Chapter 16 Amino Acids, Proteins, and Enzymes
|
|
|
- Everett Kelly Casey
- 10 years ago
- Views:
Transcription
1 Chapter 16 Amino Acids, Proteins, and Enzymes 1
2 Functions of Proteins Proteins in the body are polymers made from 20 different amino acids differ in characteristics and functions that depend on the order of amino acids that make up the protein perform many different functions in the body, such as provide structure, transport oxygen, direct biological reactions, control against infection, and even be a source of energy The horns of animals are made of proteins. 2
3 3 Functions of Proteins
4 Amino Acids Amino acids are the molecular building blocks of proteins contain a carboxylic acid group and an amino group on the alpha ( ) carbon are ionized in solution each contain a different side group (R) side chain R H 2 N C COOH H 4
5 Ionization of Amino Acids In most body fluids the carboxylic group ( COOH) and the amino group ( NH 2 ) are ionized. An ionized amino acid that has a positive and negative charge is a dipolar ion called a zwitterion. R R + H 2 N C COOH H 3 N C COO H H Zwitterion (ionized form) 5
6 Fischer Projections of Amino Acids Amino acids are chiral except for glycine have Fischer projections that are stereoisomers with the - carboxylate group at the top, - R group at the bottom, and - amino group on the left are the L isomers in proteins are all L isomers; no D isomers are found in proteins 6
7 Stereoisomers of Amino Acids COO COO + + H 3 N C H H 3 N C H CH 3 side chain CH 2 SH L Alanine L Cysteine 7
8 Types of Amino Acids Amino acids are classified as nonpolar (hydrophobic) with hydrocarbon side chains polar (hydrophilic) with polar or ionic side chains Nonpolar Valine Polar Asparagine 8
9 Types of Amino Acids Amino acids are classified as acidic (hydrophilic) with acidic side chains basic (hydrophilic) with NH 2 side chains Acidic Aspartic Acid Basic Histidine 9
10 Nonpolar Amino Acids An amino acid is nonpolar when the R group is H, alkyl, or aromatic. 10
11 Polar Amino Acids An amino acid is polar when the R group is an alcohol, thiol, or amide. 11
12 Acidic Amino Acids An amino acid is acidic when the R group is a carboxylic acid. 12
13 Basic Amino Acids An amino acid is basic when the R group is an amine. 13
14 Learning Check Identify each of the following amino acids as polar or nonpolar. + A. H 3 N CH 2 COO Glycine CH 3 CH OH + B. H 3 N CH COO Threonine 14
15 Essential Amino Acids Essential amino acids are 10 amino acids not synthesized by the body must be consumed from proteins in the diet 15
16 Amino Acid Deficiency in Selected Vegetables and Grains Diets that rely on plant foods for protein must contain a variety of protein sources to obtain all the essential amino acids. 16
17 Ionized Forms of Amino Acids An amino acid may have an overall neutral charge that forms only at a specific ph or isoelectric point (pi) can exist as a positive ion if the solution is more acidic than its pi can exist as a negative ion if the solution is more basic than its pi 17
18 Amino Acids as Bases In solution more acidic than physiological ph, the COO in the amino acid accepts a proton. + H + + H 3 N CH 2 COO H 3 N CH 2 COOH Zwitterion Positive ion at a at its pi ph lower than pi Total charge = 0 Total charge = 1+ 18
19 Amino Acids as Acids In solutions more basic than physiological ph, the NH 3 + in the amino acid donates a proton. + OH H 3 N CH 2 COO H 2 N CH 2 COO Zwitterion at its pi Total charge = 0 Negative ion at ph higher than its pi Total charge = 1 19
20 ph and Ionization H + OH + + H 3 N CH 2 COOH H 3 N CH 2 COO H 2 N CH 2 COO Positive ion Zwitterion Negative ion low ph pi high ph 20
21 Learning Check CH 3 CH 3 + H 3 N CH COOH H 2 N CH COO (1) (2) Which structure represents A. alanine at a ph above its pi? B. alanine at a ph below its pi? 21
22 Solution CH 3 CH 3 + H 3 N CH COOH H 2 N CH COO (1) (2) Which structure represents A. alanine at a ph above its pi? (2) B. alanine at a ph below its pi? (1) 22
23 Learning Check Consider the amino acid leucine that has a pi of 6.0. A. At a ph of 3.0 how does the zwitterion of leucine change? B. At a ph of 9.0 how does the zwitterion of leucine change? 23
24 Solution Consider the amino acid leucine with a pi of 6.0. A. At a ph of 3.0 how does the zwitterion of leucine change? Because the ph of 3.0 is more acidic than the pi at 6.0, the COO group gains an H + to give COOH. The remaining NH 3 + gives leucine an overall positive charge (1+). B. At a ph of 9.0 how does the zwitterion of leucine change? Because a ph of 9.0 is more basic and above the pi of leucine, the NH 3 + loses H + to give NH 2. The remaining gives leucine an overall negative charge (1-). 24
25 Formation of Peptides A peptide bond is an amide bond forms between the carboxyl group of one amino acid and the amino group of the next amino acid O CH 3 O + + H 3 N CH 2 C O + H 3 N CH C O O H CH 3 O + H 3 N CH 2 C N CH C O peptide bond 25
26 Formation of a Dipeptide 26
27 Learning Check Write the dipeptide Ser-Thr. 27
28 Solution Write the dipeptide Ser-Thr. 28
29 Naming Peptides A peptide is named with names of all amino acids in the peptide using a -yl ending for the name is named using the full amino acid name for the amino acid at the the C terminal N Terminal C Terminal 29
30 Learning Check Write the three-letter abbreviation and name for the following tetrapeptide. CH 3 CH 3 S CH CH 3 SH CH 2 CH 3 O H CH O H CH 2 O H CH 2 O + H 3 N CH C N CH C N CH C N CH C O 30
31 Solution Ala-Leu-Cys-Met Alanylleucylcysteinylmethionine CH 3 CH 3 S CH CH 3 SH CH 2 CH 3 O H CH O H CH 2 O H CH 2 O + H 3 N CH C N CH C N CH C N CH C O Alanine Leucine Cysteine Methionine 31
32 Primary Structure of Proteins The primary structure of a protein is the particular sequence of amino acids the backbone of a peptide chain or protein CH 3 CH 3 CH 3 CH SH CH 2 S CH 3 O CH O CH 2 O CH 2 O H 3 N CH C N CH C N CH C N CH C O - H H H Ala Leu Cys Met 32
33 Primary Structures The nonapeptides oxytocin and vasopressin have similar primary structures. Only the amino acids at positions 3 and 8 differ. 33
34 Primary Structure of Insulin Insulin was the first protein to have its primary structure determined has a primary structure of two polypeptide chains linked by disulfide bonds has a chain A with 21 amino acids and a chain B with 30 amino acids 34
35 Secondary Structure Alpha Helix The secondary structure of an alpha helix is a three-dimensional spatial arrangement of amino acids in a polypeptide chain held by H bonds between the H of N-H group and the O of C=O of the fourth amino acid down the chain a corkscrew shape that looks like a coiled telephone cord 35
36 Secondary Structure Beta-Pleated Sheet The secondary structure of a beta-pleated sheet consists of polypeptide chains arranged side by side has hydrogen bonds between chains has R groups above and below the sheet is typical of fibrous proteins, such as silk 36
37 Secondary Structure Beta-Pleated Sheet 37
38 Secondary Structure Triple Helix The secondary structure of a triple helix is three polypeptide chains woven together typical of collagen, connective tissue, skin, tendons, and cartilage Collagen fibers are triple helices of polypeptide chains held together by hydrogen bonds. 38
39 Learning Check Indicate the type of protein structure. primary alpha helix beta-pleated sheet triple helix A. polypeptide chains held side by side by H bonds B. sequence of amino acids in a polypeptide chain C. corkscrew shape with H bonds between amino acids D. three peptide chains woven like a rope 39
40 Solution A. polypeptide chains held side by side by H bonds beta-pleated sheet B. sequence of amino acids in a polypeptide chain primary C. corkscrew shape with H bonds between amino acids alpha helix D. three peptide chains woven like a rope triple helix 40
41 Tertiary Structure The tertiary structure of a protein is an overall threedimensional shape. is determined by crosslinks, the attractions and repulsions between the side chains (R groups) of the amino acids in a peptide chain 41
42 Cross-links in Tertiary Structures There are five types of cross-links in tertiary structures. 1. Hydrophobic interactions are interactions between two nonpolar R groups. Within a protein, the amino acids with nonpolar R groups move away from the aqueous environment to form a hydrophobic center at the interior of the protein molecule. 2. Hydrophilic interactions are attractions between the external aqueous environment and the R groups of polar amino acids moving the polar amino acids toward the outer surface of globular proteins where they form hydrogen bonds with water. 42
43 Cross-links in Tertiary Structures 3. Salt bridges are ionic bonds between ionized R groups of basic and acidic amino acids. For example, the ionized R group of arginine, which has a positive charge, can form a salt bridge (ionic bond) with the R group in aspartic acid, which has a negative charge. 4. Hydrogen bonds form between H of a polar R group and the O or N of another amino acid. For example, a hydrogen bond can form between the groups of two serines or between the of serine and the in the R group of glutamine. 5. Disulfide bonds are covalent bonds that form between the groups of cysteines in a polypeptide chain. 43
44 Learning Check Select the type of tertiary interaction. disulfide ionic H bonds hydrophobic A. leucine and valine B. two cysteines C. aspartic acid and lysine D. serine and threonine 44
45 Solution Select the type of tertiary interaction. disulfide ionic H bonds hydrophobic A. leucine and valine hydrophobic B. two cysteines disulfide C. aspartic acid and lysine ionic D. serine and threonine H bonds 45
46 Globular Proteins Globular proteins have compact, spherical shapes carry out synthesis, transport, and metabolism in the cells such as myoglobin store and transport oxygen in muscle The ribbon structure represents the tertiary structure of myoglobin. Myoglobin 46
47 Fibrous Proteins Fibrous proteins consist of long, fiber-like shapes such as alpha keratins make up hair, wool, skin, and nails such as feathers contain beta keratins with large amounts of beta-pleated sheet structures 47 The fibrous proteins of -keratin wrap together to for fibrils of hair and wool.
48 Quaternary Structure The quaternary structure is the combination of two or more protein units of hemoglobin consists of four polypeptide chains as subunits is stabilized by the same interactions found in tertiary structures In the ribbon structure of hemoglobin, the quaternary structure is made up of four polypeptide subunits, two (red) are chains and two (blue) are chains. The heme groups (green) in the four subunits bind oxygen. 48
49 Summary of Protein Structural Levels 49
50 50 Summary of Protein Structure
51 Learning Check Identify the level of protein structure. primary secondary tertiary quaternary A. beta-pleated sheet B. order of amino acids in a protein C. a protein with two or more peptide chains D. the shape of a globular protein E. disulfide bonds between R groups 51
52 Solution Identify the level of protein structure. primary secondary tertiary quaternary A. beta-pleated sheet secondary B. order of amino acids in a protein primary C. a protein with two or more peptide chains quaternary D. the shape of a globular protein tertiary E. disulfide bonds between R groups tertiary 52
53 Denaturation Denaturation involves the disruption of bonds in the secondary, tertiary, and quaternary protein structures heat and organic compounds that break apart H bonds and disrupt hydrophobic interactions acids and bases that break H bonds between polar R groups and disrupt ionic bonds heavy metal ions that react with S-S bonds to form solids agitation such as whipping that stretches peptide chains until bonds break 53
54 Applications of Denaturation Denaturation of protein occurs when an egg is cooked the skin is wiped with alcohol heat is used to cauterize blood vessels instruments are sterilized in autoclaves Denaturation of egg protein occurs when the bonds of the tertiary structure are disrupted. 54
55 55 Applications of Denaturation
56 Enzymes Are Biological Catalysts Enzymes are proteins that catalyze nearly all the chemical reactions taking place in the cells of the body increase the rate of reaction by lowering the energy of activation The enzyme carbonic anhydrase lowers the activation energy needed for the reaction of CO2 and H2O. 56
57 Names of Enzymes The name of an enzyme usually ends in ase identifies the reacting substance; for example, sucrase catalyzes the reaction of sucrose describes the function of the enzyme; for example, oxidases catalyze oxidation could be a common name, particularly for the digestion enzymes, such as pepsin and trypsin 57
58 Classification of Enzymes Enzymes are classified by the reaction they catalyze. Class Oxidoreductases Transferases Hydrolases Lyases Isomerases Ligases Type of Reactions Catalyzed Oxidation-reduction Transfer groups of atoms Hydrolysis Add atoms/remove atoms to or from a double bond Rearrange atoms Use ATP to combine small molecules 58
59 Learning Check Match the type of reaction with an enzyme. aminase dehydrogenase isomerase synthetase A. converts a cis-fatty acid to a trans-fatty acid B. removes 2 H atoms to form double bond C. combines two molecules to make a new compound D. adds NH 3 59
60 Solution Match the type of reaction with an enzyme. aminase isomerase dehydrogenase synthetase A. converts a cis-fatty acid to a trans-fatty acid isomerase B. removes 2 H atoms to form double bond dehydrogenase C. combines two molecules to make a new compound synthetase D. adds NH 3 aminase 60
61 Active Site The active site is a region within an enzyme that fits the shape of the reacting molecule called a substrate contains amino acid R groups that bind the substrate releases products when the reaction is complete On the surface of an enzyme, a small region called an active site binds a substrate and catalyzes a reaction of that substrate. 61
62 Enzyme-Catalyzed Reaction In an enzyme-catalyzed reaction, a substrate attaches to the active site an enzyme substrate (ES) complex forms reaction occurs and products are released an enzyme is used over and over E + S ES E + P Binding of a substrate occurs when it interacts with the amino acids within the active site. 62
63 Lock-and-Key Model In the lock-and-key model, the active site has a rigid shape enzyme only binds substrates that exactly fit the active site enzyme is analogous to a lock substrate is the key that fits that lock In the lock-and-key model, a substrate fits the shape of the active site and forms an enzyme substrate complex. 63
64 Induced-Fit Model In the induced-fit model, enzyme structure is flexible, not rigid enzyme and substrate adjust the shape of the active site to bind substrate the range of substrate specificity increases shape changes improve catalysis during reaction In the induced-fit model, a flexible active site and substrate adjust shape to provide the best fit for the reaction. 64
65 Example of an Enzyme-Catalyzed Reaction At the active site, sucrose is aligned for the hydrolysis reaction. The monosaccharides produced dissociate from the active site, and the enzyme is ready to bind to another sucrose molecule. 65
66 Learning Check 1. The active site is A. the enzyme B. a section of the enzyme C. the substrate 2. In the induced-fit model, the shape of the enzyme when substrate binds A. stays the same B. adapts to the shape of the substrate 66
67 Solution 1. The active site is B. a section of the enzyme 2. In the induced-fit model, the shape of the enzyme when substrate binds B. adapts to the shape of the substrate 67
68 Diagnostic Enzymes Diagnostic enzymes determine the amount of damage in tissues that are elevated may indicate damage or disease in a particular organ 68
69 Isoenzymes Isoenzymes catalyze the same reaction in different tissues in the body can be used to identify the organ or tissue involved in damage or disease such as lactate dehydrogenase (LDH), which converts lactate to pyruvate, consist of five isoenzymes such as LDH have one form more prevalent in heart muscle and another form in skeletal muscle and liver 69
70 Isoenzymes The different isoenzymes of lactate dehydrogenase (LDH) indicate damage to different organs in the body. 70
71 Temperature and Enzyme Action Enzymes are most active at an optimum temperature (usually 37 C in humans) show little activity at low temperatures. lose activity at high temperatures as denaturation occurs with loss of catalytic activity 71
72 ph and Enzyme Action Enzymes are most active at optimum ph contain R groups of amino acids with proper charges at optimum ph lose activity in low or high ph as tertiary structure is disrupted 72
73 Optimum ph Values Enzymes in the body have an optimum ph of about 7.4 certain organs operate at lower and higher optimum ph values 73
74 Substrate Concentration As substrate concentration increases, the rate of reaction increases (at constant enzyme concentration) the enzyme eventually becomes saturated, giving maximum activity 74
75 Learning Check Sucrase has an optimum temperature of 37 C and an optimum ph of 6.2. Determine the effect of the following on its rate of reaction. no change increase decrease A. Increasing the concentration of sucrase B. Changing the ph to 4 C. Running the reaction at 70 C 75
76 Solution Sucrase has an optimum temperature of 37 C and an optimum ph of 6.2. Determine the effect of the following on its rate of reaction. no change increase decrease A. Increasing the concentration of sucrase increase B. Changing the ph to 4 decrease C. Running the reaction at 70 C decrease 76
77 Enzyme Inhibition Inhibitors are molecules that cause a loss of catalytic activity prevent substrates from fitting into the active sites E + S ES E + P E + I EI no P 77
78 Competitive Inhibition A competitive inhibitor has a structure that is similar to that of the substrate competes with the substrate for the active site has its effect reversed by increasing substrate concentration 78
79 Noncompetitive Inhibition A noncompetitive inhibitor has a structure that is much different than the substrate distorts the shape of the enzyme, which alters the shape of the active site prevents the binding of the substrate cannot have its effect reversed by adding more substrate 79
80 Irreversible Inhibition An irreversible inhibitor has a molecule that causes the enzyme to lose all activity is often a toxic substance that destroys enzymes usually forms a covalent bond with an amino acid side chain preventing catalytic activity may be nerve gases or insecticides or antibiotics 80
81 Learning Check Identify each description as an inhibitor that is competitive or noncompetitive. A. Increasing substrate reverses inhibition. B. Binds to enzyme surface, but not to the active site. C. Structure is similar to substrate. D. Inhibition is not reversed by adding more substrate. 81
82 Solution A. Increasing substrate reverses inhibition. competitive B. Binds to enzyme surface, but not to the active site. noncompetitive C. Structure is similar to substrate. competitive D. Inhibition is not reversed by adding more substrate. noncompetitive 82
83 Function of Coenzymes Cofactors assist enzymes in catalytic activity may be metal ions that are bonded to one of the amino acid side chains or small organic molecules known as coenzymes 83
84 84 Metal Ions as Coenzymes
85 Water-Soluble Vitamins Water-soluble vitamins are essential for normal health and growth are soluble in aqueous solutions due to the presence of OH and COOH groups act as cofactors for many enzymes are not stored in the body and are easily destroyed by heat, oxygen, and ultraviolet light 85
86 86 Water-Soluble Vitamins
87 Ascorbic Acid (Vitamin C) Vitamin C is required in collagen synthesis deficiency can lead to weakened connective tissue, slow-healing wounds, and anemia is found in blueberries, citrus fruits, tomatoes, broccoli, and red and green vegetables 87
88 Fat-Soluble Vitamins Fat-soluble vitamins, including A, D, E, and K, are soluble in lipids, but not in aqueous solutions important in vision, bone formation, antioxidants, and blood clotting stored in the body 88
89 Vitamin A The orange pigment in carrots is used to help synthesize vitamin A in the body. Vitamin A is a fat-soluble vitamin, stored in the body and not eliminated. 89
90 Learning Check Identify the vitamin associated with each: Thiamin (B 1 ) Vitamin A Vitamin K Vitamin D Ascorbic acid A. collagen formation B. absorption of phosphorus and calcium in bone C. vision D. blood clotting 90
91 Solution Identify the vitamin associated with each: Thiamin (B 1 ) Vitamin A Vitamin K Vitamin D Ascorbic acid A. collagen formation Ascorbic acid B. absorption of phosphorus and calcium in bone Vitamin D C. vision Vitamin A D. blood clotting Vitamin K 91
92 92 Amino Acids, Proteins, and Enzymes Concept Map
Amino Acids, Proteins, and Enzymes. Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation
 Amino Acids, Proteins, and Enzymes Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation 1 Primary Structure of Proteins H 3 N The particular sequence of
Amino Acids, Proteins, and Enzymes Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation 1 Primary Structure of Proteins H 3 N The particular sequence of
Exam 4 Outline CH 105 Spring 2012
 Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
18.2 Protein Structure and Function: An Overview
 18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Built from 20 kinds of amino acids
 Built from 20 kinds of amino acids Each Protein has a three dimensional structure. Majority of proteins are compact. Highly convoluted molecules. Proteins are folded polypeptides. There are four levels
Built from 20 kinds of amino acids Each Protein has a three dimensional structure. Majority of proteins are compact. Highly convoluted molecules. Proteins are folded polypeptides. There are four levels
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon. V. Polypeptides and Proteins
 IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
Chemistry: Introduction to General, Organic & Biological Chemistry (Timberlake) Chapter 16: Amino Acids, Proteins, and Enzymes
 Chemistry: Introduction to General, Organic & Biological Chemistry (Timberlake) Chapter 16: Amino Acids, Proteins, and Enzymes MULTIPLE CHOICE 1) Which of the following is NOT a function of proteins? A)
Chemistry: Introduction to General, Organic & Biological Chemistry (Timberlake) Chapter 16: Amino Acids, Proteins, and Enzymes MULTIPLE CHOICE 1) Which of the following is NOT a function of proteins? A)
http://faculty.sau.edu.sa/h.alshehri
 http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Chapter 12 - Proteins
 Roles of Biomolecules Carbohydrates Lipids Proteins 1) Catalytic 2) Transport 3) Regulatory 4) Structural 5) Contractile 6) Protective 7) Storage Nucleic Acids 12.1 -Amino Acids Chapter 12 - Proteins Amino
Roles of Biomolecules Carbohydrates Lipids Proteins 1) Catalytic 2) Transport 3) Regulatory 4) Structural 5) Contractile 6) Protective 7) Storage Nucleic Acids 12.1 -Amino Acids Chapter 12 - Proteins Amino
Amino Acids, Peptides, Proteins
 Amino Acids, Peptides, Proteins Functions of proteins: Enzymes Transport and Storage Motion, muscle contraction Hormones Mechanical support Immune protection (Antibodies) Generate and transmit nerve impulses
Amino Acids, Peptides, Proteins Functions of proteins: Enzymes Transport and Storage Motion, muscle contraction Hormones Mechanical support Immune protection (Antibodies) Generate and transmit nerve impulses
Amino Acids and Proteins
 Amino Acids and Proteins Proteins are composed of amino acids. There are 20 amino acids commonly found in proteins. All have: N2 C α R COO Amino acids at neutral p are dipolar ions (zwitterions) because
Amino Acids and Proteins Proteins are composed of amino acids. There are 20 amino acids commonly found in proteins. All have: N2 C α R COO Amino acids at neutral p are dipolar ions (zwitterions) because
A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys
 Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
The Organic Chemistry of Amino Acids, Peptides, and Proteins
 Essential rganic Chemistry Chapter 16 The rganic Chemistry of Amino Acids, Peptides, and Proteins Amino Acids a-amino carboxylic acids. The building blocks from which proteins are made. H 2 N C 2 H Note:
Essential rganic Chemistry Chapter 16 The rganic Chemistry of Amino Acids, Peptides, and Proteins Amino Acids a-amino carboxylic acids. The building blocks from which proteins are made. H 2 N C 2 H Note:
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK
 Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. [email protected] Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. [email protected] Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
Pipe Cleaner Proteins. Essential question: How does the structure of proteins relate to their function in the cell?
 Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
H H N - C - C 2 R. Three possible forms (not counting R group) depending on ph
 Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?)
 ChemActivity 46 Amino Acids, Polypeptides and Proteins 1 ChemActivity 46 Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?) Model 1: The 20 Amino Acids at Biological p See
ChemActivity 46 Amino Acids, Polypeptides and Proteins 1 ChemActivity 46 Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?) Model 1: The 20 Amino Acids at Biological p See
8/20/2012 H C OH H R. Proteins
 Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Biological Molecules
 Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Structure of proteins
 Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
I N V E S T I C E D O R O Z V O J E V Z D Ě L Á V Á N Í
 I V E S T I E D Z V J E V Z D Ě L Á V Á Í AMIAIDS PEPTIDES AMIAIDS = substitutional/functional derivatives of carboxylic acids = basic units of proteins (2-aminoacids) General formula of 2-aminoacids (α-aminoacids):
I V E S T I E D Z V J E V Z D Ě L Á V Á Í AMIAIDS PEPTIDES AMIAIDS = substitutional/functional derivatives of carboxylic acids = basic units of proteins (2-aminoacids) General formula of 2-aminoacids (α-aminoacids):
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Shu-Ping Lin, Ph.D. E-mail: [email protected]
 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute te of Biomedical Engineering ing E-mail: [email protected] Website: http://web.nchu.edu.tw/pweb/users/splin/ edu tw/pweb/users/splin/ Date: 10.13.2010
Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute te of Biomedical Engineering ing E-mail: [email protected] Website: http://web.nchu.edu.tw/pweb/users/splin/ edu tw/pweb/users/splin/ Date: 10.13.2010
Recap. Lecture 2. Protein conformation. Proteins. 8 types of protein function 10/21/10. Proteins.. > 50% dry weight of a cell
 Lecture 2 Protein conformation ecap Proteins.. > 50% dry weight of a cell ell s building blocks and molecular tools. More important than genes A large variety of functions http://www.tcd.ie/biochemistry/courses/jf_lectures.php
Lecture 2 Protein conformation ecap Proteins.. > 50% dry weight of a cell ell s building blocks and molecular tools. More important than genes A large variety of functions http://www.tcd.ie/biochemistry/courses/jf_lectures.php
Structure and properties of proteins. Vladimíra Kvasnicová
 Structure and properties of proteins Vladimíra Kvasnicová Chemical nature of proteins biopolymers of amino acids macromolecules (M r > 10 000) Classification of proteins 1) by localization in an organism
Structure and properties of proteins Vladimíra Kvasnicová Chemical nature of proteins biopolymers of amino acids macromolecules (M r > 10 000) Classification of proteins 1) by localization in an organism
Enzymes. Enzyme Structure. Enzyme Classification. CHEM464/Medh, J.D. Reaction Rate and Enzyme Activity
 Enzymes Enzymes are biological catalysts They are not consumed or altered during the reaction They do not change the equilibrium, just reduce the time required to reach equilibrium. They increase the rate
Enzymes Enzymes are biological catalysts They are not consumed or altered during the reaction They do not change the equilibrium, just reduce the time required to reach equilibrium. They increase the rate
CHAPTER 29 AMINO ACIDS, POLYPEPTIDES, AND PROTEINS SOLUTIONS TO REVIEW QUESTIONS
 APTER 29 AMI AIDS, PLYPEPTIDES, AD PRTEIS SLUTIS T REVIEW QUESTIS 1. The designation, α, means that the amine group in common amino acids is connected to the carbon immediately adjacent to the carboxylic
APTER 29 AMI AIDS, PLYPEPTIDES, AD PRTEIS SLUTIS T REVIEW QUESTIS 1. The designation, α, means that the amine group in common amino acids is connected to the carbon immediately adjacent to the carboxylic
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7. 4. Which of the following weak acids would make the best buffer at ph = 5.0?
 Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7 4. Which of the following weak acids would make the best buffer at ph = 5.0? A) Acetic acid (Ka = 1.74 x 10-5 ) B) H 2 PO - 4 (Ka =
Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7 4. Which of the following weak acids would make the best buffer at ph = 5.0? A) Acetic acid (Ka = 1.74 x 10-5 ) B) H 2 PO - 4 (Ka =
Helices From Readily in Biological Structures
 The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS
 UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS 11.1 Types of Lipids Lipids are also biochemical compounds that contain carbon, hydrogen, and oxygen. But lipids, unlike carbohydrates, share no common
UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS 11.1 Types of Lipids Lipids are also biochemical compounds that contain carbon, hydrogen, and oxygen. But lipids, unlike carbohydrates, share no common
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl
How To Understand The Chemistry Of An Enzyme
 Chapt. 8 Enzymes as catalysts Ch. 8 Enzymes as catalysts Student Learning Outcomes: Explain general features of enzymes as catalysts: Substrate -> Product Describe nature of catalytic sites general mechanisms
Chapt. 8 Enzymes as catalysts Ch. 8 Enzymes as catalysts Student Learning Outcomes: Explain general features of enzymes as catalysts: Substrate -> Product Describe nature of catalytic sites general mechanisms
CHAPTER 6 AN INTRODUCTION TO METABOLISM. Section B: Enzymes
 CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
AP BIOLOGY 2008 SCORING GUIDELINES
 AP BIOLOGY 2008 SCORING GUIDELINES Question 1 1. The physical structure of a protein often reflects and affects its function. (a) Describe THREE types of chemical bonds/interactions found in proteins.
AP BIOLOGY 2008 SCORING GUIDELINES Question 1 1. The physical structure of a protein often reflects and affects its function. (a) Describe THREE types of chemical bonds/interactions found in proteins.
INTRODUCTION TO PROTEIN STRUCTURE
 Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
CHAPTER 4: Enzyme Structure ENZYMES
 CHAPTER 4: ENZYMES Enzymes are biological catalysts. There are about 40,000 different enzymes in human cells, each controlling a different chemical reaction. They increase the rate of reactions by a factor
CHAPTER 4: ENZYMES Enzymes are biological catalysts. There are about 40,000 different enzymes in human cells, each controlling a different chemical reaction. They increase the rate of reactions by a factor
Nafith Abu Tarboush DDS, MSc, PhD [email protected] www.facebook.com/natarboush
 Nafith Abu Tarboush DDS, MSc, PhD [email protected] www.facebook.com/natarboush α-keratins, bundles of α- helices Contain polypeptide chains organized approximately parallel along a single axis: Consist
Nafith Abu Tarboush DDS, MSc, PhD [email protected] www.facebook.com/natarboush α-keratins, bundles of α- helices Contain polypeptide chains organized approximately parallel along a single axis: Consist
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
MCAT Organic Chemistry - Problem Drill 23: Amino Acids, Peptides and Proteins
 MCAT rganic Chemistry - Problem Drill 23: Amino Acids, Peptides and Proteins Question No. 1 of 10 Question 1. Which amino acid does not contain a chiral center? Question #01 (A) Serine (B) Proline (C)
MCAT rganic Chemistry - Problem Drill 23: Amino Acids, Peptides and Proteins Question No. 1 of 10 Question 1. Which amino acid does not contain a chiral center? Question #01 (A) Serine (B) Proline (C)
Proteins. Proteins. Amino Acids. Most diverse and most important molecule in. Functions: Functions (cont d)
 Proteins Proteins Most diverse and most important molecule in living i organisms Functions: 1. Structural (keratin in hair, collagen in ligaments) 2. Storage (casein in mother s milk) 3. Transport (HAEMOGLOBIN!)
Proteins Proteins Most diverse and most important molecule in living i organisms Functions: 1. Structural (keratin in hair, collagen in ligaments) 2. Storage (casein in mother s milk) 3. Transport (HAEMOGLOBIN!)
Protein Physics. A. V. Finkelstein & O. B. Ptitsyn LECTURE 1
 Protein Physics A. V. Finkelstein & O. B. Ptitsyn LECTURE 1 PROTEINS Functions in a Cell MOLECULAR MACHINES BUILDING BLOCKS of a CELL ARMS of a CELL ENZYMES - enzymatic catalysis of biochemical reactions
Protein Physics A. V. Finkelstein & O. B. Ptitsyn LECTURE 1 PROTEINS Functions in a Cell MOLECULAR MACHINES BUILDING BLOCKS of a CELL ARMS of a CELL ENZYMES - enzymatic catalysis of biochemical reactions
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Invariant residue-a residue that is always conserved. It is assumed that these residues are essential to the structure or function of the protein.
 Chapter 6 The amino acid side chains have polar and nonpolar properties, and the relative hydrophobicity of the amino acid side chains is critical for the folding and stability of a protein. The more hydrophobic
Chapter 6 The amino acid side chains have polar and nonpolar properties, and the relative hydrophobicity of the amino acid side chains is critical for the folding and stability of a protein. The more hydrophobic
Energy & Enzymes. Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy.
 Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Myoglobin and Hemoglobin
 Myoglobin and Hemoglobin Myoglobin and hemoglobin are hemeproteins whose physiological importance is principally related to their ability to bind molecular oxygen. Myoglobin (Mb) The oxygen storage protein
Myoglobin and Hemoglobin Myoglobin and hemoglobin are hemeproteins whose physiological importance is principally related to their ability to bind molecular oxygen. Myoglobin (Mb) The oxygen storage protein
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Introduction to General, Organic and Biochemistry Chapter 22 Proteins
 hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 22 Proteins Step-growth polyamide (polypeptide) polymers or oligomers of L-α-aminoacids.
hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 22 Proteins Step-growth polyamide (polypeptide) polymers or oligomers of L-α-aminoacids.
Structures of Proteins. Primary structure - amino acid sequence
 Structures of Proteins Primary structure - amino acid sequence Secondary structure chain of covalently linked amino acids folds into regularly repeating structures. Secondary structure is the result of
Structures of Proteins Primary structure - amino acid sequence Secondary structure chain of covalently linked amino acids folds into regularly repeating structures. Secondary structure is the result of
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Two Forms of Energy
 Module 2D - Energy and Metabolism Objective # 19 All living organisms require energy for survival. In this module we will examine some general principles about chemical reactions and energy usage within
Module 2D - Energy and Metabolism Objective # 19 All living organisms require energy for survival. In this module we will examine some general principles about chemical reactions and energy usage within
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
2007 7.013 Problem Set 1 KEY
 2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Introduction to Proteins and Enzymes
 Introduction to Proteins and Enzymes Basics of protein structure and composition The life of a protein Enzymes Theory of enzyme function Not all enzymes are proteins / not all proteins are enzymes Enzyme
Introduction to Proteins and Enzymes Basics of protein structure and composition The life of a protein Enzymes Theory of enzyme function Not all enzymes are proteins / not all proteins are enzymes Enzyme
Amino Acids as Acids, Bases and Buffers:
 Amino Acids as Acids, Bases and Buffers: - Amino acids are weak acids - All have at least 2 titratable protons (shown below as fully protonated species) and therefore have 2 pka s o α-carboxyl (-COOH)
Amino Acids as Acids, Bases and Buffers: - Amino acids are weak acids - All have at least 2 titratable protons (shown below as fully protonated species) and therefore have 2 pka s o α-carboxyl (-COOH)
Figure 5. Energy of activation with and without an enzyme.
 Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Anabolic and Catabolic Reactions are Linked by ATP in Living Organisms
 Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Lecture 13-14 Conformation of proteins Conformation of a protein three-dimensional structure native state. native condition
 Lecture 13-14 Conformation of proteins Conformation of a protein refers to the three-dimensional structure in its native state. There are many different possible conformations for a molecule as large as
Lecture 13-14 Conformation of proteins Conformation of a protein refers to the three-dimensional structure in its native state. There are many different possible conformations for a molecule as large as
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM
 AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
Copyright 2000-2003 Mark Brandt, Ph.D. 54
 Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Proteins the primary biological macromolecules of living organisms
 Proteins the primary biological macromolecules of living organisms Protein structure and folding Primary Secondary Tertiary Quaternary structure of proteins Structure of Proteins Protein molecules adopt
Proteins the primary biological macromolecules of living organisms Protein structure and folding Primary Secondary Tertiary Quaternary structure of proteins Structure of Proteins Protein molecules adopt
Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain.
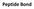 Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Enzymes and Metabolic Pathways
 Enzymes and Metabolic Pathways Enzyme characteristics Made of protein Catalysts: reactions occur 1,000,000 times faster with enzymes Not part of reaction Not changed or affected by reaction Used over and
Enzymes and Metabolic Pathways Enzyme characteristics Made of protein Catalysts: reactions occur 1,000,000 times faster with enzymes Not part of reaction Not changed or affected by reaction Used over and
Computational Systems Biology. Lecture 2: Enzymes
 Computational Systems Biology Lecture 2: Enzymes 1 Images from: David L. Nelson, Lehninger Principles of Biochemistry, IV Edition, Freeman ed. or under creative commons license (search for images at http://search.creativecommons.org/)
Computational Systems Biology Lecture 2: Enzymes 1 Images from: David L. Nelson, Lehninger Principles of Biochemistry, IV Edition, Freeman ed. or under creative commons license (search for images at http://search.creativecommons.org/)
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
Amino Acids. Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain. Alpha Carbon. Carboxyl. Group.
 Protein Structure Amino Acids Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain Alpha Carbon Amino Group Carboxyl Group Amino Acid Properties There are
Protein Structure Amino Acids Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain Alpha Carbon Amino Group Carboxyl Group Amino Acid Properties There are
Disulfide Bonds at the Hair Salon
 Disulfide Bonds at the Hair Salon Three Alpha Helices Stabilized By Disulfide Bonds! In order for hair to grow 6 inches in one year, 9 1/2 turns of α helix must be produced every second!!! In some proteins,
Disulfide Bonds at the Hair Salon Three Alpha Helices Stabilized By Disulfide Bonds! In order for hair to grow 6 inches in one year, 9 1/2 turns of α helix must be produced every second!!! In some proteins,
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
NO CALCULATORS OR CELL PHONES ALLOWED
 Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
In addition to being shorter than a single bond, the double bonds in ethylene don t twist the way single bonds do. In other words, the other atoms
 In addition to being shorter than a single bond, the double bonds in ethylene don t twist the way single bonds do. In other words, the other atoms attached to the carbons (hydrogens in this case) can no
In addition to being shorter than a single bond, the double bonds in ethylene don t twist the way single bonds do. In other words, the other atoms attached to the carbons (hydrogens in this case) can no
CSC 2427: Algorithms for Molecular Biology Spring 2006. Lecture 16 March 10
 CSC 2427: Algorithms for Molecular Biology Spring 2006 Lecture 16 March 10 Lecturer: Michael Brudno Scribe: Jim Huang 16.1 Overview of proteins Proteins are long chains of amino acids (AA) which are produced
CSC 2427: Algorithms for Molecular Biology Spring 2006 Lecture 16 March 10 Lecturer: Michael Brudno Scribe: Jim Huang 16.1 Overview of proteins Proteins are long chains of amino acids (AA) which are produced
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d.
 1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
I N V E S T I C E D O R O Z V O J E V Z D Ě L Á V Á N Í ENZYMES
 = substances that... biological reactions 1. Provide an alternative reaction route which has a lower... energy 2. Reactions catalysed by enzymes occur under mild conditions + good yield + fast 3. Enzymes
= substances that... biological reactions 1. Provide an alternative reaction route which has a lower... energy 2. Reactions catalysed by enzymes occur under mild conditions + good yield + fast 3. Enzymes
Organic Functional Groups Chapter 7. Alcohols, Ethers and More
 Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
Lecture 4 Enzymes Catalytic proteins. Enzymes. Enzymes 10/21/10. What enzymes do therefore is:
 Lecture 4 Catalytic proteins Are a type of protein that acts as a catalyst-speeding up chemical reactions A catalyst is defined as a chemical agent that changes the rate of a reaction without being consumed
Lecture 4 Catalytic proteins Are a type of protein that acts as a catalyst-speeding up chemical reactions A catalyst is defined as a chemical agent that changes the rate of a reaction without being consumed
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Enzymes. Enzymes are characterized by: Specificity - highly specific for substrates
 Enzymes Enzymes are characterized by: Catalytic Power - rates are 10 6-10 12 greater than corresponding uncatalyzed reactions Specificity - highly specific for substrates Regulation - acheived in many
Enzymes Enzymes are characterized by: Catalytic Power - rates are 10 6-10 12 greater than corresponding uncatalyzed reactions Specificity - highly specific for substrates Regulation - acheived in many
Previous lecture: Today:
 Previous lecture: The energy requiring step from substrate to transition state is an energy barrier called the free energy of activation G Transition state is the unstable (10-13 seconds) highest energy
Previous lecture: The energy requiring step from substrate to transition state is an energy barrier called the free energy of activation G Transition state is the unstable (10-13 seconds) highest energy
