Structure of Metals 110
|
|
|
- Augustine Newton
- 9 years ago
- Views:
Transcription
1 Structure of Metals 110 Welcome to the Tooling University. This course is designed to be used in conjunction with the online version of this class. The online version can be found at We offer high quality web -based e -learning that focuses on today's industrial manufacturing training needs. We deliver superior training content over the Internet using text, photos, video, audio, and illustrations. Our courses contain "roll -up -your -sleeves" content that offers real -world solutions on subjects such as Metal Cutting, Workholding, Materials, and CNC with much more to follow. Today's businesses face the challenge of maintaining a trained workforce. Companies must locate apprenticeship programs, cover travel and lodging expenses, and disrupt operations to cover training needs. Our web -based training offers low -cost, all -access courses and services to maximize your training initiatives. Class Outline
2 Class Outline Objectives The Importance of Structure The Atom Valence Electrons The Periodic Table How Elements Interact Ionic and Covalent Bonds Metallic Bonds Molecular Bonding Crystal Formation in Metals Types of Crystal Structures Effects of Crystal Structure Crystal Growth Grains and Strength Summary Lesson:1/15 Objectives l Describe the importance of metal properties. l Identify the parts of the atom. l Describe a valence electron. l Describe the usefulness of the Periodic Table. l Explain why elements react with one another. l Distinguish between an ionic and covalent bond. l Define metallic bond. l Distinguish between primary and secondary bonds. l Describe how crystals form in metals. l Describe the different types of crystal structures. l Describe the significance of crystal structure. l Explain the beginning and end of crystal growth in metals. l Identify the impact of grain size. Figure 1. An illustration of a hydrogen atom. Figure 2. A representation of grains forming in a metal.
3 Lesson:1/15 Objectives l Describe the importance of metal properties. l Identify the parts of the atom. l Describe a valence electron. l Describe the usefulness of the Periodic Table. l Explain why elements react with one another. l Distinguish between an ionic and covalent bond. l Define metallic bond. l Distinguish between primary and secondary bonds. l Describe how crystals form in metals. l Describe the different types of crystal structures. l Describe the significance of crystal structure. l Explain the beginning and end of crystal growth in metals. l Identify the impact of grain size. Figure 1. An illustration of a hydrogen atom. Figure 2. A representation of grains forming in a metal. Lesson:2/15 The Importance of Structure The study of metals requires an understanding of their internal structure. In manufacturing, materials do not stay the same. They are transformed through chemical processes or physical processes. These processes can change the strength, hardness, and ductility of a metal. Plus, when you combine materials, the result is often a new material that shares some of the properties of the original materials. The structure of any material consists of the arrangement of its inner components. Structure can be studied at several levels: from the smallest particles that make up an atom illustrated in Figure 1, to structures that are visible to the human eye. The structure of a metal usually refers to the arrangement of atoms within the metal. Figure 2 illustrates a sample arrangement of atoms. The structure of metals affects your ability to control the behavior of a metal during the manufacturing Knowing metal's structure can help you pick the best metal for a Copyright 2015process. Tooling U, LLC. AllaRights Reserved. particular application under a certain pressure, temperature, or other condition.
4 Lesson:2/15 The Importance of Structure The study of metals requires an understanding of their internal structure. In manufacturing, materials do not stay the same. They are transformed through chemical processes or physical processes. These processes can change the strength, hardness, and ductility of a metal. Plus, when you combine materials, the result is often a new material that shares some of the properties of the original materials. The structure of any material consists of the arrangement of its inner components. Structure can be studied at several levels: from the smallest particles that make up an atom illustrated in Figure 1, to structures that are visible to the human eye. The structure of a metal usually refers to the arrangement of atoms within the metal. Figure 2 illustrates a sample arrangement of atoms. The structure of metals affects your ability to control the behavior of a metal during the manufacturing process. Knowing a metal's structure can help you pick the best metal for a particular application under a certain pressure, temperature, or other condition. Figure 1. An atom consists of incredibly small particles. Figure 2. Atoms are arranged in repeating patterns within a metal. Lesson:3/15 The Atom All materials are made up of atoms, which are the basic units of matter. The structure of an atom consists of a nucleus surrounded by electrons. As you can see in Figure 1, the nucleus is located in the center, and the electrons revolve around it. The path that an electron orbits around the nucleus is called a shell. Figure 2 shows an atom with three shells. The nucleus is the biggest part of the atom, and it is made up of positively charged protons and Copyright neutrons ToolingThe U, LLC. All Rights Reserved. uncharged number of protons in the nucleus determines the type of atom. For example, every oxygen atom contains eight protons. The electrons have a very small mass, nearly 2000 times smaller than the proton or neutron. The negative charge of electrons balances the
5 Lesson:3/15 The Atom All materials are made up of atoms, which are the basic units of matter. The structure of an atom consists of a nucleus surrounded by electrons. As you can see in Figure 1, the nucleus is located in the center, and the electrons revolve around it. The path that an electron orbits around the nucleus is called a shell. Figure 2 shows an atom with three shells. The nucleus is the biggest part of the atom, and it is made up of positively charged protons and uncharged neutrons. The number of protons in the nucleus determines the type of atom. For example, every oxygen atom contains eight protons. The electrons have a very small mass, nearly 2000 times smaller than the proton or neutron. The negative charge of electrons balances the positive charge of the protons. Normally, an atom contains the same number of electrons and protons to maintain this balanced charge. Figure 1. The nucleus is located in the center, and electrons orbit around the nucleus. Figure 2. An atom with three shells. Lesson:4/15 Valence Electrons Most atoms contain more than one shell. Like any sphere, the area of the outermost shell is larger than any shell closer to the nucleus. Therefore, the further you get from the center, the more room you have to put electrons in a layer. Every shell has a maximum number of electrons that it can contain. Figure 1 illustrates the shells of a sodium atom and shows how they are filled by electrons. The electrons that are located in the outer shell of an atom are called valence electrons. If an atom s outer shell is only partially filled, its valence electrons are easily borrowed or shared during reactions with other atoms. These electrons determine the many physical and chemical properties of materials.
6 Lesson:4/15 Valence Electrons Most atoms contain more than one shell. Like any sphere, the area of the outermost shell is larger than any shell closer to the nucleus. Therefore, the further you get from the center, the more room you have to put electrons in a layer. Every shell has a maximum number of electrons that it can contain. Figure 1 illustrates the shells of a sodium atom and shows how they are filled by electrons. The electrons that are located in the outer shell of an atom are called valence electrons. If an atom s outer shell is only partially filled, its valence electrons are easily borrowed or shared during reactions with other atoms. These electrons determine the many physical and chemical properties of materials. Figure 1. A sodium atom has one valence electron. Figure 2. A carbon atom has six valence electrons. Lesson:5/15 The Periodic Table There are just over 100 elements. As you can seen in Figure 1, these elements are arranged in a periodic table according to the number of protons they contain, from lowest to highest. The hydrogen atom in Figure 2 is at the top-left of the table because it contains only one proton. This arrangement creates seven horizontal rows called periods. The vertical columns, or groups of elements, have a similar number of electrons in the outer shell. This means they respond similarly to the same chemical and physical conditions. A working knowledge of the periodic table has its practical benefits. For instance, the arrangement of groups shows which element may be used to replace another element when making a metal
7 Lesson:5/15 The Periodic Table There are just over 100 elements. As you can seen in Figure 1, these elements are arranged in a periodic table according to the number of protons they contain, from lowest to highest. The hydrogen atom in Figure 2 is at the top-left of the table because it contains only one proton. This arrangement creates seven horizontal rows called periods. The vertical columns, or groups of elements, have a similar number of electrons in the outer shell. This means they respond similarly to the same chemical and physical conditions. A working knowledge of the periodic table has its practical benefits. For instance, the arrangement of groups shows which element may be used to replace another element when making a metal alloy, so that a similar material will result. For example, molybdenum (Mo) could be considered a substitute for chromium (Cr) because they both are in Group 6 of the table. Figure 1. The periodic table groups all the elements according to the number of protons contained in each atom. Figure 2. A hydrogen atom typically has one proton and one electron. Lesson:6/15 How Elements Interact The number of electrons in the outer shell determines how an atom interacts with other atoms. Every atom has the tendency to fill its outer shell to its maximum capacity. Other atoms with very few valence electrons in the outer shell are more willing to part with these electrons. For example, a hydrogen atom, which is shown in Figure 1, has only one electron in a single shell. Hydrogen atoms easily react with oxygen to form water since both of their outer shells are incomplete. The oxygen atom borrows two hydrogen electrons to fill its outer shell, as shown in Figure 3. Copyright 2015 Tooling that U, LLC. Rights Reserved. The number of protons theallatom contains identifies its atomic number. The atomic number is unique to each element. In other words, all atoms of the same element share the same number of protons.
8 Lesson:6/15 How Elements Interact The number of electrons in the outer shell determines how an atom interacts with other atoms. Every atom has the tendency to fill its outer shell to its maximum capacity. Other atoms with very few valence electrons in the outer shell are more willing to part with these electrons. For example, a hydrogen atom, which is shown in Figure 1, has only one electron in a single shell. Hydrogen atoms easily react with oxygen to form water since both of their outer shells are incomplete. The oxygen atom borrows two hydrogen electrons to fill its outer shell, as shown in Figure 3. The number of protons that the atom contains identifies its atomic number. The atomic number is unique to each element. In other words, all atoms of the same element share the same number of protons. Figure 1. A hydrogen atom has one electron orbiting in a single shell. Figure 2. An oxygen atom has six electrons orbiting in its outer shell. Figure 3. The oxygen atom borrows two atoms from hydrogen to fill its outer shell and form a water molecule.
9 Figure 3. The oxygen atom borrows two atoms from hydrogen to fill its outer shell and form a water molecule. Lesson:7/15 Ionic and Covalent Bonds Atoms combine with one another to form molecules. These atoms are held together by strong primary bonds, which depend on the actions of the atoms valence electrons. A water molecule consists of hydrogen and oxygen atoms. The way that the electrons interact in the molecule determines the type of primary bond. An ionic bond, which is depicted in Figure 1, is formed when an atom gives up a valence electron to help another atom fill its outer shell. Together, these two atoms bond together and form a molecule. Table salt is formed by ionic bonds. The covalent bond, shown in Figure 2, is formed when atoms share electrons in their outer shell. The shared electrons act as community electrons that complete the outer shells for both atoms simultaneously, like conjoined twins. Water is held together by covalent bonds. Figure 1. An ionic bond involves the borrowing of one or more electrons. Figure 2. A covalent bond involves the sharing of electrons. Lesson:8/15 Metallic Bonds Many atoms are held together by ionic and covalent bonds. However, a different type of primary bond holds the atoms of metals together. Metallic bonds consist of an electron cloud that allows for the movement of electrons from one atom to the next. Figure 1 illustrates the arrangement of electrons in a metallic bond. In a metallic bond, electrons are neither borrowed nor shared. Instead, electrons are free to roam. Because of this unique type of bond, most metals have a high electrical conductivity. For example, copper is a popular metal for making electrical wiring, which is shown in Figure 2. Copyright involves 2015 Tooling U, LLC. All Rights Reserved. Electricity the movement of electrons, and a metallic bond permits this movement.
10 Lesson:7/15 Ionic and Covalent Bonds Atoms combine with one another to form molecules. These atoms are held together by strong primary bonds, which depend on the actions of the atoms valence electrons. A water molecule consists of hydrogen and oxygen atoms. The way that the electrons interact in the molecule determines the type of primary bond. An ionic bond, which is depicted in Figure 1, is formed when an atom gives up a valence electron to help another atom fill its outer shell. Together, these two atoms bond together and form a molecule. Table salt is formed by ionic bonds. The covalent bond, shown in Figure 2, is formed when atoms share electrons in their outer shell. The shared electrons act as community electrons that complete the outer shells for both atoms simultaneously, like conjoined twins. Water is held together by covalent bonds. Figure 1. An ionic bond involves the borrowing of one or more electrons. Figure 2. A covalent bond involves the sharing of electrons. Lesson:8/15 Metallic Bonds Many atoms are held together by ionic and covalent bonds. However, a different type of primary bond holds the atoms of metals together. Metallic bonds consist of an electron cloud that allows for the movement of electrons from one atom to the next. Figure 1 illustrates the arrangement of electrons in a metallic bond. In a metallic bond, electrons are neither borrowed nor shared. Instead, electrons are free to roam. Because of this unique type of bond, most metals have a high electrical conductivity. For example, copper is a popular metal for making electrical wiring, which is shown in Figure 2. Electricity involves the movement of electrons, and a metallic bond permits this movement.
11 Lesson:8/15 Metallic Bonds Many atoms are held together by ionic and covalent bonds. However, a different type of primary bond holds the atoms of metals together. Metallic bonds consist of an electron cloud that allows for the movement of electrons from one atom to the next. Figure 1 illustrates the arrangement of electrons in a metallic bond. In a metallic bond, electrons are neither borrowed nor shared. Instead, electrons are free to roam. Because of this unique type of bond, most metals have a high electrical conductivity. For example, copper is a popular metal for making electrical wiring, which is shown in Figure 2. Electricity involves the movement of electrons, and a metallic bond permits this movement. Figure 1. A metallic bond creates an electron cloud where electrons move freely. Figure 2. Copper is used for electrical wire because it permits the flow of electrons. Lesson:9/15 Molecular Bonding Primary bonds hold atoms together. However, molecules are also attracted to each other with weaker bonds called secondary bonds. Figures 1 and 2 compare primary bonds and secondary bonds. Secondary bonds are not as powerful as the primary bonds that hold atoms together. Primary bonds are continuous; once an electron is borrowed or shared, another force is required to change that relationship. With secondary bonds, the forces that hold the molecules together are periodic and temporary. Secondary bonds are more likely to break apart than primary bonds. The composition of chewing gum helps to explain this distinction. Primary bonds are similar to the forces that hold the separate ingredients of gum together to determine its fundamental composition: sweeteners, flavors, and gum base. Secondary forces are similar to the forces that keep the gum from splitting into separate pieces.
12 Lesson:9/15 Molecular Bonding Primary bonds hold atoms together. However, molecules are also attracted to each other with weaker bonds called secondary bonds. Figures 1 and 2 compare primary bonds and secondary bonds. Secondary bonds are not as powerful as the primary bonds that hold atoms together. Primary bonds are continuous; once an electron is borrowed or shared, another force is required to change that relationship. With secondary bonds, the forces that hold the molecules together are periodic and temporary. Secondary bonds are more likely to break apart than primary bonds. The composition of chewing gum helps to explain this distinction. Primary bonds are similar to the forces that hold the separate ingredients of gum together to determine its fundamental composition: sweeteners, flavors, and gum base. Secondary forces are similar to the forces that keep the gum from splitting into separate pieces. Figure 1. Strong primary bonds occur between atoms. Figure 2. Weaker secondary bonds occur between molecules. Lesson:10/15 Crystal Formation in Metals When a metal is a solid, the atoms that make up the metal are tightly packed together. These atoms tend to arrange themselves into a predictable, repeating pattern, which is called the metal s crystal structure. Another name for this arrangement is space lattice system. The arrangement of atoms is always the same for a particular metal. The way that atoms arrange themselves is similar to how you would pack balloons into a box. Figure 1 shows atoms that are arranged in a crystal structure. Atoms arrange themselves in the crystal structure that requires the least amount of energy. In other words, the crystal structure of a metal is the most efficient way for the atoms to pack themselves in a given space.
13 Lesson:10/15 Crystal Formation in Metals When a metal is a solid, the atoms that make up the metal are tightly packed together. These atoms tend to arrange themselves into a predictable, repeating pattern, which is called the metal s crystal structure. Another name for this arrangement is space lattice system. The arrangement of atoms is always the same for a particular metal. The way that atoms arrange themselves is similar to how you would pack balloons into a box. Figure 1 shows atoms that are arranged in a crystal structure. Atoms arrange themselves in the crystal structure that requires the least amount of energy. In other words, the crystal structure of a metal is the most efficient way for the atoms to pack themselves in a given space. Figure 1. A crystal structure is a predictable, repeating pattern of atom arrangement. Lesson:11/15 Types of Crystal Structures Metals usually settle into one of three types of crystal structures: l l l The face-centered cubic (FCC) structure contains fourteen atoms. One atom is located at each corner, and another is located in the center of each face of the cube. Figure 1 shows an FCC structure. The body-centered cubic (BCC) structure contains nine atoms. One atom is located in the center, and the rest are located at each corner. Figure 2 shows a BCC structure. The hexagonal close-packed (HCP) structure contains seventeen atoms arranged in a hexagon. Seven atoms are arranged at each end, and three atoms are sandwiched in the middle. Figure 3 shows an HCP structure. Other crystal structures than these are possible. However, almost all metals consist of one of these three crystal structures. Figure 1. A face-centered cubic structure.
14 Lesson:11/15 Types of Crystal Structures Metals usually settle into one of three types of crystal structures: l l l The face-centered cubic (FCC) structure contains fourteen atoms. One atom is located at each corner, and another is located in the center of each face of the cube. Figure 1 shows an FCC structure. The body-centered cubic (BCC) structure contains nine atoms. One atom is located in the center, and the rest are located at each corner. Figure 2 shows a BCC structure. The hexagonal close-packed (HCP) structure contains seventeen atoms arranged in a hexagon. Seven atoms are arranged at each end, and three atoms are sandwiched in the middle. Figure 3 shows an HCP structure. Other crystal structures than these are possible. However, almost all metals consist of one of these three crystal structures. Figure 1. A face-centered cubic structure. Figure 2. A body-centered cubic structure. Figure 3. A hexagonal close -packed structure.
15 Lesson:12/15 Effects of Crystal Structure The crystal structure of a metal determines its properties. Because of the way they are packed together, metals with an HCP structure tend to be very brittle. FCC metals, illustrated in Figure 1, tend to be very ductile. It is easier for the atoms in an FCC structure to "slide around" than it is in an HCP structure. Some FCC metals include aluminum, copper, gold, lead, and nickel. These metals are typically soft, ductile, and not very strong. Common HCP metals are magnesium and titanium, and both metals are very brittle. Metals with a BCC structure, shown in Figure 2, share similar properties as well. These metals tend to be hard, and they are not always ductile. Iron, chromium, and tungsten are BCC metals at room temperature. The crystal structure of a metal can change at certain temperatures. Metals form a particular crystal structure because they use the structure requiring the least amount of energy at a particular temperature. However, energy can be changed by varying the temperature or pressure. For example, iron s BCC structure changes to FCC at higher temperatures. Figure 1. An FCC structure is common for ductile metals. Figure 2. A BCC structure is common for harder metals such as iron, chromium, and tungsten. Lesson:13/15 Crystal Growth Solid metals contain a particular crystal structure. However, when a metal is in its molten state, it has no crystal structure. Instead, the arrangement of the atoms is entirely random. As a liquid metal cools, small seed crystals form at the coolest positions, as shown in Figure 1. As the temperature continues to fall, these crystals grow by incorporating other atoms from the surrounding liquid. Copyright 2015 Tooling U, LLC. All Rights Reserved. These grains grow until they are stopped by the boundary of an adjoining neighbor, as shown in
16 Lesson:12/15 Effects of Crystal Structure The crystal structure of a metal determines its properties. Because of the way they are packed together, metals with an HCP structure tend to be very brittle. FCC metals, illustrated in Figure 1, tend to be very ductile. It is easier for the atoms in an FCC structure to "slide around" than it is in an HCP structure. Some FCC metals include aluminum, copper, gold, lead, and nickel. These metals are typically soft, ductile, and not very strong. Common HCP metals are magnesium and titanium, and both metals are very brittle. Metals with a BCC structure, shown in Figure 2, share similar properties as well. These metals tend to be hard, and they are not always ductile. Iron, chromium, and tungsten are BCC metals at room temperature. The crystal structure of a metal can change at certain temperatures. Metals form a particular crystal structure because they use the structure requiring the least amount of energy at a particular temperature. However, energy can be changed by varying the temperature or pressure. For example, iron s BCC structure changes to FCC at higher temperatures. Figure 1. An FCC structure is common for ductile metals. Figure 2. A BCC structure is common for harder metals such as iron, chromium, and tungsten. Lesson:13/15 Crystal Growth Solid metals contain a particular crystal structure. However, when a metal is in its molten state, it has no crystal structure. Instead, the arrangement of the atoms is entirely random. As a liquid metal cools, small seed crystals form at the coolest positions, as shown in Figure 1. As the temperature continues to fall, these crystals grow by incorporating other atoms from the surrounding liquid. These grains grow until they are stopped by the boundary of an adjoining neighbor, as shown in Figure 2. The rate of crystalline growth is called the nucleation rate. It is related to the time
17 Lesson:13/15 Crystal Growth Solid metals contain a particular crystal structure. However, when a metal is in its molten state, it has no crystal structure. Instead, the arrangement of the atoms is entirely random. As a liquid metal cools, small seed crystals form at the coolest positions, as shown in Figure 1. As the temperature continues to fall, these crystals grow by incorporating other atoms from the surrounding liquid. These grains grow until they are stopped by the boundary of an adjoining neighbor, as shown in Figure 2. The rate of crystalline growth is called the nucleation rate. It is related to the time passed and temperature loss. Figure 1. Initial seed crystals. Figure 2. Continued crystal growth. Lesson:14/15 Grains and Strength As the metal cools and solidifies to form crystals, grains develop in the metal, as shown in Figure 1. These grains usually grow more rapidly in one direction than in the other due to small temperature variations in the cooling metal. Grains near the surface of the metal branch out in a treelike fashion. This is called dendritic growth. The surfaces where grains contact their neighbors and stop growing are called grain boundaries, as shown in Figure 2. The grain size of a metal depends on the rate at which it was cooled and by the number of nucleation sites in the molten metal. Faster cooling often results in a stronger, tougher metal with smaller grains. Slower cooling results in a softer metal with larger grains that is easier to machine, but not as strong. Figure 1. Crystal growth develops as the liquid metal cools into a solid.
18 Lesson:14/15 Grains and Strength As the metal cools and solidifies to form crystals, grains develop in the metal, as shown in Figure 1. These grains usually grow more rapidly in one direction than in the other due to small temperature variations in the cooling metal. Grains near the surface of the metal branch out in a treelike fashion. This is called dendritic growth. The surfaces where grains contact their neighbors and stop growing are called grain boundaries, as shown in Figure 2. The grain size of a metal depends on the rate at which it was cooled and by the number of nucleation sites in the molten metal. Faster cooling often results in a stronger, tougher metal with smaller grains. Slower cooling results in a softer metal with larger grains that is easier to machine, but not as strong. Figure 1. Crystal growth develops as the liquid metal cools into a solid. Figure 2. Grain boundaries form as the various grains contact each other. Lesson:15/15 Summary The structure of a metal determines the properties of that metal. Knowledge of the structure of metals can help you choose the right metal for the job. All atoms contain electrons that circulate around a nucleus. The nucleus contains neutrons and protons. Valence electrons determine properties of an element as well as bonding arrangements. Each element is distinguished by its atomic number, which indicates the number of protons in its nucleus. Atoms are held together by ionic, covalent, or metallic bonds. Bonds typically form when an atom strives to fill its outer shell with electrons. A water molecule is an example of the covalent bond formed between one oxygen and two hydrogen atoms. The metals used in manufacturing generally form one of three crystal structures: face-centered cubic (FCC), body-centered cubic (BCC), and hexagonal close-packed (HCP) structures. FCC metals are soft and ductile, but not strong. BCC metals are hard but not ductile. HCP metals are brittle. As a liquid metal cools, crystals begin to form. These grains also influence the metals strength. A smaller grain structure is stronger than a larger grain structure. Figure 1. An atom consists of protons and neutrons in the nucleus, with electrons that
19 Lesson:15/15 Summary The structure of a metal determines the properties of that metal. Knowledge of the structure of metals can help you choose the right metal for the job. All atoms contain electrons that circulate around a nucleus. The nucleus contains neutrons and protons. Valence electrons determine properties of an element as well as bonding arrangements. Each element is distinguished by its atomic number, which indicates the number of protons in its nucleus. Atoms are held together by ionic, covalent, or metallic bonds. Bonds typically form when an atom strives to fill its outer shell with electrons. A water molecule is an example of the covalent bond formed between one oxygen and two hydrogen atoms. The metals used in manufacturing generally form one of three crystal structures: face-centered cubic (FCC), body-centered cubic (BCC), and hexagonal close-packed (HCP) structures. FCC metals are soft and ductile, but not strong. BCC metals are hard but not ductile. HCP metals are brittle. As a liquid metal cools, crystals begin to form. These grains also influence the metals strength. A smaller grain structure is stronger than a larger grain structure. Figure 1. An atom consists of protons and neutrons in the nucleus, with electrons that orbit around the nucleus. Figure 2. A water molecule holds hydrogen and oxygen atoms together by covalent bonds. Figure 3. A BCC crystal structure is common in harder metals.
20 Figure 3. A BCC crystal structure is common in harder metals. Class Vocabulary Term Definition Alloy A metal consisting of two or more materials. One of these materials must be a metal. Atom The smallest distinguishable unit of a material that maintains that material's characteristics. Atomic Number Body-Centered Cubic Brittle Chemical Processes Covalent Bond The number of protons that are contained within the nucleus of an element's atoms. The crystal structure that contains an atom in the center and one atom in each corner of a cube. A metal's unwillingness to be drawn, stretched, or formed. Brittle metals tend to break if subjected to these forces. A process that changes the atomic or molecular structure of the involved materials by altering the bonds that hold together their atoms and molecules. Chemical processes result in a new substance. A type of atomic bond that occurs when two atoms share electrons. Crystal Structure A repeating arrangement of the same type of atom that creates a uniform, repeating structure. Dendritic Growth Grain development that resembles the increasingly smaller branches of a tree. Ductility Electrical Conductivity Electron Electron Cloud Element Face-Centered Cubic Grain Group Hardness Hexagonal Close-Packed Ionic Bond A metal's ability to be drawn, stretched, or formed without breaking or cracking. A material's ability to act as a medium for conveying electricity. The smallest part of the atom that revolves around the nucleus. Electrons have a negative charge, and they are the basic charge of electricity. The arrangement of electrons within a metallic bond that permits the free movement of electrons from one atom to the next. One of the basic materials out of which all matter is made. Elements are the simplest of substances, and each element contains atoms with an identical number of protons. The crystal structure that contains one atom in the center of the six sides of a cube and one atom in each corner of the cube. A repeating arrangement of either the same type of atom or different atoms that create a uniform, repeating structure. A vertical column in the periodic table that contains elements with similar properties and chemical reactions. The ability of a material to resist penetration, indentation, or scratching. The crystal structure that contains a collection of atoms that are closely packed into the shape of a hexagon. Metals with a hexagonal close-packed crystal structure are very difficult to form. A type of atomic bond that occurs when one atom "borrows" one or more electrons from another atom. Mass The amount of matter that is contained within an object. Metal A hard, crystalline solid that conducts electricity and heat. It is shiny when polished, and it can be hammered, bent, formed, and machined. Metallic Bond A type of atomic bond that occurs when atoms "share" electrons that float about in a general electron cloud. Metals are held together by metallic bonds. Molecule TheRights smallest unit into which a material can be divided without changing its properties. A molecule consists of a Copyright 2015 Tooling U, LLC. All Reserved. group of atoms held together by strong primary bonds.
21 Class Vocabulary Term Definition Alloy A metal consisting of two or more materials. One of these materials must be a metal. Atom The smallest distinguishable unit of a material that maintains that material's characteristics. Atomic Number Body-Centered Cubic Brittle Chemical Processes Covalent Bond The number of protons that are contained within the nucleus of an element's atoms. The crystal structure that contains an atom in the center and one atom in each corner of a cube. A metal's unwillingness to be drawn, stretched, or formed. Brittle metals tend to break if subjected to these forces. A process that changes the atomic or molecular structure of the involved materials by altering the bonds that hold together their atoms and molecules. Chemical processes result in a new substance. A type of atomic bond that occurs when two atoms share electrons. Crystal Structure A repeating arrangement of the same type of atom that creates a uniform, repeating structure. Dendritic Growth Grain development that resembles the increasingly smaller branches of a tree. Ductility Electrical Conductivity Electron Electron Cloud Element Face-Centered Cubic Grain Group Hardness Hexagonal Close-Packed Ionic Bond A metal's ability to be drawn, stretched, or formed without breaking or cracking. A material's ability to act as a medium for conveying electricity. The smallest part of the atom that revolves around the nucleus. Electrons have a negative charge, and they are the basic charge of electricity. The arrangement of electrons within a metallic bond that permits the free movement of electrons from one atom to the next. One of the basic materials out of which all matter is made. Elements are the simplest of substances, and each element contains atoms with an identical number of protons. The crystal structure that contains one atom in the center of the six sides of a cube and one atom in each corner of the cube. A repeating arrangement of either the same type of atom or different atoms that create a uniform, repeating structure. A vertical column in the periodic table that contains elements with similar properties and chemical reactions. The ability of a material to resist penetration, indentation, or scratching. The crystal structure that contains a collection of atoms that are closely packed into the shape of a hexagon. Metals with a hexagonal close-packed crystal structure are very difficult to form. A type of atomic bond that occurs when one atom "borrows" one or more electrons from another atom. Mass The amount of matter that is contained within an object. Metal A hard, crystalline solid that conducts electricity and heat. It is shiny when polished, and it can be hammered, bent, formed, and machined. Metallic Bond Molecule A type of atomic bond that occurs when atoms "share" electrons that float about in a general electron cloud. Metals are held together by metallic bonds. The smallest unit into which a material can be divided without changing its properties. A molecule consists of a group of atoms held together by strong primary bonds. Neutron A particle with a neutral charge that is located in the nucleus of an atom. Nucleation Rate The rate at which small particles within a liquid metal begin to cool and form a solid.
22 group of atoms held together by strong primary bonds. Neutron Nucleation Rate Nucleation Site Nucleus Period Periodic Table Physical Processes Pressure Primary Bond Proton Secondary Bond Seed Crystal A particle with a neutral charge that is located in the nucleus of an atom. The rate at which small particles within a liquid metal begin to cool and form a solid. The locations at which seed crystals develop. The core of an atom around which electrons rotate. A horizontal row within the periodic table. The table containing all the elements arranged according to their atomic numbers. Vertical columns in the table contain elements with similar properties. A process that transforms metal by manufacturing processes including forming, machining, casting, and joining. A force or stress which, when applied, causes changes to the properties of the material. A bond that forms between atoms and that involves the exchanging or sharing of electrons. A particle with a positive charge that is located in the nucleus of an atom. A bond that involves attractions between molecules. Unlike primary bonding, there is no transfer or sharing of electrons. The origin of crystal growth that develops at one of the coolest points within a metal. Shell The approximately circular level in which electrons travel around the nucleus. Each shell consists of subshells, or orbitals. Space Lattice System The stacking of atoms into compact, symmetrical, three-dimensional arrangements that occurs as crystals form in a metal. Strength Temperature Valence Electron The ability of a material to resist forces that attempt to bend, stretch, or compress. The degree of heat within a material. An electron that is located in the outermost shell of an atom. Valence electrons are easily shared or transferred during a chemical reaction.
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Ionic and Metallic Bonding
 Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
The mechanical properties of metal affected by heat treatment are:
 Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
ANSWER KEY. Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take!
 ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
KINETIC MOLECULAR THEORY OF MATTER
 KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
Unit 2 Periodic Behavior and Ionic Bonding
 Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
CHAPTER 6 Chemical Bonding
 CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
All about Chemical Bonding Ionic
 Program Support Notes by: Peter Gribben BA BSc (Hons) PGCE Produced by: VEA Pty Ltd Commissioning Editor: Darren Gray Cert IV Training & Assessment Executive Producer: Simon Garner B.Ed, Dip Management
Program Support Notes by: Peter Gribben BA BSc (Hons) PGCE Produced by: VEA Pty Ltd Commissioning Editor: Darren Gray Cert IV Training & Assessment Executive Producer: Simon Garner B.Ed, Dip Management
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Metals are located on the left side of the periodic table and are generally shiny, malleable, ductile, and good conductors.
 Section 1: are located on the left side of the periodic table and are generally shiny, malleable, ductile, and good conductors. K What I Know W What I Want to Find Out L What I Learned Essential Questions
Section 1: are located on the left side of the periodic table and are generally shiny, malleable, ductile, and good conductors. K What I Know W What I Want to Find Out L What I Learned Essential Questions
Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding
 Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding Key Concepts The attractions between the protons and electrons of atoms can cause an electron to move completely from one atom to the other.
Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding Key Concepts The attractions between the protons and electrons of atoms can cause an electron to move completely from one atom to the other.
Experiment: Crystal Structure Analysis in Engineering Materials
 Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras
 Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
A pure covalent bond is an equal sharing of shared electron pair(s) in a bond. A polar covalent bond is an unequal sharing.
 CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
hij GCSE Additional Science Chemistry 2 Higher Tier Chemistry 2H SPECIMEN MARK SCHEME Version 1.0
 hij GCSE Additional Science Chemistry 2 Higher Tier Chemistry 2H SPECIMEN MARK SCHEME Version.0 Copyright 20 AQA and its licensors. All rights reserved. The Assessment and Qualifications Alliance (AQA)
hij GCSE Additional Science Chemistry 2 Higher Tier Chemistry 2H SPECIMEN MARK SCHEME Version.0 Copyright 20 AQA and its licensors. All rights reserved. The Assessment and Qualifications Alliance (AQA)
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
CHAPTER 6 REVIEW. Chemical Bonding. Answer the following questions in the space provided.
 Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Unit 12 Practice Test
 Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
NaCl Lattice Science Activities
 NaCl Lattice Science Activities STEM: The Science of Salt Using a Salt Lattice Model Teacher Notes Science Activities A Guided-Inquiry Approach Using the 3D Molecular Designs NaCl Lattice Model Classroom
NaCl Lattice Science Activities STEM: The Science of Salt Using a Salt Lattice Model Teacher Notes Science Activities A Guided-Inquiry Approach Using the 3D Molecular Designs NaCl Lattice Model Classroom
Chem 106 Thursday Feb. 3, 2011
 Chem 106 Thursday Feb. 3, 2011 Chapter 13: -The Chemistry of Solids -Phase Diagrams - (no Born-Haber cycle) 2/3/2011 1 Approx surface area (Å 2 ) 253 258 Which C 5 H 12 alkane do you think has the highest
Chem 106 Thursday Feb. 3, 2011 Chapter 13: -The Chemistry of Solids -Phase Diagrams - (no Born-Haber cycle) 2/3/2011 1 Approx surface area (Å 2 ) 253 258 Which C 5 H 12 alkane do you think has the highest
(1) e.g. H hydrogen that has lost 1 electron c. anion - negatively charged atoms that gain electrons 16-2. (1) e.g. HCO 3 bicarbonate anion
 GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
Trends of the Periodic Table Basics
 Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Unit 3.2: The Periodic Table and Periodic Trends Notes
 Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Chapter 3 Student Reading
 Chapter 3 Student Reading If you hold a solid piece of lead or iron in your hand, it feels heavy for its size. If you hold the same size piece of balsa wood or plastic, it feels light for its size. The
Chapter 3 Student Reading If you hold a solid piece of lead or iron in your hand, it feels heavy for its size. If you hold the same size piece of balsa wood or plastic, it feels light for its size. The
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
ELECTRICAL FUNDAMENTALS
 General Electricity is a form of energy called electrical energy. It is sometimes called an "unseen" force because the energy itself cannot be seen, heard, touched, or smelled. However, the effects of
General Electricity is a form of energy called electrical energy. It is sometimes called an "unseen" force because the energy itself cannot be seen, heard, touched, or smelled. However, the effects of
H 2O gas: molecules are very far apart
 Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together is called a(n)
 Chemistry I ATOMIC BONDING PRACTICE QUIZ Mr. Scott Select the best answer. 1) A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together is
Chemistry I ATOMIC BONDING PRACTICE QUIZ Mr. Scott Select the best answer. 1) A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together is
Chapter Outline. How do atoms arrange themselves to form solids?
 Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Simple cubic Face-centered cubic Body-centered cubic Hexagonal close-packed
Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Simple cubic Face-centered cubic Body-centered cubic Hexagonal close-packed
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
Bohr s Model of the Atom
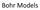 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
ME 612 Metal Forming and Theory of Plasticity. 1. Introduction
 Metal Forming and Theory of Plasticity Yrd.Doç. e mail: [email protected] Makine Mühendisliği Bölümü Gebze Yüksek Teknoloji Enstitüsü In general, it is possible to evaluate metal forming operations
Metal Forming and Theory of Plasticity Yrd.Doç. e mail: [email protected] Makine Mühendisliği Bölümü Gebze Yüksek Teknoloji Enstitüsü In general, it is possible to evaluate metal forming operations
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance
 Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Study the following diagrams of the States of Matter. Label the names of the Changes of State between the different states.
 Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Class Notes Standards Addressed: 8.3.11
 Name: Period #: Class Notes Standards Addressed: 8.3.11 History of the Periodic Table: Demitri Mendeleev = Russian chemist who discovered a pattern to the in 1869. o How did he discovery a pattern to the
Name: Period #: Class Notes Standards Addressed: 8.3.11 History of the Periodic Table: Demitri Mendeleev = Russian chemist who discovered a pattern to the in 1869. o How did he discovery a pattern to the
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
Name Date Class CHAPTER 1 REVIEW. Answer the following questions in the space provided.
 CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
Type of Chemical Bonds
 Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds.
 Problem 1 Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds. Ionic Bonds Two neutral atoms close to each can undergo an ionization process in order
Problem 1 Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds. Ionic Bonds Two neutral atoms close to each can undergo an ionization process in order
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
3 Atomic Structure 15
 3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Chapter 1 Student Reading
 Chapter 1 Student Reading Chemistry is the study of matter You could say that chemistry is the science that studies all the stuff in the entire world. A more scientific term for stuff is matter. So chemistry
Chapter 1 Student Reading Chemistry is the study of matter You could say that chemistry is the science that studies all the stuff in the entire world. A more scientific term for stuff is matter. So chemistry
Chapter 8 Basic Concepts of the Chemical Bonding
 Chapter 8 Basic Concepts of the Chemical Bonding 1. There are paired and unpaired electrons in the Lewis symbol for a phosphorus atom. (a). 4, 2 (b). 2, 4 (c). 4, 3 (d). 2, 3 Explanation: Read the question
Chapter 8 Basic Concepts of the Chemical Bonding 1. There are paired and unpaired electrons in the Lewis symbol for a phosphorus atom. (a). 4, 2 (b). 2, 4 (c). 4, 3 (d). 2, 3 Explanation: Read the question
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Science 20. Unit A: Chemical Change. Assignment Booklet A1
 Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
Chapter 6 Assessment. Name: Class: Date: ID: A. Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
PERIODIC TABLE. reflect
 reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s)
 BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
Warm-Up 9/9. 1. Define the term matter. 2. Name something in this room that is not matter.
 Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
Cutting Tool Materials
 Training Objectives After watching the video and reviewing this printed material, the viewer will gain knowledge and understanding of cutting tool metallurgy and specific tool applications for various
Training Objectives After watching the video and reviewing this printed material, the viewer will gain knowledge and understanding of cutting tool metallurgy and specific tool applications for various
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
WHERE DID ALL THE ELEMENTS COME FROM??
 WHERE DID ALL THE ELEMENTS COME FROM?? In the very beginning, both space and time were created in the Big Bang. It happened 13.7 billion years ago. Afterwards, the universe was a very hot, expanding soup
WHERE DID ALL THE ELEMENTS COME FROM?? In the very beginning, both space and time were created in the Big Bang. It happened 13.7 billion years ago. Afterwards, the universe was a very hot, expanding soup
 Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
(b) Formation of calcium chloride:
 Chapter 2: Chemical Compounds and Bonding Section 2.1: Ionic Compounds, pages 22 23 1. An ionic compound combines a metal and a non-metal joined together by an ionic bond. 2. An electrostatic force holds
Chapter 2: Chemical Compounds and Bonding Section 2.1: Ionic Compounds, pages 22 23 1. An ionic compound combines a metal and a non-metal joined together by an ionic bond. 2. An electrostatic force holds
neutrons are present?
 AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
States of Matter and the Kinetic Molecular Theory - Gr10 [CAPS]
![States of Matter and the Kinetic Molecular Theory - Gr10 [CAPS] States of Matter and the Kinetic Molecular Theory - Gr10 [CAPS]](/thumbs/40/21739317.jpg) OpenStax-CNX module: m38210 1 States of Matter and the Kinetic Molecular Theory - Gr10 [CAPS] Free High School Science Texts Project This work is produced by OpenStax-CNX and licensed under the Creative
OpenStax-CNX module: m38210 1 States of Matter and the Kinetic Molecular Theory - Gr10 [CAPS] Free High School Science Texts Project This work is produced by OpenStax-CNX and licensed under the Creative
The Properties of Water
 1 Matter & Energy: Properties of Water, ph, Chemical Reactions EVPP 110 Lecture GMU Dr. Largen Fall 2003 2 The Properties of Water 3 Water - Its Properties and Its Role in the Fitness of Environment importance
1 Matter & Energy: Properties of Water, ph, Chemical Reactions EVPP 110 Lecture GMU Dr. Largen Fall 2003 2 The Properties of Water 3 Water - Its Properties and Its Role in the Fitness of Environment importance
EML 2322L MAE Design and Manufacturing Laboratory. Welding
 EML 2322L MAE Design and Manufacturing Laboratory Welding Intro to Welding A weld is made when separate pieces of material to be joined combine and form one piece when heated to a temperature high enough
EML 2322L MAE Design and Manufacturing Laboratory Welding Intro to Welding A weld is made when separate pieces of material to be joined combine and form one piece when heated to a temperature high enough
KINETIC THEORY OF MATTER - molecules in matter are always in motion - speed of molecules is proportional to the temperature
 1 KINETIC TERY F MATTER - molecules in matter are always in motion - speed of molecules is proportional to the temperature TE STATES F MATTER 1. Gas a) ideal gas - molecules move freely - molecules have
1 KINETIC TERY F MATTER - molecules in matter are always in motion - speed of molecules is proportional to the temperature TE STATES F MATTER 1. Gas a) ideal gas - molecules move freely - molecules have
In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and oxygen. [Include any charges or partial charges.
 Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Bonding in Elements and Compounds. Covalent
 Bonding in Elements and Compounds Structure of solids, liquids and gases Types of bonding between atoms and molecules Ionic Covalent Metallic Many compounds between metals & nonmetals (salts), e.g. Na,
Bonding in Elements and Compounds Structure of solids, liquids and gases Types of bonding between atoms and molecules Ionic Covalent Metallic Many compounds between metals & nonmetals (salts), e.g. Na,
