Department of Physics and Geology The Elements and the Periodic Table
|
|
|
- Marian Fletcher
- 7 years ago
- Views:
Transcription
1 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev and Lothar Meyer from Germany independently established an arrangement of elements similar to our present day periodic table. Mendeleev produced a chart summarizing the known properties of the elements for his students. When the elements were arranged in order of increasing atomic weight, Mendeleev noticed that the chemical properties of the elements repeated. Lithium and sodium, for example, are similar not only in their general physical properties (appearance, the conduction of electricity, etc.), but also in how they combine with other elements (chemical properties). Any element that combines with lithium will combine in exactly the same proportion with sodium. In order to properly align elements down a column, Mendeleev occasionally had to leave a blank space that was not filled by any known element. Instead of looking upon these blank spaces as a defect with his chart, Mendeleev predicted that new elements were still waiting to be discovered! By studying the properties of the elements around the gap and the variation of these properties across the rows and down the columns, he went on to predict the properties of these unknown elements. These predictions helped lead to the eventual discovery of those elements. Figure 1. Mendeleev s Periodic Table. (Source: web.fccj.org/~ethall/2045/ch5/mendelev.htm) Mendeleev's periodic table helped confirm that all matter is made of atoms. At the time though he had no idea why the properties of the elements repeated themselves. Today we know that this is because of the structure of the atom. The periodic tables designed by Mendeleev and Meyer were virtually identical but since Mendeleev drafted his table earlier in 1869 and because his table included blanks for yet to be discovered elements to fit, Mendeleev is considered the father of the modern periodic table.
2 The structure of the atom and the modern periodic table The atom is composed of a small, heavy nucleus that is surrounded by probability clouds of negatively charged particles called electrons. The nucleus is composed of protons (a heavy, positivelycharged particle) and neutrons (a heavy particle with no charge). The number of protons in an atom is known as its atomic number. This number defines each element. For example, hydrogen is the element that has one proton and lithium is the element that has three protons. Because all atoms under normal conditions are neutral, the number of electrons in an atom is equal to the number of protons. Unlike the number of protons, the number of neutrons in the nucleus can vary for a given element. For example, hydrogen can have no neutrons (most common hydrogen), one neutron (a hydrogen isotope called deuterium) or two neutrons (a hydrogen isotope called tritium). These different forms of an element are called isotopes. Although different isotopes of a given chemical element will have slightly different weights, the isotopes of the given element will all have the same chemical behavior. Each element is normally a mixture of several isotopes. This is why the atomic mass of most elements is not exactly an integer. Each element in the periodic table has a name and a symbol. In most cases the symbol is just an abbreviation. For example the symbol for aluminum is Al. Those elements, which have been known for a very long time, usually have a symbol that is related to their Latin name. For example the Latin word for gold is aurus (Au), and iron is ferrus (Fe). In the modern periodic table, the rows are called periods and the columns are called groups. Elements are arranged strictly in order of increasing atomic number. Atomic structure As indicated above, the number of protons determines how many electrons an element will have. In turn, the number of electrons determines the chemical behavior of an element. The possible states of each electron in an atom are described by a set of four quantum numbers: n, l, ml, and ms. Two different electrons cannot share the same state. Thus, two different electrons in an atom have to be represented by two different sets of values of quantum numbers. The principle quantum number n (n = 1, 2, 3,...) corresponds approximately to the average distance of an electron from the nucleus. Electrons with the same value of n have about the same energy and are said to occupy the same shell. The orbital angular momentum quantum number l (l = 0, 1, 2,..., n-1) corresponds approximately to the shape of the orbital (probability cloud distribution) of an electron around the nucleus. Electrons with the same values of n and l are said to be in the same subshell. Standard notation represents the values of the quantum number l with letters: l designation s p d f g h i p. 2
3 That is, l=0 is known as the s-orbital, l=l is the p-orbital, l=2 is the d-orbital, l=3 is the f-orbital, and so on. When n=1, there is only one possible subshell (l=0); for n=2 there are two possible subshells (l=0 and l=l); and so on. That is, for a given value of n, there are n possible values for l. When denoting a subshell, the standard notation is to use a number (principal quantum number) accompanied with a letter (orbital angular momentum quantum number); for example the subshell 3p corresponds to subshell with quantum numbers n=3 and l=1. Quantum number ml (ml = -l, -l+1,, -2, -1, 0, 1, 2,, l-1, l ) corresponds approximately to the orientation in space of the orbital ( probability cloud distribution) of an electron around the nucleus. When l=0, there is only one possible value for ml (ml = 0). When l=1, there are three possible values for ml (ml = -1,0,1). In general, for a given value of l, there are 2l+1 possible values for ml. Quantum number spin ms corresponds to the spin orientation of the electron. ms has two possible values -1/2 and +1/2. Since two different electrons cannot be in the same state, for each subshell there is a maximum number of electrons. The maximum number of electrons that can be present in a given subshell is determined by the orbital quantum number l. For example, for an s-subshell (l=0, for a given n), the only possible value for ml is 0. Considering that ms has two possible values, the total number of electrons that can be in an s- subshell is 2. Principal quantum number: n Orbital Angular Momentum: 0 (s-orbital) Possible states: n=principal, l=0, ml = 0, ms = -1/2 n=principal, l=0, ml = 0, ms = +1/2 Total # states: 2 Similarly for a p-subshell (l=1, for a given n), one finds that the total number of electrons that can be there is 6: Principal quantum number: n Orbital Angular Momentum: 1 (p-orbital) Possible states: n=principal, l=1, ml = -1, ms = -1/2 n=principal, l=1, ml = -1, ms = +1/2 n=principal, l=1, ml = 0, ms = -1/2 n=principal, l=1, ml = 0, ms = +1/2 n=principal, l=1, ml = +1, ms = -1/2 n=principal, l=1, ml = +1, ms = +1/2 Total # states: 6 The larger the value of l, the more electrons a particular subshell can hold. In general, for a subshell with orbital quantum atomic number l, the total number of possible states in the subshell is given by 2(2l+1), equal to 4l +2. Therefore every time we increase l by one, the number of possible states increases by four. Thus a d-subshell (l=2) has 10 possible states (6+4); an f-subshell (l=3) has 14 possible states (10+4); and so on. p. 3
4 The sequence in which electron subshells are filled in an atom corresponds to the order of increasing electron energy: ls, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s Most periodic tables give the electron configuration of each element - this describes how the electrons are arranged around the nucleus. Each subshell is identified by its principle quantum number n followed by the letter corresponding to its orbital quantum number l (standard notation). A superscript after the letter indicates the number of electrons in that subshell. For example, nitrogen has seven electrons and its electron configuration is 1s 2 2s 2 2p 3. This tells us that this element contains two 1s electrons (n=1, l=0), two 2s electrons (n=2, l=0), and three 2p electrons (n=2, l=l). We can visually describe this arrangement by placing a straight line above a subshell for each possible value of quantum number ml, and by representing each electron with an up or down arrow indicating its spin (up indicating ms = +1/2, down indicating ms = -1/2). For example, beryllium has 4 electrons, and thus its electron configuration is 1s 2 2s 2, which can be represented as: 1 2 Another example would be the electronic configuration for sodium (atomic number = 11) which is 1s 2 2s 2 2p 6 3s 1 and can be represented as: A shell or a subshell that contains its full quota of electrons is said to be closed. In sodium the 1s, 2s and 2p subshells are closed, but 3s is considered open since it can still have one more electron. Chemical Activity To understand how electrons determine the chemical activity of an atom, you need to remember electrons in closed shells do not contribute to chemical reactions. Only the electrons in the outermost subshell contribute to chemical reactions. All metal atoms have one or more electrons outside a closed shell or subshells. Since these electrons are weakly bound, metal atoms combine chemically to nonmetal atoms by losing these electrons. All nonmetal atoms need one or more electrons to achieve a closed shell or a subshell and the maximum stability. In chemical reactions, these atoms pick up electrons from metal atoms or from sharing electrons with other nonmetal atoms. The inert gases have closed shells or subshells. This makes it hard for them to gain or lose electrons. As a result, they have almost no ability to react chemically. Answer the questions in the Lab Report section. p. 4
5 Name Class Date Part 3: Lab Report The Elements and the Periodic Table Questions 1. What is the name of the element with 6 protons? 2. What is the name of the element with 16 protons? 3. What is the name of the element with 36 electrons? 4. What element has the symbol Pb? 5. What element has the symbol Ar? 6. Elements in the same group have similar chemical properties. A) True B) False 7. Phosphorus and Sulfur belong to the same... A) Group B) Period 8. Two atoms have the same number of neutrons, but a different number of protons. Can they be the same element? A) Yes B) No Put the correct number of electrons in each orbital for: Boron: Aluminum: Oxygen: Argon: Sodium: p. 5
6 14. In Fluorine, which orbitals are closed? 15. Which orbital has quantum numbers n=2 and l=0? A) 1s B) 2s C) 2p 16. Which orbital has a higher energy than the 4s orbital? A) 2p B) 3d C) 1s 17. Give the electronic configuration of cobalt. 18. Give the electronic configuration of magnesium. 19. How many electrons in a silicon atom can contribute to chemical reactions (i.e., are not in closed shells)? 20. How many electrons in a neon atom can contribute to chemical reactions (i.e., are not in closed shells)? p. 6
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Student Exploration: Electron Configuration
 Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Worksheet: Electron Configurations. I Heart Chemistry!
 1. Which electron configuration represents an atom in an excited state? 1s 2 2s 2 2p 6 3p 1 1s 2 2s 2 2p 6 3s 2 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name:
1. Which electron configuration represents an atom in an excited state? 1s 2 2s 2 2p 6 3p 1 1s 2 2s 2 2p 6 3s 2 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name:
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
PERIODIC TABLE. reflect
 reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Multi-electron atoms
 Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Chemistry - Elements Electron Configurations The Periodic Table. Ron Robertson
 Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
neutrons are present?
 AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
Properties of Atoms and the Periodic Table
 Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Student Exploration: Electron Configuration
 www.explorelearning.com Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund
www.explorelearning.com Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
13- What is the maximum number of electrons that can occupy the subshell 3d? a) 1 b) 3 c) 5 d) 2
 Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
THE PERIODIC TABLE O F T H E E L E M E N T S. The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27)
 THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
Unit 3.2: The Periodic Table and Periodic Trends Notes
 Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW
 CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Practice Questions - Chapter 7 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following represents an impossible set of
Practice Questions - Chapter 7 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following represents an impossible set of
Chemistry: The Periodic Table and Periodicity
 Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
Electron Configurations, Isoelectronic Elements, & Ionization Reactions. Chemistry 11
 Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Arrangement of Electrons in Atoms
 CHAPTER 4 PRE-TEST Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the following orbital
CHAPTER 4 PRE-TEST Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the following orbital
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 7. Electron Structure of the Atom. Chapter 7 Topics
 Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
Chapter 4, Lesson 2: The Periodic Table
 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
Question: Do all electrons in the same level have the same energy?
 Question: Do all electrons in the same level have the same energy? From the Shells Activity, one important conclusion we reached based on the first ionization energy experimental data is that electrons
Question: Do all electrons in the same level have the same energy? From the Shells Activity, one important conclusion we reached based on the first ionization energy experimental data is that electrons
Chapter Test. Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a.
 Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Bohr s Model of the Atom
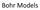 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
5.4 Trends in the Periodic Table
 5.4 Trends in the Periodic Table Think about all the things that change over time or in a predictable way. For example, the size of the computer has continually decreased over time. You may become more
5.4 Trends in the Periodic Table Think about all the things that change over time or in a predictable way. For example, the size of the computer has continually decreased over time. You may become more
Molecular Models & Lewis Dot Structures
 Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Ions & Their Charges Worksheet
 Ions & Their Charges Worksheet Name Date Teacher Diagram of charges based on groups on the periodic table including transition metals and noble gases: IA IIA Transition IIIA IVA VA VIA VIIA VIIIA metals
Ions & Their Charges Worksheet Name Date Teacher Diagram of charges based on groups on the periodic table including transition metals and noble gases: IA IIA Transition IIIA IVA VA VIA VIIA VIIIA metals
Chapter 3. Elements, Atoms, Ions, and the Periodic Table
 Chapter 3. Elements, Atoms, Ions, and the Periodic Table The Periodic Law and the Periodic Table In the early 1800's many elements had been discovered and found to have different properties. In 1817 Döbreiner's
Chapter 3. Elements, Atoms, Ions, and the Periodic Table The Periodic Law and the Periodic Table In the early 1800's many elements had been discovered and found to have different properties. In 1817 Döbreiner's
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Copyrighted by Gabriel Tang B.Ed., B.Sc.
 Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
Chapter 3, Elements, Atoms, Ions, and the Periodic Table
 1. Which two scientists in 1869 arranged the elements in order of increasing atomic masses to form a precursor of the modern periodic table of elements? Ans. Mendeleev and Meyer 2. Who stated that the
1. Which two scientists in 1869 arranged the elements in order of increasing atomic masses to form a precursor of the modern periodic table of elements? Ans. Mendeleev and Meyer 2. Who stated that the
P. Table & E Configuration Practice TEST
 P. Table & E Configuration Practice TEST Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A line spectrum is produced when an electron moves from one energy
P. Table & E Configuration Practice TEST Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A line spectrum is produced when an electron moves from one energy
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
Trends of the Periodic Table Basics
 Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Ionic and Metallic Bonding
 Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D
 Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
The Advanced Placement Examination in Chemistry. Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010
 The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
About the course GENERAL CHEMISTRY. Recommended literature: Chemistry: science of the matter. Responsible for the course: Dr.
 About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations.
 The Periodic Table Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations. Vertical Rows are called Families or Groups.
The Periodic Table Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations. Vertical Rows are called Families or Groups.
Unit 2 Atomic Structure
 Unit 2 Atomic Structure Big Idea: Atomic structure explains patterns in the behavior of elements and allows us to predict the chemical and physical behavior of a given element. The organization of elements
Unit 2 Atomic Structure Big Idea: Atomic structure explains patterns in the behavior of elements and allows us to predict the chemical and physical behavior of a given element. The organization of elements
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Noble Gases. Outline Nobel Gas Elements Radon and Health Chemistry Homework
 Radon and Other Noble Gases The elements in the last column of the periodic table are all very stable, mono-atomic gases. Until 1962, they were called inert gases because they did not react with other
Radon and Other Noble Gases The elements in the last column of the periodic table are all very stable, mono-atomic gases. Until 1962, they were called inert gases because they did not react with other
3 Atomic Structure 15
 3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Chapter 7 Periodic Properties of the Elements
 Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004. Name (print) SSN
 CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Note: Please use the actual date you accessed this material in your citation.
 MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science, Fall 2005 Please use the following citation format: Sylvia Ceyer and Catherine Drennan, 5.111 Principles of Chemical Science,
MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science, Fall 2005 Please use the following citation format: Sylvia Ceyer and Catherine Drennan, 5.111 Principles of Chemical Science,
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
WAVES AND ELECTROMAGNETIC RADIATION
 WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
Section 1: Arranging the Elements Pages 106-112
 Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
