Myosin II-Dependent Cortical Movement Is Required for Centrosome Separation and Positioning during Mitotic Spindle Assembly
|
|
|
- Collin Shaw
- 9 years ago
- Views:
Transcription
1 Cell, Vol. 117, , April 30, 2004, Copyright 2004 by Cell Press Myosin II-Dependent Cortical Movement Is Required for Centrosome Separation and Positioning during Mitotic Spindle Assembly Jody Rosenblatt, 1, * Louise P. Cramer, 1 Buzz Baum, 2 and Karen M. McGee 1 1 MRC Laboratory for Molecular Cell Biology University College London Gower Street London SC1E 6BT 2 Ludwig Institute for Cancer Research University College London Branch 91 Riding House Street London W1W 7BS United Kingdom Summary The role of myosin II in mitosis is generally thought to be restricted to cytokinesis. We present surprising new evidence that cortical myosin II is also required for spindle assembly in cells. Drug- or RNAi-mediated disruption of myosin II in cells interferes with normal spindle assembly and positioning. Time-lapse movies reveal that these treatments block the separation and positioning of duplicated centrosomes after nuclear envelope breakdown (NEBD), thereby preventing the migration of the microtubule asters to opposite sides of chromosomes. Immobilization of cortical move- ment with tetravalent lectins produces similar spindle defects to myosin II disruption and suggests that myo- sin II activity is required within the cortex. Latex beads bound to the cell surface move in a myosin II-depen- dent manner in the direction of the separating asters. We propose that after NEBD, completion of centrosome separation and positioning around chromo- somes depends on astral microtubule connections to a moving cell cortex. Introduction Further analysis of these findings, however, questions the conclusion that myosin II is not involved in other steps of mitosis. For instance, at later time points of myosin II inhibition by antibody injection, the nuclei appeared to fuse together (Kiehart et al., 1982). While these nuclei may have fused after the chromosomes segregated, another possibility is that the chromosomes never initially segregated properly. More recent studies have shown that myosin II disruption after metaphase blocks anaphase as well as cytokinesis and suggest that chromosomes may also have failed to segregate in the earlier myosin II inhibition studies (Komatsu et al., 2000; Silverman-Gavrila and Forer, 2001). Whereas myosin II does not appear to be required for spindle formation in yeast and Dictyostelium, thorough examination of RNAi-depleted myosin II light chain (MLC) from cultured Drosophila cells suggests that it is important for mitosis (Somma et al., 2002). Although DNA duplicated in all cells, only half of the cells became binucleate, sug- gesting that the chromosomes in the other half never segregated. Furthermore, while spindle assembly in ex- tracts has been useful in dissecting many aspects of mitosis, this cell-free system does not replicate all steps in mitosis, such as early prophase centrosomal migra- tion along the nuclear envelope, anaphase B, and cytoki- nesis. One important difference from spindle assembly in a cell is that, in extracts, the spindle lacks a cell cortex and the astral microtubules that normally connect to this cortex. Interactions of astral microtubules with the cell cortex are thought to play an important role in centering and aligning the spindle within the dividing cell. There has been intensive study of spindle alignment during asymmetric cell division, but correct spindle alignment is also important for symmetrically dividing cells. The spindle must be aligned correctly along the length of the cell so that cytokinesis occurs through the short axis or so that daughters remain in the same plane, such as in an epithelium or tissue culture. Further, spindles must be centered within a symmetrically dividing cell so that resulting daughter cells will be the same size. Moreover, because contacts between astral microtubules and the cortex are extensive during prophase, they may be im- portant for spindle assembly as well as for correct spindle positioning within the cell. For these reasons, we have reexamined the role of the cell cortex during spindle assembly. We find that addition of actin-depolymerizing drugs that disrupt the cell cortex (and thus the astral microtubule connections to the cortex) prevent proper spindle formation. Using improved methods to inhibit myosin II within the cortex rapidly and completely, we show that myosin II is re- quired for normal centrosome migration and positioning Microtubules and actin filaments are thought to be re- quired for separate steps in cell division. Microtubules and microtubule motors are required for mitotic spindle formation and chromosome segregation, while actin filaments and myosin II motors are required for cytokinesis. Three lines of evidence have suggested that myosin II is needed only for cytokinesis. First, inhibition of myosin II in amphibian eggs using either anti-myosin II antibod- ies or myosin II fragments allows one cell division and then blocks all further divisions, but these treatments have no apparent effect on spindle formation (Meeusen et al., 1980; Kiehart et al., 1982). Second, myosin II mutations in both fission yeast and Dictyostelium show no mitotic defects (Bezanilla et al., 1997; de Hostos et al., 1993), and RNAi depletion of myosin II presumably only around chromosomes during spindle assembly. We find blocks cytokinesis (Somma et al., 2002). Third, mitotic that cortical myosin II activity is required for the directed spindles can form in concentrated Xenopus egg extracts movement of the cortex during spindle assembly, and, containing high concentrations of actin depolymerising thus, attachment of the two asters to the moving cortex drugs (Sawin and Mitchison, 1991). separates and positions them around the chromosomes during prophase. Without this activity, the spindle fails *Correspondence: [email protected] to correctly assemble and align within the cell.
2 Cell 362 Results mately 50% of cells initiated and completed centrosome movement around the nuclear envelope prior to NEBD. Actin and Myosin II Inhibitory Drugs Disrupt In the other 50% of cells, centrosomes began movement Spindle Assembly along the nuclear envelope but completed migration by To examine whether the actin/myosin-based cell cortex nuclear envelope-independent movement after NEBD. is required for spindle assembly, we disrupted it by de- Nuclear envelope-dependent and -independent pathways pleting actin using the actin-depolymerising drug Latrunculin for centrosome separation have been seen prepleting A. Treatment of PtK2 cells with Latrunculin A viously (Rattner and Berns, 1976; Whitehead et al., 1996). for only 20 min resulted in 48% of spindles forming In control cells, we focused on the second movement, aberrantly (n 1039). These cells did not recover to in which centrosomal migration initiated before and continued form normal spindles while in drug, as longer treatments after NEBD. Here, the two centrosomes migrated in Latrunculin A caused more defective spindles to accu- around the nucleus as the chromosomes condensed mulate (72% after 120 min where n 200). (Figure 2A and Supplemental Movie S1 at We next determined whether the actin cortex is cell.com/cgi/content/full/117/3/361/dc1). Once the nuclear needed for spindle assembly simply because it acts as envelope broke down (0:00), the asters continued a scaffold for astral microtubules or whether myosin to move around the chromosomes (0:31) and aligned II activity within the cortex was also required. To test within the cell (0:48). Soon after the chromosomes whether myosin II is required for spindle formation, we aligned at the metaphase plate, the cell underwent anaphase treated PtK2 cells with drugs that specifically inactivate and then cytokinesis (at 1:20 in Supplemental myosin II within 2 3 min of addition. Y inhibits Movie S1). Rho kinase (ROCK), which in turn blocks myosin II light We discovered that myosin II activity was required for chain kinase (MLCK) and thus myosin II activity. Bleb- centrosome migration around the chromosomes only bistatin specifically inhibits the ATPase of nonmuscle after NEBD. Unfortunately, we could only film cells using myosin II directly (Straight et al., 2003). We treated the Y-27632, as blebbistatin rapidly photo bleached the GFP cells for either 20 min or 120 min with these inhibitors signal. However, because both inhibitors gave similar (or DMSO as a control) and then fixed and immuno- defects in the fixed-cell assay, we expect blebbistatinmediated stained them for nonmuscle myosin II, tubulin, and DNA. inhibition of myosin II would cause defects After 20 min in the presence of drug, 35% 50% of all using a similar mechanism. In a cell treated with Y spindles analyzed had defects (Figure 1D). Compared to at time 0:05 (Figure 2B and see Supplemental Movie S2 a normal control spindle (Figure 1A), defective spindles on Cell website), the two centrosomes halted migration commonly fell into two major categories: lopsided around the chromatin as soon as NEBD occurred (0:00), chromosomes that remain on one side of the asters then the asters remained stuck on one side of the chromosomes instead of aligning between them (Figure 1B) and at (0:15), causing abnormal spindles to form with poles, in which unaligned chromosomes stay at one or lopsided chromosomes (0:51, see Supplemental Movie both centrosomes (Figure 1C). Note that when myosin S2). In this and other movies with Y-27632, the chromo- II activity is inhibited with either blebbistatin or Y-27632, somes would decondense again after 2 4 hr, even astral microtubules still retain contacts with the edge of though asters remained on one side of the chromo- the cell (Figures 1B and 1C). In addition, occasionally somes, and spindle assembly never completed. Furthermore, monoasters or multiple asters also resulted from myosin in the Y treated movies, other events II drug inactivation (not shown). The proportions of ab- normally seen during mitosis still occur, such as cell normal spindles seen after 20 and 120 min of treatment rounding, NEBD, chromosome condensation, and de- are shown in Figure 1D, with the proportions of the four condensation, and normal microtubule dynamics-only types of spindle defects in Figure 1E. In addition to positioning of the two asters around the chromosomes abnormalities in spindle morphology, approximately is blocked. In movies, the spindle defects that typically 19% of the defective spindles were not centered or resulted from Y were lopsided spindles, as seen aligned correctly within the cell. This was probably an in fixed cells (Figure 1B). Sometimes, Y disruption underestimate, because most cells were not large resulted in chromosomes that became stuck at one pole, enough or morphologically polarized enough to detect as seen in Supplemental Movie S3. Here too, centroabnormal spindle positioning (see Figure 1C), but, when somes did not complete migration around the chromothey were, spindle positioning was typically aberrant. somes completely. Although it is not clear why the two These defective spindles did not recover with time, as chromosomes did not align, a likely possibility is that the percentage of spindles with defects accumulated when asters are not symmetrically aligned on either side with longer drug treatments (see Figure 1E, 120 ). As of the chromosomes, the microtubules from one of the myosin II appears to be required for correct spindle asters do not have access to all of the chromosomes. formation, we focused on its role in spindle assembly. The end of Supplemental Movie S3 represents the at poles defect seen in fixed time points in Figure 1C. The Myosin II Is Required for Centrosome Separation at poles defects may eventually recover to form normal and Positioning after NEBD spindles; however, we predict that they typically do not, To assess the role of myosin II during spindle assembly, as long treatments of cells with Y (2 hr) resulted we studied mitotic PtK2 cells expressing EGFP-tubulin in a higher frequency of chromosomal nondisjunction by time-lapse microscopy and disrupted myosin II activthe (18% compared to 4% in DMSO-treated cells). Thus, ity at different times during mitosis. In control cells, there movies demonstrated that both of the main types were two types of centrosomal movement. Approxi- of defects observed in fixed cells (lopsided and at poles)
3 Cortical Myosin II Is Required for Spindle Assembly 363 Figure 1. Drug Inhibition of Myosin II Interferes with Normal Spindle Assembly Confocal projections of PtK2 cells treated for 20 min with 0.1% DMSO (A), 10 M Y (B), or 100 M blebbistatin and immunostained with -tubulin (green) and nonmuscle myosin II antibodies (red) and with Hoechst for DNA. Percentage of abnormal spindles from each drug treatment after 20 and 120 min (D), represented as the mean ( SD) of 1,000 spindles over four experiments. In (E), the types of spindle defects are represented as the mean averaged from over 800 spindles in three experiments resulting from 20 min treatment with DMSO, Y-27632, or blebbistatin. Scale bar, 10 m. appeared to result from a primary defect in completion for the maintenance as well as the formation of a spindle, of centrosome separation and positioning. we added Y to a preformed metaphase spindle Significantly, we found that the Y dependent (Figure 2D and Supplemental Movie S5). The spindle spindle defects were dependent on when NEBD occurred. maintained normal morphology; however, it failed to In six out of six movies where NEBD occurred enter anaphase (1:37), as documented previously (Ko- prior to completion of centrosome separation, spindles matsu et al., 2000). The slight shrinking of the whole did not form correctly, whereas, in seven out of seven spindle seen by 1:37 was due to rounding of the cell, movies where NEBD occurred after centrosomes had as normally occurs during progress through mitosis. completed separation around the nuclear envelope, the Interestingly, at low concentrations of Y (5 25 spindles appeared to form normally. Figure 2C and Supplemental M), spindle assembly and anaphase could be inhibited Movie S4 show an example of a Y without affecting cytokinesis, in which case the cell treated prophase cell ( 0:15) where NEBD occurred would often pinch off between unsegregated chromo- after the centrosomes had completed their migration to somes. The timing from NEBD to cytokinesis was similar opposite sides of the nucleus (0:00), and spindle assembly to control cells, suggesting that the defects did not elicit was apparently normal (0:45). Thus, if centrosomes a checkpoint. At higher concentrations of Y-27632, cyto- could position symmetrically around the chromosomes kinesis was also inhibited. by migrating around the nuclear envelope, myosin II activity was no longer important for spindle assembly. Inhibition of Myosin II Disrupts Spindle Assembly Furthermore, while myosin II activity may contribute to in Round Cells centrosome movement prior to NEBD, its role does not Disruption of myosin II results in cell flattening, which become important until after NEBD occurs. In support of might impede spindle assembly. To circumvent this pos- this, addition of Y had the same effect on spindle sibility, we repeated our drug studies in round cells, assembly, whether it was added 1 hr before NEBD or whose shape was not affected by myosin II disruption. just prior to NEBD. Round B6-8 hybridoma cells were treated with DMSO To determine whether myosin II activity was required (Figure 3A), Y (Figure 3B), or blebbistatin (Figure
4 Cell 364 Figure 2. Drug Inhibition of Myosin II Prevents Completion of Centrosome Separation after NEBD Individual frames from movies show spindle assembly in PtK2 cells expressing GFP-tubulin (green) and Hoechst dye (blue) (A D). In an untreated cell (A) (see Supplemental Movie S1), the centrosomes migrated around the nuclear envelope until it broke down (0:00). The centrosomes were then pulled further around the condensed chromosomes and the spindle aligned (0:48). In a Y treated cell (B) (see Supplemental Movie S2), the centrosomes separated along the nuclear envelope until NEBD occurred (0:00), then centrosome migration halted, and the spindle failed to form normally (0:51). In another Y treated cell (C) (see Supplemental Movie S4), NEBD occurred after completion of centrosome separation around the nuclear envelope (0:00), and the spindle appeared to form normally (0:45). If Y is added to a spindle after it has assembled (D) (see Supplemental Movie S5), the spindle maintains its morphology; however, it does not enter anaphase or cytokinesis. Thus, myosin II activity is essential for the formation of a spindle after NEBD but not for maintenance of preformed spindle. Time in hr:min. 3C) and fixed and stained for actin, tubulin, and DNA. cells, at poles, is difficult to detect in these round cells, While the drug treatments had no effect on cell shape as the condensed chromosomes fill the volume of the (Figures 3B and 3C), they caused more than half of the cell (see Figure 3A for a normal polarized spindle). Thus, spindles to form aberrantly (Figure 3D), suggesting that we suspect that the defects scored in Figures 3D and the effects on spindle formation were direct and not 3E may underestimate the actual numbers of spindle secondary to effects on cell shape. Importantly, disruption defects produced by myosin II inhibition in round cells. of myosin II with either drug mainly produced lop- sided spindles (Figure 3E), the most common type of Inhibition of Myosin II by RNAi Also Disrupts defect seen when spindle assembly is disrupted by myosin Spindle Assembly II inhibition in PtK2 cells (Figures 1B and 1E). The To independently test whether myosin II is required for other predominant spindle defect normally seen in PtK2 normal spindle formation, we specifically knocked down
5 Cortical Myosin II Is Required for Spindle Assembly 365 Figure 3. Drug Inhibition of Myosin II Interferes with Normal Spindle Assembly in Round Cells Confocal projections of B6-8 hybridoma cells treated with 0.1% DMSO (A), 10 M Y (B), or 100 M blebbistatin (C) and immunostained with -tubulin antibody (turquoise), Hoechst for DNA (blue), and phalloidin for actin (red). Scale bar, 10. The defects in both (B) and (C) show lopsided chromosome spindles, as seen in the flatter PtK2 cells. Because of the angle viewed and the roundness of the cell, the poor separation of the two centrosomes can be seen more clearly in (C) than in (B). Percentage of abnormal spindles from each drug treatment after 4 hr represented as the mean ( SD) of 100 spindles over three experiments (D). As in other cells, lopsided chromosomes are the most common spindle defects seen in round cells (E). Crosslinking the Cell Surface with Lectins Also Inhibits Completion of Centrosome Migration Although myosin II localizes mainly to the cortex during mitosis, some of it also localizes diffusely within the cytoplasm (see Figure 1A). To test whether the spindle defects seen above resulted from inhibiting myosin II within the cortex, we added extracellular tetravalent lectins to inhibit myosin II-dependent movement specifi- cally in the cortex. Wheat germ agglutinin (WGA) and concanavilin A (Con A), when added at high concentra- tions to the cell medium, bind to cell surface glycoproteins and act to effectively crosslink the cell cortex. These lectins have been shown to block the actin- and myosin II-dependent cortical flow seen in Xenopus the expression of myosin II using RNAi in the Drosophila melanogaster S2R cell line. Double-stranded RNAi encoding GFP (control, Figure 4A), myosin II heavy chain (zipper, Figure 4B), or MLC (squash, Figure 4C) was added to the culture medium for 2, 4, 6, 8, or 10 days. The cells were then fixed and stained for actin, tubulin, and DNA. The immunoblot in Figure 4C shows that approximately 96% of cellular myosin II heavy chain was depleted by 6 days of treatment of myosin II heavy chain RNAi. It was also clear that myosin II heavy chain and light chain were depleted by RNAi, as cytokinesis was blocked in these cells compared to the untreated and GFP RNAi-treated control cells (Figure 4E). To avoid scoring aberrant spindle structures that resulted from excess centrosomes due to cytokinesis failure, we scored the spindle defects on day 4 or 6, when the first cytokinesis defects became apparent. We also omitted spindles containing multiple but well-formed asters typically seen in multinucleate cells. At day 4 6 of RNAi disruption of myosin II heavy chain or light chain, 65% 70% of spindles were defective in morphology (Figure 4E). As with drug disruption of myosin II in hybridoma or PtK2 cells, the most common spindle defect seen with either myosin II heavy or light chain RNAi was lopsided chromosomes (see Figures 4B and 4F). The range of spindle defects was also similar, except that multiple asters were more frequent in cells resulting from myosin II heavy chain RNAi treatment (Figure 4F). This was likely due to the fact that depletion of myosin II, rather than inactivation caused by drug inhibition or depletion of the light chain, structurally compromised the integrity of the cortex (notice decreased cortical actin staining in the cell with multiaster in Figure 4C). We suspect the multiaster defect is due to the ability of microtubules to self organize into asters in mitotic cytoplasm when not constrained by cortical contacts, as seen in in vitro spindle assembly assays using concentrated extracts (Sawin and Mitchison, 1991). Controls cells where no RNAi (data not shown) or GFP RNAi (Figure 4A) was added to S2R cells produced normal spindles (Figure 4E), suggesting that the spindle defects were caused from knocking down myosin II specifically.
6 Cell 366 Figure 4. RNAi Depletion of Myosin II Disrupts Spindle Formation in Drosophila Cells in Culture SR2 cells were treated with dsrna encoding GFP (A), myosin II light chain (MLC) (B), or myosin II heavy chain (MHC) (C) for 4 6 days and immunostained with antibody to -tubulin (green), phalloidin for actin (red), and Hoechst for DNA (blue). Scale bar, 5 m. The most common defects seen were lopsided chromosomes (B) and multiasters (C). Note that with the multiaster defect, the cortex is typically depleted of actin, as in (C). Immunoblot showing MHC is largely depleted after 6 days of RNAi treatment compared to the control lane, where tubulin antibodies are used for loading control (D). Percentage of spindle and cytokinesis defects seen after 4 6 days of RNAi treatment with each dsrna (E) shown as the mean ( SD) of three experiments from over 150 spindles. RNAi depletion of MHC and MLC causes spindle defects similar to myosin II disruption by drugs (F). (Figure 5C). That 50% 60% of total spindles were defective when cells were treated with tetravalent lectins is consistent with the analysis of live cells (see above) in which spindle defects only occurred the 50% of the time that NEBD occurred prior to completion of centrosome migration. Consistent with the idea that the lectins interfered with spindle assembly by crosslinking the cortex, the tetravalent lectins WGA and Con A were more effective at disrupting spindle formation than was the same concentration of succinyl Con A, a divalent lectin that is less efficient at crosslinking the cortex (Figure 5B). The Cortex Moves in the Direction of Centrosome Migration during Spindle Assembly and Positioning To examine how cortical myosin II activity might contrib- ute to the movement of centrosomes during spindle assembly, we visualized cortical movement by adding fluorescent latex beads to the surface of PtK2 cells expressing EGFP-tubulin. Fluorescent latex beads have been shown to bind cell surface receptors associated with the underlying cortical actin cytoskeleton during mitosis and have therefore been used to track cortical movement, or flow (Wang et al., 1994). In the control cell in Figure 6A and Supplemental Movie S7, red latex beads on the cell surface moved in the direction of centrosome migration during spindle formation and po- laevis oocytes (Canman and Bement, 1997; Tencer, 1978). Figure 5A and Supplemental Movie S6 show an EGFP-tubulin-expressing PtK2 cell treated with WGA, where NEBD has occurred prior to completion of centrosome migration. As with Y treated cells, centrosomes do not complete migration around the chromosomes after NEBD but instead stay stuck on one side, producing a lopsided chromosome spindle defect. Similarly, the inhibition of cortical movement with lectins only inhibited spindle assembly in cases in which the centrosomes had not completed migration at the time of NEBD. In movies of spindle assembly with WGA, when NEBD occurred prior to completion of centrosome migration, eight out of eight spindles were disrupted, whereas zero out of nine spindles were disrupted when NEBD occurred after centrosome migration was complete. To quantify the spindle defects resulting from lectin treatments, we analyzed the percentage of spindle defects and the frequency of different types of spindle defects in fixed PtK2 cells pretreated with either WGA or Con A (Figures 5B and 5C). As with myosin II inhibition, approximately 50% of spindles were defective (Figure 5B), and the lopsided chromosome defect was the most common type of defect detected, comprising 33% of all WGA-treated and 41% of all Con A-treated spindles, or greater than 66% of the total spindle defects seen
7 Cortical Myosin II Is Required for Spindle Assembly 367 Figure 5. Inhibition of Cortical Flow by Addition of Tetravalent Lectins Inhibits Centrosome Separation around Chromosomes Stills from Supplemental Movie S6 show defective spindle assembly in the presence of 200 g/ml WGA (A). Once NEBD occurs at 0:00, centrosomes halt migration around the condensed chromosomes and produce a lopsided chromosome defect (0:20), which is maintained as the chromosomes begin to decondense (1:04). The percentage of spindle defects after addition of the tetravalent lectins WGA and Con A and the divalent lectin Succinyl Con A (B), shown as the mean ( SD) of three experiments from over 400 spindles. As with myosin II inhibition, the most common defect scored with either tetravalent lectin was lopsided chromosomes (C). sitioning after NEBD. Bead movement is best illustrated by the bead tracks (yellow arrows) made throughout the course of spindle formation. The end points are marked by arrowheads, and the outline of the cell is traced in yellow at the beginning frame of the movie (Figure 6B). Note that the greatest cortical movement is restricted to the middle region of the cell (yellow arrows), where the centrosomes (white arrows) are also moving, and that there is little movement outside of this zone (blue arrows). Movement of the beads along the length of the cell does not reflect a stretching of the cell, as the cell actually contracts slightly over time. Instead, it reflects the movement of the cortex in the direction of centrosome separation and positioning. From the movie (see Supplemental Movie S7), there appear to be two phases of cortical movement: a rapid, early movement perpendicular to the length of the cell that correlates with spindle formation and then a slower movement parallel to the length of the cell that coincides with placement of the spindle within the cell. These two phases of cortical movement are also illustrated in the yellow bead tracks (Figure 6B), which, during spindle assembly, move to the left and up, near the left centrosome, and to the right and down, near the right centrosome. Then, during spindle positioning, all the lower yellow tracks move downward along the length of the cell (Figure 6B). The length of the tracks from the beads and the centrosomes are approximately the same length, suggesting that the rates of movement from the two are also similar. When myosin II is blocked by addition of Y-27632, centrosome movement after NEBD is blocked, as is movement of the cortex (marked by the red latex beads) (Figure 6C and Supplemental Movie S8). The track marks in Figure 6D show that very little movement of the beads occurs throughout the period filmed, either close (yellow arrows) or distant (blue arrows) to the centrosomes (white arrows). Kymographs also depict the movement of beads within one xy plane. Here, the position of beads through a given line (represented by hatched lines in Figures 6A [0:00] and 6C [0:00]) is tracked in space and time (Figures 6E and 6F, where distance x axis, and time y axis, from top to bottom). Thus, a diagonal line represents movement of a bead or bead cluster, whereas a vertical line represents a static bead. Slower movement produces a diagonal line with a steeper slope, and faster movement produces a shallower slope. Kymographs of the beads on the control cell in Figure 6A show that beads within the middle region near centrosomes migrate outward from the middle of the cell first rapidly at 0.6 m/min for about 20 min during spindle formation and then more slowly at 0.2 m/min for about 40
8 Cell 368 Figure 6. Myosin II-Dependent Cortical Contraction Is Localized during Mitosis and Results in Movement of the Cortex in the Direction of Centrosome Migration Stills from movies of spindle assembly in PtK2 cells, expressing EGFP-tubulin (green) with Hoechst for chromosome staining (blue) and red fluorescent latex beads added to the cell surface to mark cortical movement (A and C). In the control cell (A and Supplemental Movie S7), red beads migrate in the same direction as centrosomes during spindle assembly ([A], 0:00 0:20) and positioning ([A], 0:20 0:60), where representative beads are marked by white stars (during assembly) and yellow stars (during positioning). Stills from Supplemental Movie S8 of a Y treated cell show no movement of centrosomes or beads (C) where representative beads are marked by white stars. Tracks made by individual bead (yellow and blue arrows) and centrosome (white arrows) movements in the control (B) and Y treated cell in (D) have been normalized for cell rounding, where the yellow outline traces the cell at time 0:00. In the control (B), centrosomes (white arrows) move in the same direction and at the same rate (i.e., arrows have similar length) as beads near centrosomes (yellow arrows), whereas beads distant to centrosomes (blue arrows) in control and the beads in the Y treated cell (D) show little movement. Kymographs (positiontime plots) showing the movement of beads on the cells in (A) and (C) ([E] and [F], respectively). Beads near centrosomes (along the dashed line in middle of cell in [A]) move rapidly during spindle assembly (0:00 0:20) and slowly during spindle positioning (0:20 0:60) (E). Reduced bead movement in both the cortex distant to centrosomes in the control cell (right dashed line in [A]) and in a Y treated cell ([C], dashed line) produce comparatively vertical lines in kymographs ([E] and [F], respectively). A kymograph through a typical interphase cell shows no bead movement (G). Contraction/expansion of cell cortex, marked with beads during spindle assembly after NEBD in a control cell (H and I). Stills of Supplemental Movie S9 indicate the direction of centrosome separation during spindle assembly (H). Kymographs show beads on the side of the cortex nearest separating centrosomes ([H], blue hashed line) move apart ([I], top left), while beads on the other side of the separating centrosomes ([H], yellow hashed line) contract inward ([I], bottom right). Scale bars, 10 m. found that in four out of four control movies, bead movement occurred in the same direction and time as centro- some movement, whereas in three out of three movies, when spindle assembly was blocked with Y-27632, bead movement was also blocked. In addition, we found that cortical movement, as measured by bead kymographs, was restricted to mitotic PtK2 cells. In Figure 6G, we show a typical kymograph through the middle of an min during subsequent positioning of the spindle within the cell (Figure 6E, middle). By contrast, the kymograph from the top right of the cell, distant to centrosomes, in Figure 6A indicates little movement of beads within this zone. Kymographs of the regions indicated in the myosin II-inhibited cell in Figure 6F (0:00), near centrosomes, with only vertical lines, show no bead movement. By measuring movement of beads using kymographs, we
9 Cortical Myosin II Is Required for Spindle Assembly 369 interphase PtK2 cell, where vertical lines indicate no bead movement. Findings were similar in five other interphase cells. Coordinated Cortical Expansion and Contraction Directs Cortical Movement How could movement of the cortex be regulated? One possibility is that movement occurs only locally at sites near the centrosomes. Another possibility is that the cortex is circumferentially moving. Expansion of the cor- tex on the side of the cell near centrosomes is driven by contraction on the opposing side. Because Figure 6A shows bead movement on the top of the cell, cortical movement cannot be viewed on the opposing side of the cell that is attached to the glass substrate. Supplemental Movie S9 and representative stills in Figure 6H show cortical movement on both sides of the cell during spin- dle formation. The bead movement is difficult to detect over the short time sequence (10 min) of Supplemental Movie S9 but, rather, provides a reference to show the direction of centrosome migration as the spindle assem- bles and for the kymographs. The kymograph in Figure 6I depicting bead movement from the top left region of the cell (indicated by the blue hatched line in frame 0:00 of Figure 6H) shows that the cortex is expanding in the half of the cell encompassing the separating centro- somes (from centrosome to centrosome). By contrast, the kymograph of bead movement from the bottom right half of the cell (indicated by the yellow hatched line) shows that the cortex is contracting on the side opposite to where the centrosomes separate. Coordinate cortical expansion and contraction were seen consistently dur- ing spindle assembly after NEBD in three out of three movies where both sides of the cell could be viewed as the spindle formed. These results are consistent with myosin II activity, acting to cause a sink of focused cortical contraction on one side of the cell, which then pulls apart the cortex on the opposing side of cell, nearest to centrosomes. Because the centrosomes are attached to the cortex via astral microtubules, the resulting expansion of the cortex drives centrosome separation. Discussion Cortical Actin and Myosin II Are Required for Centrosome Separation after NEBD As shown in the model in Figure 7A, centrosomes can separate along the nuclear envelope in the absence of myosin II activity before NEBD. The cortical-independent centrosome separation along the nuclear envelope presumably relies on dynein and dynactin, which localize to the nuclear envelope prior to NEBD (Busson et al., 1998), as well as on the Eg5/BimC class of kinesins. While the forces required for centrosome migration around the nucleus are not well understood, some evidence for the importance of dynein and dynactin comes from studies in which inhibition of dynein results in monopolar spindles and detachment of the centro- somes from the nuclear membrane (Robinson et al., 1999; Vaisberg et al., 1993). In addition, inhibition of the BimC/Eg5 motors results in monoaster formation and While it was previously established that mitotic spindle assembly depends on microtubules and their associated motor proteins, our findings show that assembly also depends on cortical myosin II. Using several methods to inhibit myosin II and cortical movement, we find that continued separation and positioning of centrosomes around the chromosomes depends on myosin II in the cortex. Cortical myosin II activity drives circumferential movement of the cortex away from attached asters, which acts to separate these asters around the condensed chromosomes. This cortex-dependent movement becomes critical when other mechanisms of separating centrosomes along the nuclear envelope are lost upon NEBD. Because we have seen this dependence in human, rat, and Drosophila cell lines, we think that it is likely to be important for all cells in which the nuclear envelope breaks down during mitosis. Myosin II Inhibition It is surprising that a requirement for myosin II in spindle formation has not been previously detected. Previous genetic studies showed that myosin II mutations in S. pombe (Bezanilla et al., 1997), D. discoidum (de Hostos et al., 1993), and C. elegans (Shelton et al., 1999) produced only cytokinesis defects. However, the nuclear envelope does not break down during mitosis in S. pombe and D. discoidum, and it does so only just prior to anaphase in C. elegans (Lee et al., 2000). Since the spindle defects we see are dependent on NEBD, we would not expect myosin II mutations to cause spindle defects in any of these organisms. In other multicellular organisms where NEBD occurs prior to metaphase, we would expect about half the cells with myosin II muta- tions to be able to form normal spindles and then to fail at cytokinesis. The remaining cells would form abnormal spindles, which would then be removed by apoptosis and phagocytosis, making a spindle defect phenotype difficult to score. Indeed, in cells subjected to long-term treatment with myosin II RNAi, we found that few cells survived but that those that did were very large and contained many nuclei and engulfed apoptotic bodies (our unpublished data). Additionally, maternal wild-type protein might confound the analysis of such mutants; the building of new structures such as the cytokinetic ring may require more myosin II than the maintenance of a preformed cell cortex. Thus, embryos with myosin II mutations would most likely display a primarily cytoki- nesis-defective phenotype. A directed study of myosin II in single cells used inhibi- tory antibodies (Kiehart et al., 1982), which inhibited cytokinesis only after 1 2 cell divisions and may inhibit myosin II less effectively in a preformed structure such as the cortex than in an assembling contractile ring. Furthermore, as nuclei were seen to fuse at later times, it is likely that this inhibition eventually blocked mitosis. Although the myosin II inhibitors Y and blebbi- statin are relatively new drugs and have not previously been used in spindle-assembly studies, RNAi depletion confirms that these defects result specifically from myo- sin II inhibition. Thus, discovery of a new role for myosin in spindle formation has been enabled by new techniques that more rapidly and effectively inhibit myosin II.
10 Cell 370 Figure 7. Model for the Role of Myosin II-Dependent Cortical Movement in Aster Separation and Spindle Positioning during Spindle Assembly Before NEBD, the two asters can separate by moving along the nuclear envelope (A). This movement likely depends on dynein/dynactin complexes at the nuclear envelope and the pushing apart of antiparallel microtubules by Eg5-type motors. Once NEBD occurs, further separation of the asters around the chromosomes and correct spindle placement within the cell require myosin II activity within the cell cortex (B). Localized cortical contraction away from separating asters (indicated by straight black arrows) drives expansion of the cortex nearest asters, which via astral microtubule connections to this expanding cortex acts to separate the asters around the chromosomes (curved black arrows). As microtubule contacts to the cortex are inhibitory to cortical contraction, contraction would be limited near astral microtubules, thereby allowing net expansion of the cortex at this site (arrowheads). Cortical contraction becomes balanced and stops once the number of astral microtubules becomes equally balanced on either side of the cell, which consequently stops migration of asters. Blocking cortical movement still maintains astral microtubule connections to the cortex but prevents aster separation so that spindle assembly typically halts in the lopsided chromosomes position seen in (B). myosin II-based purse-string contraction at the wound site acts to pull in cortical actin filaments as well as the microtubules that are attached to the actin filaments. In our studies using beads to visualize the location and direction of cortical movement during spindle assembly, contraction of the cortex on the side of the cell opposite to where centrosomes are separating causes a coordinate expansion of the cortex on the side where centro- somes are migrating apart (Figure 7B). Although we know that the cortex moves in conjunc- tion with centrosome separation and that this separation is dependent on cortical movement, we do not yet un- derstand what regulates the direction of cortical movement or what stops this movement once centrosome migration and positioning are complete. One attractive model is that the astral microtubules might be responsi- ble for this regulation. Microtubule binding to the cortex has been shown to decrease cortical flow rates (Benink et al., 2000; Canman and Bement, 1997; Hird and White, 1993). Thus, in our model (Figure 7B), astral microtubule connections to the cortex would locally dampen myosin II activity and thereby cortical contraction at that loca- tion, whereas contraction elsewhere would be unaffected. Hence, localized cortical contraction distal to the asters (inward arrows) would drag astral microtubules (arrowheads) toward the site of contraction (inward arrows) and thereby separate centrosomes around the condensed chromosomes (curved arrows). Drag force would cause compensatory expansion of the cortex proximal to the asters (arrowheads), as we have ob- served. As cortical contraction is suppressed near astral microtubules and enhanced away from them, the circumferential contractile force of the cortex would be weak between the separating asters and strong away from them (arrows). When the spindle poles reach the opposite ends of the cell, astral microtubule contacts to the cortex would become equal and opposite so that the cortical contraction would be balanced, thereby stopping further centrosomal migration. As suggested previously, this same mechanism may also act later dur- suggests that these motors contribute to centrosome separation by pushing antiparallel microtubules apart (Kapoor et al., 2000; Sawin et al., 1992). After NEBD, dynein and Eg5 motors are no longer sufficient for centrosome separation, which now depends upon astral microtubule connections to the cortex (Figure 7B). Although actin and myosin II may also contribute to centrosome separation prior to NEBD, this contribution only becomes apparent after NEBD. It is also possible that at approximately the same time as NEBD occurs, Eg5 motors run out of antiparallel microtubules to push against, an event less obvious than NEBD. In support of this possibility, we find that if we produce monoasters with no nuclear envelope by adding the Eg5 inhibitor monastrol (Kapoor et al., 2000), these monoasters can be rescued only to the lopsided chromosome defect if monastrol is washed out in the presence of a myosin II inhibitor (our unpublished data). How might cortical actin and myosin II contraction bring about centrosome separation? One possibility is that myosin II directly moves astral microtubules along cortical actin filaments. However, to date there are no clear examples of myosin II motors directly moving microtubules on actin filaments (Rodriguez et al., 2003). An alternative model is that astral microtubules bind to a moving cell cortex (Figure 7B). This model is supported by our findings that cell surface bound fluorescent latex beads that mark the cell cortex move in the direction of the separating centrosomes. Furthermore, myosin II inhibitors or tetravalent lectins that block this cortical movement also block centrosome separation. From previous studies on directed cortical movement, termed cortical flow, we predict that myosin II-based contraction acts to pull astral microtubules in the plane of the cortex toward the site of contraction (Figure 7B) (Hird and White, 1993; Mandato and Bement, 2003). This type of mechanism has been shown to cause the directed movement of microtubules along cortical actin filaments during wound healing experiments in Xenopus oocytes (Mandato and Bement, 2003). In these studies,
11 Cortical Myosin II Is Required for Spindle Assembly 371 Experimental Procedures ing cytokinesis to position actin and myosin II contraction between the spindle poles, where fewer microtu- bules contact the cortex (Hird and White, 1993; Mandato et al., 2000). While regulation of cortical movement requires further investigation, our results underscore the importance of cortical myosin II forces for assembling a spindle and provide a new mechanism that accounts for how this newly forming spindle becomes correctly positioned within the framework of a cell. microscope using a Bio-Rad multiphoton confocal. Tracking of beads and kymograph analysis were done using Metamorph software (Universal Imaging). Acknowledgments Cell Culture PtK2 cells were cultured in DMEM and B6-8 hybridoma cells in RPMI medium with 10% fetal bovine serum and 100 g/ml penicillin/ streptomycin (all from GIBCO BRL) at 5% CO 2 and 37 C. Drosophila S2R cells were cultured in 40% S2 and 60% SFM medium with 10% fetal bovine serum and 100 g/ml penicillin/streptomycin (all, GIBCO/BRL) at 25 C. Received: May 27, 2003 Transfections Revised: February 25, 2004 An EGFP-tubulin plasmid (Clontech) was transfected into PtK2 cells Accepted: February 25, 2004 using a Lipofectamine Plus Kit (GIBCO BRL) and selected using 100 Published: April 29, 2004 g/ml G418 (GIBCO BRL). We thank Martin Raff, as always, for his generous support and lively discussions about the work and manuscript. We also thank Timothy Mitchison for helpful discussions about experiments and the manu- script and (also Aaron Straight) for generously contributing blebbi- statin. We thank Veronica Dominguez for help with RNAi preparation; Christine Field for Drosophila myosin II antibody; and James Blyth, Helen Dawe, Alison Lloyd, and Mike Redd for comments on the manuscript. This work was supported by an American Cancer Society postdoctoral fellowship, a Cancer Research UK project grant, and a BBSRC project grant to J.R.; by Royal Society University Research Fellowships to both B.B. and L.P.C.; and by a Wellcome Trust project grant (to L.P.C.). J.R. wishes to dedicate this work in loving memory to Frances Turner my love is with you always. References Cell Staining Cells were fixed with 100% methanol at 20 C for 2 min, rinsed Benink, H.A., Mandato, C.A., and Bement, W.M. (2000). Analysis of three times in phosphate buffered saline (PBS), permeabilized for cortical flow models in vivo. Mol. Biol. Cell 11, min with PBS containing 0.5% Triton X-100, and blocked in AbDil (PBS with 0.1% Triton X-100 and 2% bovine serum albumin). Cells Bezanilla, M., Forsburg, S.L., and Pollard, T.D. (1997). Identification were stained with 1:100 anti-tubulin ascites (Sigma, DM1 ), 1 g/ of a second myosin-ii in Schizosaccharomyces pombe: Myp2p is ml Hoechst (Sigma), and either 1:50 nonmuscle myosin II polyclonal conditionally required for cytokinesis. Mol. Biol. Cell 8, antibody (Biogenesis) or 1:200 actin polyclonal antibody (Sigma, Busson, S., Dujardin, D., Moreau, A., Dompierre, J., and De Mey, A2066). J.R. (1998). Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8, RNAi Treatment Canman, J.C., and Bement, W.M. (1997). Microtubules suppress RNAi depletion of GFP, MHC, and MLC was carried out according actomyosin-based cortical flow in Xenopus oocytes. J. Cell Sci. to the methods in Kiger et al. (2003). For protein detection on immu- 110, noblots, approximately 5 g of control or 6 day RNAi-depleted MHC de Hostos, E.L., Rehfuess, C., Bradtke, B., Waddell, D.R., Albrecht, lysates were loaded on gels and probed with antibodies to D. mela- R., Murphy, J., and Gerisch, G. (1993). Dictyostelium mutants lacking nogaster myosin II heavy chain (1:5000, a generous gift from Christhe cytoskeletal protein coronin are defective in cytokinesis and cell tine Field) and to tubulin (1:1000 DM1 [Sigma]). motility. J. Cell Biol. 120, Drug Treatment Hird, S.N., and White, J.G. (1993). Cortical and cytoplasmic flow Cells were treated with 100 M blebbistatin (a generous gift from polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Aaron Straight and Timothy Mitchison), 5 M Latrunculin A (Calbiochem), Biol. 121, and 10 M Y (Tocris) for the times indicated. Kapoor, T.M., Mayer, T.U., Coughlin, M.L., and Mitchison, T.J. (2000). Probing spindle assembly mechanisms with monastrol, a small mol- Image and Movie Acquisition ecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150, Fluorescence micrographs were obtained using a Nikon Eclipse Kiehart, D.P., Mabuchi, I., and Inoue, S. (1982). Evidence that myosin e800 microscope and captured using a SynSys cooled charge cou- does not contribute to force production in chromosome movement. pled device (CCD) camera (Roper Scientific). Time-lapse movies J. Cell Biol. 94, were made using an Axiovert microscope (Zeiss) and captured using Kiger, A., Baum, B., Jones, S., Jones, M., Coulson, A., Echeverri, a Micromax CCD camera (Roper Scientific). Metamorph software C., and Perrimon, N. (2003). A functional genomic analysis of cell (Universal Imaging) was used to control the camera and to process morphology using RNA interference. J. Biol. 2, 27. images. Confocal micrographs were obtained using a Bio-Rad multiphoton and Nikon microscope, and Z sections were recon- Komatsu, S., Yano, T., Shibata, M., Tuft, R.A., and Ikebe, M. (2000). structed using Confocal Assistant (BioRad). All images were promitosis Effects of the regulatory light chain phosphorylation of myosin II on cessed further using Adobe Photoshop and Illustrator. and cytokinesis of mammalian cells. J. Biol. Chem. 275, Latex Bead Movies Lee, K.K., Gruenbaum, Y., Spann, P., Liu, J., and Wilson, K.L. (2000). A coverslip of PtK2 cells expressing EGFP-tubulin was briefly C. elegans nuclear envelope proteins emerin, MAN1, lamin, and washed with PBS and incubated with a mixture of sonicated red nucleoporins reveal unique timing of nuclear envelope breakdown fluorescent latex beads (50 l of 1 red fluorescent latex beads during mitosis. Mol. Biol. Cell 11, [Sigma] in 400 l 10mg/ml BSA in PBS) for 2 min and washed twice Mandato, C.A., and Bement, W.M. (2003). Actomyosin transports with chamber medium (1:1 D-MEM:F-12 medium without phenol red microtubules and microtubules control actomyosin recruitment durand HEPES buffered 10% fetal calf serum 100 g/ml penicillin/ ing Xenopus oocyte wound healing. Curr. Biol. 13, streptomycin [all from GIBCO BRL] 1 g/ml Hoechst 33342). The cover slip was inverted on a glass slide in chamber medium with Mandato, C.A., Benink, H.A., and Bement, W.M. (2000). Microtubule- or without 10 M Y using a silicone fitting (Molecular Probes), actomyosin interactions in cortical flow and cytokinesis. Cell Motil. and red, green, and blue channels were filmed on three separate Cytoskeleton 45, Z planes approximately m apart every minute on a Nikon Meeusen, R.L., Bennett, J., and Cande, W.Z. (1980). Effect of mi-
12 Cell 372 croinjected N-ethylmaleimide-modified heavy meromyosin on cell division in amphibian eggs. J. Cell Biol. 86, Rattner, J.B., and Berns, M.W. (1976). Centriole behavior in early mitosis of rat kangaroo cells (PTK2). Chromosoma 54, Robinson, J.T., Wojcik, E.J., Sanders, M.A., McGrail, M., and Hays, T.S. (1999). Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J. Cell Biol. 146, Rodriguez, O.C., Schaefer, A.W., Mandato, C.A., Forscher, P., Bement, W.M., and Waterman-Storer, C.M. (2003). Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5, Sawin, K.E., and Mitchison, T.J. (1991). Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol. 112, Sawin, K.E., LeGuellec, K., Philippe, M., and Mitchison, T.J. (1992). Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359, Shelton, C.A., Carter, J.C., Ellis, G.C., and Bowerman, B. (1999). The nonmuscle myosin regulatory light chain gene mlc-4 is required for cytokinesis, anterior-posterior polarity, and body morphology during Caenorhabditis elegans embryogenesis. J. Cell Biol. 146, Silverman-Gavrila, R.V., and Forer, A. (2001). Effects of anti-myosin drugs on anaphase chromosome movement and cytokinesis in crane-fly primary spermatocytes. Cell Motil. Cytoskeleton 50, Somma, M.P., Fasulo, B., Cenci, G., Cundari, E., and Gatti, M. (2002). Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Mol. Biol. Cell 13, Straight, A.F., Cheung, A., Limouze, J., Chen, I., Westwood, N.J., Sellers, J.R., and Mitchison, T.J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299, Tencer, R. (1978). Transmembrane effects of lectins. I. Effects of wheat germ agglutinin and soybean agglutinin on furrow formation and cortical wound healing in Xenopus laevis eggs. Exp. Cell Res. 116, Vaisberg, E.A., Koonce, M.P., and McIntosh, J.R. (1993). Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 123, Wang, Y.L., Silverman, J.D., and Cao, L.G. (1994). Single particle tracking of surface receptor movement during cell division. J. Cell Biol. 127, Whitehead, C.M., Winkfein, R.J., and Rattner, J.B. (1996). The relationship of HsEg5 and the actin cytoskeleton to centrosome separation. Cell Motil. Cytoskeleton 35,
Interphase (A) and mitotic (B) D16 cells were plated on concanavalin A and processed for
 E07-10-1069 Rogers SUPPLEMENTARY DATA Supplemental Figure 1. Interphase day-7 control and γ-tubulin23c RNAi-treated S2 cells stained for MTs (green), γ-tubulin (red), and DNA (blue). Scale, 5μm. Supplemental
E07-10-1069 Rogers SUPPLEMENTARY DATA Supplemental Figure 1. Interphase day-7 control and γ-tubulin23c RNAi-treated S2 cells stained for MTs (green), γ-tubulin (red), and DNA (blue). Scale, 5μm. Supplemental
J. Cell Sci. 128: doi:10.1242/jcs.164566: Supplementary Material
 Figure S1. Microtubule and microfilament drug treatments severely interfere with mitotic spindle morphology, length and positioning. (A) GFP-fused lamin-a/c, LAP2 or BAF1 localizes to the spindle and all
Figure S1. Microtubule and microfilament drug treatments severely interfere with mitotic spindle morphology, length and positioning. (A) GFP-fused lamin-a/c, LAP2 or BAF1 localizes to the spindle and all
If and when cancer cells stop dividing, they do so at random points, not at the normal checkpoints in the cell cycle.
 Cancer cells have escaped from cell cycle controls Cancer cells divide excessively and invade other tissues because they are free of the body s control mechanisms. Cancer cells do not stop dividing when
Cancer cells have escaped from cell cycle controls Cancer cells divide excessively and invade other tissues because they are free of the body s control mechanisms. Cancer cells do not stop dividing when
Cell Division Mitosis and the Cell Cycle
 Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Chapter 12: The Cell Cycle
 Name Period Chapter 12: The Cell Cycle Overview: 1. What are the three key roles of cell division? State each role, and give an example. Key Role Reproduction Growth and development Tissue removal Example
Name Period Chapter 12: The Cell Cycle Overview: 1. What are the three key roles of cell division? State each role, and give an example. Key Role Reproduction Growth and development Tissue removal Example
Cellular Reproduction
 9 Cellular Reproduction section 1 Cellular Growth Before You Read Think about the life cycle of a human. On the lines below, write some of the stages that occur in the life cycle of a human. In this section,
9 Cellular Reproduction section 1 Cellular Growth Before You Read Think about the life cycle of a human. On the lines below, write some of the stages that occur in the life cycle of a human. In this section,
CHAPTER 9 CELLULAR REPRODUCTION P. 243-257
 CHAPTER 9 CELLULAR REPRODUCTION P. 243-257 SECTION 9-1 CELLULAR GROWTH Page 244 ESSENTIAL QUESTION Why is it beneficial for cells to remain small? MAIN IDEA Cells grow until they reach their size limit,
CHAPTER 9 CELLULAR REPRODUCTION P. 243-257 SECTION 9-1 CELLULAR GROWTH Page 244 ESSENTIAL QUESTION Why is it beneficial for cells to remain small? MAIN IDEA Cells grow until they reach their size limit,
Guided Notes: Chapter 9 Cellular Reproduction
 Guided Notes: Cellular Reproduction When do cells divide? Cells grow and function normally until they become too. Cell size is because increases faster than This means that there is not enough area on
Guided Notes: Cellular Reproduction When do cells divide? Cells grow and function normally until they become too. Cell size is because increases faster than This means that there is not enough area on
Biology 3A Laboratory MITOSIS Asexual Reproduction
 Biology 3A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
Biology 3A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
The Huntington Library, Art Collections, and Botanical Gardens
 The Huntington Library, Art Collections, and Botanical Gardens Rooting for Mitosis Overview Students will fix, stain, and make slides of onion root tips. These slides will be examined for the presence
The Huntington Library, Art Collections, and Botanical Gardens Rooting for Mitosis Overview Students will fix, stain, and make slides of onion root tips. These slides will be examined for the presence
Cell Growth and Reproduction Module B, Anchor 1
 Cell Growth and Reproduction Module B, Anchor 1 Key Concepts: - The larger a cell becomes, the more demands the cell places on its DNA. In addition, a larger cell is less efficient in moving nutrients
Cell Growth and Reproduction Module B, Anchor 1 Key Concepts: - The larger a cell becomes, the more demands the cell places on its DNA. In addition, a larger cell is less efficient in moving nutrients
Lecture 7 Mitosis & Meiosis
 Lecture 7 Mitosis & Meiosis Cell Division Essential for body growth and tissue repair Interphase G 1 phase Primary cell growth phase S phase DNA replication G 2 phase Microtubule synthesis Mitosis Nuclear
Lecture 7 Mitosis & Meiosis Cell Division Essential for body growth and tissue repair Interphase G 1 phase Primary cell growth phase S phase DNA replication G 2 phase Microtubule synthesis Mitosis Nuclear
Biology Behind the Crime Scene Week 4: Lab #4 Genetics Exercise (Meiosis) and RFLP Analysis of DNA
 Page 1 of 5 Biology Behind the Crime Scene Week 4: Lab #4 Genetics Exercise (Meiosis) and RFLP Analysis of DNA Genetics Exercise: Understanding how meiosis affects genetic inheritance and DNA patterns
Page 1 of 5 Biology Behind the Crime Scene Week 4: Lab #4 Genetics Exercise (Meiosis) and RFLP Analysis of DNA Genetics Exercise: Understanding how meiosis affects genetic inheritance and DNA patterns
The cell cycle, mitosis and meiosis
 The cell cycle, mitosis and meiosis Learning objective This learning material is about the life cycle of a cell and the series of stages by which genetic materials are duplicated and partitioned to produce
The cell cycle, mitosis and meiosis Learning objective This learning material is about the life cycle of a cell and the series of stages by which genetic materials are duplicated and partitioned to produce
Lecture 11 The Cell Cycle and Mitosis
 Lecture 11 The Cell Cycle and Mitosis In this lecture Cell division Chromosomes The cell cycle Mitosis PPMAT Apoptosis What is cell division? Cells divide in order to reproduce themselves The cell cycle
Lecture 11 The Cell Cycle and Mitosis In this lecture Cell division Chromosomes The cell cycle Mitosis PPMAT Apoptosis What is cell division? Cells divide in order to reproduce themselves The cell cycle
The Somatic Cell Cycle
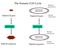 The Somatic Cell Cycle Maternal chromosome Diploid Zygote Diploid Zygote Paternal chromosome MITOSIS MITOSIS Maternal chromosome Diploid organism Diploid organism Paternal chromosome Int terpha ase The
The Somatic Cell Cycle Maternal chromosome Diploid Zygote Diploid Zygote Paternal chromosome MITOSIS MITOSIS Maternal chromosome Diploid organism Diploid organism Paternal chromosome Int terpha ase The
Cell Division CELL DIVISION. Mitosis. Designation of Number of Chromosomes. Homologous Chromosomes. Meiosis
 Cell Division CELL DIVISION Anatomy and Physiology Text and Laboratory Workbook, Stephen G. Davenport, Copyright 2006, All Rights Reserved, no part of this publication can be used for any commercial purpose.
Cell Division CELL DIVISION Anatomy and Physiology Text and Laboratory Workbook, Stephen G. Davenport, Copyright 2006, All Rights Reserved, no part of this publication can be used for any commercial purpose.
Chapter 3. Cell Division. Laboratory Activities Activity 3.1: Mock Mitosis Activity 3.2: Mitosis in Onion Cells Activity 3.
 Chapter 3 Cell Division Laboratory Activities Activity 3.1: Mock Mitosis Activity 3.2: Mitosis in Onion Cells Activity 3.3: Mock Meiosis Goals Following this exercise students should be able to Recognize
Chapter 3 Cell Division Laboratory Activities Activity 3.1: Mock Mitosis Activity 3.2: Mitosis in Onion Cells Activity 3.3: Mock Meiosis Goals Following this exercise students should be able to Recognize
Mitosis in Onion Root Tip Cells
 Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
CD3/TCR stimulation and surface detection Determination of specificity of intracellular detection of IL-7Rα by flow cytometry
 CD3/TCR stimulation and surface detection Stimulation of HPB-ALL cells with the anti-cd3 monoclonal antibody OKT3 was performed as described 3. In brief, antibody-coated plates were prepared by incubating
CD3/TCR stimulation and surface detection Stimulation of HPB-ALL cells with the anti-cd3 monoclonal antibody OKT3 was performed as described 3. In brief, antibody-coated plates were prepared by incubating
Appendix C DNA Replication & Mitosis
 K.Muma Bio 6 Appendix C DNA Replication & Mitosis Study Objectives: Appendix C: DNA replication and Mitosis 1. Describe the structure of DNA and where it is found. 2. Explain complimentary base pairing:
K.Muma Bio 6 Appendix C DNA Replication & Mitosis Study Objectives: Appendix C: DNA replication and Mitosis 1. Describe the structure of DNA and where it is found. 2. Explain complimentary base pairing:
Cell Cycle in Onion Root Tip Cells (IB)
 Cell Cycle in Onion Root Tip Cells (IB) A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules,
Cell Cycle in Onion Root Tip Cells (IB) A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules,
1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells
 Cell Growth and Reproduction 1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells A. is half of that of the parent cell. B. remains the same as in the
Cell Growth and Reproduction 1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells A. is half of that of the parent cell. B. remains the same as in the
www.njctl.org PSI Biology Mitosis & Meiosis
 Mitosis and Meiosis Mitosis Classwork 1. Identify two differences between meiosis and mitosis. 2. Provide an example of a type of cell in the human body that would undergo mitosis. 3. Does cell division
Mitosis and Meiosis Mitosis Classwork 1. Identify two differences between meiosis and mitosis. 2. Provide an example of a type of cell in the human body that would undergo mitosis. 3. Does cell division
Cell Biology Questions and Learning Objectives
 Cell Biology Questions and Learning Objectives (with hypothetical learning materials that might populate the objective) The topics and central questions listed here are typical for an introductory undergraduate
Cell Biology Questions and Learning Objectives (with hypothetical learning materials that might populate the objective) The topics and central questions listed here are typical for an introductory undergraduate
LAB 09 Cell Division
 LAB 09 Cell Division Introduction: One of the characteristics of living things is the ability to replicate and pass on genetic information to the next generation. Cell division in individual bacteria and
LAB 09 Cell Division Introduction: One of the characteristics of living things is the ability to replicate and pass on genetic information to the next generation. Cell division in individual bacteria and
LAB 8 EUKARYOTIC CELL DIVISION: MITOSIS AND MEIOSIS
 LAB 8 EUKARYOTIC CELL DIVISION: MITOSIS AND MEIOSIS Los Angeles Mission College Biology 3 Name: Date: INTRODUCTION BINARY FISSION: Prokaryotic cells (bacteria) reproduce asexually by binary fission. Bacterial
LAB 8 EUKARYOTIC CELL DIVISION: MITOSIS AND MEIOSIS Los Angeles Mission College Biology 3 Name: Date: INTRODUCTION BINARY FISSION: Prokaryotic cells (bacteria) reproduce asexually by binary fission. Bacterial
Chapter 12: The Cell Cycle
 Name Period Chapter 12: The Cell Cycle Overview: 1. What are the three key roles of cell division? State each role, and give an example. Key Role Example 2. What is meant by the cell cycle? Concept 12.1
Name Period Chapter 12: The Cell Cycle Overview: 1. What are the three key roles of cell division? State each role, and give an example. Key Role Example 2. What is meant by the cell cycle? Concept 12.1
From DNA to Protein
 Nucleus Control center of the cell contains the genetic library encoded in the sequences of nucleotides in molecules of DNA code for the amino acid sequences of all proteins determines which specific proteins
Nucleus Control center of the cell contains the genetic library encoded in the sequences of nucleotides in molecules of DNA code for the amino acid sequences of all proteins determines which specific proteins
CHROMOSOME STRUCTURE CHROMOSOME NUMBERS
 CHROMOSOME STRUCTURE 1. During nuclear division, the DNA (as chromatin) in a Eukaryotic cell's nucleus is coiled into very tight compact structures called chromosomes. These are rod-shaped structures made
CHROMOSOME STRUCTURE 1. During nuclear division, the DNA (as chromatin) in a Eukaryotic cell's nucleus is coiled into very tight compact structures called chromosomes. These are rod-shaped structures made
List, describe, diagram, and identify the stages of meiosis.
 Meiosis and Sexual Life Cycles In this topic we will examine a second type of cell division used by eukaryotic cells: meiosis. In addition, we will see how the 2 types of eukaryotic cell division, mitosis
Meiosis and Sexual Life Cycles In this topic we will examine a second type of cell division used by eukaryotic cells: meiosis. In addition, we will see how the 2 types of eukaryotic cell division, mitosis
1. Why is mitosis alone insufficient for the life cycle of sexually reproducing eukaryotes?
 Chapter 13: Meiosis and Sexual Life Cycles 1. Why is mitosis alone insufficient for the life cycle of sexually reproducing eukaryotes? 2. Define: gamete zygote meiosis homologous chromosomes diploid haploid
Chapter 13: Meiosis and Sexual Life Cycles 1. Why is mitosis alone insufficient for the life cycle of sexually reproducing eukaryotes? 2. Define: gamete zygote meiosis homologous chromosomes diploid haploid
MITOSIS IN ONION ROOT TIP CELLS: AN INTRODUCTION TO LIGHT MICROSCOPY
 MITOSIS IN ONION ROOT TIP CELLS: AN INTRODUCTION TO LIGHT MICROSCOPY Adapted from Foundations of Biology I; Lab 6 Introduction to Microscopy Dr. John Robertson, Westminster College Biology Department,
MITOSIS IN ONION ROOT TIP CELLS: AN INTRODUCTION TO LIGHT MICROSCOPY Adapted from Foundations of Biology I; Lab 6 Introduction to Microscopy Dr. John Robertson, Westminster College Biology Department,
CHAPTER 10 CELL CYCLE AND CELL DIVISION
 CHAPTER 10 CELL CYCLE AND CELL DIVISION Cell division is an inherent property of living organisms. It is a process in which cells reproduce their own kind. The growth, differentiation, reproduction and
CHAPTER 10 CELL CYCLE AND CELL DIVISION Cell division is an inherent property of living organisms. It is a process in which cells reproduce their own kind. The growth, differentiation, reproduction and
Mitotic Membrane Turnover Coordinates Differential Induction of the Heart Progenitor Lineage
 Developmental Cell Supplemental Information Mitotic Membrane Turnover Coordinates Differential Induction of the Heart Progenitor Lineage Christina D. Cota and Brad Davidson Supplemental Figures S1-S7 S1.
Developmental Cell Supplemental Information Mitotic Membrane Turnover Coordinates Differential Induction of the Heart Progenitor Lineage Christina D. Cota and Brad Davidson Supplemental Figures S1-S7 S1.
LABORATORY 2 THE CELL CYCLE AND THE STAGES OF MITOSIS LEARNING OBJECTIVES AFTER COMPLETING THIS LABORATORY, YOU SHOULD BE ABLE TO:
 LABORATORY 2 THE CELL CYCLE AND THE STAGES OF MITOSIS LEARNING OBJECTIVES AFTER COMPLETING THIS LABORATORY, YOU SHOULD BE ABLE TO: 1. Describe the cell cycle. 2. Identify stages of mitosis from prepared
LABORATORY 2 THE CELL CYCLE AND THE STAGES OF MITOSIS LEARNING OBJECTIVES AFTER COMPLETING THIS LABORATORY, YOU SHOULD BE ABLE TO: 1. Describe the cell cycle. 2. Identify stages of mitosis from prepared
Lab 3: Testing Hypotheses about Mitosis
 Lab 3: Testing Hypotheses about Mitosis Why do cells divide? Lab today focuses on cellular division, also known as cellular reproduction. To become more familiar with why cells divide, the types of cell
Lab 3: Testing Hypotheses about Mitosis Why do cells divide? Lab today focuses on cellular division, also known as cellular reproduction. To become more familiar with why cells divide, the types of cell
Test Two Study Guide
 Test Two Study Guide 1. Describe what is happening inside a cell during the following phases (pictures may help but try to use words): Interphase: : Consists of G1 / S / G2. Growing stage, cell doubles
Test Two Study Guide 1. Describe what is happening inside a cell during the following phases (pictures may help but try to use words): Interphase: : Consists of G1 / S / G2. Growing stage, cell doubles
Chapter 18: Applications of Immunology
 Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Modelling cell motion: From microscopic to macroscopic scale. Luigi Preziosi [email protected] calvino.polito.it/~preziosi
 Modelling cell motion: From microscopic to macroscopic scale Luigi Preziosi [email protected] calvino.polito.it/~preziosi Modelling cell-ecm interaction: An opportunity to present different modelling
Modelling cell motion: From microscopic to macroscopic scale Luigi Preziosi [email protected] calvino.polito.it/~preziosi Modelling cell-ecm interaction: An opportunity to present different modelling
5. The cells of a multicellular organism, other than gametes and the germ cells from which it develops, are known as
 1. True or false? The chi square statistical test is used to determine how well the observed genetic data agree with the expectations derived from a hypothesis. True 2. True or false? Chromosomes in prokaryotic
1. True or false? The chi square statistical test is used to determine how well the observed genetic data agree with the expectations derived from a hypothesis. True 2. True or false? Chromosomes in prokaryotic
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/339/ra80/dc1 Supplementary Materials for Manipulation of receptor oligomerization as a strategy to inhibit signaling by TNF superfamily members Julia T. Warren,
www.sciencesignaling.org/cgi/content/full/7/339/ra80/dc1 Supplementary Materials for Manipulation of receptor oligomerization as a strategy to inhibit signaling by TNF superfamily members Julia T. Warren,
An Overview of Cells and Cell Research
 An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
The illustrations below reflect other scientists results in identifying and counting the stages of the onion root tip and the whitefish blastula.
 Abstract: The purpose of this laboratory experiment was to identify in what stage of mitosis viewed cells were in. The stages of mitosis include prophase, metaphase, anaphase and telophase. Although the
Abstract: The purpose of this laboratory experiment was to identify in what stage of mitosis viewed cells were in. The stages of mitosis include prophase, metaphase, anaphase and telophase. Although the
Lecture 2: Mitosis and meiosis
 Lecture 2: Mitosis and meiosis 1. Chromosomes 2. Diploid life cycle 3. Cell cycle 4. Mitosis 5. Meiosis 6. Parallel behavior of genes and chromosomes Basic morphology of chromosomes telomere short arm
Lecture 2: Mitosis and meiosis 1. Chromosomes 2. Diploid life cycle 3. Cell cycle 4. Mitosis 5. Meiosis 6. Parallel behavior of genes and chromosomes Basic morphology of chromosomes telomere short arm
Outline. 1. Experiment. 2. Sample analysis and storage. 3. Image analysis and presenting data. 4. Probemaker
 Tips and tricks Note: this is just an informative document with general recommendations. Please contact [email protected] should you have any queries. Document last reviewed 2011-11-17 Outline 1. Experiment
Tips and tricks Note: this is just an informative document with general recommendations. Please contact [email protected] should you have any queries. Document last reviewed 2011-11-17 Outline 1. Experiment
4.2 Meiosis. Meiosis is a reduction division. Assessment statements. The process of meiosis
 4.2 Meiosis Assessment statements State that meiosis is a reduction division of a diploid nucleus to form haploid nuclei. Define homologous chromosomes. Outline the process of meiosis, including pairing
4.2 Meiosis Assessment statements State that meiosis is a reduction division of a diploid nucleus to form haploid nuclei. Define homologous chromosomes. Outline the process of meiosis, including pairing
CYCLIN DEPENDENT KINASES AND CELL CYCLE CONTROL
 CYCLIN DEPENDENT KINASES AND CELL CYCLE CONTROL Nobel Lecture, December 9, 2001 by PAUL M. NURSE Cancer Research UK, PO Box 123, 61 Lincoln s Inn Fields, London WC2A 3PK, United Kingdom. The cell is the
CYCLIN DEPENDENT KINASES AND CELL CYCLE CONTROL Nobel Lecture, December 9, 2001 by PAUL M. NURSE Cancer Research UK, PO Box 123, 61 Lincoln s Inn Fields, London WC2A 3PK, United Kingdom. The cell is the
protocol handbook 3D cell culture mimsys G hydrogel
 handbook 3D cell culture mimsys G hydrogel supporting real discovery handbook Index 01 Cell encapsulation in hydrogels 02 Cell viability by MTS assay 03 Live/Dead assay to assess cell viability 04 Fluorescent
handbook 3D cell culture mimsys G hydrogel supporting real discovery handbook Index 01 Cell encapsulation in hydrogels 02 Cell viability by MTS assay 03 Live/Dead assay to assess cell viability 04 Fluorescent
Trasposable elements: P elements
 Trasposable elements: P elements In 1938 Marcus Rhodes provided the first genetic description of an unstable mutation, an allele of a gene required for the production of pigment in maize. This instability
Trasposable elements: P elements In 1938 Marcus Rhodes provided the first genetic description of an unstable mutation, an allele of a gene required for the production of pigment in maize. This instability
PROTOCOL. Immunocytochemistry (ICC) MATERIALS AND EQUIPMENT REQUIRED
 PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
Protein Analysis. -Detection and quantification. Toby M Holmes
 Protein Analysis -Detection and quantification Toby M Holmes Clinical Research Unit UCD school of Medicine and Medical Sciences Mater Misericordiae University Hospital Dublin Protein structure General
Protein Analysis -Detection and quantification Toby M Holmes Clinical Research Unit UCD school of Medicine and Medical Sciences Mater Misericordiae University Hospital Dublin Protein structure General
Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides
 Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides Polyacrylamide gel electrophoresis (PAGE) and subsequent analyses are common tools in biochemistry and molecular biology. This Application
Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides Polyacrylamide gel electrophoresis (PAGE) and subsequent analyses are common tools in biochemistry and molecular biology. This Application
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis
 ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
Predict Reactivity Note Chicken (100%), Sheep (100%), Rhesus Monkey (100%), Chimpanzee (100%), Bovine (100%), Guinea pig (100%)
 Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 [email protected] GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 [email protected] Date :
Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 [email protected] GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 [email protected] Date :
PROTOCOL. Immunostaining for Flow Cytometry. Background. Materials and equipment required.
 PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
Radius 24-Well Cell Migration Assay (Laminin Coated)
 Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
CELL DIVISION. STAGES OF MITOTIC DIVISION (Diag. C1)
 1 CELL DIVISION Cell division is the process by which cells replicate in order to replace cell loss, repair tissue damage and reproduce the organism. Two types of cell division are encountered in the Eukaryotic
1 CELL DIVISION Cell division is the process by which cells replicate in order to replace cell loss, repair tissue damage and reproduce the organism. Two types of cell division are encountered in the Eukaryotic
AP Biology 2011 Scoring Guidelines Form B
 AP Biology 2011 Scoring Guidelines Form B The College Board The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded
AP Biology 2011 Scoring Guidelines Form B The College Board The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded
!!!!!!!!!!!!!!!!!!!!!!!!!!
 Figure S7 cdl-1 3'UTR gld-1 binding site (4..10) let-7 binding site (326..346) Figure Legends Figure S1. gld-1 genetically interacts with nhl-2 and vig-1 during germline development. (A) nhl-2(ok818) enhances
Figure S7 cdl-1 3'UTR gld-1 binding site (4..10) let-7 binding site (326..346) Figure Legends Figure S1. gld-1 genetically interacts with nhl-2 and vig-1 during germline development. (A) nhl-2(ok818) enhances
Meiosis is a special form of cell division.
 Page 1 of 6 KEY CONCEPT Meiosis is a special form of cell division. BEFORE, you learned Mitosis produces two genetically identical cells In sexual reproduction, offspring inherit traits from both parents
Page 1 of 6 KEY CONCEPT Meiosis is a special form of cell division. BEFORE, you learned Mitosis produces two genetically identical cells In sexual reproduction, offspring inherit traits from both parents
Germ cell formation / gametogenesis And Fertilisation
 Developmental Biology BY1101 P. Murphy Lecture 3 The first steps to forming a new organism Descriptive embryology I Germ cell formation / gametogenesis And Fertilisation Why bother with sex? In terms of
Developmental Biology BY1101 P. Murphy Lecture 3 The first steps to forming a new organism Descriptive embryology I Germ cell formation / gametogenesis And Fertilisation Why bother with sex? In terms of
Classify chromosomes in a karyotype according to size and centromere position. Identify metacentric, submetacentric and acrocentric chromosomes
 Mitosis, Meiosis and the Cell Cycle Prof. Alfred Cuschieri University of Malta Department of Anatomy Objectives By the end of the session the student shoud be able to: Define the meaning of chromosomes
Mitosis, Meiosis and the Cell Cycle Prof. Alfred Cuschieri University of Malta Department of Anatomy Objectives By the end of the session the student shoud be able to: Define the meaning of chromosomes
EXTRACTION OF DNA FROM CALF THYMUS CELLS Revised 2/1/96 Introduction
 Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small
 The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small molecules that can elicit an immune response when linked
The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small molecules that can elicit an immune response when linked
Sample Questions for Exam 3
 Sample Questions for Exam 3 1. All of the following occur during prometaphase of mitosis in animal cells except a. the centrioles move toward opposite poles. b. the nucleolus can no longer be seen. c.
Sample Questions for Exam 3 1. All of the following occur during prometaphase of mitosis in animal cells except a. the centrioles move toward opposite poles. b. the nucleolus can no longer be seen. c.
Control of Gene Expression
 Home Gene Regulation Is Necessary? Control of Gene Expression By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection favoring
Home Gene Regulation Is Necessary? Control of Gene Expression By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection favoring
BME 42-620 Engineering Molecular Cell Biology. Lecture 02: Structural and Functional Organization of
 BME 42-620 Engineering Molecular Cell Biology Lecture 02: Structural and Functional Organization of Eukaryotic Cells BME42-620 Lecture 02, September 01, 2011 1 Outline A brief review of the previous lecture
BME 42-620 Engineering Molecular Cell Biology Lecture 02: Structural and Functional Organization of Eukaryotic Cells BME42-620 Lecture 02, September 01, 2011 1 Outline A brief review of the previous lecture
Crime Scenes and Genes
 Glossary Agarose Biotechnology Cell Chromosome DNA (deoxyribonucleic acid) Electrophoresis Gene Micro-pipette Mutation Nucleotide Nucleus PCR (Polymerase chain reaction) Primer STR (short tandem repeats)
Glossary Agarose Biotechnology Cell Chromosome DNA (deoxyribonucleic acid) Electrophoresis Gene Micro-pipette Mutation Nucleotide Nucleus PCR (Polymerase chain reaction) Primer STR (short tandem repeats)
Chapter 7. Physical interaction between Jamb and Jamc is necessary for myoblast fusion. Summary
 Chapter 7 Physical interaction between Jamb and Jamc is necessary for myoblast fusion Summary In this chapter I describe a series of transplant experiments designed to further elucidate the function and
Chapter 7 Physical interaction between Jamb and Jamc is necessary for myoblast fusion Summary In this chapter I describe a series of transplant experiments designed to further elucidate the function and
Investigating the role of a Cryptosporidium parum apyrase in infection
 Investigating the role of a Cryptosporidium parum apyrase in infection David Riccardi and Patricio Manque Abstract This project attempted to characterize the function of a Cryptosporidium parvum apyrase
Investigating the role of a Cryptosporidium parum apyrase in infection David Riccardi and Patricio Manque Abstract This project attempted to characterize the function of a Cryptosporidium parvum apyrase
The Cell Cycle: A series of modeling activities
 The Cell Cycle: A series of modeling activities Cancer Education Project University of Rochester Premise: Students learn best when exposed to a variety of activities Overview 1. Information Gathering:
The Cell Cycle: A series of modeling activities Cancer Education Project University of Rochester Premise: Students learn best when exposed to a variety of activities Overview 1. Information Gathering:
Protease Peptide Microarrays Ready-to-use microarrays for protease profiling
 Protocol Protease Peptide Microarrays Ready-to-use microarrays for protease profiling Contact us: InfoLine: +49-30-97893-117 Order per fax: +49-30-97893-299 Or e-mail: [email protected] www: www.jpt.com
Protocol Protease Peptide Microarrays Ready-to-use microarrays for protease profiling Contact us: InfoLine: +49-30-97893-117 Order per fax: +49-30-97893-299 Or e-mail: [email protected] www: www.jpt.com
Lesson 3 Reading Material: Oncogenes and Tumor Suppressor Genes
 Lesson 3 Reading Material: Oncogenes and Tumor Suppressor Genes Becoming a cancer cell isn t easy One of the fundamental molecular characteristics of cancer is that it does not develop all at once, but
Lesson 3 Reading Material: Oncogenes and Tumor Suppressor Genes Becoming a cancer cell isn t easy One of the fundamental molecular characteristics of cancer is that it does not develop all at once, but
How To Align A Spindle In A Cell
 Research Article 1973 Interphase microtubule bundles use global cell shape to guide spindle alignment in fission yeast Rafael R. Daga 1,2, * and Paul Nurse 1 1 Rockefeller University, 1234 York Avenue,
Research Article 1973 Interphase microtubule bundles use global cell shape to guide spindle alignment in fission yeast Rafael R. Daga 1,2, * and Paul Nurse 1 1 Rockefeller University, 1234 York Avenue,
Xinnan Wang Stanford University School of Medicine
 Xinnan Wang Stanford University School of Medicine Cellular Organelles are Highly Dynamic COS7 Cell, MitoTracker Fission-fusion, changing morphology and locabon To meet ever-changing energy demand Electrical
Xinnan Wang Stanford University School of Medicine Cellular Organelles are Highly Dynamic COS7 Cell, MitoTracker Fission-fusion, changing morphology and locabon To meet ever-changing energy demand Electrical
Running protein gels and detection of proteins
 Running protein gels and detection of proteins 1. Protein concentration determination using the BIO RAD reagent This assay uses a colour change reaction to give a direct measurement of protein concentration.
Running protein gels and detection of proteins 1. Protein concentration determination using the BIO RAD reagent This assay uses a colour change reaction to give a direct measurement of protein concentration.
Carbon Hydrogen Oxygen Nitrogen
 Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Lecture 4 Cell Membranes & Organelles
 Lecture 4 Cell Membranes & Organelles Structure of Animal Cells The Phospholipid Structure Phospholipid structure Encases all living cells Its basic structure is represented by the fluidmosaic model Phospholipid
Lecture 4 Cell Membranes & Organelles Structure of Animal Cells The Phospholipid Structure Phospholipid structure Encases all living cells Its basic structure is represented by the fluidmosaic model Phospholipid
Use of the Microscope and Cytology
 Use of the Microscope and Cytology Introduction: A true study of anatomy not only considers the large, visible structures of an organism, but also the small structures that provide the organism its form
Use of the Microscope and Cytology Introduction: A true study of anatomy not only considers the large, visible structures of an organism, but also the small structures that provide the organism its form
Chapter 13: Meiosis and Sexual Life Cycles
 Name Period Chapter 13: Meiosis and Sexual Life Cycles Concept 13.1 Offspring acquire genes from parents by inheriting chromosomes 1. Let s begin with a review of several terms that you may already know.
Name Period Chapter 13: Meiosis and Sexual Life Cycles Concept 13.1 Offspring acquire genes from parents by inheriting chromosomes 1. Let s begin with a review of several terms that you may already know.
The Making of the Fittest: Evolving Switches, Evolving Bodies
 OVERVIEW MODELING THE REGULATORY SWITCHES OF THE PITX1 GENE IN STICKLEBACK FISH This hands-on activity supports the short film, The Making of the Fittest:, and aims to help students understand eukaryotic
OVERVIEW MODELING THE REGULATORY SWITCHES OF THE PITX1 GENE IN STICKLEBACK FISH This hands-on activity supports the short film, The Making of the Fittest:, and aims to help students understand eukaryotic
Supplemental Information. McBrayer et al. Supplemental Data
 1 Supplemental Information McBrayer et al. Supplemental Data 2 Figure S1. Glucose consumption rates of MM cell lines exceed that of normal PBMC. (A) Normal PBMC isolated from three healthy donors were
1 Supplemental Information McBrayer et al. Supplemental Data 2 Figure S1. Glucose consumption rates of MM cell lines exceed that of normal PBMC. (A) Normal PBMC isolated from three healthy donors were
Workshop: Cellular Reproduction via Mitosis & Meiosis
 Workshop: Cellular Reproduction via Mitosis & Meiosis Introduction In this workshop you will examine how cells divide, including how they partition their genetic material (DNA) between the two resulting
Workshop: Cellular Reproduction via Mitosis & Meiosis Introduction In this workshop you will examine how cells divide, including how they partition their genetic material (DNA) between the two resulting
MEF Starter Nucleofector Kit
 page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
ab185915 Protein Sumoylation Assay Ultra Kit
 ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
Pure-IP Western Blot Detection Kit
 Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
The Cell: Organelle Diagrams
 The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
CCR Biology - Chapter 5 Practice Test - Summer 2012
 Name: Class: Date: CCR Biology - Chapter 5 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. If a cell cannot move enough material
Name: Class: Date: CCR Biology - Chapter 5 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. If a cell cannot move enough material
The Need for a PARP in vivo Pharmacodynamic Assay
 The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
TOOLS sirna and mirna. User guide
 TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
Materials and Methods. Ribonuclease.--Removal of ribonucleic acid from meristematic onion cells did not show any qualitative differences
 B~U~ Noz~s 129 Alterations in Nuclear Ribonucleic Acid Metabolism Induced by l~inetin.* BY RUTH GUNMAN. From the Department of Genetics, University of California, Be, kezey.~ Kinetin, a substance recently
B~U~ Noz~s 129 Alterations in Nuclear Ribonucleic Acid Metabolism Induced by l~inetin.* BY RUTH GUNMAN. From the Department of Genetics, University of California, Be, kezey.~ Kinetin, a substance recently
ArC Amine Reactive Compensation Bead Kit
 ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
