THE DISTRIBUTION OF THE WATER-SOLUBLE INORGANIC ORTHOPHOSPHATE IONS WITHIN THE CELL : ACCUMULATION IN THE NUCLEUS. Electron Probe Microanalysis
|
|
|
- Lynne Wood
- 8 years ago
- Views:
Transcription
1 THE DISTRIBUTION OF THE WATER-SOLUBLE INORGANIC ORTHOPHOSPHATE IONS WITHIN THE CELL : ACCUMULATION IN THE NUCLEUS Electron Probe Microanalysis CESAR M. LIBANATI and CARLOS J. TANDLER From the Coinision Nacional de Energia Atoniica, Buenos Aires, and the Instituto de Anatomia General y Embriologia, Facultad de Medicina, Buenos Aires, Argentina ABSTRACT Lead acetate treatment of unfixed cells immobilizes the intracellular water-soluble, inorganic orthophosphate ions as microcrystalline lead hydroxyapatite precipitates (see reference 1). These precipitates have been analyzed with the electron microprobe. A much higher concentration of phosphorus has been found in the nucleoli of maize root tip cells fixed in lead acetate-glutaraldehyde (organic phosphorus plus inorganic orthophosphate), as compared to the nucleoli of roots fixed in glutaraldehyde alone (organic phosphorus). The concentration of the inorganic orthophosphate pool in these nucleoli is three to five times as high as the concentration of the macromolecular organic phosphate. Since nearly all of the latter is in RNA, the concentration of inorganic phosphate in the nucleolus is calculated to be roughly M. About 30 17o-and up to 50%-of the total cellular inorganic phosphate is accumulated in the nucleolus since the mean concentration per cell is about 10-2 M. In the extranucleolar part of the nucleus the mean concentration was estimated by densitometry to be roughly six times less than in the nucleolus ( < 0.1 M), and appears more concentrated in the nucleoplasm than in the condensed chromatin. While there is no direct evidence for the concentration in the cytoplasm, it certainly must be much lower than the mean cellular level (i.e., < 10-2 M) since the nucleus is about 10% of the total cell volume. The implications of this compartmentation in the intact cell are discussed in connection with (A) the availability of orthophosphate ions for the cytoplasm in those processes in which these ions affect the rate of enzymatic reactions, and (B) protein-nucleic acid interactions within the nucleus and nucleolus. INTRODUCTION Inorganic orthophosphate, as a water-soluble anion, is well known to be an important constituent of all living plant and animal cells, Previous work in our laboratory (1) has lent support to the concept that the distribution of this ion within the cell is not homogeneous. The nucleus and especially the nucleolus have been demonstrated to possess a relatively high concentration of watersoluble inorganic phosphate ions (1). An exact interpretation of this work, however, was hampered by lack of quantitative data. The following questions remained to be answered : What is the concentration of inorganic phosphate in the nucleolus and nucleus? How much of the 7 54
2 total inorganic phosphate of the whole cell is present in the nucleolus and nucleus? To resolve the problem, we investigated the quantitative distribution of phosphorus with the electron probe X-ray microanalyzer. The evidence presented here conclusively demonstrates that the concentration of inorganic phosphate in the nucleolus is, indeed, very high and that the amount present in the whole nucleus probably accounts for a large (more than 50 i0 ) proportion of the total inorganic phosphate of the whole cell. MATERIAL AND METHODS Electron Microscope Maize root tip cells were selected for this work because of the large size of the nucleolus. The fixatives used were : (A) a 4.5% solution of lead acetate, Pb (C2H3O2)2.3H20 (Merck Chemical Division, Rahway, N.J., analytical reagent) in 0.2 M cacodylate-hci buffer (ph 7.0) containing 2 0%o glutaraldehyde (50% ; Fisher Scientific Company, Pittsburgh, Pa., biological grade), or (B) a 7.5% solution of lead acetate in glass-distilled water (ph 6.0). The roots were fixed by immersion, washed, and embedded in Maraglas as described before (1). Control sections were obtained from roots fixed in glutaraldehyde alone (in 0.2 M cacodylate-hcl buffer, ph 7.0), immersed afterwards into lead acetate, washed, and embedded as before. Thin and thick (1-µ) sections were cut with glass knives on a Porter-Blum microtome, mounted on formvar-coated copper grids, and examined unstained with a Siemens Elmiskop I electron microscope. Electron Microprobe The X-ray microanalyzer used in this work is a sensitive instrument, although its resolution is limited to about 1 s diameter. The atomic elements can be determined qualitatively and, in our case, also quantitatively if identical preparation procedures are followed. The instrument has been described in the literature (2, See also reference 3), and its application to biological samples is illustrated in more than 80 papers published since 1959 (4). The procedures used in this work were as follows : the Maraglas-embedded 1-p sections of maize root tips mounted on copper grids as described above were covered, in vacuum, on both sides with an aluminum film about 100 A thick. The high thermal and electrical conductivity of this simple arrangement made possible the scanning for long periods during m easureinent without burning of the specimen. The distribution of the elements in the sections was studied with a CAMECA model MS 46 microprobe with an accelerating voltage of 10 kv (for phosphorus and sulfur) and 30 kv (for lead) and a probe current of na. The Kat-radiation was used for studying the distribution of phosphorus and sulfur while lead was scanned with the La-radiation. As the electron beam sweeps across the tissue section the emission of characteristic X-rays by the element present in the section is picked up by a crystal spectrometer equipped with a suitable counting system, and the output is displayed on an oscilloscope ; in this manner, the relative distribution of phosphorus, lead, and sulfur in a given area is recorded in micrographs (e.g., Figs. 8-10). It is also possible to display the electrons transmitted by the section on an oscilloscope and to photograph the image obtained (e.g., Fig. 7). Thus, by comparison, the topography can be correlated with the presence of the particular elements. Scans are obtained by moving the specimen slowly along a line ; the amplitude of the trace is proportional to the concentration of the element present at different points in the traverse (Figs. 11 and 12). The emission from a given element is independent of the crystal structure of the compound containing it (5). Thus, the phosphorus in the crystalline lead hydroxyapatite precipitate can be measured accurately in our sections, as well as the phosphorus in the organic material. The degree of penetration of the beam varies with the density of the specimen ; however, the thickness of the section is certainly less than the depth of penetration. RESULTS Electron Microscope The experimental basis of these studies lies in the finding that lead acetate fixation effectively immobilizes the intracellular inorganic orthophosphate anions as microcrystalline lead precipitates, and that these precipitates accurately reflect the localization which exists in the living cell (1). Electron and X-ray diffraction data have demonstrated the chemical uniformity of the precipitates, which is due exclusively to lead hydroxyapatite Pb 5(P04) 3 OH (1). Fig. 1 illustrates the striking specificity of localization in an unstained section of maize root tip cells fixed in lead acetate-glutaraldehyde. The lead phosphate crystals are massively deposited inside the nucleoli, and the nucleolus boundary is quite sharply delimited by them. The rest of the precipitate is found within the nucleus and is delimited by the nuclear membrane. These precipitates were completely absent in roots fixed in glutaraldehyde first and treated afterwards with lead acetate, indicating the washing out of the water-soluble C. M. LIBANATI AND C. J. TANDLER Water-Soluble Ions in Nucleus 7 55
3 FIGURE 1 Electron micrograph of a thin section of maize root tip fixed in lead acetate-glutaraldehyde, ph 7.0. The electron-opaque microcrystalline precipitate of lead orthophosphate is massively deposited in the nucleoli. The rest of the precipitate is in the nuclei and is delimited by the nuclear membrane. Unstained. Scale mark, 1 k.. X 6,000. FIGURE 2 Electron micrograph of a thin section of maize root tip fixed as for Fig. 1. The cell in the center of the micrograph is in mitosis (metaphase or early anaphase) ; the cytoplasm is filled with the lead phosphate crystals. A part of two interphase nuclei can be seen at left of the micrograph. Unstained. Scale mark, 1.i. X 6,900. orthophosphate pool by the aqueous glutaraldehyde fixative. Fig. 2 shows an unstained thin section through several cells, one of which is in mitosis (metaphase or early anaphase, as shown by uranyl-acetate staining of this same section). In this case-and contrary to what is found in interphase cells-the cytoplasm is filled with lead phosphate crystals. This evidence, that the watersoluble inorganic phosphate ions are detectable in the cytoplasm only when the nuclear membrane is absent (or incomplete), strongly suggests that the concentration of these ions in the cytoplasm of the interphase cell is quite low, and probably below the sensitivity range of the reagent. An independent confirmation, however, is needed to prove this point conclusively, and the quantitative analysis with the electron microprobe was, therefore, made with these same sections. Electron Microprobe QUALITATIVE DETERMINATIONS : Fig. 3 shows the absorbed electron image from a lead acetateglutaraldehyde-fixed section. The outlines of three nucleoli with their nuclei are evident in Fig. 3. This image also shows a nucleus without a nucleolus, owing to the plane of sectioning, and two "light" cellulose walls crossing at about the middle of the micrograph. This topography can be visualized in more detail in Fig. 1, which shows an image through the electron microscope. Fig. 4 shows the lead La-emission image of the same specimen. The white specks are clearly distributed at the site of the nucleoli. The background specks over the cytoplasmic areas are similar in density to those found over an area free of tissue and are attributable to "noise" in the THE JOURNAL OF CELL BIOLOGY. VOLUME 42, 1969
4 FIGURE 3 Absorbed electron image taken with the microprobe from a thick 1-is section of maize root tip fixed in lead acetate-glutaraldehyde, ph 7.0. The outlines of three nucleoli with their nuclei are evident. The image also shows a nucleus without a nucleolus, and two "light" cellulose walls crossing at about the middle of the micrograph. X 3,800. FIGURE -1 The image for the L«-emission wavelength of lead on the same area of the section shown in Fig. 3. X 3,800. instrument ; they do not indicate presence of any element. Evidently the lead distribution closely corresponds to the electron-opaque microcrystalline precipitate found in the nucleoli of Fig. 1, as observed with the electron microscope. Fig. 5 shows the phosphorus Ka I-emission image of the same specimen. In this case, the white specks are more concentrated at the sites of the nucleoli than they are over the cytoplasm. The outlines of the cellulose walls can be discerned in this micrograph, more or less delimited by the white specks. It is evident that this emission image corresponds to the phosphorus distribution of both the lead phosphate and the organic phosphate (nucleic acids, etc.). Fig. 6 shows the phosphorus Kcal-emission image of a "control" section obtained from roots fixed in glutaraldehyde alone. This section represents the phosphorus distribution due to the organic material only : the white specks are more or less evenly distributed and there is no difference in phosphorus concentration over nucleoli and cytoplasm. It is evident, therefore, that the great concentration of phosphorus at the nucleoli in Fig. 5 is due mainly to the lead microcrystalline precipitate. This is the direct proof for the presence of phosphorus in the nucleolar lead precipitate and constitutes a further confirmation of the electron and X-ray diffraction evidence which showed that this crystalline precipitate is due exclusively to lead hydroxyapatite (1). Moreover, the sulfur Ka I-emission images of the same section as Fig. 3 failed to show any difference in concentration at the nucleoli, indicating the absence of this element in the lead precipitate. Fig. 10 shows the sulfur Ka,-emission image of the same "control" section as Fig. 6 and shows that, as is the case with phosphorus, sulfur is more or less evenly distributed over nucleolus and cytoplasm. Fig. 7 shows the absorbed electron image of a section from roots fixed in lead acetate alone (omitting the glutaraldehyde in the fixative). One large nucleolus is outlined lying inside the elongated nucleus. Figs. 8 and 9 show, respectively, the lead and phosphorus distribution in this same specimen. There is no doubt that with this fixative, too, the phosphorus is heavily concentrated C. M. LIBANATI AND C. J. TANDLER Water-Soluble Ions in Nucleus 757
5 FIGURE 5 The image for the KaI-emission wave length of phosphorus on the same area of the section shown in Figs. 3 and 4. It represents the phosphorus distribution due to both the organic material plus the lead orthophosphate precipitate ; the highest concentration is in the nucleoli (compare with Fig. 6). The outline of the cellulose walls can be seen delimited by the white specks. X 3,800. FIGURE 6 The image for the KaI-emission wave length of phosphorus of a "control" section fixed in glutaraldehyde alone. It represents the phosphorus distribution due to the organic material only, and there is no difference in concentration over nucleoli and cytoplasm. X 3,800. over the nucleolus and that the larger part of it corresponds to the lead orthophosphate precipitate. There is no doubt that the good intracellular localization obtained with the probe in Figs. 5 and 9 can be attributed both to the relatively large size of the nucleoli (2-5 µ in diameter) and to the high concentration and specificity of localization of the lead phosphate precipitate. QUANTITATIVE DETERMINATIONS : Fig. 11 shows the phosphorus concentration along a line traversing several nucleoli. This is the same specimen as Fig. 3 and corresponds to roots fixed in lead acetate-glutaraldehyde. It is interesting that the intensity in the peaks is approximately equal, indicating that about the same amount of lead phosphate was precipitated per unit volume in all these nucleoli. The average ratio counts per second (cps) of nucleolus to counts per second of cytoplasm was 5.2, with values ranging between 4.0 and 6.3 (corrected for background of 2.8 cps over the nucleolus and 1.0 cps over the cytoplasm). Fig. 12 shows the phosphorus concentration along a similar traverse line of the same "control" section as Fig. 6 and corresponds to roots fixed in glutaraldehyde alone. It is evident that the intensity due to organic phosphorus does not differ greatly along the trace. This indicates that the phosphorus concentration due to the organic material (per unit area) at the nucleolus and at the cytoplasm is nearly the same. Of course, this result applies to cells of the meristematic zone : in these cells-and contrary to those located in the vacuolized zone-the cytoplasm shows a strong basophilia due to RNA and is very rich in ribonucleoprotein granules, as observed with the electron microscope (1). By comparing Figs. 11 and 12, the relative concentration of phosphorus as lead phosphate precipitate can be determined. In both cases, the concentration of organic phosphorus must be the same and the lead phosphate crystals are absent from the cytoplasm of the interphase cells (Fig. 1). Therefore, the relative concentration of phosphorus as lead phosphate precipitate can be expressed as the intensity ratio between nucleolus and cytoplasm of Fig. 11 and subtracting from it the intensity ratio for organic phosphorus (equal to 1, Fig. 12). This gives an average ratio of about THE JOURNAL OF CELL BIOLOGY - VOLUME 42, 1969
6 FIGURE 7 Absorbed electron image taken with the microprobe from a thick 1-µ section of maize root tip fixed in lead acetate, ph 6.0. The outline of one large nucleolus lying inside the elongated nucleus is evident. X 3,800. FIGURE 8 The image for the La-emission wavelength of lead on the same area of the section shown in Fig. 7. X 3,800. FIGURE 9 The image for the KaI-emission wavelength of phosphorus on the same area of the section shown in Figs. 7 and 8. X 3,800. FIGURE 10 The image for the KaI-emission wavelength of sulfur of the same area of the "control" section shown in Fig. 6. X 3,800. C. M. LIBANATI AND C. J. TANDLER Water-Soluble Ions in Nucleus 7 59
7 J I N i I, M(' S ) FIGURE 11 Phosphorus concentration along a 39-µ traverse line on the same area as Figs. 3 and 5. The peaks correspond to a much higher concentration in the nucleoli which is due mainly to lead orthophosphate (compare with Fig. 12 ). The height of the trace corresponds to the relative concentration of element present. I : X-ray intensity (counts per second). I FIGURE 12 Phosphorus concentration along a 39-µ traverse line on the same "control" area as Fig. 6, and corresponds to the organic phosphorus only. I : X-ray intensity (counts per second). (with values ranging from ) for inorganic phosphorus relative to the organic phosphorus. CALCULATIONS : There is no doubt, therefore, that lead acetate fixation preserves a large amount of phosphate, which is not retained by the conventional fixatives, e.g., glutaraldehyde alone versus glutaraldehyde plus lead acetate (1). In the nucleolus, the phosphate due to a water-soluble inorganic orthophosphate pool is three to five times greater than the phosphate due to the macromolecular organic phosphate. Some calculations can be made, assuming that practically all the organic phosphate in the nucleolus is due to RNA (6) and taking published values from the literature. The concentration of protein in the nucleolus is about 500 mg/cm3 (7) (which corresponds roughly to a dry matter of 40%0 ). The RNA concentration is about 10% of the total dry weight, that is, about 50 mg/cm3, or 5 mg/cm3 as RNA phosphorus (taking 10% as the average phosphorus content of RNA ; 8). Therefore, the concentration of the nucleolar inorganic phosphate pool, which is three to five times higher, gives a value of mg/cm 3 (as phosphorus) or about mg/cm3 (as phosphate), which corresponds roughly to a molarity of M. Although the exact value is not known, owing to the uncertainties in the value taken as representative for organic phosphate (or 760 THE JOURNAL OF CELL BIOLOGY. VOLUME 42, 1969
8 for RNA, too), there is no doubt that the concentration is indeed very high. The concentration of inorganic phosphate in the rest of the nucleus is somewhat difficult of estimation with certainty. The lead phosphate crystals, although delimited by the nuclear membrane (Fig. 1), are unevenly precipitated within the nucleus (1). An approximate value was calculated, from the micrograph of Fig. 1, by integration of the height of the densitometric tracings per unit length over nucleolus and nucleus (and over the nucleus without a nucleolus, Fig. 1), using a Chromoscan MK II Densitometer (Joyce, Loebl & Co., Ltd., England) (magnification of the micrograph, X 10,000 ; slit, 0.5 mm in width). The uneven dispersion of the phosphate crystals affected the values obtained. The average concentration ratio of nucleus to nucleolus found by this procedure was about?%, ranging from % to % at different sites within the nucleus. Therefore, the average inorganic phosphate concentration in the nucleus would correspond roughly to < 0.1 M. The water-soluble inorganic orthophosphate concenti ation of the tissue, estimated by direct chemical analysis of the undiluted sap, is about 10-2 M (9). Taking this value as representative for the mean concentration of the whole cell, one can calculate approximately how much inorganic phosphate is present in the nucleolus and nucleus. These structures constitute, respectively, about I and 10% of the total cell volume, and their inorganic phosphate concentration is roughly M and < 0.1 M, respectively. Therefore, about 30 %o and up to 50 %-of the inorganic phosphate of the whole cell is accumulated in the nucleolus. This means that more than 50% of the inorganic orthophosphate ions of the cell are accumulatedand unevenly distributed-in the nucleus. Of course, this is a very rough estimate, but there is no doubt that the concentration in the cytoplasm is quite low compared to that in the nucleus. There is no direct evidence for its exact value, but it is presumably much lower (< 10-2 M) than the mean concentration, since this phosphate concentration should have been detected. What can be said with certainty is that the cytochemical test truly represents the sites in which important amounts of inorganic phosphate are concentrated in the living cell. DISCUSSION Significance of Inorganic Phosphate Compartmentalization in the Intact Cell NUCLEUS AND CYTOPLASM : The present paper, together with the evidence presented in a previous paper (1), demonstrates that the cell nucleus is intimately involved in the accumulation of water-soluble inorganic phosphate ions. The mean intracellular concentration of inorganic orthophosphate is rather high in both plant and animal cells, as indicated by direct chemical analysis of total cell extracts (9, 10). We have found that in maize root tips the average concentration in the nucleus is roughly five to ten times higher than the mean level per total cell. Therefore, the conclusion seems inescapable that the concentration of inorganic phosphate in the cytoplasm must be much lower than the mean cellular level since the nucleus represent about 1070 of the total cell volume. The nuclear membrane-its inner layer (1)- marks the boundary between a nuclear, phosphaterich compartment, and the cytoplasmic compartment with a low orthophosphate level. This finding carries biochemical implications for a number of enzymatic reactions which take place in the cytoplasm, particularly those in which the concentration of orthophosphate controls the reaction rate. Indeed it has been suggested already that compartmentation could control the availability of intracellular orthophosphate (11, 12). Our results provide the evidence for the existence of such a regulation process in the living cell. Of course, other mechanisms for the regulation of enzymatic activity are also operational (13). The importance of inorganic orthophosphate in the economy of the intact cell is clearly evidenced by the well known fact that the concentration of this anion is one of the factors which control the rate of both glycolysis (13, 14) and respiration (15). Some of the other factors are metabolites (AMP, ADP, ATP) which exist in (enzymatic) equilibrium with orthophosphate itself (14, 15). Respiration occurs in mitochondria, while glycolysis apparently takes place in both the cytoplasmic matrix and the nucleus. The distribution of glycolytic enzymes between nucleus and cytoplasm is reported to be similar (16). However, the contribution of the nucleus to the glycolytic ATP formation in the intact cell should be considered also in relation to the availability of both ortho- C. M. LIBANATI AND C. J. TANDLER Water-Soluble Ions in Nucleus 76 1
9 phosphate and glucose, among other parameters. In this connection, it is interesting to quote here that diphosphopyridine nucleotide (DPN), a key coenzyme for both glycolysis and respiration, has been definitely proven to be synthesized in the nucleus (16), and is compartmentalized between the nucleus and cytoplasm (17). The unequal distribution of orthophosphate ions, as reported here, strongly suggests that the nucleus has a regulatory or control function over the phosphate metabolism of the whole cell. We do not have any evidence on the mechanism of entrance of the phosphate ions into the nucleus and whether some type of active transport through the nuclear membrane is involved in building up a concentration gradient. What is evident is that the total cellular inorganic phosphate is not fully available to the cytoplasm. This structural nonavailability presumably is a limiting factor in the rate of glycolysis and respiration, and probably of other reactions in which orthophosphate exists in enzymatic equilibrium with organic phosphates in the cytoplasm. This regulation of the metabolic processes occurring in the cytoplasm could certainly control the phosphate uptake by the intact cell since the translocation of inorganic phosphate ions through the cellular membrane is known to be an active process, i.e., dependent on a functional energyyielding process such as respiration or glycolysis (11, 14, 15). In this connection, it is very suggestive that anucleate cell fragments obtained experimentally from nonphotosynthetic cells such as Amoeba and Stentor showed an extraordinary decline in P uptake, followed by altered levels of ATP and carbohydrate utilization (18, 19). From the high level of inorganic phosphate found within the nucleus, as reported in this paper, a similarly high concentration of cation(s) as counterions is predictable. Fragmentary reports in the literature strongly suggest that the cell nucleus may, indeed, be involved in the accumulation of other ions too. As early as 1949, Abelson and Duryee (20), using the large frog ovarian oocyte, demonstrated a striking accumulation of Na 24 in the nucleus. These experiments were confirmed and extended by Naora et al. (21) who showed an accumulation of both sodium and potassium in the nucleus of the oocyte, the respective concentrations in the nucleus being about five and three times higher than in the cytoplasm. In this large specialized cell, a striking nuclear accumulation was also found for inorganic phosphate and sulfate ions as well as for several aminoacids (21). A relationship between the nucleus and iron uptake has also been described in liver (22) and pea root (23) cells. It is of special interest that all these ions have been traced into the nucleus in the intact cell ; this fact shows that the nuclear membrane is permeable to these ions and, more significantly, that their (mean) concentration in the nucleus is much higher than that in the cytoplasm. In this connection, it is interesting that the cytoplasmic components and the nucleus have different ph values ; the nucleus has a slightly alkaline ph, which is about one unit higher than the ph of the cytoplasmic matrix (24). Rat liver nuclei isolated by the Behrens's nonaqueous procedure were reported to contain also considerable quantities of sodium and potassium (16) as well as inorganic phosphate (25). The ratio nuclei/cytoplasm for sodium and potassium obtained by this technic in rat liver was about 10 and 1.3, respectively (16). INTRANUCLEAR COMPARTMENTATION : The second point demonstrated is that the distribution of water-soluble inorganic orthophosphate ions within the nucleus is not homogeneous. The condensed chromatin appears relatively free of these anions, whereas they are dispersed through the nucleoplasm and are heavily concentrated in the nucleoli (1). The nucleolus boundary itself clearly delimits the regions between a nucleolar, very rich compartment and the extranucleolar compartment with much lower orthophosphate level (Fig. 1). Particularly striking is the accumulation of phosphate ions inside the nucleolus since this structure does not have a limiting membrane. Evidence for the probable existence of a "binder" in the nucleolus itself has been pointed out before (1). Diffusion artifacts as a source of false location for the nucleolus have been eliminated (I) and will not be referred to here. The nucleolus has been demonstrated to supply the cell with the two major ribosomal RNA's found in the cytoplasmic ribosomal subunits (26). The inorganic phosphate level in the nucleoli of maize root tips, as determined with the electron microprobe, represents three to five times the amount of the macromolecular organic phosphate (which is mainly in RNA ; 6). This is roughly of the order expected for a "local" synthesis of RNA, i.e., starting mith the ribonucleotide triphosphates and releasing two phosphate groups (as pyrophosphate). In fact, RNA polymerase (as well as DPN pyrophosphorylase) is concentrated and TIIE JOURNAL OF CELL BIOLOGY VOLUME 42, 1969
10 firmly bound in the nucleolus (27, 28). In this respect, it is interesting that we have not detected in the nucleolus pyrophosphate which would be expected to accumulate through the action of RNA polymerase. This finding probably points to a more complex enzymatic situation, resulting in hydrolysis to inorganic orthophosphate. Indeed, both biochemical and cytochemical evidence clearly has indicated the presence of ATPase(s) (27, 29, 30), ribonuclease (27), and several phosphatases, including pyrophosphatase, (31) in the nucleolus. Of course, other explanations for the accumulation of orthophosphate are possible since we do not have any evidence on the mechanism of entrance of phosphate ions into the nucleolus. For instance, we have found that about 30 %o and up to 50 %-of the total cellular orthophosphate is accumulated in the nucleolus of maize root tip cells, and it is quite possible that this particular pool reflects a general situation found in the phosphorus metabolism of the whole cell. In this phosphorus metabolism, although both inorganic ortho- and pyrophosphate would be expected to exist in enzymatic equilibrium with organic phosphates, the high level corresponds only to the orthophosphate. The amount of inorganic pyrophosphate is negligible, and the orthophosphate accounts for more than half of the total soluble phosphates in maize root tips (9, 32). A similar high level for inorganic orthophosphate has been found also in the liver (10). A second problem that should be considered is the possibility that the inorganic orthophosphate ions of the nucleolus are the immediate precursors of the nucleoside triphosphates. Whether some phosphorylative system (27) or glycolysis (which results in ATP formation) occurs in the nucleolus itself is not known. In fact, it has not been determined whether ATP in the nucleus-which is needed for synthesis of nucleic acid, DPN, or any other reaction-stems from nuclear synthesized ATP or is provided by the cytoplasm (27). The high ionic strength found in the nucleolus could also affect the latency of several enzymes (e.g., ribonuclease ; 27, 33). Craig et al. (34) have found that a high ionic strength suppresses the nonspecific degradation of RNA in isolated nucleoli. From the unequal distribution of orthophosphate anions within the nucleus, a similar distribution of cation(s) as counterions is expected. Some experimental evidence supports such an expectation. Spicer et al. (35), using the pyroantimonate-osmium tetroxide procedure, found electron-opaque deposits, presumably of sodium pyroantimonate, localized mainly in the nucleus ; these precipitates were limited by the nuclear membrane and were heavily deposited in the nucleoli. This pattern of Na+ localization would be consistent with the pattern of localization found for orthophosphate, although some of the counterparts for phosphate could be basic groups of proteins. Regarding this subject, it is interesting to remark that dense electron-opaque inclusions-the "foamy particles"-have been reported in the nucleoli of some cells. Particularly striking are the cases of onion and broad bean root tip cells, in which these "particles" were reported by Lafontaine (36) ; in later work, he could not find them (37). An examination of his papers reveals that these "particles" were found in those cases in which the plants were grown in tissue medium culture, which contains relatively large amounts of divalent cations (e.g., calcium). It is possible that the "foamy particles" represent a precipitation reaction between the high concentration of endogenous nucleolar inorganic orthophosphate and the divalent cations of the culture medium. Whether this takes place in vivo or occurs during the fixation process deserves further study. The high level of inorganic orthophosphate anions in the nucleolus (roughly M), together with their cationic counterparts, strongly suggests a role in the solubilization of ribosomal proteins and other types of protein located in the nucleolus which are known to be rather insoluble in dilute aqueous salt solutions, but soluble in salt solutions of high ionic strength (38, 39). The relatively high intranuclear level of orthophosphate ions certainly could affect the association and dissociation of nucleic acids with proteins. An interesting correlation was suggested (1) with the virtual absence of completed ribosomes in the nucleoli (40-42) or even in the whole nucleus (41) and the dissociating effect of phosphate ions on ribosomes (43). High levels of inorganic phosphate cause also reversible disaggregation of chromatin structures (44). Such a process has been related to activation of repressed chromatin (45). Therefore, regulation of the ionic intranuclear environment may be involved in genetic regulatory mechanisms. It is very suggestive that after partial hepatectomy, in which changes of the regulatory mechanisms seem to be involved, increases in the intracellular C. M. LIBANATI AND C. J. TANDLER Water-Soluble Ions in Nucleus
11 content of inorganic orthophosphate and sodium have been observed that become maximal within 5 or 10 min after hepatectomy (46). In the liver, as well as in a number of other animal tissues, the pattern of inorganic phosphate localization is essentially similar to that in the plant cells (see reference 1). The authors wish to express their thanks to Mr. Tulio Palacios for assistance in the operation of the microanalyzer. Dr. Tandler is an established Investigator of the Consejo Nacional de Investigaciones Cientificas y Tecnicas, Argentine. Received for publication 5 March 1969, and in revised form 21 April REFERENCES 1. TANDLER, C. J., and A. J. SOLARI Nucleolar orthophosphate ions. Electron microscope and diffraction studies. J. Cell Biol. 41 : CASTAING, R Application des sondes electroniques a une methode d'analyse punctuelle 13. chimique et cristallographique. ONERA (Office National d'etudes et de Recherches 14. Aeronautiques), publication No. 55, Chatillonsous-Bagneux, France. 3. ENGEL, W. K., J. S. RESNICK, and E. MARTIN The electron probe in enzyme histo- 15. chemistry. J. Histochem. Cytochem. 16 : HALL, T Some aspects of the Microprobe 16. analysis of biological specimens (1) ; Bibliography, biological applications of electron probe X-Ray microanalysis (II). Cavendish 17. Laboratory, Cambridge (England). Unpublished. 5. HALE, A. J Identification of cytochemical 18. reaction products by scanning X-Ray emission microanalysis. J. Cell Biol. 15 : STERN, H., F. B. JOHNSTON, and G. SETTERFIELD Some chemical properties of isolated pea nucleoli. J. Biophys. Biochem. Cytol. 6 : STENRAM, U Interferometric dry matter 20. determinations on liver nucleoli of protein-fed, protein-deprived, and thyroid-fed rats. Exp. Cell Res. 12 : MAGASANIK, B Isolation and composition of the pentose nucleic acids and of the corresponding nucleoproteins. In The Nucleic Acids. E. Chargaff and J. N. Davidson, editors. Academic Press Inc., New York Ts'o, P. O. P., and C. S. SATO Synthesis of ribonucleic acid in plants. Exp. Cell Res. 17 : SACKS, J Phosphate transport and turnover in the liver. Arch. Biochem. 30 : RACKER, E., and R. Wu Limiting factors in 24. glycolysis of ascites tumour cells and the Pasteur effect. In Ciba Foundation Symposium- Regulation of Cell Metabolism. G. E. Wolstenholme and C. M. O'Connor, editors. J. & A. 25. Churchill Ltd., London LYNEN, F., G. HARTMANN, K. F. NETTER, and A. SCHUEGRAF Phosphate turnover and Pasteur effect. In Ciba Foundation Symposium-Regulation of Cell Metabolism. G. E. Wolstenholme and C. M. O'Connor, editors. J. & A. Churchill Ltd., London STADTMAN, E. R Allosteric regulation of enzyme activity. Advan. Enzymol. 28 :41. MAHLER, H. R., and E. H. CORDES Biological Chemistry. Harper & Row, New York, Evanston, and London, and John Weatherhill, Inc., Tokyo. LEHNINGER, A. L The Mitochondrion. W. A. Benjamin, Inc., New York. SIEBERT, G., and G. B. HUMPHREY Enzymology of the nucleus. Advan. Enzymol. 27 :239. KOHEN, E Pyridine nucleotide compartmentalization in glass-grown ascites cells. Exp. Cell Res. 35 :303. BRACHET, J Biochemical Cytology. Academic Press Inc., New York MAZIA, D Mitosis and the physiology of cell division. In The Cell. J. Brachet and A. E. Mirsky, editors. Academic Press Inc., New York. 3 :362. ABELSON, P. H., and W. R. DURYEE Radioactive sodium permeability and exchange in frog eggs. Biol. Bull. 96 :205. NAORA, H., H. NAORA, M. IZAWA, V. G. ALLFREY, and A. E. MIRSKY Some observations on differences in composition between the nucleus and cytoplasm of the frog oocyte. Proc. Nat. Acad. Sci. U.S.A. 48 :853. BASS, R., and P. SALTMAN The accumulation of iron by rat liver cell suspensions. Exp. Cell Res. 18 :560. POSSINOHAM, J. V., and R. BROWN Intracellular incorporation of Iron-59 into the root cells of Pisum. Nature. 180 :653. CHAMBERS, R., and E. L. CHAMBERS Explorations Into the Nature of the Living Cell. Harvard University Press, Cambridge, Mass. 152 and 177. SIEBERT, G Nuclear enzymes, especially those of energy metabolism. In The Cell Nucleus. Butterworths & Co., Ltd., London THE JOURNAL OF CELL BIOLOGY. VOLUME 42, 1969
12 26. The Nucleolus. Its Structure and Function W. S. Vincent and O. L. Miller, editors. Nat. Cancer Inst. Monogr SIEBERT, G Nucleolar enzymes of isolated rat liver nucleoli. Nat. Cancer Inst. Monogr. 23 : BALTUS, E Observations sur le role biochemique du nucl6ole. Biochim. Biophys. Acta. 15 : COLEMAN, J. R Biochemical and cytochemical demonstration of adenosine triphosphatase activity in nuclei. J. Cell Biol. 27 :20A. (Abstr.) 30. SHIFRIN, N., and L. LEVINE Cytochemical adenosinetriphosphatase in plant root meristem. J. Cell Sci. 3 : PENNIALL, R., J. P. HOLBROOK, N. C. DAVIDIAN, and W. B. ELLIOTT Studies of phosphorus metabolism by isolated nuclei. X. Nucleolar nucleosidediphosphatase activity. Biochim. Biophys. Acta. 157 : TANDLER, C. J The localization of intracellular orthophosphate. The role of the nucleoli. Biochim. Biophys. Acta. 44 : SPITNIK-ELSON, P Fractionation of the proteins of Escherichia coli ribosomes. Biochim. Biophys. Acta. 61 : CRAIG, N. C., M. C. LIAU, and R. P. PERRY In vitro studies of the maturation of ribosomal RNA. J. Cell Biol. 39 :29A. (Abstr.) 35. SPICER, S. S., J. H. HARDIN, and W. B. GREENE Nuclear precipitates in pyroantimonateosmium tetroxide-fixed tissues. J. Cell Biol. 39 : LAFONTAINE, J. G Structure and mode of formation of the nucleolus in meristematic cells of Vicia faba and Allium cepa. J. Biophys. Biochem. Cytol. 4 : LAFONTAINE, J. G., and L. A. CHOUINARD A correlated light and electron microscope study of the nucleolar material during mitosis in Vicia faba. J. Cell Biol. 17 : VINCENT, W. S., E. BALTUS, A. LovLIE, and R. E. MUNDELL Proteins and nucleic acids of starfish oocyte nucleoli and ribosomes. Cancer Inst. Monogr. 23 :235. Nat. 39. MUNDELL, R. E Studies on nucleolar and ribosomal basic proteins and their relationship to nucleolar function. Exp. Cell Res. 53 : NARAYAN, S. K., W. J. STEELE, and H. BuscH Evidence that the granular and fibrillar components of nucleoli contain 28 and 6S RNA, respectively. Exp. Cell Res. 43 : PENMAN, S., I. SMITH, and E. HOLTZMAN Ribosomal RNA synthesis and processing in a particulate site in the HeLa cell nucleus. Science. 154 : PERRY, R. P On ribosome biogenesis. Nat. Cancer Inst. Monogr. 23 : PETERMANN, M. L The Physical and Chemical Properties of Ribosomes. American Elsevier Publishing Co., Inc., New York WHITFIELD, J. F., and A. D. PERRIS Dissolution of the condensed chromatin structures of isolated thymocyte nuclei and the disruption of deoxyribonucleoprotein by inorganic phosphate and a phosphoprotein. Cell Res. 49 :359. Exp. 45. HISTONES. Their Role in the Transfer of Genetic Information Ciba Foundation Study Groups No. 24. A. V. S. de Reuck and J. Knight, editors. J. & A. Churchill, Ltd., London. 46. TSUKADA, K., and I. LIEBERMAN Synthesis of ribonucleic acid by liver nuclear and nucleolar preparations after partial hepatectomy. J. Biol. Chem. 239 :2952. C. M. LIBANATI AND C. J. TANDLER Water-Soluble Ions in Nucleus 765
Materials and Methods. Ribonuclease.--Removal of ribonucleic acid from meristematic onion cells did not show any qualitative differences
 B~U~ Noz~s 129 Alterations in Nuclear Ribonucleic Acid Metabolism Induced by l~inetin.* BY RUTH GUNMAN. From the Department of Genetics, University of California, Be, kezey.~ Kinetin, a substance recently
B~U~ Noz~s 129 Alterations in Nuclear Ribonucleic Acid Metabolism Induced by l~inetin.* BY RUTH GUNMAN. From the Department of Genetics, University of California, Be, kezey.~ Kinetin, a substance recently
ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS. II. Ultrastructure of the Beta Granule
 ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS II Ultrastructure of the Beta Granule MARIE H GREIDER, S L HOWELL, and P E LACY From the Department of Pathology, Washington
ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS II Ultrastructure of the Beta Granule MARIE H GREIDER, S L HOWELL, and P E LACY From the Department of Pathology, Washington
(From the Kerckhoff Laboratories of Biology, California Institute of Technology, Pasadena, and Sacramento State College, Sacramento)
 THE CYTOCHEMICAL LOCALIZATION OF ASCORBIC ACID IN ROOT TIP CELLS BY WILLIAM A. JENSEN,* PH.D., AND LEROY G. KAVALJ-IAN,$ PH.D. (From the Kerckhoff Laboratories of Biology, California Institute of Technology,
THE CYTOCHEMICAL LOCALIZATION OF ASCORBIC ACID IN ROOT TIP CELLS BY WILLIAM A. JENSEN,* PH.D., AND LEROY G. KAVALJ-IAN,$ PH.D. (From the Kerckhoff Laboratories of Biology, California Institute of Technology,
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
the plant & animal cell
 6.1 Basic unit of life Biology Biology Structure & functions of 06 the plant & animal cell In 1665, Robert Hooke observed a section of a cork using a microscope prepared by him. He discovered a structure
6.1 Basic unit of life Biology Biology Structure & functions of 06 the plant & animal cell In 1665, Robert Hooke observed a section of a cork using a microscope prepared by him. He discovered a structure
Methods of Grading S/N Style of grading Percentage Score 1 Attendance, class work and assignment 10 2 Test 20 3 Examination 70 Total 100
 COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
EXTRACTION OF DNA FROM CALF THYMUS CELLS Revised 2/1/96 Introduction
 Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
Copyright 2000-2003 Mark Brandt, Ph.D. 54
 Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Introduction, Noncovalent Bonds, and Properties of Water
 Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline
 AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
CELLS: PLANT CELLS 20 FEBRUARY 2013
 CELLS: PLANT CELLS 20 FEBRUARY 2013 Lesson Description In this lesson we will discuss the following: The Cell Theory Terminology Parts of Plant Cells: Organelles Difference between plant and animal cells
CELLS: PLANT CELLS 20 FEBRUARY 2013 Lesson Description In this lesson we will discuss the following: The Cell Theory Terminology Parts of Plant Cells: Organelles Difference between plant and animal cells
LABORATORY 2 THE CELL CYCLE AND THE STAGES OF MITOSIS LEARNING OBJECTIVES AFTER COMPLETING THIS LABORATORY, YOU SHOULD BE ABLE TO:
 LABORATORY 2 THE CELL CYCLE AND THE STAGES OF MITOSIS LEARNING OBJECTIVES AFTER COMPLETING THIS LABORATORY, YOU SHOULD BE ABLE TO: 1. Describe the cell cycle. 2. Identify stages of mitosis from prepared
LABORATORY 2 THE CELL CYCLE AND THE STAGES OF MITOSIS LEARNING OBJECTIVES AFTER COMPLETING THIS LABORATORY, YOU SHOULD BE ABLE TO: 1. Describe the cell cycle. 2. Identify stages of mitosis from prepared
Investigating cells. Cells are the basic units of living things (this means that all living things are made up of one or more cells).
 SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
The correct answer is d C. Answer c is incorrect. Reliance on the energy produced by others is a characteristic of heterotrophs.
 1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
Cell Unit Practice Test #1
 ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Introduction to the Cell: Plant and Animal Cells
 Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Comparing Plant And Animal Cells
 Comparing Plant And Animal Cells http://khanacademy.org/video?v=hmwvj9x4gny Plant Cells shape - most plant cells are squarish or rectangular in shape. amyloplast (starch storage organelle)- an organelle
Comparing Plant And Animal Cells http://khanacademy.org/video?v=hmwvj9x4gny Plant Cells shape - most plant cells are squarish or rectangular in shape. amyloplast (starch storage organelle)- an organelle
The Nucleus: DNA, Chromatin And Chromosomes
 The Nucleus: DNA, Chromatin And Chromosomes Professor Alfred Cuschieri Department of Anatomy, University of Malta. Objectives By the end of this unit the student should be able to: 1. List the major structural
The Nucleus: DNA, Chromatin And Chromosomes Professor Alfred Cuschieri Department of Anatomy, University of Malta. Objectives By the end of this unit the student should be able to: 1. List the major structural
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Cell Division CELL DIVISION. Mitosis. Designation of Number of Chromosomes. Homologous Chromosomes. Meiosis
 Cell Division CELL DIVISION Anatomy and Physiology Text and Laboratory Workbook, Stephen G. Davenport, Copyright 2006, All Rights Reserved, no part of this publication can be used for any commercial purpose.
Cell Division CELL DIVISION Anatomy and Physiology Text and Laboratory Workbook, Stephen G. Davenport, Copyright 2006, All Rights Reserved, no part of this publication can be used for any commercial purpose.
PART I: Neurons and the Nerve Impulse
 PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
Todays Outline. Metabolism. Why do cells need energy? How do cells acquire energy? Metabolism. Concepts & Processes. The cells capacity to:
 and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Plant and Animal Cells
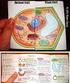 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES. Bio 171 Week 6
 RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 171 Week 6 Procedure Label test tubes well, including group name 1) Add solutions listed to small test tubes 2) For
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 171 Week 6 Procedure Label test tubes well, including group name 1) Add solutions listed to small test tubes 2) For
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
MITOSIS IN ONION ROOT TIP CELLS: AN INTRODUCTION TO LIGHT MICROSCOPY
 MITOSIS IN ONION ROOT TIP CELLS: AN INTRODUCTION TO LIGHT MICROSCOPY Adapted from Foundations of Biology I; Lab 6 Introduction to Microscopy Dr. John Robertson, Westminster College Biology Department,
MITOSIS IN ONION ROOT TIP CELLS: AN INTRODUCTION TO LIGHT MICROSCOPY Adapted from Foundations of Biology I; Lab 6 Introduction to Microscopy Dr. John Robertson, Westminster College Biology Department,
Biology 3A Laboratory MITOSIS Asexual Reproduction
 Biology 3A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
Biology 3A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
Cell Cycle in Onion Root Tip Cells (IB)
 Cell Cycle in Onion Root Tip Cells (IB) A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules,
Cell Cycle in Onion Root Tip Cells (IB) A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules,
RAD 223. Radiography physiology. Lecture Notes. First lecture: Cell and Tissue
 RAD 223 Radiography physiology Lecture Notes First lecture: Cell and Tissue Physiology: the word physiology derived from a Greek word for study of nature. It is the study of how the body and its part work
RAD 223 Radiography physiology Lecture Notes First lecture: Cell and Tissue Physiology: the word physiology derived from a Greek word for study of nature. It is the study of how the body and its part work
Student name ID # 2. (4 pts) What is the terminal electron acceptor in respiration? In photosynthesis? O2, NADP+
 1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
Cell and Membrane Practice. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Summary of Metabolism. Mechanism of Enzyme Action
 Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Carbon Hydrogen Oxygen Nitrogen
 Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
B2 1 Cells, Tissues and Organs
 B2 Cells, Tissues and Organs 5 minutes 5 marks Page of 7 Q. The diagram shows a bacterium. On the drawing, name the structures labelled A, B, C and D. (Total 4 marks) Q2. (a) The diagrams show cells containing
B2 Cells, Tissues and Organs 5 minutes 5 marks Page of 7 Q. The diagram shows a bacterium. On the drawing, name the structures labelled A, B, C and D. (Total 4 marks) Q2. (a) The diagrams show cells containing
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Biology I. Chapter 7
 Biology I Chapter 7 Interest Grabber NOTEBOOK #1 Are All Cells Alike? All living things are made up of cells. Some organisms are composed of only one cell. Other organisms are made up of many cells. 1.
Biology I Chapter 7 Interest Grabber NOTEBOOK #1 Are All Cells Alike? All living things are made up of cells. Some organisms are composed of only one cell. Other organisms are made up of many cells. 1.
Chapter 2 Phosphorus in the Organic Life: Cells, Tissues, Organisms
 Chapter 2 Phosphorus in the Organic Life: Cells, Tissues, Organisms As already mentioned (see Chap. 1 ), in the living cell phosphorus plays a decisive role in three different essential structures: In
Chapter 2 Phosphorus in the Organic Life: Cells, Tissues, Organisms As already mentioned (see Chap. 1 ), in the living cell phosphorus plays a decisive role in three different essential structures: In
Cells. Introduction WSBCTC 1
 Cells Cells are the fundamental unit of life. All living things are composed of cells. While there are several characteristics that are common to all cells, such as the presence of a cell membrane, cytoplasm,
Cells Cells are the fundamental unit of life. All living things are composed of cells. While there are several characteristics that are common to all cells, such as the presence of a cell membrane, cytoplasm,
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
3.1 AS Unit: Cells, Exchange and Transport
 3.1 AS Unit: Cells, Exchange and Transport Module 1: Cells 1.1.1 Cell Structure Candidates should be able to: (a) state the resolution and magnification that can be achieved by a light microscope, a transmission
3.1 AS Unit: Cells, Exchange and Transport Module 1: Cells 1.1.1 Cell Structure Candidates should be able to: (a) state the resolution and magnification that can be achieved by a light microscope, a transmission
Compartmentalization of the Cell. Objectives. Recommended Reading. Professor Alfred Cuschieri. Department of Anatomy University of Malta
 Compartmentalization of the Cell Professor Alfred Cuschieri Department of Anatomy University of Malta Objectives By the end of this session the student should be able to: 1. Identify the different organelles
Compartmentalization of the Cell Professor Alfred Cuschieri Department of Anatomy University of Malta Objectives By the end of this session the student should be able to: 1. Identify the different organelles
Electron Transport Generates a Proton Gradient Across the Membrane
 Electron Transport Generates a Proton Gradient Across the Membrane Each of respiratory enzyme complexes couples the energy released by electron transfer across it to an uptake of protons from water in
Electron Transport Generates a Proton Gradient Across the Membrane Each of respiratory enzyme complexes couples the energy released by electron transfer across it to an uptake of protons from water in
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT
 BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
AP Bio Photosynthesis & Respiration
 AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Multiple Choice Questions
 Chapter 5 THE FUNDAMENTAL UNIT OF LIFE Multiple Choice Questions 1. Which of the following can be made into crystal? (a) A Bacterium (b) An Amoeba (c) A Virus (d) A Sperm 2. A cell will swell up if (a)
Chapter 5 THE FUNDAMENTAL UNIT OF LIFE Multiple Choice Questions 1. Which of the following can be made into crystal? (a) A Bacterium (b) An Amoeba (c) A Virus (d) A Sperm 2. A cell will swell up if (a)
MCAS Biology. Review Packet
 MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
Chapter 9 Cellular Respiration
 Chapter 9 Cellular Respiration Electrons carried in NADH Mitochondrion Glucose Glycolysis Pyruvic acid Krebs Cycle Electrons carried in NADH and FADH 2 Electron Transport Chain Cytoplasm Mitochondrion
Chapter 9 Cellular Respiration Electrons carried in NADH Mitochondrion Glucose Glycolysis Pyruvic acid Krebs Cycle Electrons carried in NADH and FADH 2 Electron Transport Chain Cytoplasm Mitochondrion
Cellular Structure and Function
 Chapter Test A CHAPTER 7 Cellular Structure and Function Part A: Multiple Choice In the space at the left, write the letter of the term or phrase that best answers each question. 1. Which defines a cell?
Chapter Test A CHAPTER 7 Cellular Structure and Function Part A: Multiple Choice In the space at the left, write the letter of the term or phrase that best answers each question. 1. Which defines a cell?
called a cell wall. The cell wall protects against mechanical stress and keeps the cell from becoming over-filled with water.
 What are Cells? By: Byron Norelius About Cells A cell is the basic unit of life. All living organisms are composed of one (unicellular) or more (multicellular) cells. In unicellular organisms, like many
What are Cells? By: Byron Norelius About Cells A cell is the basic unit of life. All living organisms are composed of one (unicellular) or more (multicellular) cells. In unicellular organisms, like many
How To Make A Tri Reagent
 TRI Reagent For processing tissues, cells cultured in monolayer or cell pellets Catalog Number T9424 Store at room temperature. TECHNICAL BULLETIN Product Description TRI Reagent is a quick and convenient
TRI Reagent For processing tissues, cells cultured in monolayer or cell pellets Catalog Number T9424 Store at room temperature. TECHNICAL BULLETIN Product Description TRI Reagent is a quick and convenient
Cell Structure & Function!
 Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d.
 1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
1. A covalent bond between two atoms represents what kind of energy? a. Kinetic energy b. Potential energy c. Mechanical energy d. Solar energy A. Answer a is incorrect. Kinetic energy is the energy of
ANATOMY AND PHYSIOLOGY I (BIO 2311) SYLLABUS
 ANATOMY AND PHYSIOLOGY I (BIO 2311) SYLLABUS NEW YORK CITY COLLEGE OF TECHNOLOGY The City University Of New York School of Arts and Sciences Department of Biological Sciences Course Information Course
ANATOMY AND PHYSIOLOGY I (BIO 2311) SYLLABUS NEW YORK CITY COLLEGE OF TECHNOLOGY The City University Of New York School of Arts and Sciences Department of Biological Sciences Course Information Course
Mitosis in Onion Root Tip Cells
 Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Photosynthesis and Cellular Respiration. Stored Energy
 Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
TOTAL PROTEIN FIBRINOGEN
 UNIT: Proteins 16tproteins.wpd Task Determination of Total Protein, Albumin and Globulins Objectives Upon completion of this exercise, the student will be able to: 1. Explain the ratio of albumin and globulin
UNIT: Proteins 16tproteins.wpd Task Determination of Total Protein, Albumin and Globulins Objectives Upon completion of this exercise, the student will be able to: 1. Explain the ratio of albumin and globulin
DURABILITY OF MORTAR LININGS IN DUCTILE IRON PIPES Durability of mortar linings
 DURABILITY OF MORTAR LININGS IN DUCTILE IRON PIPES Durability of mortar linings I. S. MELAND SINTEF Civil and Environmental Engineering, Cement and Concrete, Trondheim, Norway Durability of Building Materials
DURABILITY OF MORTAR LININGS IN DUCTILE IRON PIPES Durability of mortar linings I. S. MELAND SINTEF Civil and Environmental Engineering, Cement and Concrete, Trondheim, Norway Durability of Building Materials
Cellular Respiration An Overview
 Why? Cellular Respiration An Overview What are the phases of cellular respiration? All cells need energy all the time, and their primary source of energy is ATP. The methods cells use to make ATP vary
Why? Cellular Respiration An Overview What are the phases of cellular respiration? All cells need energy all the time, and their primary source of energy is ATP. The methods cells use to make ATP vary
(From the Department of Biochemistry, University of Nebraska College of Medicine, Omaha)
 THE INHIBITION OF THE ADENOSINE TRIPHOSPHATASE ACTIVITY OF ACTOMYOSIN BY MAGNESIUM IONS BY IRVIN BRAVERMAN A.~ SERGIUS MORGULIS (From the Department of Biochemistry, University of Nebraska College of Medicine,
THE INHIBITION OF THE ADENOSINE TRIPHOSPHATASE ACTIVITY OF ACTOMYOSIN BY MAGNESIUM IONS BY IRVIN BRAVERMAN A.~ SERGIUS MORGULIS (From the Department of Biochemistry, University of Nebraska College of Medicine,
Chapter 9 Mitochondrial Structure and Function
 Chapter 9 Mitochondrial Structure and Function 1 2 3 Structure and function Oxidative phosphorylation and ATP Synthesis Peroxisome Overview 2 Mitochondria have characteristic morphologies despite variable
Chapter 9 Mitochondrial Structure and Function 1 2 3 Structure and function Oxidative phosphorylation and ATP Synthesis Peroxisome Overview 2 Mitochondria have characteristic morphologies despite variable
Look for these related items from Learning Resources :
 Look for these related items from Learning Resources : LER 1901 Cross Section Plant Cell LER 1902 Cross Section Heart Model LER 1903 Cross Section Brain Model LER 2437 Cross Section Earth Model For a dealer
Look for these related items from Learning Resources : LER 1901 Cross Section Plant Cell LER 1902 Cross Section Heart Model LER 1903 Cross Section Brain Model LER 2437 Cross Section Earth Model For a dealer
The Cell: Organelle Diagrams
 The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND
 #3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
#3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
Biology Chapter 7 Practice Test
 Biology Chapter 7 Practice Test Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. The work of Schleiden and Schwann can be summarized by
Biology Chapter 7 Practice Test Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. The work of Schleiden and Schwann can be summarized by
The Huntington Library, Art Collections, and Botanical Gardens
 The Huntington Library, Art Collections, and Botanical Gardens Rooting for Mitosis Overview Students will fix, stain, and make slides of onion root tips. These slides will be examined for the presence
The Huntington Library, Art Collections, and Botanical Gardens Rooting for Mitosis Overview Students will fix, stain, and make slides of onion root tips. These slides will be examined for the presence
7.2 Cells: A Look Inside
 CHAPTER 7 CELL STRUCTURE AND FUNCTION 7.2 Cells: A Look Inside Imagine a factory that makes thousands of cookies a day. Ingredients come into the factory, get mixed and baked, then the cookies are packaged.
CHAPTER 7 CELL STRUCTURE AND FUNCTION 7.2 Cells: A Look Inside Imagine a factory that makes thousands of cookies a day. Ingredients come into the factory, get mixed and baked, then the cookies are packaged.
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman
 SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
HiPer Ion Exchange Chromatography Teaching Kit
 HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
7 Answers to end-of-chapter questions
 7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
Marmara Üniversitesi Fen-Edebiyat Fakültesi Kimya Bölümü / Biyokimya Anabilim Dalı PURIFICATION AND CHARACTERIZATION OF PROTEINS
 EXPERIMENT VI PURIFICATION AND CHARACTERIZATION OF PROTEINS I- Protein isolation and dialysis In order to investigate its structure and properties a protein must be obtained in pure form. Since proteins
EXPERIMENT VI PURIFICATION AND CHARACTERIZATION OF PROTEINS I- Protein isolation and dialysis In order to investigate its structure and properties a protein must be obtained in pure form. Since proteins
The Cell Teaching Notes and Answer Keys
 The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
Biology 101 Chapter 4 Cells as the Basic Unit of Life. The Cell Theory Major Contributors: Galileo = first observations made with a microscope
 Biology 101 Chapter 4 Cells as the Basic Unit of Life The Cell Theory Major Contributors: Galileo = first observations made with a microscope Robert Hooke = first to observe small compartments in dead
Biology 101 Chapter 4 Cells as the Basic Unit of Life The Cell Theory Major Contributors: Galileo = first observations made with a microscope Robert Hooke = first to observe small compartments in dead
III. THE MICROBIAL BIOMASS
 III. THE MICROBIAL BIOMASS Required Readings: Ley, R.E., D.A. Lipson and S.K. Schmidt. 2001. Microbial biomass levels in barren and vegetated high altitude talus soils. Soil Sci. Soc. Am. J. 65:111 117.
III. THE MICROBIAL BIOMASS Required Readings: Ley, R.E., D.A. Lipson and S.K. Schmidt. 2001. Microbial biomass levels in barren and vegetated high altitude talus soils. Soil Sci. Soc. Am. J. 65:111 117.
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
008 Chapter 8. Student:
 008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
VLLM0421c Medical Microbiology I, practical sessions. Protocol to topic J10
 Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
COMPARISON OF PLANT AND ANIMAL CELLS SIMILARITIES IN PLANT & ANIMAL CELLS
 COMPARISON OF PLANT AND ANIMAL CELLS Cells vary widely in structure and function, even within the same organism. The human body, for example, has more than 200 different types of cells, each with a specialized
COMPARISON OF PLANT AND ANIMAL CELLS Cells vary widely in structure and function, even within the same organism. The human body, for example, has more than 200 different types of cells, each with a specialized
TEACHER ACTIVITY GUIDE
 Page 1/5 TEACHER ACTIVITY GUIDE EFFECT OF HEAT & ph ON COLOR & TEXTURE OF GREEN VEGETABLES Taken from IFT Experiments in Food Science Series Color plays a key role in establishing consumer acceptability
Page 1/5 TEACHER ACTIVITY GUIDE EFFECT OF HEAT & ph ON COLOR & TEXTURE OF GREEN VEGETABLES Taken from IFT Experiments in Food Science Series Color plays a key role in establishing consumer acceptability
Review of the Cell and Its Organelles
 Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
Cellular Energy: ATP & Enzymes. What is it? Where do organism s get it? How do they use it?
 Cellular Energy: ATP & Enzymes What is it? Where do organism s get it? How do they use it? Where does Energy come from? Ultimately, from the sun. It is transferred between organisms in the earth s lithosphere,
Cellular Energy: ATP & Enzymes What is it? Where do organism s get it? How do they use it? Where does Energy come from? Ultimately, from the sun. It is transferred between organisms in the earth s lithosphere,
Cells & Cell Organelles
 Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Unit I: Introduction To Scientific Processes
 Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
The Cell. Grade 8 Activity Plan
 The Cell Grade 8 Activity Plan Plant Cell Project Objectives: 1. To identify cell organelles and their functions. 2. To demonstrate the difference between plant and animal cells. Keywords/concepts: cells,
The Cell Grade 8 Activity Plan Plant Cell Project Objectives: 1. To identify cell organelles and their functions. 2. To demonstrate the difference between plant and animal cells. Keywords/concepts: cells,
NUTRITION AND GROWTH OF BACTERIA
 3 NUTRITION AND GROWTH OF BACTERIA 3.1 INTRODUCTION Bacteria are prokaryotic organisms that do not contain chlorophyll. They are unicellular and do not show true branching. They differ from eukaryotes
3 NUTRITION AND GROWTH OF BACTERIA 3.1 INTRODUCTION Bacteria are prokaryotic organisms that do not contain chlorophyll. They are unicellular and do not show true branching. They differ from eukaryotes
 SETSCO SERVICES PTE LTD TEST REPORT MICROSCOPIC ANALYSIS ON THE CONCRETE CORES FROM RETAINING WALL AT CHANGI AIRPORT TERMINAL 3 REVERTON ENGINEERING(S) PTE LTD 1. INTRODUCTION 2. MICROSCOPIC ANALYSIS 3.
SETSCO SERVICES PTE LTD TEST REPORT MICROSCOPIC ANALYSIS ON THE CONCRETE CORES FROM RETAINING WALL AT CHANGI AIRPORT TERMINAL 3 REVERTON ENGINEERING(S) PTE LTD 1. INTRODUCTION 2. MICROSCOPIC ANALYSIS 3.
CLIL lesson for TKT CLIL Chiara Cappa Liceo Scientifico Respighi - Piacenza. CLIL lesson on cells
 CLIL lesson on cells Time: 1 hour Number of students: 20 Age: 14-15 Level: Pre-intermediate (B1) Subject: Biology Learning outcomes: at the end of the lesson students should be able to: o describe the
CLIL lesson on cells Time: 1 hour Number of students: 20 Age: 14-15 Level: Pre-intermediate (B1) Subject: Biology Learning outcomes: at the end of the lesson students should be able to: o describe the
Anabolic and Catabolic Reactions are Linked by ATP in Living Organisms
 Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
