Topics in Fluorescence Spectroscopy Volume 7 DNA Technology
|
|
|
- Audra Lang
- 8 years ago
- Views:
Transcription
1
2 Topics in Fluorescence Spectroscopy Volume 7 DNA Technology
3 Topics in Fluorescence Spectroscopy Edited by JOSEPH R. LAKOWICZ Volume 1: Techniques Volume 2: Principles Volume 3: Biochemical Applications Volume 4: Probe Design and Chemical Sensing Volume 5: Nonlinear and Two-Photon-Induced Fluorescence Volume 6: Protein Fluorescence Volume 7: DNA Technology
4 Topics in Fluorescence Spectroscopy Volume 7 DNA Technology Edited by JOSEPH R. LAKOWICZ Center for Fluorescence Spectroscopy and Department of Biochemistry and Molecular Biology University of Maryland School of Medicine Baltimore, Maryland KLUWER ACADEMIC PUBLISHERS NEW YORK, BOSTON, DORDRECHT, LONDON, MOSCOW
5 ebook ISBN: Print ISBN: Springer Science + Business Media, Inc. Print 2003 Kluwer Academic/Plenum Publishers New York All rights reserved No part of this ebook may be reproduced or transmitted in any form or by any means, electronic, mechanical, recording, or otherwise, without written consent from the Publisher Created in the United States of America Visit Springer's ebookstore at: and the Springer Global Website Online at:
6 Contributors W. Patrick Ambrose Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Current address: Lawrence Livermore National Laboratory, Livermore, California Hong Cai Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Sabato D Auria Center for Fluorescence Spectroscopy and Department of Biochemistry and Molecular Biology, University of Maryland Medical School, Baltimore, Maryland 21201; and Institute of Protein Biochemistry and Enzymology, National Research Council, Naples, Italy Michael J. Difilippantonio Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland Alexander N. Glazer Department of Molecular and Cell Biology, University of California, Berkeley, California Peter M. Goodwin Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico W. Kevin Grace Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Ignacy Gryczynski Center for Fluorescence Spectroscopy and Department of Biochemistry and Molecular Biology, University of Maryland Medical School, Baltimore, Maryland Zygmunt Gryczynski Center for Fluorescence Spectroscopy and Department of Biochemistry and Molecular Biology, University of Maryland Medical School, Baltimore, Maryland Robert C. Habbersett Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Mary E. Hawkins Pharmacology and Experimental Therapeutics Section, National Cancer Institute, Bethesda, Maryland v
7 vi Contributors Su-Chun Hung Department of Molecular and Cell Biology, University of California, Berkeley, California James H. Jett Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Richard A. Keller Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Ulrich J. Krull Chemical Sensors Group, Department of Chemistry, University of Toronto at Mississauga, Mississauga, Ontario, Canada L5L 1C6 Erica J. Larson Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Current address: Engineering Science and Applications Division, Los Alamos National Laboratory, Los Alamos, New Mexico Suzanne Lassiter Department of Chemistry, Louisiana State University, Baton Rouge, Louisiana Joanna Malicka Center for Fluorescence Spectroscopy and Department of Biochemistry and Molecular Biology, University of Maryland Medical School, Baltimore, Maryland Richard A. Mathies Department of Chemistry, University of California, Berkeley, California Linda B. McGown Carolina Department of Chemistry, Duke University, Durham, North Larry E. Morrison Vysis Inc., Downers Grove, Illinois Clyde Owens Department of Chemistry, Louisiana State University, Baton Rouge, Louisiana Paul A. E. Piunno N2V 2K1 FONA Technologies, Inc., Waterloo, Ontario, Canada, Thomas Ried Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland Mosè Rossi Institute of Protein Biochemistry and Enzymology, National Research Council, Naples, Italy
8 Contributors vii Paul R. Selvin Physics Department and Biophysics Center, University of Illinois, Urbana, Illinois Steven A. Soper Department of Chemistry, Louisiana State University, Baton Rouge, Louisiana Emanuel Waddell Department of Chemistry, Louisiana State University, Baton Rouge, Louisiana James H. Werner Bioscience Division, Los Alamos National Laboratory, Los Alamos, New Mexico Jin Xie Department of Molecular and Cell Biology, University of California, Berkeley, California Yichuan Xu Department of Chemistry, Louisiana State University, Baton Rouge, Louisiana 70803
9 This page intentionally left blank
10 Preface During the past fifteen years, DNA technology has advanced at an astounding pace. The pioneering papers on the use of fluorescence in sequencing appeared in , resulting in publication of the human genome sequence in February of Fluorescence detection has played a dominant role not only in sequencing, but in genetics research and diagnostics. In addition to sequencing, fluorescence is used in essentially all measurements of DNA amplification by PCR and other methods. Molecular beacons are used to light up specific sequences in mixtures of DNA, even at the intracellular level. In the past several years, DNA arrays or gene chips have become an important research tool in developmental biology, and drug discovery, and targeted treatments. It is now possible to place an excess of 30,000 DNA sequences on a single microscope slide, and to identify the amounts of complimentary DNA by fluorescence ratiometric measurements. In this volume, we attempted to provide an overview of the fundamental principles associated with these modern applications of fluorescence to nucleic acids. Because of the rapid rate of development in this field, it is not possible for any volume to be completely up to date on the latest details. Hopefully the fundamental principles will remain useful to scientists using modern DNA methodology. Joseph R. Lakowicz Center for Fluorescence Spectroscopy March 2002 ix
11 This page intentionally left blank
12 Contents 1. DNA Sequencing Using Fluorescence Detection Steven A. Soper, Clyde Owens, Suzanne Lassiter, Yichuan Xu, and Emanuel Waddell 1.1. General Considerations What Is DNA? Organization of Genome Functions of Genes What Is DNA Sequencing? DNA Sequencing Factories Structure of DNA Nucleotide Bases Watson Crick Base Pairing Methods for Determining the Primary Structure of DNA Maxam Gilbert Sequencing Sanger Chain Termination Method Modes of Electrophoresis Slab Gel Electrophoresis Capillary Gel Electrophoresis Detection Methods for DNA Sequencing Autoradiographic Detection Fluorescence Detection 1.2. Fluorescent Dyes for DNA Labeling and Sequencing Visible Fluorescence Dyes for DNA Labeling ET Dyes for DNA Sequencing Near-Infrared Dyes for DNA Sequencing 1.3. Instrumental Formats for Fluorescence Detection in DNA Sequencing Fluorescence Scanning Instruments Fluorescence Imaging Systems for DNA Sequencing 1.4. Dye Primer/Terminator Chemistry and Fluorescence Detection Formats Single Color/Four Lane Single Color/Single Lane Two Color/Single Lane Four Color/Single Lane xi
13 xii Contents So Which Sequencing Format It Better? Single Color/Four Lifetime Sequencing 1.5. Single Molecule DNA Sequencing Using Fluorescence Detection References Fluorescence in Nucleic Acid Hybridization Assays Larry E. Morrison 2.1. Introduction Heterogeneous Versus Homogeneous Hybridization Assays Amplified Hybridization Assays 2.2. Heterogeneous Hybridization Assays Non-Amplified Heterogeneous Hybridization Assays Amplified Heterogeneous Hybridization Assays Signal Amplification in Heterogeneous Hybridization Assays Target Amplification in Heterogeneous Hybridization Assays 2.3. Homogeneous Hybridization Assays Non-Amplified Homogeneous Hybridization Assays Dual Label Formats Single Label Formats Amplified Homogeneous Hybridization Assays Dual Interacting Label Formats Single Label Formats Nucleic Acid Binding Dyes 2.4. Conclusions References Energy Transfer Fluorescent Labels for DNA Sequencing and Analysis Jin Xie, Su-Chun Hung, Alexander N. Glazer, and Richard A. Mathies 3.1. Introduction 3.2. Theory of Fluorescence Resonance Energy Transfer 3.3. Sanger Dideoxy Chain-Termination Sequencing 3.4. Energy-Transfer Fluorescent Primers
14 Contents 3.5. ET Cassette for Construction of Energy-Transfer Fluorescent Primers 3.6. Energy-Transfer Fluorescent Terminators 3.7. Short Tandem Repeat Analysis with Capillary Array Electrophoresis and ET Labels 3.8. Biotinylated Energy Transfer Cassettes 3.9. Template-Directed Dye-Terminator Incorporation Conclusions Acknowledgments References xiii On-the-Fly Fluorescence Lifetime Detection in Capillary Electrophoresis for DNA Analysis Linda B. McGown 4.1. Introduction Measuring Fluorescence Lifetime On-the-Fly in CE 4.2. Analysis of On-the-Fly Lifetime Data 4.3. Lifetime Detection of Labeled DNA Primers 4.4. DNA Sequencing Restriction Fragment Length Polymorphism Analysis 4.5. Conclusions References Fluorescent Nucleoside Analogues as DNA Probes Mary E. Hawkins 5.1. Introduction 5.2. Pteridine Nucleoside Analogues Background Effect of ph on Fluorescence Emission Fluorescence of Pteridine Analogue- Combining Oligonucleotides Melting Temperatures 5.3. Applications Using Pteridine Integrase Assay Bulge Hybridization Probes Anisotropy Intracellular Transport of Oligonucleotides DNA Conformation
15 xiv Contents Amino Purine Background Applications ,N 6 -Ethenoadenosine 5.6. Summary and Outlook Acknowledgments References Lanthanide-Labeled DNA Paul R. Selvin 6.1. Overview 6.2. Lanthanide as Luminescent Labels Representative Chelats Sensitivity Multiple Labeling and Selected Applications 6.3. Imaging Alternative Time-Resolved Probes 6.4. Lanthanides as Donors in Resonance Energy Transfer 6.5. Advantages of LRET 6.6. LRET Applied to Protein DNA Interactions Protein-Induced DNA Bends 6.7. New DNA Dyes Based on LRET with Tuneable Emission Color and Excited-State Lifetime 6.8. Conclusion References DNA Arrays for Genetic Analyses and Medical Diagnosis Sabato D Auria, Mosè Rossi, Joanna Malicka, Zygmunt Gryczynski, and Ignacy Gryczynski 7.1. Introduction 7.2. Sample Preparation Spotting Method In Situ Syntheses Method 7.3. Fluorescence Probes 7.4. Arrayers 7.5. Image Analysis Array Target Segmentation Background Intensity Extraction Target Detection
16 Contents Target Intensity Extraction Ratio Analysis Multiple Image Analysis 7.6. Applications 7.7. Pespectives Acknowledgment References xv Flow Cytometric Sizing of DNA Fragments W. Patrick Ambrose, Hong Cai, Peter M. Goodwin, James H. Jett, Robert C. Habbersett, Erica J. Larson, W. Kevin Grace, James H. Werner, and Richard A. Keller 8.1. Introduction 8.2. Single Molecule Detection 8.3. DNA Fragment Sizing Apparatus Data Analysis Sample Preparation Sizing of DNA, Digests, and Concatamers Applications PAC Clones PCR Fragments Bacteria Species and Strain Discrimination 8.4. Summary Acknowledgments References Fluorimetric DNA Biosensors Paul A. E. Piunno and Ulrich J. Krull 9.1. Introduction 9.2. Fluorimetric Fiber Optic Biosensors 9.3. Evanescent Wave Biosensors 9.4. Nonevanescent Intrinsic Mode Biosensors 9.5. Selectivity and Calibrations Issues 9.6. A Reagentless Biosensor 9.7. Future References
17 xvi Contents 10. Technicolor Genome Analysis Michael J. Difilippantonio and Thomas Ried Introduction Historical Perspective Principles behind FISH Preparation of Cytological Specimens Preparation of DNA Probes Labeling of DNA Probes Hybridization of Probe to Speciment Detection and Visualization High Sensitivity Detection Procedures Applications of FISH Gene Mapping Somatic Hybrid Analysis Clinical and Cancer Cytogenetics Chromosome Evolution Karyotype Analysis Comparative Genome Hybridization Spectral Karyotyping References Index 317
18 Topics in Fluorescence Spectroscopy Volume 7 DNA Technology
19 This page intentionally left blank
20 1 DNA Sequencing Using Fluorescence Detection Steven A. Soper, Clyde Owens, Suzanne Lassiter, Yichuan Xu, and Emanuel Waddell 1.1. General Considerations What Is DNA? Organization of Genome The blueprint for all cellular structures and functions is encoded in the genome of any organism. The genome consists of deoxyribonucleic acid (DNA) which is tightly coiled into narrow threads that, when completely stretched, reach a length of approximately 1.5 m yet possesses a width of only ~2.0 nm The threads of DNA are typically associated with many different types of proteins and are organized into structures called chromosomes that are housed within the nucleus of cells in most eukaryotic organisms. Within the human genome, there are 23 pairs of chromosomes. DNA is comprised of several different chemical units, which are a deoxyribose sugar unit, a phosphate group and one of four different nucleotide bases (adenine (A), guanine (G), cytosine (C), or thymine (T)). At a molecular level, it is the order of these bases which carries the code to build proteins within the cell that inevitably control the function of various cells and also determine the organism s physical characteristics. In the human genome, 3 billion bases are contained within the 23 pairs of chromosomes. The length of the chromosomes (in base pairs) varies greatly, with the smallest chromosome containing 50 million bases (chromosome Y) and the longest containing 250 million bases (chromosome 1). It is the primary function of DNA sequencing to determine the order of these nucleotide bases. Steven A. Soper, Clyde Owens, Suzanne Lassiter, Yichuan Xu, and Emanuel Waddell Department of Chemistry, Louisiana State University, Baton Rouge, Louisiana, Topics in Fluorescence Spectroscopy, Volume 7: DNA Technology, edited by Joseph R. Lakowicz, Kluwer Academic/Plenum Publishers, New York,
21 2 Steven A. Soper et al Functions of Genes It should be noted that the coding regions (regions which carry the information for the construction of proteins) of the genome are contained within genes. Each gene consists of a specific sequence of nucleotide bases, with three nucleotide bases (codon) directing the cells protein-synthesizing machinery to add a specific amino acid to the target protein. It is estimated that within the human genome, there are approximately 30,000 genes, with the length of the genes highly variable. However, only approximately 10% of the human genome is thought to contain protein-coding sequences What Is DNA Sequencing? A flow chart depicting the important steps involved in the process of DNA sequencing is shown in Figure 1.1. As can be seen, there are three primary steps: mapping, sequencing, and finally, assembly. Within each of these three general steps are a number ofsub-steps, which include such processes as cloning and subcloning (mapping), template preparation and gel electrophoresis (sequencing), and the computer algorithms required to assemble the small bits of sequencing data into contiguous strings which comprise the intact chromosome. Each chromosome may consist of hundreds of millions of nucleotide bases and, unfortunately, the sequencing phase of the intricate process can only handle pieces of DNA that vary from 1000 base pairs (bp) to 2000 bp in length. Therefore, the chromosome must typically be broken down into manageable pieces using either restriction enzymes or mechanical shearing, and, then cloned into bacterium in order to increase the copy number of the individual pieces of DNA. Following sequencing of each cloned fragment, the individual pieces must be reassembled into a contiguous strand representing the entire chromosome. This process typically involves sophisticated computer algorithms to look for commonalties in the small fragments and overlap them to build the sequence ofthe entire chromosome DNA Sequencing Factories In order to accomplish the lofty goal of sequencing the entire human genome, sequencing factories have been assembled to produce large amounts of data and deposit this data into public databases for easy accessibility by the general scientific and medical communities. One such production-scale sequencing center is located at the Baylor College of Medicine ( To put the sequencing demands into perspective, it is informative to give some statistics on this particular sequencing center. As of May, 1999, the Baylor sequencing center
22 DNA Sequencing Using Fluorescence Detection 3 had deposited over 26 Mbp base pairs, 0.7% of the human genome) of sequence data into the public data bases and typically runs approximately 14 automated fluorescence-based DNA sequencing machines 12 hours a day. This amounts to performing 50,000 sequencing reactions a month. In this chapter, we will focus on the sequencing phase of the genome processing steps. It is in this particular step that fluorescence both hardware and probe development has had a profound impact on augmenting the throughput of acquiring sequencing data. We will begin by briefly introducing (or refreshing) the reader on the molecular and geometrical structure of DNA and review the common schemes used to sequence DNA. In addition, we will discuss the various electrophoretic modes for fractionating DNA based on size, an integral component in high throughput sequencing. We will then discuss common strategies of
23 4 Steven A. Soper et al.
24 DNA Sequencing Using Fluorescence Detection 5 fluorescence detection used in many sequencing machines and include in this discussion, hardware developments as well as probe (labeling dye) developments Structure of DNA Nucleotide Bases As stated in the previous section, the basic building blocks of DNA are a deoxyribose sugar unit, a phosphate group, and the nucleotide base, of which there are four, and when combined chemically they form an individual nucleotide. In Figure 1.2 is shown the chemical structure of the four nucleotide units which comprise DNA. The bases are grouped into two different classes: the pyrimidines (T, C) and the purines (A, G). In the case of RNA (ribonucleic acid) the structural differences include the incorporation of a ribose sugar (inclusion of a hydroxyl group at the 2' position) and also the substitution of uracil for thymine. Since DNA, more specifically chromosomes, are composed of many of these individual nucleotide units strung together, the nucleotides are covalently attached via phosphodiester linkages, which occur between the phosphate group on the 5' site of one sugar unit and the 3' hydroxyl group on another nucleotide (see Figure 1.2). Therefore, DNA exists as a biopolymer, with the repeating units being deoxyribose and phosphate residues that are always linked together by the same type of linkage and form the backbone of the DNA molecule. However, the order of bases along the biopolymer backbone can vary greatly and impart a high degree of individuality to any particular DNA molecule Watson Crick Base Pairing The three-dimensional structure of DNA is known to differ greatly from that of proteins, which are also biopolymers composed of different amino acid residues. It was discovered by James Watson and Francis Crick in 1953 that DNA exists in a double helical structure (see Figure 1.2) with the sugar phosphate backbone oriented on the outside of the molecule and the bases positioned on the inside of the double helix. 1 They also surmised that the two strands of the doublestranded molecule were held together via hydrogen bonds between a pair of bases on opposing strands. From modeling, they found that A could pair only with T and C with G (see Figure 1.2). Each of these base pairs possesses a symmetry that permits it to be placed into the double helix in two different ways (A to T and T Figure 1.2. Chemical structures of deoxyribose nucleic acid (DNA). The computer image shows the double helical nature of DNA. Also shown are the hydrogen bonding between nucleotide bases (Watson Crick base pairing) and the structures of the nucleotide building blocks.
25 6 Steven A. Soper et al. to A; C to G and G to C). Thus, all possible permutations (4) of sequence can exist for all four bases. In spite of the irregular sequence of bases along each strand, the sugar phosphate backbone assumes a very regular helical structure, with each turn of the double helix comprised of 10 nucleotide units Methods for Determining the Primary Structure of DNA The important factor to consider when developing a sequencing strategy for whole genomes is to remember that one can sequence only small sections of DNA ( bp) and that entire chromosomes are comprised of well over bp. The actual process of generating DNAs that can be handled by sequencing machines is typically involved and requires a number of cloning and purification steps followed by actual sequencing and then assembly of the pieces into contiguous regions of the target chromosome. While these specific processes will not be covered in this chapter, Figure 1.3 gives a schematic diagram of a typical strategy that is used in many sequencing laboratories. This strategy is termed an ordered shotgun approach and starts with the mechanical shearing (breaking apart) of
26 DNA Sequencing Using Fluorescence Detection 7 intact chromosomes into pieces composed of 100,000 to 200,000 These sheared DNAs are then cloned into yeast artificial chromosomes (YACs), one sheared section per YAC that allows amplification of the number of copies of the insert. Cloning provides an unlimited amount of material for sequencing and serves as the basis for construction of libraries, which are random sets of cloned DNA fragments. Genomic libraries are sets of overlapping fragments encompassing an entire genome. Once these libraries have been constructed, single inserts are extracted from the library and further sheared into fragments ranging from bp. These fragments are then sub-cloned into M13 vectors to produce high quality single-stranded DNAs appropriate for actual sequencing. Typically, M13 sub-clones are sequenced from both ends to allow construction of maps of the single YAC inserts, which are then used to provide a scaffold for the complete sequence analysis of the YAC insert. The important procedures that will be the focus of this chapter will be the procedures that are actually used to produce the sequence data of the M13 sub-clones. The two most common procedures used are the Maxam Gilbert chemical degradation method and the Sanger dideoxy-chain termination method. 3,4 The commonality in both of these methods is the production of a nested set of fragments that are all terminated (cleaved) at a common base(s) Maxam Gilbert Sequencing The Maxam Gilbert sequencing method uses chemical cleavage methods to break single-stranded DNA molecules at either one or two bases, which is followed by a size fractionation step to sort the cleaved products. The cleavage reaction involves two different reactions; one that cleaves at G and A (purine) residues and the other that cleaves at C and T (pyrimidine) residues. The first reaction can be slightly modified to cleave at G only and the second at T only. Therefore, one can run four separate cleavage reactions, G only, A + G, T only, T + C, from which the sequence can be deduced. In each cleavage reaction, the general process involves chemically modifying a single base, removing the modified base from its sugar, and finally breaking the bond of the exposed sugar in the DNA backbone. The chemical steps involved in G cleavage are shown in Figure 1.4a. In this step, dimethylsulfate is used to methylate G. After eviction of the modified base via heating, the strand is broken at the exposed sugar by subjecting the DNA to alkaline conditions. To cleave at both A and G residues, the procedure is identical to the G cleavage reaction except that a dilute acid is added after the methylation step (see Figure 1.4b). The reaction that cleaves at either a C or C and T residue is carried out by subjecting the DNA to hydrazine to remove the base and piperidine to cleave the sugar phosphate backbone. The extent of each reaction can be carefully limited so that each strand is cleaved at only one site. The entire Maxam Gilbert process is depicted in Figure 1.4c. As can be seen,
27 8 Steven A. Soper et al.
28 DNA Sequencing Using Fluorescence Detection 9
29 10 Steven A. Soper et al. four cleavage reactions are run: G, G + A, T, T + C. Prior to chemical cleavage, the intact DNA strand is labeled (typically with radiolabel for detection at the 5' end). Following chemical cleavage, the reactions are run in an electrophoresis gel (polyacrylamide) and separated based on size. The actual sequence of the strand is then deduced from the generated gel pattern Sanger Chain Termination Method Contrary to the Maxam Gilbert method, the Sanger procedure is an enzymatic method and involves construction of a DNA complement to the template whose sequence is to be determined. The complement is a strand of DNA that is constructed with a polymerase enzyme, which incorporates single nucleotide bases according to Watson Crick base pairing rules (A T; G C) (see Figure 1.5). The nested set of fragments is produced by interrupting the polymerization by inserting into the reaction cocktail a base that has a structural modification, that modification being the lack of a hydroxyl group at the 3' position of the deoxyribose sugar (dideoxy nucleotide, ddntp). Figure 1.5A shows the chemical structure of a ddntp. When mixed with the deoxynucleotides (dntp), the polymerization proceeds until the ddntp is incorporated. The entire Sanger, chain-termination protocol is shown in Figure 1.5C. The process is typically carried out by adding to the template DNA a primer which has a known sequence and which anneals (binds) to a complementary site on the unknown template. This primer can carry some type of label, for example a covalently attached fluorochrome. However, situating the fluorescent label on the ddntp can be done as well. Following annealing of the primer to the template, the reaction is carried out by the addition of a polymerase enzyme, all four dntps, and one particular ddntp. Therefore, four reactions are carried out, each containing a particular ddntp. Following polymerization, the reactions are loaded onto an electrophoresis gel and size fractionated. The final step involves reading the sequence from the gel. The Sanger method has been the preferred sequencing method for most large-scale sequencing projects due to its ease in preparing the nested set of fragments. Many times reactions can be run under standard conditions, without the need for chemical additions at timed intervals. In addition, the process is very conducive to automation Modes of Electrophoresis Whatever fluorescence detection protocol is used for calling bases, the important analytical technique that is required is a fractionation step, in which the
30 DNA Sequencing Using Fluorescence Detection 11 DNA molecules are sorted by size. While the focus of this chapter is on fluorescence-based detection in sequencing applications, it is informative to briefly discuss some of the common gel electrophoresis platforms that are used, since the fluorescence detector is integrated with the sizing step to provide on-line detection. The commonality in all electrophoresis formats is the use of an electric field to shuttle the DNAs through a maze which consists of a polymer, either static or dynamic, that possesses pores of various sizes. In all electrophoresis experiments, the mobility of the molecule defined as the steady state velocity per unit electric field strength) in an electric field is determined by where is the net charge on the molecule and is the frictional property of the molecule and is related to its conformational state as well as the molecular weight (MW) of the molecule. For example, proteins can be considered solid spheres and thus, Inthe case of DNAs, either single stranded or double stranded, because the DNA molecule acts as a free-draining coil. Since is also related to the length of the DNA molecule, one finds that where indicates that the mobility (in free solution) is independent of the number of bases comprising the DNA molecule. Because of this property of DNAs, the electrophoresis step must include some type of sieving medium, which can be a polymer consisting of pores with a definitive size. For any type of analytical separation, resolution is a key parameter which is optimized to improve the performance of the separation. The resolution (R) for electrophoretic separations can be calculated from the simple relationship where is the difference in mobility between two neighboringbands, is the average electrophoretic mobility for the same neighboring bands, and N is the plate number which represents the efficiency (bandwidth) for the electrophoresis. As can be seen from this equation, the resolution can be improved by increasing the difference in the mobility of the two bands (increase selectivity, gel property) or increasing the plate numbers (narrower bands). As a matter of reference, when R = 0.75, two bands are baseline resolved. For DNA sequencing, the accuracy in the base call depends intimately on the resolution obtained during gel fractionation. Common polymers used for DNA sequencing are linear or crosslinked polyacry lamides or polyethylene oxides, both of which possess the appropriate pore size for sorting single-stranded DNAs. Polyacrylamides are prepared from the acrylamide monomer which is copolymerized with a certain per-
31 12 Steven A. Soper et al.
32 DNA Sequencing Using Fluorescence Detection 13
33 14 Steven A. Soper et al. centage of crosslinker, N,N'-methylenebisacrylamide in the presence of a catalyst accelerator-chain initiator mixture. The porosity of the gel is determined by the relative proportion of acrylamide monomer to crosslinking agent. For reproducible fractionation of DNAs, they must be maintained in a single-strand conformation, which is accomplished by adding denaturants, such as urea or formamide, to the sieving gel. The existence of a gel network contributes significantly to the electrophoretic migration pattern (i.e., electrophoretic mobility) observed in the sequencing process. Any DNA fragments to be fractionated will inevitably encounter the gel network of polymer threads. This encounter increases the effective friction and consequently lowers the velocity of movement of the molecules. It is obvious that the retardation will be most pronounced when the mean diameter of gel pores is comparable to the size of the DNA fragments. Size therefore plays a critical role in determining the relative electrophoresis mobility and the degree of separation of different DNA fragments. It is this sieving effect that partly determines the resolution obtained with polyacrylamide gels. The gel network also minimizes convection currents caused by small temperature gradients. The main characteristic property of DNA is that the value of the negative charge on the molecule is almost independent of the ph of the medium. Therefore, their electrophoretic mobility is due mainly to differences in molecular size, not in charge. For a particular gel, the electrophoretic mobility of a DNA fragment is inversely proportional to the logarithm of the number of bases up to a certain limit. Since each DNA fragment is expected to possess a unique size, its electrophoretic mobility is unique and it migrates to a unique position within the electric field in a given length of time. Therefore, if a mixture of DNA fragments is subjected to electrophoresis, each of the fragments would be expected to concentrate into a tight migrating band at unique positions in the electric field Slab Gel Electrophoresis DNA sequencing is usually performed with slab gels. In slab gel electrophoresis, polymerization of acrylamide as well as the electrophoresis are conducted in a mold formed by two glass plates with a thin spacer. When analytical electrophoresis is performed, several samples are usually run simultaneously in the same slab. Since all samples are present in the same gel, the conditions of electrophoresis are quite constant from sample to sample. In slab gel electrophoresis, the sample is loaded using a pipette into wells formed into the gel during polymerization. A loading volume of is typical for slab gel electrophoresis. The field strength that can be applied to these types of gels range from V/cm, with the upper limit determined by heating (Joule) caused by current flow through the gel. Due to the thick nature of the gel, this heat is not efficiently dissipated, causing convective mixing and thus, zone broadening, which limits the upper level on the electric field strength that can be effectively used.
34 DNA Sequencing Using Fluorescence Detection Capillary Gel Electrophoresis In order to reduce the development time in the electrophoresis, many sequencing applications now use capillary gel electrophoresis, in which a glass tube (i.d. = length = cm) contains the gel sieving matrix and an electric field is applied to this capillary column. Due to the higher surface-tovolume ratio afforded by the thin glass capillary, large electric fields can be applied (~300 V/cm), which results in shorter electrophoresis development times (2 3 hrs) and also enhanced plate numbers compared to slab gel electrophoresis. The glass capillary contains silanol groups on its surface, which at high ph are deprotonated. As such, the glass capillary is negatively charged above ph = 7.0. Cations in the electrolyte solution build up at the wall of the glass tube, producing an electrical double layer. When a voltage is applied across the capillary, the cations migrate to the wall and exert a force on the surrounding fluid causing a bulk flow of solution toward the cathode (negative terminal). This electrically induced flow is called an electroosmotic flow and, unfortunately, it can interfere with the electrophoresis of the DNAs. Therefore, in DNA separations using glass capillaries, the wall is coated with some type of polymer (for example a linear polyacrylamide) to suppress this electroosmostic flow. After coating the wall, the capillary can then be filled with the sieving gel, which can be a linear polyacrylamide (no crosslinking) or some other type of gel, such as a hydroxyl cellulose of poly (ethylene oxide). 5 In addition, crosslinked gels may also be used in these small capillaries, but since crosslinked gels are not free flowing like their noncrosslinked counterparts, they must be polymerized directly within the capillary tube. Most capillary-based DNA sequencers use linear gels, since they can be easily replaced using high pressure pumping allowing the capillary to be used for multiple sequencing runs. The crosslinked gels cannot be removed from the capillary once polymerized. The electrophoresis is performed following filling the column with the gel by inserting one end of the capillary into a sample containing the sequencing mix and applying an electric field to the capillary tube. The injection end is typically cathodic (negative), with the opposite end being anodic (positive). By applying a fixed voltage for a certain time period, a controlled amount of sample can be inserted into the column with the injection volume ranging from 1 to 10 nl. Since the capillary is made of fused silica, fluorescence detection can be performed directly from within the capillary tube. In most cases, the capillary column is coated with a polyimide coating (non-transparent) to give it strength and the optical detection window can be produced by simply burning off a section of the polymer using a low temperature flame. The higher plate numbers (i.e., better resolution) that is obtained in capillary gel electrophoresis compared to slab gel is a direct consequence of the ability to use higher electric fields. Since the development time is significantly shorter in capillary gel electrophoresis due to the ability to apply higher electric fields, band spreading due to longitudinal diffusion is reduced resulting in higher plate num-
35 16 Steven A. Soper et al. bers. The ability to use higher electric fields in capillary gel electrophoresis results from the fact that Joule heating is suppressed since the heat can be effectively dissipated by the high surface-to-volume ratio capillary Detection Methods for DNA Sequencing Autoradiographic Detection Following electrophoresis, the individual DNA bands separated on the gel must be detected and subsequently analyzed. One of the earlier methods implemented to detect DNA bands in gels was autoradiography. In this mode, one of the phosphates of an individual nucleotide is replaced with a radioisotope, typically or both or which are radioprobes that emit When Sanger methods are used to prepare the sequencing reactions, either the primer or the dideoxynucleotide can contain the radiolabel. The labeling is done using an enzyme (T4 polynucleotide kinase), which catalyzes the transfer of a group from ATP to the 5'-hydroxy terminus of a sequencing primer. After the electrophoresis has been run, the gel is dried and then situated on an X-ray film. The film is developed (exposure to radiation from radioprobes) and dark bands are produced on the film where the DNA was resident. This is then followed by reading the sequence from the gel manually. The primary advantage of this approach is the inexpensive nature of the required equipment to perform the measurement. It basically requires only a gel dryer, film holder, and film. The difficulties associated with this approach are numerous. One important issue is the fact that radioisotopes are used, and therefore waste disposal becomes a difficult problem to contend with. Throughput issues (data production rates) are also a primary concern when using autoradiographic detection. For example, radiography can sometimes require several days to expose the film in order to get strong signals to read the bases from the gel. In addition, the detection is done after the electrophoresis and not during the electrophoretic run. Also, since there is no means to identify the individual bases using radioprobes, each base must be analyzed in a different lane of the gel. And finally, the bases must be called manually, which many times leads to frequent errors in the sequence reconstruction. Therefore, the inability to obtain data production rates sufficient to accommodate large sequencing projects has made radiographic detection obsolete for high throughput applications Fluorescence Detection For most DNA sequencing applications, irrespective of the separation platform used, fluorescence is the accepted detection protocol for several important
36 DNA Sequencing Using Fluorescence Detection 17 reasons. Fluorescence allows one to perform the base calling and detection in an automated fashion and alleviates the need for manual base calling. In addition, fluorescence can be carried out during the separation, eliminating long film development times. More importantly, due to the ability to implement multiple probes possessing unique spectral properties, the four bases comprising the DNA molecule can be identified in a single gel lane, potentially increasing throughput by a factor of four compared to radiographic detection. All of these important advantages associated with fluorescence allow for higher throughputs in DNA sequencing applications. As such, fluorescence can be considered as one of the most important recent technical innovations in DNA sequencing and has made it feasible to consider tackling large genome sequencing projects, such as the human genome. The first demonstrations on the use of fluorescence in DNA sequencing came with the work of Smith et al., 6 Prober et al., 7 and Ansorge et al. 8 In these works, slab gels or large gel tubes were used to fractionate the DNA ladders produced during enzymatic polymerization using Sanger sequencing strategies. The fluorescence detection was accomplished using four spectroscopically unique probes, which allowed the DNA sequence reconstruction to be done in a single electrophoresis lane of the gel. The chemical structures of the dye labels used in the Smith et al. 6 and Prober et al. 7 experiments are shown in Figures 1.6 and 1.7,
37 18 Steven A. Soper et al. respectively. As can be seen, the dyes were either attached (covalently) to the sequencing primer or to the dideoxynucleotides. The advantage of using dyelabeled dideoxynucleotides is that the sequencing reactions can be performed in a single reaction tube, whereas the dye-labeled primer reactions must be performed in four separate tubes and pooled prior to electrophoresis. In the case of the dyelabeled terminators, succinylfluorescein analogs were used with slight structural modifications to alter the absorption/emission maxima. The dyes were attached either to the 5 position of the pyrimidine bases or the 7 position of the 7-deazapurines, both of which are non-hydrogen bonding sites on the nucleotide base. The linker structure is also important, which in this case was a propargylamine, since the presence of the dye onto the terminator radically affects its ability to be incorporated by the polymerase enzyme. For dye-labeled primers, the oligonucleotides possessing the appropriate sequence were prepared on a standard DNA synthesizer. For Smith et al., 6 a thymidine derivative was prepared, which contained a phosphoramidite at the 3' carbon and a protected alkyl amino group at
38 DNA Sequencing Using Fluorescence Detection 19 the 5' carbon (typically a 6-carbon linker structure). During the final addition cycle of the oligonucleotide prepared via solid-phase synthesis using phosphoramidite chemistry the thymidineresidue is added and, following deprotection of the alkyl amino group and cleavage from the support, a free primary amine group results which can be reacted with any amino-reactive fluorescent dye to produce the oligonucleotide derivative. Also shown in Figures 1.6 and 1.7 are the absorption and emission profiles for the dye sets used in these experiments. The major attributes of the dye sets are that they can be efficiently excited with either 488 and/or nm lines of the Ar ion laser. In addition, there is minimal separation between the emission maxima of the dyes, which allows processing of the fluorescence on as few detection channels as possible. However, there is significant overlap in the emission spectra of the four dyes, producing severe spectral leakage into other detection channels, which must be corrected by software. The optical hardware used to process the four-color fluorescence for both systems are depicted in Figure 1.8. For the Smith et al. experiment, 6 a single laser was used as well as a single detection channel, which in this case consisted of a multiline Ar ion laser and a conventional photomultiplier tube (PMT). Placed in front of the laser and PMT were filter wheels to select the appropriate excitation wavelength (488 or nm) and emission color. The filter pairs used during fluorescence readout were 488/520 nm; 488/550 nm; 514/580 nm; 514/610 nm. In the case of the Prober et al. experiment, 7 due to the narrow distribution between the excitation maxima of the dyes, only excitation using the 488 nm line from the Ar ion laser was required as well as two PMT tubes to process the emission from the four colors. Discrimination of the four colors was accomplished by monitoring the intensity of each dye on both detectors simultaneously. By histograming the ratio of the fluorescence intensity of each dye (produced from an electrophoresis band) on the two detection channels, a discrete value was obtained that allowed facile discrimination of the four different fluorescent dyes (i.e., terminal base). In order to determine the limit of detection of these fluorescence systems, injections of known concentrations of dye labeled sequencing primers were electrophoresed. In both cases, the mass detection limit was estimated to be moles. In Figure 1.9 is shown the data output from these systems. Unfortunately, reading the sequence directly from the raw gel data becomes problematic due to several non-idealities, including signal from a single dye appearing on multiple detection channels due to the broad and closely spaced emission bands, dyedependent electrophoretic mobility shifts, and non-uniformity in the intensity of the electrophoresis bands due to the enzymatic reaction used to construct the individual DNA size-ladders. As such, several post-electrophoresis processing steps were required to augment sequence reconstruction of the test template. In the case of the Smith et al. example, 6 these steps involved:
39 20 Steven A. Soper et al.
40 DNA Sequencing Using Fluorescence Detection High frequency noise removal using a low-pass Fourier filter. A time-delay between measurements at different wavelengths corrected by linear interpolation between successive measurements. A multicomponent analysis performed on each set of four data points, which produced the amount of the four dyes present in the detector as a function of time. The peaks present in the data stream located. The mobility corrected for the dye attached to each DNA fragment. In this case, it was empirically determined that fluorescein and rhodaminelabeled DNA fragments moved as if they were 1 base longer than the NBD-labeled fragments and the Texas Red fragments moved as if they were 1¼ bases longer. The important performance criteria in any type ofautomated DNA sequencer is its throughput, the number of bases it can process in a single gel read and its accuracy in calling bases. In terms of base calling accuracy, these early instruments demonstrated an error rate of approximately 1% with a read length approaching 500 bases. The throughput of the instrument described by Prober et al. 7 was estimated to give a raw throughput of 600 bases per hour (12 electrophoresis lanes). Interestingly, many present-day commercial automated sequencers still use similar technology in their machines and the throughput can be as high as 16,000 bases per hour (96 electrophoresis lanes) Fluorescent Dyes for DNA Labeling and Sequencing As stated above, fluorescence detection has had a tremendous impact in the area of DNA sequencing because of the speed of the readout process as well as the ability to discriminate amongst the four nucleotide bases in a single gel lane due to the unique spectral properties of the target dye molecules. There are a variety of dye sets (typically four dyes per set, one for each nucleotide base) that have been developed for DNA sequencing applications and whichever dye set one wishes to
41 22 Steven A. Soper et al.
42 DNA Sequencing Using Fluorescence Detection 23 consider, certain properties associated with the dyes for DNA sequencing applications are important. These properties are listed below: Each dye in the set must be spectroscopically distinct. The ideal situation from a throughput point of view, is to identify each base in a single gel lane instead of four gel lanes. In most cases, the discrimination is based on differences in the emission properties of each dye (distinct emission maxima), however, other fluorescence properties can be used as well, such as fluorescence lifetimes. The dye set should preferably be excited by a single laser source. It becomes instrumentally difficult to implement multiple excitation sources since lasers are typically used as the source of excitation to improve the limit of detection in the measurement and the upkeep on multiple lasers becomes problematic. The dye set should possess high extinction at the excitation frequency and also large quantum yields in the gel matrix used to fractionate the DNAs. Good photophysical properties are necessary in order to improve detectability. In addition, the dye set should show reasonable quantum yields in denaturing gels, which consist of high concentrations of urea and/or formamide. The dye set should show favorable chemical stability at high temperatures. A common procedure in most Sanger sequencing strategies is to implement a thermostable polymerase and then subject the sequencing reactions to multiple temperature cycles (55 C 95 C) in order to amplify the amount of product generated (cycle sequencing). Therefore, the dyes must be able to withstand high temperature conditions for extended periods of time.
How many of you have checked out the web site on protein-dna interactions?
 How many of you have checked out the web site on protein-dna interactions? Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. Find and be ready to discuss
How many of you have checked out the web site on protein-dna interactions? Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. Find and be ready to discuss
1. Molecular computation uses molecules to represent information and molecular processes to implement information processing.
 Chapter IV Molecular Computation These lecture notes are exclusively for the use of students in Prof. MacLennan s Unconventional Computation course. c 2013, B. J. MacLennan, EECS, University of Tennessee,
Chapter IV Molecular Computation These lecture notes are exclusively for the use of students in Prof. MacLennan s Unconventional Computation course. c 2013, B. J. MacLennan, EECS, University of Tennessee,
DNA Separation Methods. Chapter 12
 DNA Separation Methods Chapter 12 DNA molecules After PCR reaction produces many copies of DNA molecules Need a way to separate the DNA molecules from similar sized molecules Only way to genotype samples
DNA Separation Methods Chapter 12 DNA molecules After PCR reaction produces many copies of DNA molecules Need a way to separate the DNA molecules from similar sized molecules Only way to genotype samples
2. True or False? The sequence of nucleotides in the human genome is 90.9% identical from one person to the next. False (it s 99.
 1. True or False? A typical chromosome can contain several hundred to several thousand genes, arranged in linear order along the DNA molecule present in the chromosome. True 2. True or False? The sequence
1. True or False? A typical chromosome can contain several hundred to several thousand genes, arranged in linear order along the DNA molecule present in the chromosome. True 2. True or False? The sequence
- In 1976 1977, Allan Maxam and walter Gilbert devised the first method for sequencing DNA fragments containing up to ~ 500 nucleotides.
 DNA Sequencing - DNA sequencing includes several methods and technologies that are used for determining the order of the nucleotide bases adenine, guanine, cytosine, and thymine in a molecule of DNA. -
DNA Sequencing - DNA sequencing includes several methods and technologies that are used for determining the order of the nucleotide bases adenine, guanine, cytosine, and thymine in a molecule of DNA. -
Forensic DNA Testing Terminology
 Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
The Biotechnology Education Company
 EDVTEK P.. Box 1232 West Bethesda, MD 20827-1232 The Biotechnology 106 EDV-Kit # Principles of DNA Sequencing Experiment bjective: The objective of this experiment is to develop an understanding of DNA
EDVTEK P.. Box 1232 West Bethesda, MD 20827-1232 The Biotechnology 106 EDV-Kit # Principles of DNA Sequencing Experiment bjective: The objective of this experiment is to develop an understanding of DNA
DNA Sequencing. Contents. Introduction. Maxam-Gilbert
 DNA Sequencing Contents Introduction... 1 Maxam-Gilbert... 1 Sanger... 3 Automated Fluorescence Sequencing... 5 References... 8 Introduction Prior to the mid-1970 s no method existed by which DNA could
DNA Sequencing Contents Introduction... 1 Maxam-Gilbert... 1 Sanger... 3 Automated Fluorescence Sequencing... 5 References... 8 Introduction Prior to the mid-1970 s no method existed by which DNA could
Chapter 11: Molecular Structure of DNA and RNA
 Chapter 11: Molecular Structure of DNA and RNA Student Learning Objectives Upon completion of this chapter you should be able to: 1. Understand the major experiments that led to the discovery of DNA as
Chapter 11: Molecular Structure of DNA and RNA Student Learning Objectives Upon completion of this chapter you should be able to: 1. Understand the major experiments that led to the discovery of DNA as
STRUCTURES OF NUCLEIC ACIDS
 CHAPTER 2 STRUCTURES OF NUCLEIC ACIDS What is the chemical structure of a deoxyribonucleic acid (DNA) molecule? DNA is a polymer of deoxyribonucleotides. All nucleic acids consist of nucleotides as building
CHAPTER 2 STRUCTURES OF NUCLEIC ACIDS What is the chemical structure of a deoxyribonucleic acid (DNA) molecule? DNA is a polymer of deoxyribonucleotides. All nucleic acids consist of nucleotides as building
Sanger Sequencing and Quality Assurance. Zbigniew Rudzki Department of Pathology University of Melbourne
 Sanger Sequencing and Quality Assurance Zbigniew Rudzki Department of Pathology University of Melbourne Sanger DNA sequencing The era of DNA sequencing essentially started with the publication of the enzymatic
Sanger Sequencing and Quality Assurance Zbigniew Rudzki Department of Pathology University of Melbourne Sanger DNA sequencing The era of DNA sequencing essentially started with the publication of the enzymatic
Name Date Period. 2. When a molecule of double-stranded DNA undergoes replication, it results in
 DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA. Discovery of the DNA double helix
 DNA Replication DNA Discovery of the DNA double helix A. 1950 s B. Rosalind Franklin - X-ray photo of DNA. C. Watson and Crick - described the DNA molecule from Franklin s X-ray. What is DNA? Question:
DNA Replication DNA Discovery of the DNA double helix A. 1950 s B. Rosalind Franklin - X-ray photo of DNA. C. Watson and Crick - described the DNA molecule from Franklin s X-ray. What is DNA? Question:
2. The number of different kinds of nucleotides present in any DNA molecule is A) four B) six C) two D) three
 Chem 121 Chapter 22. Nucleic Acids 1. Any given nucleotide in a nucleic acid contains A) two bases and a sugar. B) one sugar, two bases and one phosphate. C) two sugars and one phosphate. D) one sugar,
Chem 121 Chapter 22. Nucleic Acids 1. Any given nucleotide in a nucleic acid contains A) two bases and a sugar. B) one sugar, two bases and one phosphate. C) two sugars and one phosphate. D) one sugar,
DNA, RNA, Protein synthesis, and Mutations. Chapters 12-13.3
 DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
DNA and Forensic Science
 DNA and Forensic Science Micah A. Luftig * Stephen Richey ** I. INTRODUCTION This paper represents a discussion of the fundamental principles of DNA technology as it applies to forensic testing. A brief
DNA and Forensic Science Micah A. Luftig * Stephen Richey ** I. INTRODUCTION This paper represents a discussion of the fundamental principles of DNA technology as it applies to forensic testing. A brief
Technical Note. Roche Applied Science. No. LC 18/2004. Assay Formats for Use in Real-Time PCR
 Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure
 Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure Nucleic acids play an important role in the storage and expression of genetic information. They are divided into
Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure Nucleic acids play an important role in the storage and expression of genetic information. They are divided into
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
DNA Replication & Protein Synthesis. This isn t a baaaaaaaddd chapter!!!
 DNA Replication & Protein Synthesis This isn t a baaaaaaaddd chapter!!! The Discovery of DNA s Structure Watson and Crick s discovery of DNA s structure was based on almost fifty years of research by other
DNA Replication & Protein Synthesis This isn t a baaaaaaaddd chapter!!! The Discovery of DNA s Structure Watson and Crick s discovery of DNA s structure was based on almost fifty years of research by other
Nucleotides and Nucleic Acids
 Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Replication Study Guide
 Replication Study Guide This study guide is a written version of the material you have seen presented in the replication unit. Self-reproduction is a function of life that human-engineered systems have
Replication Study Guide This study guide is a written version of the material you have seen presented in the replication unit. Self-reproduction is a function of life that human-engineered systems have
Recombinant DNA & Genetic Engineering. Tools for Genetic Manipulation
 Recombinant DNA & Genetic Engineering g Genetic Manipulation: Tools Kathleen Hill Associate Professor Department of Biology The University of Western Ontario Tools for Genetic Manipulation DNA, RNA, cdna
Recombinant DNA & Genetic Engineering g Genetic Manipulation: Tools Kathleen Hill Associate Professor Department of Biology The University of Western Ontario Tools for Genetic Manipulation DNA, RNA, cdna
Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism )
 Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
Structure and Function of DNA
 Structure and Function of DNA DNA and RNA Structure DNA and RNA are nucleic acids. They consist of chemical units called nucleotides. The nucleotides are joined by a sugar-phosphate backbone. The four
Structure and Function of DNA DNA and RNA Structure DNA and RNA are nucleic acids. They consist of chemical units called nucleotides. The nucleotides are joined by a sugar-phosphate backbone. The four
Answer: 2. Uracil. Answer: 2. hydrogen bonds. Adenine, Cytosine and Guanine are found in both RNA and DNA.
 Answer: 2. Uracil Adenine, Cytosine and Guanine are found in both RNA and DNA. Thymine is found only in DNA; Uracil takes its (Thymine) place in RNA molecules. Answer: 2. hydrogen bonds The complementary
Answer: 2. Uracil Adenine, Cytosine and Guanine are found in both RNA and DNA. Thymine is found only in DNA; Uracil takes its (Thymine) place in RNA molecules. Answer: 2. hydrogen bonds The complementary
Transcription and Translation of DNA
 Transcription and Translation of DNA Genotype our genetic constitution ( makeup) is determined (controlled) by the sequence of bases in its genes Phenotype determined by the proteins synthesised when genes
Transcription and Translation of DNA Genotype our genetic constitution ( makeup) is determined (controlled) by the sequence of bases in its genes Phenotype determined by the proteins synthesised when genes
The Techniques of Molecular Biology: Forensic DNA Fingerprinting
 Revised Fall 2011 The Techniques of Molecular Biology: Forensic DNA Fingerprinting The techniques of molecular biology are used to manipulate the structure and function of molecules such as DNA and proteins
Revised Fall 2011 The Techniques of Molecular Biology: Forensic DNA Fingerprinting The techniques of molecular biology are used to manipulate the structure and function of molecules such as DNA and proteins
DNA Sequence Analysis
 DNA Sequence Analysis Two general kinds of analysis Screen for one of a set of known sequences Determine the sequence even if it is novel Screening for a known sequence usually involves an oligonucleotide
DNA Sequence Analysis Two general kinds of analysis Screen for one of a set of known sequences Determine the sequence even if it is novel Screening for a known sequence usually involves an oligonucleotide
DNA is found in all organisms from the smallest bacteria to humans. DNA has the same composition and structure in all organisms!
 Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
Chapter 6 DNA Replication
 Chapter 6 DNA Replication Each strand of the DNA double helix contains a sequence of nucleotides that is exactly complementary to the nucleotide sequence of its partner strand. Each strand can therefore
Chapter 6 DNA Replication Each strand of the DNA double helix contains a sequence of nucleotides that is exactly complementary to the nucleotide sequence of its partner strand. Each strand can therefore
Translation Study Guide
 Translation Study Guide This study guide is a written version of the material you have seen presented in the replication unit. In translation, the cell uses the genetic information contained in mrna to
Translation Study Guide This study guide is a written version of the material you have seen presented in the replication unit. In translation, the cell uses the genetic information contained in mrna to
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Real-Time PCR Vs. Traditional PCR
 Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
Next Generation Sequencing
 Next Generation Sequencing DNA sequence represents a single format onto which a broad range of biological phenomena can be projected for high-throughput data collection Over the past three years, massively
Next Generation Sequencing DNA sequence represents a single format onto which a broad range of biological phenomena can be projected for high-throughput data collection Over the past three years, massively
Technical Note. Roche Applied Science. No. LC 19/2004. Color Compensation
 Roche Applied Science Technical Note No. LC 19/2004 Purpose of this Note Color The LightCycler System is able to simultaneously detect and analyze more than one color in each capillary. Due to overlap
Roche Applied Science Technical Note No. LC 19/2004 Purpose of this Note Color The LightCycler System is able to simultaneously detect and analyze more than one color in each capillary. Due to overlap
Concepts and methods in sequencing and genome assembly
 BCM-2004 Concepts and methods in sequencing and genome assembly B. Franz LANG, Département de Biochimie Bureau: H307-15 Courrier électronique: Franz.Lang@Umontreal.ca Outline 1. Concepts in DNA and RNA
BCM-2004 Concepts and methods in sequencing and genome assembly B. Franz LANG, Département de Biochimie Bureau: H307-15 Courrier électronique: Franz.Lang@Umontreal.ca Outline 1. Concepts in DNA and RNA
1/12 Dideoxy DNA Sequencing
 1/12 Dideoxy DNA Sequencing Dideoxy DNA sequencing utilizes two steps: PCR (polymerase chain reaction) amplification of DNA using dideoxy nucleoside triphosphates (Figures 1 and 2)and denaturing polyacrylamide
1/12 Dideoxy DNA Sequencing Dideoxy DNA sequencing utilizes two steps: PCR (polymerase chain reaction) amplification of DNA using dideoxy nucleoside triphosphates (Figures 1 and 2)and denaturing polyacrylamide
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Automated DNA Sequencing. Chemistry Guide
 Automated DNA Sequencing Chemistry Guide Copyright 2000, Applied Biosystems For Research Use Only. Not for use in diagnostic procedures. ABI PRISM and its design, Applied Biosystems, and MicroAmp are registered
Automated DNA Sequencing Chemistry Guide Copyright 2000, Applied Biosystems For Research Use Only. Not for use in diagnostic procedures. ABI PRISM and its design, Applied Biosystems, and MicroAmp are registered
Lab 5: DNA Fingerprinting
 Lab 5: DNA Fingerprinting You are about to perform a procedure known as DNA fingerprinting. The data obtained may allow you to determine if the samples of DNA that you will be provided with are from the
Lab 5: DNA Fingerprinting You are about to perform a procedure known as DNA fingerprinting. The data obtained may allow you to determine if the samples of DNA that you will be provided with are from the
IDTutorial: DNA Sequencing
 IDTutorial: DNA Sequencing Introduction Prior to the mid-1970 s no method existed by which DNA could be directly sequenced. Knowledge about gene and genome organization was based upon studies of prokaryotic
IDTutorial: DNA Sequencing Introduction Prior to the mid-1970 s no method existed by which DNA could be directly sequenced. Knowledge about gene and genome organization was based upon studies of prokaryotic
DNA Detection. Chapter 13
 DNA Detection Chapter 13 Detecting DNA molecules Once you have your DNA separated by size Now you need to be able to visualize the DNA on the gel somehow Original techniques: Radioactive label, silver
DNA Detection Chapter 13 Detecting DNA molecules Once you have your DNA separated by size Now you need to be able to visualize the DNA on the gel somehow Original techniques: Radioactive label, silver
Fluorescent dyes for use with the
 Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
Bio 102 Practice Problems Chromosomes and DNA Replication
 Bio 102 Practice Problems Chromosomes and DNA Replication Multiple choice: Unless otherwise directed, circle the one best answer: 1. Which one of the following enzymes is NT a key player in the process
Bio 102 Practice Problems Chromosomes and DNA Replication Multiple choice: Unless otherwise directed, circle the one best answer: 1. Which one of the following enzymes is NT a key player in the process
Nucleic Acid Techniques in Bacterial Systematics
 Nucleic Acid Techniques in Bacterial Systematics Edited by Erko Stackebrandt Department of Microbiology University of Queensland St Lucia, Australia and Michael Goodfellow Department of Microbiology University
Nucleic Acid Techniques in Bacterial Systematics Edited by Erko Stackebrandt Department of Microbiology University of Queensland St Lucia, Australia and Michael Goodfellow Department of Microbiology University
Introduction To Real Time Quantitative PCR (qpcr)
 Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Molecular Genetics. RNA, Transcription, & Protein Synthesis
 Molecular Genetics RNA, Transcription, & Protein Synthesis Section 1 RNA AND TRANSCRIPTION Objectives Describe the primary functions of RNA Identify how RNA differs from DNA Describe the structure and
Molecular Genetics RNA, Transcription, & Protein Synthesis Section 1 RNA AND TRANSCRIPTION Objectives Describe the primary functions of RNA Identify how RNA differs from DNA Describe the structure and
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Genetics Module B, Anchor 3
 Genetics Module B, Anchor 3 Key Concepts: - An individual s characteristics are determines by factors that are passed from one parental generation to the next. - During gamete formation, the alleles for
Genetics Module B, Anchor 3 Key Concepts: - An individual s characteristics are determines by factors that are passed from one parental generation to the next. - During gamete formation, the alleles for
CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA
 CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA INTRODUCTION DNA : DNA is deoxyribose nucleic acid. It is made up of a base consisting of sugar, phosphate and one nitrogen base.the
CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA INTRODUCTION DNA : DNA is deoxyribose nucleic acid. It is made up of a base consisting of sugar, phosphate and one nitrogen base.the
Essentials of Real Time PCR. About Sequence Detection Chemistries
 Essentials of Real Time PCR About Real-Time PCR Assays Real-time Polymerase Chain Reaction (PCR) is the ability to monitor the progress of the PCR as it occurs (i.e., in real time). Data is therefore collected
Essentials of Real Time PCR About Real-Time PCR Assays Real-time Polymerase Chain Reaction (PCR) is the ability to monitor the progress of the PCR as it occurs (i.e., in real time). Data is therefore collected
VLLM0421c Medical Microbiology I, practical sessions. Protocol to topic J10
 Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Genetics Test Biology I
 Genetics Test Biology I Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Avery s experiments showed that bacteria are transformed by a. RNA. c. proteins.
Genetics Test Biology I Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Avery s experiments showed that bacteria are transformed by a. RNA. c. proteins.
Molecular Spectroscopy
 Molecular Spectroscopy UV-Vis Spectroscopy Absorption Characteristics of Some Common Chromophores UV-Vis Spectroscopy Absorption Characteristics of Aromatic Compounds UV-Vis Spectroscopy Effect of extended
Molecular Spectroscopy UV-Vis Spectroscopy Absorption Characteristics of Some Common Chromophores UV-Vis Spectroscopy Absorption Characteristics of Aromatic Compounds UV-Vis Spectroscopy Effect of extended
DNA Fingerprinting. Unless they are identical twins, individuals have unique DNA
 DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Gene Mapping Techniques
 Gene Mapping Techniques OBJECTIVES By the end of this session the student should be able to: Define genetic linkage and recombinant frequency State how genetic distance may be estimated State how restriction
Gene Mapping Techniques OBJECTIVES By the end of this session the student should be able to: Define genetic linkage and recombinant frequency State how genetic distance may be estimated State how restriction
DNA PROFILING IN FORENSIC SCIENCE
 DA PROFILIG I FORESIC SCIECE DA is the chemical code that is found in every cell of an individual's body, and is unique to each individual. Because it is unique, the ability to examine DA found at a crime
DA PROFILIG I FORESIC SCIECE DA is the chemical code that is found in every cell of an individual's body, and is unique to each individual. Because it is unique, the ability to examine DA found at a crime
DNA Replication in Prokaryotes
 OpenStax-CNX module: m44488 1 DNA Replication in Prokaryotes OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44488 1 DNA Replication in Prokaryotes OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
2007 7.013 Problem Set 1 KEY
 2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
Objectives: Vocabulary:
 Introduction to Agarose Gel Electrophoresis: A Precursor to Cornell Institute for Biology Teacher s lab Author: Jennifer Weiser and Laura Austen Date Created: 2010 Subject: Molecular Biology and Genetics
Introduction to Agarose Gel Electrophoresis: A Precursor to Cornell Institute for Biology Teacher s lab Author: Jennifer Weiser and Laura Austen Date Created: 2010 Subject: Molecular Biology and Genetics
Crime Scenes and Genes
 Glossary Agarose Biotechnology Cell Chromosome DNA (deoxyribonucleic acid) Electrophoresis Gene Micro-pipette Mutation Nucleotide Nucleus PCR (Polymerase chain reaction) Primer STR (short tandem repeats)
Glossary Agarose Biotechnology Cell Chromosome DNA (deoxyribonucleic acid) Electrophoresis Gene Micro-pipette Mutation Nucleotide Nucleus PCR (Polymerase chain reaction) Primer STR (short tandem repeats)
Mitochondrial DNA Analysis
 Mitochondrial DNA Analysis Lineage Markers Lineage markers are passed down from generation to generation without changing Except for rare mutation events They can help determine the lineage (family tree)
Mitochondrial DNA Analysis Lineage Markers Lineage markers are passed down from generation to generation without changing Except for rare mutation events They can help determine the lineage (family tree)
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Basic Concepts of DNA, Proteins, Genes and Genomes
 Basic Concepts of DNA, Proteins, Genes and Genomes Kun-Mao Chao 1,2,3 1 Graduate Institute of Biomedical Electronics and Bioinformatics 2 Department of Computer Science and Information Engineering 3 Graduate
Basic Concepts of DNA, Proteins, Genes and Genomes Kun-Mao Chao 1,2,3 1 Graduate Institute of Biomedical Electronics and Bioinformatics 2 Department of Computer Science and Information Engineering 3 Graduate
Troubleshooting Sequencing Data
 Troubleshooting Sequencing Data Troubleshooting Sequencing Data No recognizable sequence (see page 7-10) Insufficient Quantitate the DNA. Increase the amount of DNA in the sequencing reactions. See page
Troubleshooting Sequencing Data Troubleshooting Sequencing Data No recognizable sequence (see page 7-10) Insufficient Quantitate the DNA. Increase the amount of DNA in the sequencing reactions. See page
Name Class Date. Figure 13 1. 2. Which nucleotide in Figure 13 1 indicates the nucleic acid above is RNA? a. uracil c. cytosine b. guanine d.
 13 Multiple Choice RNA and Protein Synthesis Chapter Test A Write the letter that best answers the question or completes the statement on the line provided. 1. Which of the following are found in both
13 Multiple Choice RNA and Protein Synthesis Chapter Test A Write the letter that best answers the question or completes the statement on the line provided. 1. Which of the following are found in both
DNA (genetic information in genes) RNA (copies of genes) proteins (functional molecules) directionality along the backbone 5 (phosphate) to 3 (OH)
 DNA, RNA, replication, translation, and transcription Overview Recall the central dogma of biology: DNA (genetic information in genes) RNA (copies of genes) proteins (functional molecules) DNA structure
DNA, RNA, replication, translation, and transcription Overview Recall the central dogma of biology: DNA (genetic information in genes) RNA (copies of genes) proteins (functional molecules) DNA structure
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
DNA Sequencing & The Human Genome Project
 DNA Sequencing & The Human Genome Project An Endeavor Revolutionizing Modern Biology Jutta Marzillier, Ph.D Lehigh University Biological Sciences November 13 th, 2013 Guess, who turned 60 earlier this
DNA Sequencing & The Human Genome Project An Endeavor Revolutionizing Modern Biology Jutta Marzillier, Ph.D Lehigh University Biological Sciences November 13 th, 2013 Guess, who turned 60 earlier this
HiPer RT-PCR Teaching Kit
 HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
PRACTICE TEST QUESTIONS
 PART A: MULTIPLE CHOICE QUESTIONS PRACTICE TEST QUESTIONS DNA & PROTEIN SYNTHESIS B 1. One of the functions of DNA is to A. secrete vacuoles. B. make copies of itself. C. join amino acids to each other.
PART A: MULTIPLE CHOICE QUESTIONS PRACTICE TEST QUESTIONS DNA & PROTEIN SYNTHESIS B 1. One of the functions of DNA is to A. secrete vacuoles. B. make copies of itself. C. join amino acids to each other.
a. Ribosomal RNA rrna a type ofrna that combines with proteins to form Ribosomes on which polypeptide chains of proteins are assembled
 Biology 101 Chapter 14 Name: Fill-in-the-Blanks Which base follows the next in a strand of DNA is referred to. as the base (1) Sequence. The region of DNA that calls for the assembly of specific amino
Biology 101 Chapter 14 Name: Fill-in-the-Blanks Which base follows the next in a strand of DNA is referred to. as the base (1) Sequence. The region of DNA that calls for the assembly of specific amino
26 - Introduction The DNA chain and its sense The double helix Figure 5 -
 26 - Introduction The DNA chain and its sense The DNA chain forms as phosphate groups are shared by subsequent nucleotides, creating phosphodiester bonds (see Figure 4) between them. This sharing is possible
26 - Introduction The DNA chain and its sense The DNA chain forms as phosphate groups are shared by subsequent nucleotides, creating phosphodiester bonds (see Figure 4) between them. This sharing is possible
Welcome to Pacific Biosciences' Introduction to SMRTbell Template Preparation.
 Introduction to SMRTbell Template Preparation 100 338 500 01 1. SMRTbell Template Preparation 1.1 Introduction to SMRTbell Template Preparation Welcome to Pacific Biosciences' Introduction to SMRTbell
Introduction to SMRTbell Template Preparation 100 338 500 01 1. SMRTbell Template Preparation 1.1 Introduction to SMRTbell Template Preparation Welcome to Pacific Biosciences' Introduction to SMRTbell
Introduction to next-generation sequencing data
 Introduction to next-generation sequencing data David Simpson Centre for Experimental Medicine Queens University Belfast http://www.qub.ac.uk/research-centres/cem/ Outline History of DNA sequencing NGS
Introduction to next-generation sequencing data David Simpson Centre for Experimental Medicine Queens University Belfast http://www.qub.ac.uk/research-centres/cem/ Outline History of DNA sequencing NGS
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Lecture 13: DNA Technology. DNA Sequencing. DNA Sequencing Genetic Markers - RFLPs polymerase chain reaction (PCR) products of biotechnology
 Lecture 13: DNA Technology DNA Sequencing Genetic Markers - RFLPs polymerase chain reaction (PCR) products of biotechnology DNA Sequencing determine order of nucleotides in a strand of DNA > bases = A,
Lecture 13: DNA Technology DNA Sequencing Genetic Markers - RFLPs polymerase chain reaction (PCR) products of biotechnology DNA Sequencing determine order of nucleotides in a strand of DNA > bases = A,
Experiment #5: Qualitative Absorption Spectroscopy
 Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
An Overview of DNA Sequencing
 An Overview of DNA Sequencing Prokaryotic DNA Plasmid http://en.wikipedia.org/wiki/image:prokaryote_cell_diagram.svg Eukaryotic DNA http://en.wikipedia.org/wiki/image:plant_cell_structure_svg.svg DNA Structure
An Overview of DNA Sequencing Prokaryotic DNA Plasmid http://en.wikipedia.org/wiki/image:prokaryote_cell_diagram.svg Eukaryotic DNA http://en.wikipedia.org/wiki/image:plant_cell_structure_svg.svg DNA Structure
12.1 The Role of DNA in Heredity
 12.1 The Role of DNA in Heredity Only in the last 50 years have scientists understood the role of DNA in heredity. That understanding began with the discovery of DNA s structure. In 1952, Rosalind Franklin
12.1 The Role of DNA in Heredity Only in the last 50 years have scientists understood the role of DNA in heredity. That understanding began with the discovery of DNA s structure. In 1952, Rosalind Franklin
1.5 page 3 DNA Replication S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.5 Nucleic Acids and their functions Page 3 l. DNA Replication 1. Go through PowerPoint 2. Read notes p2 and then watch the animation
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.5 Nucleic Acids and their functions Page 3 l. DNA Replication 1. Go through PowerPoint 2. Read notes p2 and then watch the animation
How is genome sequencing done?
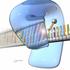 How is genome sequencing done? Using 454 Sequencing on the Genome Sequencer FLX System, DNA from a genome is converted into sequence data through four primary steps: Step One DNA sample preparation; Step
How is genome sequencing done? Using 454 Sequencing on the Genome Sequencer FLX System, DNA from a genome is converted into sequence data through four primary steps: Step One DNA sample preparation; Step
Genetic Analysis. Phenotype analysis: biological-biochemical analysis. Genotype analysis: molecular and physical analysis
 Genetic Analysis Phenotype analysis: biological-biochemical analysis Behaviour under specific environmental conditions Behaviour of specific genetic configurations Behaviour of progeny in crosses - Genotype
Genetic Analysis Phenotype analysis: biological-biochemical analysis Behaviour under specific environmental conditions Behaviour of specific genetic configurations Behaviour of progeny in crosses - Genotype
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Basic Concepts Recombinant DNA Use with Chapter 13, Section 13.2
 Name Date lass Master 19 Basic oncepts Recombinant DN Use with hapter, Section.2 Formation of Recombinant DN ut leavage Splicing opyright lencoe/mcraw-hill, a division of he Mcraw-Hill ompanies, Inc. Bacterial
Name Date lass Master 19 Basic oncepts Recombinant DN Use with hapter, Section.2 Formation of Recombinant DN ut leavage Splicing opyright lencoe/mcraw-hill, a division of he Mcraw-Hill ompanies, Inc. Bacterial
Troubleshooting Guide for DNA Electrophoresis
 Troubleshooting Guide for Electrophoresis. ELECTROPHORESIS Protocols and Recommendations for Electrophoresis electrophoresis problem 1 Low intensity of all or some bands 2 Smeared bands 3 Atypical banding
Troubleshooting Guide for Electrophoresis. ELECTROPHORESIS Protocols and Recommendations for Electrophoresis electrophoresis problem 1 Low intensity of all or some bands 2 Smeared bands 3 Atypical banding
Academic Nucleic Acids and Protein Synthesis Test
 Academic Nucleic Acids and Protein Synthesis Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Each organism has a unique combination
Academic Nucleic Acids and Protein Synthesis Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Each organism has a unique combination
Co Extra (GM and non GM supply chains: Their CO EXistence and TRAceability) Outcomes of Co Extra
 GM and non GM supply chains: Their CO EXistence and TRAceability Outcomes of Co Extra Comparison of different real time PCR chemistries and their suitability for detection and quantification of genetically
GM and non GM supply chains: Their CO EXistence and TRAceability Outcomes of Co Extra Comparison of different real time PCR chemistries and their suitability for detection and quantification of genetically
Next Generation Sequencing
 Next Generation Sequencing Technology and applications 10/1/2015 Jeroen Van Houdt - Genomics Core - KU Leuven - UZ Leuven 1 Landmarks in DNA sequencing 1953 Discovery of DNA double helix structure 1977
Next Generation Sequencing Technology and applications 10/1/2015 Jeroen Van Houdt - Genomics Core - KU Leuven - UZ Leuven 1 Landmarks in DNA sequencing 1953 Discovery of DNA double helix structure 1977
Just the Facts: A Basic Introduction to the Science Underlying NCBI Resources
 1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
From DNA to Protein. Proteins. Chapter 13. Prokaryotes and Eukaryotes. The Path From Genes to Proteins. All proteins consist of polypeptide chains
 Proteins From DNA to Protein Chapter 13 All proteins consist of polypeptide chains A linear sequence of amino acids Each chain corresponds to the nucleotide base sequence of a gene The Path From Genes
Proteins From DNA to Protein Chapter 13 All proteins consist of polypeptide chains A linear sequence of amino acids Each chain corresponds to the nucleotide base sequence of a gene The Path From Genes
K'NEX DNA Models. Developed by Dr. Gary Benson Department of Biomathematical Sciences Mount Sinai School of Medicine
 KNEX DNA Models Introduction Page 1 of 11 All photos by Kevin Kelliher. To download an Acrobat pdf version of this website Click here. K'NEX DNA Models Developed by Dr. Gary Benson Department of Biomathematical
KNEX DNA Models Introduction Page 1 of 11 All photos by Kevin Kelliher. To download an Acrobat pdf version of this website Click here. K'NEX DNA Models Developed by Dr. Gary Benson Department of Biomathematical
RNA and Protein Synthesis
 Name lass Date RN and Protein Synthesis Information and Heredity Q: How does information fl ow from DN to RN to direct the synthesis of proteins? 13.1 What is RN? WHT I KNOW SMPLE NSWER: RN is a nucleic
Name lass Date RN and Protein Synthesis Information and Heredity Q: How does information fl ow from DN to RN to direct the synthesis of proteins? 13.1 What is RN? WHT I KNOW SMPLE NSWER: RN is a nucleic
Appendix 2 Molecular Biology Core Curriculum. Websites and Other Resources
 Appendix 2 Molecular Biology Core Curriculum Websites and Other Resources Chapter 1 - The Molecular Basis of Cancer 1. Inside Cancer http://www.insidecancer.org/ From the Dolan DNA Learning Center Cold
Appendix 2 Molecular Biology Core Curriculum Websites and Other Resources Chapter 1 - The Molecular Basis of Cancer 1. Inside Cancer http://www.insidecancer.org/ From the Dolan DNA Learning Center Cold
Combinatorial Chemistry and solid phase synthesis seminar and laboratory course
 Combinatorial Chemistry and solid phase synthesis seminar and laboratory course Topic 1: Principles of combinatorial chemistry 1. Introduction: Why Combinatorial Chemistry? Until recently, a common drug
Combinatorial Chemistry and solid phase synthesis seminar and laboratory course Topic 1: Principles of combinatorial chemistry 1. Introduction: Why Combinatorial Chemistry? Until recently, a common drug
Illumina Sequencing Technology
 Illumina Sequencing Technology Highest data accuracy, simple workflow, and a broad range of applications. Introduction Figure 1: Illumina Flow Cell Illumina sequencing technology leverages clonal array
Illumina Sequencing Technology Highest data accuracy, simple workflow, and a broad range of applications. Introduction Figure 1: Illumina Flow Cell Illumina sequencing technology leverages clonal array
