sucrose Sucrose, or table sugar, is obtained from sugar cane and sugar beets. is hydrolyzed in the body by the enzyme sucrase to form glucose and fructose. It is estimated that each person in the U.S. consumes an average of 150 lbs. of sucrose every year. Sucrose + 2 O sucrase glucose + fructose
14 Sucrose
15 Learning Check Draw the aworth structures and give the names of the two monosaccharides that form when sucrose is hydrolyzed.
16 Solution 2013 Pearson Education, Inc. Chapter 15, Section 2 16
17 Polysaccharides Polysaccharides are polymers of many monosaccharides linked together. differ in the type of links between monosaccharides, and the monosaccharides in the polymer. differ by the amount of branching in the polymer Pearson Education, Inc. Chapter 15, Section 1 17
18 Important Polysaccharides Important polysaccharides include starches made of -D-glucose molecules, amylose, and amylopectin. glycogen (animal starch in muscle), which is made of -Dglucose. cellulose (plants and wood), which is made of -D-glucose Pearson Education, Inc. Chapter 15, Section 1 18
19 Structures of Amylose and Amylopectin amylose amylopectin
20 Glycogen Animal starch or glycogen is stored in the liver and muscles of animals. hydrolyzed in our cells to maintain glucose and energy levels between meals. similar to amylopectin, but is more highly branched Pearson Education, Inc. Chapter 15, Section 2 20
21 Cellulose Cellulose is a major structural unit of wood and plants. Cotton is almost pure cellulose. Structure is similar to amylose where the glucose forms a long unbranched chain. The difference is that amylose makes α-1,4-glycosidic bonds and cellulose makes β-1,4- glycosidic bonds. Therefore, cellulose does not coil into a helical shape Pearson Education, Inc. Chapter 15, Section 1 21
22 Cellulose The polysaccharide cellulose is composed of glucose units connected by β-1,4-glycosidic bonds Pearson Education, Inc. Chapter 15, Section 1 22
23 Learning Check Identify the polysaccharides and types of glycosidic bonds in each of the following. A. B Pearson Education, Inc. Chapter 15, Section 1 23
24 Solution Identify the polysaccharides and types of glycosidic bonds in each of the following. A. Cellulose -1,4-glycosidic bonds B. Amylose -1,4-glycosidic bonds Amylopectin -1,4- and -1,6-glycosidic bonds
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Solid Phase Peptide Synthesis
 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Qualitative Testing for Carbohydrates
 R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
How To Eat Healthily
 Pierce College Putman/NUTR& 101 Unit 04 Practice Exam: Carbohydrates 1. Which is not a monosaccharide? a. lactose b. glucose c. fructose d. galactose 2. Used for immediate energy in the body: a. carbohydrates
Pierce College Putman/NUTR& 101 Unit 04 Practice Exam: Carbohydrates 1. Which is not a monosaccharide? a. lactose b. glucose c. fructose d. galactose 2. Used for immediate energy in the body: a. carbohydrates
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
What happens to the food we eat? It gets broken down!
 Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Photo Cell Resp Practice. A. ATP B. oxygen C. DNA D. water. The following equation represents the process of photosynthesis in green plants.
 Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Respiration Worksheet. Respiration is the controlled release of energy from food. Types of Respiration. Aerobic Respiration
 Respiration Worksheet Respiration is the controlled release of energy from food The food involved in respiration is usually Internal respiration is controlled by which allow energy to be released in The
Respiration Worksheet Respiration is the controlled release of energy from food The food involved in respiration is usually Internal respiration is controlled by which allow energy to be released in The
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Digestive System Functions
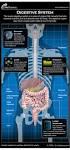 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Bulletin 887B. HPLC Carbohydrate Column Selection Guide
 9 North Harrison Road Bellefonte, PA -00 USA Telephone 00-- -9- Fax 00--0-9-0 email: supelco@sial.com sigma-aldrich.com/supelco HPLC Carbohydrate Column Selection Guide Because carbohydrates exhibit a
9 North Harrison Road Bellefonte, PA -00 USA Telephone 00-- -9- Fax 00--0-9-0 email: supelco@sial.com sigma-aldrich.com/supelco HPLC Carbohydrate Column Selection Guide Because carbohydrates exhibit a
PHOTOSYNTHESIS AND CELLULAR RESPIRATION
 reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
Get It Right. Answers. Chapter 1: The Science of Life. A biologist studies all living things.
 Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Chapter 4. Carbohydrates: Bountiful Sources of Energy and Nutrients
 hapter 4 arbohydrates: Bountiful Sources of Energy and Nutrients hapter Objectives After reading this chapter, you will be able to: 1. Describe the difference between simple and complex carbohydrates,
hapter 4 arbohydrates: Bountiful Sources of Energy and Nutrients hapter Objectives After reading this chapter, you will be able to: 1. Describe the difference between simple and complex carbohydrates,
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
T F T F in childr T F
 T F T F in childr T F 108 Plant-derived energy nutrients 3 mon. 123 124. 110 Glucose is the preferred source of energy for the brain. What As noted earlier (in Chapter 1), carbohydrates are one of the
T F T F in childr T F 108 Plant-derived energy nutrients 3 mon. 123 124. 110 Glucose is the preferred source of energy for the brain. What As noted earlier (in Chapter 1), carbohydrates are one of the
The Human Digestive System
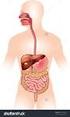 The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Biology for Science Majors
 Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
lt\ Nutrition a-glncose www.cambridge.org in this web service Cambridge University Press Glucose+ fructose Glucose+ galactose
 P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
chapter 5 Carbohydrates
 part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
Chapter 15 Lecture Notes: Metabolism
 Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
8. Be able to label a diagram of an earthworm. Know the function of each of the major parts of the earthworm.
 Review for Unit Test: The Digestive System 1. Know the meaning of these terms: heterotrophs digestion peristalsis microvilli autotrophs chemical digestion chyme lacteal intracellular digestion mechanical
Review for Unit Test: The Digestive System 1. Know the meaning of these terms: heterotrophs digestion peristalsis microvilli autotrophs chemical digestion chyme lacteal intracellular digestion mechanical
Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps):
 Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps): 1) How many ATP molecules are produced for each glucose molecule used in fermentation?
Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps): 1) How many ATP molecules are produced for each glucose molecule used in fermentation?
Carbohydrate Digestion and Absorption NASPGHAN Physiology Series. Christine Waasdorp Hurtado, MD, MSCS, FAAP Christine.Waasdorp@childrenscolorado.
 Carbohydrate Digestion and Absorption NASPGHAN Physiology Series Christine Waasdorp Hurtado, MD, MSCS, FAAP Christine.Waasdorp@childrenscolorado.org Expert Reviewers: Richard Grand, MD and Jeremiah Levine,
Carbohydrate Digestion and Absorption NASPGHAN Physiology Series Christine Waasdorp Hurtado, MD, MSCS, FAAP Christine.Waasdorp@childrenscolorado.org Expert Reviewers: Richard Grand, MD and Jeremiah Levine,
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
CHEM 2353 Fundamentals of Organic Chemistry. Carbohydrates. Organic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R.
 hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
Photosynthesis and Sucrose Production
 Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Metabolism: Cellular Respiration, Fermentation and Photosynthesis
 Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
The Digestive System. You are what you eat!
 The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
ANNEX-1 EXEMPTIONS FROM ARTICLES 7 AND 8. 1-The following substances which occur in nature, if they are not chemically modified.
 ANNEX-1 EXEMPTIONS FROM ARTICLES 7 AND 8 1-The following substances which occur in nature, if they are not chemically modified. Minerals, ores, ore concentrates, cement clinker, natural gas, liquefied
ANNEX-1 EXEMPTIONS FROM ARTICLES 7 AND 8 1-The following substances which occur in nature, if they are not chemically modified. Minerals, ores, ore concentrates, cement clinker, natural gas, liquefied
Disclosure. Diet in Irritable Bowel Syndrome. Objectives. FGIDs and Dietary Complaints. Diet in IBS. Pathogenesis of Symptoms
 Disclosure Diet in Irritable Bowel Syndrome Robert J. Shulman, MD Professor of Pediatrics I have the following financial relationships to disclose: Gerson-Lehrman (consultant) Mead-Johnson (consultant)
Disclosure Diet in Irritable Bowel Syndrome Robert J. Shulman, MD Professor of Pediatrics I have the following financial relationships to disclose: Gerson-Lehrman (consultant) Mead-Johnson (consultant)
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
SMALL AND LARGE INTESTINE SECRETIONS
 SMALL AND LARGE INTESTINE SECRETIONS Objectives At the end of lecture student should be able to know, Digestive system Digestive system secretions Small intestine Component of small intestine Intestinal
SMALL AND LARGE INTESTINE SECRETIONS Objectives At the end of lecture student should be able to know, Digestive system Digestive system secretions Small intestine Component of small intestine Intestinal
Beer Styles for the Novice Your Brew Day Sanitation
 Concepts for Novice Brewers Curtis Eulberg 4/2013 Beer Beer is a fermented beverage made most commonly from water, malted barley, hops, and yeast. These items when skillfully combined with proper time
Concepts for Novice Brewers Curtis Eulberg 4/2013 Beer Beer is a fermented beverage made most commonly from water, malted barley, hops, and yeast. These items when skillfully combined with proper time
Enzymes. Chapter 3. 3.1 Enzymes and catalysts. Vital mistake. What is an enzyme?
 Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
McMush. Testing for the Presence of Biomolecules
 Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Unit B Understanding Animal Body Systems. Lesson 1 Understanding Animal Digestion
 Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
Absorption and Transport of Nutrients
 Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Carbohydrate Analysis: Column Chemistries and Detection
 Carbohydrate Analysis: Column Chemistries and Detection Joe Romano Waters Corporation Carbohydrates in Feeds Methodology Forum AOAC 2007 Annual Meeting Anaheim, CA September 18, 2007 2007 Waters Corporation
Carbohydrate Analysis: Column Chemistries and Detection Joe Romano Waters Corporation Carbohydrates in Feeds Methodology Forum AOAC 2007 Annual Meeting Anaheim, CA September 18, 2007 2007 Waters Corporation
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Plants: The Ultimate Green Machines Science, Grade Level 7
 DRAFT 1 Lesson Title: Plants: The Ultimate Green Machines Grade Level: 7 Subject Area: Science Setting: Garden, Classroom, Laboratory Instructional Time: 1-2 class periods Plants: The Ultimate Green Machines
DRAFT 1 Lesson Title: Plants: The Ultimate Green Machines Grade Level: 7 Subject Area: Science Setting: Garden, Classroom, Laboratory Instructional Time: 1-2 class periods Plants: The Ultimate Green Machines
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Multi-Enzyme Supplement for Intensive Digestive Support
 Specially formulated for those with complex GI issues. Vital-Zymes Complete is a high-potency, multi-enzyme supplement formulated for individuals with compromised gastrointestinal function who require
Specially formulated for those with complex GI issues. Vital-Zymes Complete is a high-potency, multi-enzyme supplement formulated for individuals with compromised gastrointestinal function who require
- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.
![- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells. - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.](/thumbs/40/21014327.jpg) Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Biology 13A Lab #13: Nutrition and Digestion
 Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
Metabolism: Manometric Measurement of the Fermentation of Sucrose by Saccharomyces cerevisiae. Introduction
 Metabolism: Manometric Measurement of the Fermentation of Sucrose by Saccharomyces cerevisiae Introduction Man has been brewing alcoholic beverages for thousands of years (longer than man has been making
Metabolism: Manometric Measurement of the Fermentation of Sucrose by Saccharomyces cerevisiae Introduction Man has been brewing alcoholic beverages for thousands of years (longer than man has been making
Chapter 9 Review Worksheet Cellular Respiration
 1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
