Rheology of polymer systems/ Reologia dos sistemas poliméricos
|
|
|
- Isabella Brooks
- 10 years ago
- Views:
Transcription
1 Rheology of polymer systems/ Reologia dos sistemas poliméricos 1. Viscosity/Viscosidade Jorge Morgado, IST
2 Polymers Molecular materials in nature COMPLEX behaviour
3 Viscosity of concentrated solu1ons and polymer melts 1. DefiniMon of viscosity (η). 2. Factors affecmng the viscosity of polymer solumons and melts: i. ConcentraMon ii. Molecular weight iii. Pressure iv. Temperature. WLF (Williams, Landel, Ferry) equamon. 3. VariaMon of the viscosity with Mme and with strain/ deformamon rate. Non- newtonian behaviour
4 Viscosity A force per unit area (F/A) is required to maintain a constant velocity gradient τ Shear stress γ rate of shear η shear viscosity Newton law - viscosity is independent of stress and shear rate Dynamic (absolute) viscosity, η [poise, Pa.s or ML - 1 T - 1 ] and kinemamc viscosity, ν=η/ρ [L 2 T - 1 ]
5 Low viscosity liquids Capillary viscometer/viscosímetro de capilar KinemaMc viscosity/viscosidade cinemámca η ρ = At B t 2 CorrecMon factor (losses of kinemc energy at the capillary)
6 For flow when viscosity is not constant Use of externally pressurized capillary and rotamonal viscometers/viscosímetros de capilar pressurizados ou rotacionais
7 Newtonian flow Increasing viscosity Non- newtonian flow η=f(γ,t)
8 Non- newtonian flow i) Effect of shear rate/ velocidade de deformação Dilatant behaviour may be found in parmculate dispersions, latexes and concentrated suspensions Pseudopas:c behaviour (polymer solumons and melts) Upon increase of the molecular weight (expected for M > 1-2 x 10 6 ), the non- newtonian behaviour occurs at lower shear rate.
9 Non- newtonian flow i) Effect of shear rate/ velocidade de deformação In simple liquids viscosity is constant but in polymers it may depend on shear rate Zero shear viscosity Newtonian Plateau (viscosity constant) Linear viscous Power law Shear thinning
10 Different types of Viscosity Shear- thickening liquids- low viscosity when smrred slowly, but viscosity increases when mixed quickly Shear thinning liquids- high viscosity when mixed slowly, easier to mix quickly Bingham liquids- at low stress behaves like a solid, high stress behaves like a liquid Granular materials- like Bingham, but do not have a well- defined viscosity
11 The role of molecular orientamon in viscosity vs. shear rate relamon Newtonian Plateau (viscosity constant) Linear viscous Power Law Linear in log-log plot Significant alignment results in a decrease in viscosity Steady state alignment level is achieved. More alignment for fast flow No structure develops Any alignment is destroyed by random thermal motion Slow flow High strain rate plateau Fully aligned molecules Flow is so fast that thermal motion is not fast enough to destroy it. (difficult to measure)
12 Non- newtonian flow Models i) Power- law Valid for melts over few decades of shear rate, but fails when approaching the newtonian behaviour Also: at zero shear rate - > infinite viscosity at infinite shear rate - > zero viscosity
13 Non- newtonian flow Models ii) Ellis Model η 0 Se γ 0; η η - 1 = kτ a 0 η Se γ ; η 0 Combines newtonian behaviour at low shear stress (τ) and power law at high τ
14 Non- newtonian flow ii) Effect of Ame Upon shearing at a steady state, the viscosity may increase or decrease with Mme Shearing starts e.g. ionic polymers in water Mme/ Expected when viscosity is parmally due to intermolecular structures that take some Mme to be formed or destroyed
15 Viscosity of polymers (solu:ons and melts)
16 Viscosimetric terms/designações Name Symbol Units Solvent viscosity/visc. do solvente η s Poise= =g.cm - 1.s - 1 SoluMon vicosity/visc. da solução η Poise RelaMve viscosity/viscosidade relamva η rel =η / η s Adimens. Specific viscosity/visc. específica Reduced viscosity/visc. reduzida Inherent viscosity/visc. inerente Intrinsic viscosity/visc. intrínseca η = η- η s sp η s =η rel - 1 η = η sp red C η = ln η rel inh C [η] η =lim sp C C 0 Adimens. dl/g dl/g dl/g
17 Factors affecmng the viscosity of polymer solumons and melts: 1. Intrinsic factors Intermolecular interacmons Side groups Molecular weight 2. Extrinsic factors SoluMon concentramon Pressure Temperature Shear rate/velocidade de escoamento Shearing Mme/Tempo de escoamento
18 2.1 Effect of concentramon on η Dilute solu1ons molecules act independently from each others. Concentrated solu1ons limitaaons to movement; existence of some cooperaaon during flow
19 i) Dilute solumons (c 0.5 g/dl)
20 i) Dilute solumons (c 0.5 g/dl) An example of viscosity versus concentramon plots for polystyrene (Mw= g/ mol) in benzene at 30 C. White circles: plot of η sp / c vs. c; black circles: plot of (lnη r )/c vs. c. T. Kotaka et al., J. Chem. Phys. 45, (1966).
21 ii) Concentrated solumons and polymer melts Increase of concentra:on leads to the forma:on of entanglements/emaranhados The molecules must disentangle first effect or shear rate/ flow on the viscosity non- newtonian behaviour: Low shear rate /baixas velocidades de escoamento(fluxo) thermal movement that tends to bring the molecules back to the entangled configuramon (equilibrium configuramon) prevails and the viscosity approaches that of the solumon at rest. High shear rate/elevadas velocidades de escoamento the orientamon of the molecules prevails over the increase of their interacmon with the solvent, so the viscosity decreases
22 ii) Concentrated solumons and melts To determine the intrinsic viscosity: i) C 0 ii) def. rate 0 When approaching the melt situa1on (c>50 g/dl): η solumon = η pol.. v 2 v 2 = volume fracmon of polymer
23 2.2 Effect of molecular weight on η Dilute solu1ons - (Mark- Houwink- Sakurada) [η] = K M a a=0.5-1; K= ml/g Polymer melts: Presence of side groups or branched chains makes the molecules flow more difficult/dificulta o deslizamento das cadeias; The molecular weight has a significant effect on the viscosity because the molecules have to disentangle in order to flow.
24 2.2 Effect of molecular weight on η Melts:
25 2.2 Effect of molecular weight Linear polymer melts: Molecular weight, M w Zero-shear viscosity, η 0 Relaxation time, τ < M c ~ M w ~ M w 2 > M c ~ M w 3.4 ~ M w 3 [G. C. Berry and T. G. Fox, Adv. Polym. Sci. 5, (1968).]
26 2.2 Effect of molecular weight on η Concentrated solu1ons: Mechanism is similar to that of the melts Concentrated solumon
27 2.2 Effect of molecular weight on η Concentrated solu1ons
28 2.2 Effect of molecular weight on η Entaglement theory or repta1on theory/ teoria dos emaranhados ou teoria da reptação
29 ReptaMon theory/teoria da reptação Polymer chain is pictured as being confined into a tube by the entanglements of its neighbours. The chains escapes its tube by a repmle type movement_diffusion (brownian momon) Chain surrounded by frozen chains Movement by diffusion only Chain surrounded by unfrozen chains diffusion is faster than disentanglement relaxamon/relaxação dos constrangimentos
30 2.3 Effect of temperature on η Viscous flow- jumps of molecules or molecule segments to holes (free space) (specific free volume, V f ). DooliSle- melts: log η= A+ B V 0 V f with V f =V- V 0 Upon increase of pressure, V f decreases, increasing the viscosity of melts
31 2.4 Effect of temperature on η The increase of temperature facilitates the creamon of free volume where a molecule or a molecule segment can jump to (jumps from a posimon in a lawce to a vacant hole) i) At high temperatures: ii) For melts of glassy polymers between T g and T g +100, WLF (Williams, Landel and Ferry) empirical rela:on_melts:
32 2.4 Effect of temperature on η WLF temperature shift parameters J. D. Ferry, Viscoelastic Properties of Polymers, 3rd ed., Wiley: New York (1980).
33 2.4 Effect of temperature on η If T 0 =T g, a 1 = ; a 2 =51.6 When T- T g >100 o C, the behaviour is beyer described by the more general equamon B and β are fiyed constants, M- molecular weight
34 2.4 Effect of temperature on η
35 References Principles of Polymer Systems, 2 nd ed., F. Rodriguez, McGraw- Hill- Int. Student Ed., 1983: IntroducMon to Macromolecular Science, P. Munk, John Wiley & Sons, 1989: Giant Molecules- Here, There and Everywhere, A. Yu. Grosberg, A.R. Khokhlov, Academic Press, 1997.
Notes on Polymer Rheology Outline
 1 Why is rheology important? Examples of its importance Summary of important variables Description of the flow equations Flow regimes - laminar vs. turbulent - Reynolds number - definition of viscosity
1 Why is rheology important? Examples of its importance Summary of important variables Description of the flow equations Flow regimes - laminar vs. turbulent - Reynolds number - definition of viscosity
3.3. Rheological Behavior of Vinyl Ester Resins
 3.3. Rheological Behavior of Vinyl Ester Resins 3.3.1. Introduction Rheology is the study of the deformation and flow of matter 1. There has been significant appreciation of the importance of the rheological
3.3. Rheological Behavior of Vinyl Ester Resins 3.3.1. Introduction Rheology is the study of the deformation and flow of matter 1. There has been significant appreciation of the importance of the rheological
1. Fluids Mechanics and Fluid Properties. 1.1 Objectives of this section. 1.2 Fluids
 1. Fluids Mechanics and Fluid Properties What is fluid mechanics? As its name suggests it is the branch of applied mechanics concerned with the statics and dynamics of fluids - both liquids and gases.
1. Fluids Mechanics and Fluid Properties What is fluid mechanics? As its name suggests it is the branch of applied mechanics concerned with the statics and dynamics of fluids - both liquids and gases.
Viscoelasticity of Polymer Fluids.
 Viscoelasticity of Polymer Fluids. Main Properties of Polymer Fluids. Entangled polymer fluids are polymer melts and concentrated or semidilute (above the concentration c) solutions. In these systems polymer
Viscoelasticity of Polymer Fluids. Main Properties of Polymer Fluids. Entangled polymer fluids are polymer melts and concentrated or semidilute (above the concentration c) solutions. In these systems polymer
Polymer Melt Rheology. Introduction to Viscoelastic Behavior. Time-Temperature Equivalence
 Topics to be Covered Polymer Melt Rheology Introduction to Viscoelastic Behavior Time-Temperature Equivalence Chapter 11 in CD (Polymer Science and Engineering) Polymer Melt Rheology δ τ xy Newton s Law
Topics to be Covered Polymer Melt Rheology Introduction to Viscoelastic Behavior Time-Temperature Equivalence Chapter 11 in CD (Polymer Science and Engineering) Polymer Melt Rheology δ τ xy Newton s Law
FLUID DYNAMICS. Intrinsic properties of fluids. Fluids behavior under various conditions
 FLUID DYNAMICS Intrinsic properties of fluids Fluids behavior under various conditions Methods by which we can manipulate and utilize the fluids to produce desired results TYPES OF FLUID FLOW Laminar or
FLUID DYNAMICS Intrinsic properties of fluids Fluids behavior under various conditions Methods by which we can manipulate and utilize the fluids to produce desired results TYPES OF FLUID FLOW Laminar or
Praktikum III. Experiment P4. Simulation of Polymer Behavior in Step Shear Rate Flow using the Reptation Model
 Praktikum III Fall Term 211 Experiment P4 Simulation of Polymer Behavior in Step Shear Rate Flow using the Reptation Model A lab report written by: Baumli Philipp [email protected] Date of the experiment:
Praktikum III Fall Term 211 Experiment P4 Simulation of Polymer Behavior in Step Shear Rate Flow using the Reptation Model A lab report written by: Baumli Philipp [email protected] Date of the experiment:
Diffusion and Fluid Flow
 Diffusion and Fluid Flow What determines the diffusion coefficient? What determines fluid flow? 1. Diffusion: Diffusion refers to the transport of substance against a concentration gradient. ΔS>0 Mass
Diffusion and Fluid Flow What determines the diffusion coefficient? What determines fluid flow? 1. Diffusion: Diffusion refers to the transport of substance against a concentration gradient. ΔS>0 Mass
HW 10. = 3.3 GPa (483,000 psi)
 HW 10 Problem 15.1 Elastic modulus and tensile strength of poly(methyl methacrylate) at room temperature [20 C (68 F)]. Compare these with the corresponding values in Table 15.1. Figure 15.3 is accurate;
HW 10 Problem 15.1 Elastic modulus and tensile strength of poly(methyl methacrylate) at room temperature [20 C (68 F)]. Compare these with the corresponding values in Table 15.1. Figure 15.3 is accurate;
 RHEOLOGY RHEOLOGY Science describing the flow and deformation of matter under stress. Rheo = the flow Viscosity (η) is the resistance of a fluid material to flow under stress. The higher the viscosity,
RHEOLOGY RHEOLOGY Science describing the flow and deformation of matter under stress. Rheo = the flow Viscosity (η) is the resistance of a fluid material to flow under stress. The higher the viscosity,
Fluid Mechanics: Static s Kinematics Dynamics Fluid
 Fluid Mechanics: Fluid mechanics may be defined as that branch of engineering science that deals with the behavior of fluid under the condition of rest and motion Fluid mechanics may be divided into three
Fluid Mechanics: Fluid mechanics may be defined as that branch of engineering science that deals with the behavior of fluid under the condition of rest and motion Fluid mechanics may be divided into three
Rheological Properties of Topical Formulations
 Rheological Properties of Topical Formulations Hemi Nae, PhD Hydan Technologies, Inc. Key Words Complex Modulus, Creep/Recovery, Dilatant Flow, Dynamic Viscosity, Flow, Flow Curve, Flow Models, Frequency
Rheological Properties of Topical Formulations Hemi Nae, PhD Hydan Technologies, Inc. Key Words Complex Modulus, Creep/Recovery, Dilatant Flow, Dynamic Viscosity, Flow, Flow Curve, Flow Models, Frequency
4 Microscopic dynamics
 4 Microscopic dynamics In this section we will look at the first model that people came up with when they started to model polymers from the microscopic level. It s called the Oldroyd B model. We will
4 Microscopic dynamics In this section we will look at the first model that people came up with when they started to model polymers from the microscopic level. It s called the Oldroyd B model. We will
Basic Principles in Microfluidics
 Basic Principles in Microfluidics 1 Newton s Second Law for Fluidics Newton s 2 nd Law (F= ma) : Time rate of change of momentum of a system equal to net force acting on system!f = dp dt Sum of forces
Basic Principles in Microfluidics 1 Newton s Second Law for Fluidics Newton s 2 nd Law (F= ma) : Time rate of change of momentum of a system equal to net force acting on system!f = dp dt Sum of forces
Scalars, Vectors and Tensors
 Scalars, Vectors and Tensors A scalar is a physical quantity that it represented by a dimensional number at a particular point in space and time. Examples are hydrostatic pressure and temperature. A vector
Scalars, Vectors and Tensors A scalar is a physical quantity that it represented by a dimensional number at a particular point in space and time. Examples are hydrostatic pressure and temperature. A vector
CE 204 FLUID MECHANICS
 CE 204 FLUID MECHANICS Onur AKAY Assistant Professor Okan University Department of Civil Engineering Akfırat Campus 34959 Tuzla-Istanbul/TURKEY Phone: +90-216-677-1630 ext.1974 Fax: +90-216-677-1486 E-mail:
CE 204 FLUID MECHANICS Onur AKAY Assistant Professor Okan University Department of Civil Engineering Akfırat Campus 34959 Tuzla-Istanbul/TURKEY Phone: +90-216-677-1630 ext.1974 Fax: +90-216-677-1486 E-mail:
Fluids and Solids: Fundamentals
 Fluids and Solids: Fundamentals We normally recognize three states of matter: solid; liquid and gas. However, liquid and gas are both fluids: in contrast to solids they lack the ability to resist deformation.
Fluids and Solids: Fundamentals We normally recognize three states of matter: solid; liquid and gas. However, liquid and gas are both fluids: in contrast to solids they lack the ability to resist deformation.
10.7 Kinetic Molecular Theory. 10.7 Kinetic Molecular Theory. Kinetic Molecular Theory. Kinetic Molecular Theory. Kinetic Molecular Theory
 The first scheduled quiz will be given next Tuesday during Lecture. It will last 5 minutes. Bring pencil, calculator, and your book. The coverage will be pp 364-44, i.e. Sections 0.0 through.4. 0.7 Theory
The first scheduled quiz will be given next Tuesday during Lecture. It will last 5 minutes. Bring pencil, calculator, and your book. The coverage will be pp 364-44, i.e. Sections 0.0 through.4. 0.7 Theory
Ch 2 Properties of Fluids - II. Ideal Fluids. Real Fluids. Viscosity (1) Viscosity (3) Viscosity (2)
 Ch 2 Properties of Fluids - II Ideal Fluids 1 Prepared for CEE 3500 CEE Fluid Mechanics by Gilberto E. Urroz, August 2005 2 Ideal fluid: a fluid with no friction Also referred to as an inviscid (zero viscosity)
Ch 2 Properties of Fluids - II Ideal Fluids 1 Prepared for CEE 3500 CEE Fluid Mechanics by Gilberto E. Urroz, August 2005 2 Ideal fluid: a fluid with no friction Also referred to as an inviscid (zero viscosity)
Surface Tension. the surface tension of a liquid is the energy required to increase the surface area a given amount
 Tro, Chemistry: A Molecular Approach 1 Surface Tension surface tension is a property of liquids that results from the tendency of liquids to minimize their surface area in order to minimize their surface
Tro, Chemistry: A Molecular Approach 1 Surface Tension surface tension is a property of liquids that results from the tendency of liquids to minimize their surface area in order to minimize their surface
CBE 6333, R. Levicky 1 Review of Fluid Mechanics Terminology
 CBE 6333, R. Levicky 1 Review of Fluid Mechanics Terminology The Continuum Hypothesis: We will regard macroscopic behavior of fluids as if the fluids are perfectly continuous in structure. In reality,
CBE 6333, R. Levicky 1 Review of Fluid Mechanics Terminology The Continuum Hypothesis: We will regard macroscopic behavior of fluids as if the fluids are perfectly continuous in structure. In reality,
Name Class Date. In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Sizes, shapes and interactions of molecules in solution
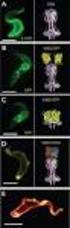 Sizes, shapes and interactions of molecules in solution Light Scattering Analytical Viscometry Ultracentrifugation Steve Harding, NCMH University of Nottingham 1. Molecular weight distribution analysis
Sizes, shapes and interactions of molecules in solution Light Scattering Analytical Viscometry Ultracentrifugation Steve Harding, NCMH University of Nottingham 1. Molecular weight distribution analysis
FLUID FLOW STREAMLINE LAMINAR FLOW TURBULENT FLOW REYNOLDS NUMBER
 VISUAL PHYSICS School of Physics University of Sydney Australia FLUID FLOW STREAMLINE LAMINAR FLOW TURBULENT FLOW REYNOLDS NUMBER? What type of fluid flow is observed? The above pictures show how the effect
VISUAL PHYSICS School of Physics University of Sydney Australia FLUID FLOW STREAMLINE LAMINAR FLOW TURBULENT FLOW REYNOLDS NUMBER? What type of fluid flow is observed? The above pictures show how the effect
Lecture 24 - Surface tension, viscous flow, thermodynamics
 Lecture 24 - Surface tension, viscous flow, thermodynamics Surface tension, surface energy The atoms at the surface of a solid or liquid are not happy. Their bonding is less ideal than the bonding of atoms
Lecture 24 - Surface tension, viscous flow, thermodynamics Surface tension, surface energy The atoms at the surface of a solid or liquid are not happy. Their bonding is less ideal than the bonding of atoms
SIZE OF A MOLECULE FROM A VISCOSITY MEASUREMENT
 Experiment 8, page 1 Version of April 25, 216 Experiment 446.8 SIZE OF A MOLECULE FROM A VISCOSITY MEASUREMENT Theory Viscous Flow. Fluids attempt to minimize flow gradients by exerting a frictional force,
Experiment 8, page 1 Version of April 25, 216 Experiment 446.8 SIZE OF A MOLECULE FROM A VISCOSITY MEASUREMENT Theory Viscous Flow. Fluids attempt to minimize flow gradients by exerting a frictional force,
Determining the impact of electrical loss in coaxial cable
 Determining the impact of electrical loss in coaxial cable Here's a discussion on cable attenuation based on the differences in dielectric loss of low density polyethylene resins and quantify the consequent
Determining the impact of electrical loss in coaxial cable Here's a discussion on cable attenuation based on the differences in dielectric loss of low density polyethylene resins and quantify the consequent
Molar Mass of Polyvinyl Alcohol by Viscosity
 Molar Mass of Polyvinyl Alcohol by Viscosity Introduction Polyvinyl Alcohol (PVOH) is a linear polymer (i. e., it has little branching) of Ethanol monomer units: -CH 2 -CHOH- Unlike most high molar mass
Molar Mass of Polyvinyl Alcohol by Viscosity Introduction Polyvinyl Alcohol (PVOH) is a linear polymer (i. e., it has little branching) of Ethanol monomer units: -CH 2 -CHOH- Unlike most high molar mass
Fluid Dynamics Viscosity. Dave Foster Department of Chemical Engineering University of Rochester Email: dafoster@che
 Fluid Dynamics Viscosity Dave Foster Department of Chemical Engineering University of Rochester Email: dafoster@che che.rochester.eduedu 1 Chemical Engineering What do Chemical Engineers Do? Manufacturing
Fluid Dynamics Viscosity Dave Foster Department of Chemical Engineering University of Rochester Email: dafoster@che che.rochester.eduedu 1 Chemical Engineering What do Chemical Engineers Do? Manufacturing
PUMPS STEAM TURBINES BUILDING & FIRE WASTEWATER SERVICE PUMP CLINIC 22 VISCOSITY
 PUMP CLINIC 22 VISCOSITY The viscosity of a fluid is that property which tends to resist a shearing force. It can be thought of as the internal friction resulting when one layer of fluid is made to move
PUMP CLINIC 22 VISCOSITY The viscosity of a fluid is that property which tends to resist a shearing force. It can be thought of as the internal friction resulting when one layer of fluid is made to move
VISUAL PHYSICS School of Physics University of Sydney Australia. Why do cars need different oils in hot and cold countries?
 VISUAL PHYSICS School of Physics University of Sydney Australia FLUID FLOW VISCOSITY POISEUILLE'S LAW? Why do cars need different oils in hot and cold countries? Why does the engine runs more freely as
VISUAL PHYSICS School of Physics University of Sydney Australia FLUID FLOW VISCOSITY POISEUILLE'S LAW? Why do cars need different oils in hot and cold countries? Why does the engine runs more freely as
Viscosity. Desmond Schipper Andrew R. Barron. 1 Introduction
 OpenStax-CNX module: m50215 1 Viscosity Desmond Schipper Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract This module discusses
OpenStax-CNX module: m50215 1 Viscosity Desmond Schipper Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract This module discusses
1. The Kinetic Theory of Matter states that all matter is composed of atoms and molecules that are in a constant state of constant random motion
 Physical Science Period: Name: ANSWER KEY Date: Practice Test for Unit 3: Ch. 3, and some of 15 and 16: Kinetic Theory of Matter, States of matter, and and thermodynamics, and gas laws. 1. The Kinetic
Physical Science Period: Name: ANSWER KEY Date: Practice Test for Unit 3: Ch. 3, and some of 15 and 16: Kinetic Theory of Matter, States of matter, and and thermodynamics, and gas laws. 1. The Kinetic
CONSTITUTIVE MODELING OF VISCOELASTIC FLUIDS
 CONSTITUTIVE MODELING OF VISCOELSTIC FLUIDS Faith. Morrison Department of Chemical Engineering, Michigan Technological University, Houghton, MI US Keywords: rheology, viscoelasticity, deformation, flow,
CONSTITUTIVE MODELING OF VISCOELSTIC FLUIDS Faith. Morrison Department of Chemical Engineering, Michigan Technological University, Houghton, MI US Keywords: rheology, viscoelasticity, deformation, flow,
Elastic Properties of Polymer Melts Filled with Nanoparticles
 Elastic Properties of Polymer Melts Filled with Nanoparticles Helmut Münstedt and Christian Triebel Citation: AIP Conf. Proc. 1375, 21 (211); doi: 1.163/1.364479 View online: http://dx.doi.org/1.163/1.364479
Elastic Properties of Polymer Melts Filled with Nanoparticles Helmut Münstedt and Christian Triebel Citation: AIP Conf. Proc. 1375, 21 (211); doi: 1.163/1.364479 View online: http://dx.doi.org/1.163/1.364479
Lecture 5 Hemodynamics. Description of fluid flow. The equation of continuity
 1 Lecture 5 Hemodynamics Description of fluid flow Hydrodynamics is the part of physics, which studies the motion of fluids. It is based on the laws of mechanics. Hemodynamics studies the motion of blood
1 Lecture 5 Hemodynamics Description of fluid flow Hydrodynamics is the part of physics, which studies the motion of fluids. It is based on the laws of mechanics. Hemodynamics studies the motion of blood
Lava Flows. Most lava flows are basaltic in composition. Basalt 90% Andesite 8% Dacite/Rhyolite 2%
 Lava Flows Most lava flows are basaltic in composition Basalt 90% Andesite 8% Dacite/Rhyolite 2% This is because most silicic and intermediate magmas erupt explosively (higher gas content and viscocity)
Lava Flows Most lava flows are basaltic in composition Basalt 90% Andesite 8% Dacite/Rhyolite 2% This is because most silicic and intermediate magmas erupt explosively (higher gas content and viscocity)
Solidification, Crystallization & Glass Transition
 Solidification, Crystallization & Glass Transition Cooling the Melt solidification Crystallization versus Formation of Glass Parameters related to the formaton of glass Effect of cooling rate Glass transition
Solidification, Crystallization & Glass Transition Cooling the Melt solidification Crystallization versus Formation of Glass Parameters related to the formaton of glass Effect of cooling rate Glass transition
The Viscosity of Fluids
 Experiment #11 The Viscosity of Fluids References: 1. Your first year physics textbook. 2. D. Tabor, Gases, Liquids and Solids: and Other States of Matter (Cambridge Press, 1991). 3. J.R. Van Wazer et
Experiment #11 The Viscosity of Fluids References: 1. Your first year physics textbook. 2. D. Tabor, Gases, Liquids and Solids: and Other States of Matter (Cambridge Press, 1991). 3. J.R. Van Wazer et
PHYSICS FUNDAMENTALS-Viscosity and flow
 PHYSICS FUNDAMENTALS-Viscosity and flow The origin of viscosity When a force is applied to a solid, it will yield slightly, and then resist further movement. However, when we apply force to a fluid, it
PHYSICS FUNDAMENTALS-Viscosity and flow The origin of viscosity When a force is applied to a solid, it will yield slightly, and then resist further movement. However, when we apply force to a fluid, it
Introduction to Microfluidics. Date: 2013/04/26. Dr. Yi-Chung Tung. Outline
 Introduction to Microfluidics Date: 2013/04/26 Dr. Yi-Chung Tung Outline Introduction to Microfluidics Basic Fluid Mechanics Concepts Equivalent Fluidic Circuit Model Conclusion What is Microfluidics Microfluidics
Introduction to Microfluidics Date: 2013/04/26 Dr. Yi-Chung Tung Outline Introduction to Microfluidics Basic Fluid Mechanics Concepts Equivalent Fluidic Circuit Model Conclusion What is Microfluidics Microfluidics
VISCOSITY OF A LIQUID. To determine the viscosity of a lubricating oil. Time permitting, the temperature variation of viscosity can also be studied.
 VISCOSITY OF A LIQUID August 19, 004 OBJECTIVE: EQUIPMENT: To determine the viscosity of a lubricating oil. Time permitting, the temperature variation of viscosity can also be studied. Viscosity apparatus
VISCOSITY OF A LIQUID August 19, 004 OBJECTIVE: EQUIPMENT: To determine the viscosity of a lubricating oil. Time permitting, the temperature variation of viscosity can also be studied. Viscosity apparatus
Lecture 9, Thermal Notes, 3.054
 Lecture 9, Thermal Notes, 3.054 Thermal Properties of Foams Closed cell foams widely used for thermal insulation Only materials with lower conductivity are aerogels (tend to be brittle and weak) and vacuum
Lecture 9, Thermal Notes, 3.054 Thermal Properties of Foams Closed cell foams widely used for thermal insulation Only materials with lower conductivity are aerogels (tend to be brittle and weak) and vacuum
Proposed Nomenclature for Steady Shear Flow and Linear Viscoelastic Behavior
 TRANSACTIONS OF THE SOCIETY OF RHEOLOGY 20:2, 311317 (1976) Proposed Nomenclature for Steady Shear Flow and Linear Viscoelastic Behavior C. L. SIEGLAFF, Diamond Shamrock Co., inesville, Ohio 44077 Eighteen
TRANSACTIONS OF THE SOCIETY OF RHEOLOGY 20:2, 311317 (1976) Proposed Nomenclature for Steady Shear Flow and Linear Viscoelastic Behavior C. L. SIEGLAFF, Diamond Shamrock Co., inesville, Ohio 44077 Eighteen
KINETIC MOLECULAR THEORY OF MATTER
 KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
TEMPERATURE, CONCENTRATION, AND PUMPING EFFECTS ON PAM VISCOSITY
 TEMPERATURE, CONCENTRATION, AND PUMPING EFFECTS ON PAM VISCOSITY D. L. Bjorneberg ABSTRACT. As polyacrylamide (PAM) use in irrigated agriculture increases, new methods are being sought to accurately and
TEMPERATURE, CONCENTRATION, AND PUMPING EFFECTS ON PAM VISCOSITY D. L. Bjorneberg ABSTRACT. As polyacrylamide (PAM) use in irrigated agriculture increases, new methods are being sought to accurately and
Teil I. Student Laboratory Manuals
 Teil I Student Laboratory Manuals 1 IR1 5. Fluid friction in liquids 5.1 Introduction Generally the term fluid is understood to be matter either in the gaseous or liquid state. The physics involved on
Teil I Student Laboratory Manuals 1 IR1 5. Fluid friction in liquids 5.1 Introduction Generally the term fluid is understood to be matter either in the gaseous or liquid state. The physics involved on
10.7 Kinetic Molecular Theory. 10.7 Kinetic Molecular Theory. Kinetic Molecular Theory. Kinetic Molecular Theory. Kinetic Molecular Theory
 Week lectures--tentative 0.7 Kinetic-Molecular Theory 40 Application to the Gas Laws 0.8 Molecular Effusion and Diffusion 43 Graham's Law of Effusion Diffusion and Mean Free Path 0.9 Real Gases: Deviations
Week lectures--tentative 0.7 Kinetic-Molecular Theory 40 Application to the Gas Laws 0.8 Molecular Effusion and Diffusion 43 Graham's Law of Effusion Diffusion and Mean Free Path 0.9 Real Gases: Deviations
Lecture 13: The Fictive and Glass Transition Temperatures
 Lecture 13: The Fictive and Glass Transition Temperatures March 2, 2010 Dr. Roger Loucks Alfred University Dept. of Physics and Astronomy [email protected] Consider some property, p, of a liquid. p may
Lecture 13: The Fictive and Glass Transition Temperatures March 2, 2010 Dr. Roger Loucks Alfred University Dept. of Physics and Astronomy [email protected] Consider some property, p, of a liquid. p may
Viscosity experiments: physical controls and implications for volcanic hazards. Ben Edwards Dept of Geology, Dickinson College
 Viscosity experiments: physical controls and implications for volcanic hazards Student Name: Ben Edwards Dept of Geology, Dickinson College OBJECTIVES OF LAB Learn about the rheological property called
Viscosity experiments: physical controls and implications for volcanic hazards Student Name: Ben Edwards Dept of Geology, Dickinson College OBJECTIVES OF LAB Learn about the rheological property called
Long term performance of polymers
 1.0 Introduction Long term performance of polymers Polymer materials exhibit time dependent behavior. The stress and strain induced when a load is applied are a function of time. In the most general form
1.0 Introduction Long term performance of polymers Polymer materials exhibit time dependent behavior. The stress and strain induced when a load is applied are a function of time. In the most general form
CHAPTER 12. Gases and the Kinetic-Molecular Theory
 CHAPTER 12 Gases and the Kinetic-Molecular Theory 1 Gases vs. Liquids & Solids Gases Weak interactions between molecules Molecules move rapidly Fast diffusion rates Low densities Easy to compress Liquids
CHAPTER 12 Gases and the Kinetic-Molecular Theory 1 Gases vs. Liquids & Solids Gases Weak interactions between molecules Molecules move rapidly Fast diffusion rates Low densities Easy to compress Liquids
Understanding Rheology
 Understanding Rheology Ross Clark Distinguished Research Fellow San Diego R&D Page 1 Background CP Kelco makes carbohydrate based water soluble polymers Fermentation Xanthan Gellan Plant derived Pectin
Understanding Rheology Ross Clark Distinguished Research Fellow San Diego R&D Page 1 Background CP Kelco makes carbohydrate based water soluble polymers Fermentation Xanthan Gellan Plant derived Pectin
Differential Relations for Fluid Flow. Acceleration field of a fluid. The differential equation of mass conservation
 Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
XI / PHYSICS FLUIDS IN MOTION 11/PA
 Viscosity It is the property of a liquid due to which it flows in the form of layers and each layer opposes the motion of its adjacent layer. Cause of viscosity Consider two neighboring liquid layers A
Viscosity It is the property of a liquid due to which it flows in the form of layers and each layer opposes the motion of its adjacent layer. Cause of viscosity Consider two neighboring liquid layers A
02/21/2006 10:13 AM. Viscosity. The Physics Hypertextbook 1998-2005 by Glenn Elert All Rights Reserved -- Fair Use Encouraged.
 Viscosity The Physics Hypertextbook 1998-2005 by Glenn Elert All Rights Reserved -- Fair Use Encouraged prev up next Discussion definitions Informally, viscosity is the quantity that describes a fluid's
Viscosity The Physics Hypertextbook 1998-2005 by Glenn Elert All Rights Reserved -- Fair Use Encouraged prev up next Discussion definitions Informally, viscosity is the quantity that describes a fluid's
Chapter 5: Diffusion. 5.1 Steady-State Diffusion
 : Diffusion Diffusion: the movement of particles in a solid from an area of high concentration to an area of low concentration, resulting in the uniform distribution of the substance Diffusion is process
: Diffusion Diffusion: the movement of particles in a solid from an area of high concentration to an area of low concentration, resulting in the uniform distribution of the substance Diffusion is process
Chapter Outline. Diffusion - how do atoms move through solids?
 Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
Thermodynamics of Mixing
 Thermodynamics of Mixing Dependence of Gibbs energy on mixture composition is G = n A µ A + n B µ B and at constant T and p, systems tend towards a lower Gibbs energy The simplest example of mixing: What
Thermodynamics of Mixing Dependence of Gibbs energy on mixture composition is G = n A µ A + n B µ B and at constant T and p, systems tend towards a lower Gibbs energy The simplest example of mixing: What
Laminar Flow and Heat Transfer of Herschel-Bulkley Fluids in a Rectangular Duct; Finite-Element Analysis
 Tamkang Journal of Science and Engineering, Vol. 12, No. 1, pp. 99 107 (2009) 99 Laminar Flow and Heat Transfer of Herschel-Bulkley Fluids in a Rectangular Duct; Finite-Element Analysis M. E. Sayed-Ahmed
Tamkang Journal of Science and Engineering, Vol. 12, No. 1, pp. 99 107 (2009) 99 Laminar Flow and Heat Transfer of Herschel-Bulkley Fluids in a Rectangular Duct; Finite-Element Analysis M. E. Sayed-Ahmed
Lecture 4 Classification of Flows. Applied Computational Fluid Dynamics
 Lecture 4 Classification of Flows Applied Computational Fluid Dynamics Instructor: André Bakker http://www.bakker.org André Bakker (00-006) Fluent Inc. (00) 1 Classification: fluid flow vs. granular flow
Lecture 4 Classification of Flows Applied Computational Fluid Dynamics Instructor: André Bakker http://www.bakker.org André Bakker (00-006) Fluent Inc. (00) 1 Classification: fluid flow vs. granular flow
The Viscosity of Fluids
 Experiment #11 The Viscosity of Fluids References: 1. Your first year physics textbook. 2. D. Tabor, Gases, Liquids and Solids: and Other States of Matter (Cambridge Press, 1991). 3. J.R. Van Wazer et
Experiment #11 The Viscosity of Fluids References: 1. Your first year physics textbook. 2. D. Tabor, Gases, Liquids and Solids: and Other States of Matter (Cambridge Press, 1991). 3. J.R. Van Wazer et
Define the notations you are using properly. Present your arguments in details. Good luck!
 Umeå Universitet, Fysik Vitaly Bychkov Prov i fysik, Thermodynamics, 0-0-4, kl 9.00-5.00 jälpmedel: Students may use any book(s) including the textbook Thermal physics. Minor notes in the books are also
Umeå Universitet, Fysik Vitaly Bychkov Prov i fysik, Thermodynamics, 0-0-4, kl 9.00-5.00 jälpmedel: Students may use any book(s) including the textbook Thermal physics. Minor notes in the books are also
Temperature. Temperature
 Chapter 8 Temperature Temperature a number that corresponds to the warmth or coldness of an object measured by a thermometer is a per-particle property no upper limit definite limit on lower end Temperature
Chapter 8 Temperature Temperature a number that corresponds to the warmth or coldness of an object measured by a thermometer is a per-particle property no upper limit definite limit on lower end Temperature
Solution for Homework #1
 Solution for Homework #1 Chapter 2: Multiple Choice Questions (2.5, 2.6, 2.8, 2.11) 2.5 Which of the following bond types are classified as primary bonds (more than one)? (a) covalent bonding, (b) hydrogen
Solution for Homework #1 Chapter 2: Multiple Choice Questions (2.5, 2.6, 2.8, 2.11) 2.5 Which of the following bond types are classified as primary bonds (more than one)? (a) covalent bonding, (b) hydrogen
Ch 8 Potential energy and Conservation of Energy. Question: 2, 3, 8, 9 Problems: 3, 9, 15, 21, 24, 25, 31, 32, 35, 41, 43, 47, 49, 53, 55, 63
 Ch 8 Potential energ and Conservation of Energ Question: 2, 3, 8, 9 Problems: 3, 9, 15, 21, 24, 25, 31, 32, 35, 41, 43, 47, 49, 53, 55, 63 Potential energ Kinetic energ energ due to motion Potential energ
Ch 8 Potential energ and Conservation of Energ Question: 2, 3, 8, 9 Problems: 3, 9, 15, 21, 24, 25, 31, 32, 35, 41, 43, 47, 49, 53, 55, 63 Potential energ Kinetic energ energ due to motion Potential energ
EXAMPLE: Water Flow in a Pipe
 EXAMPLE: Water Flow in a Pipe P 1 > P 2 Velocity profile is parabolic (we will learn why it is parabolic later, but since friction comes from walls the shape is intuitive) The pressure drops linearly along
EXAMPLE: Water Flow in a Pipe P 1 > P 2 Velocity profile is parabolic (we will learn why it is parabolic later, but since friction comes from walls the shape is intuitive) The pressure drops linearly along
Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134)
 Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134) 1. Helium atoms do not combine to form He 2 molecules, What is the strongest attractive
Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134) 1. Helium atoms do not combine to form He 2 molecules, What is the strongest attractive
CHAPTER 2: LIQUID VISCOSITY MEASUREMENT
 CHAPTER 2: LIQUID VISCOSITY MEASUREMENT Objective Calculate viscosity (dynamic or absolute, and kinematic) and determine how this property varies with changes in temperature for a constant-composition
CHAPTER 2: LIQUID VISCOSITY MEASUREMENT Objective Calculate viscosity (dynamic or absolute, and kinematic) and determine how this property varies with changes in temperature for a constant-composition
Experiment 1: Colligative Properties
 Experiment 1: Colligative Properties Determination of the Molar Mass of a Compound by Freezing Point Depression. Objective: The objective of this experiment is to determine the molar mass of an unknown
Experiment 1: Colligative Properties Determination of the Molar Mass of a Compound by Freezing Point Depression. Objective: The objective of this experiment is to determine the molar mass of an unknown
ZETA POTENTIAL ANALYSIS OF NANOPARTICLES
 ZETA POTENTIAL ANALYSIS OF NANOPARTICLES SEPTEMBER 2012, V 1.1 4878 RONSON CT STE K SAN DIEGO, CA 92111 858-565 - 4227 NANOCOMPOSIX.COM Note to the Reader: We at nanocomposix have published this document
ZETA POTENTIAL ANALYSIS OF NANOPARTICLES SEPTEMBER 2012, V 1.1 4878 RONSON CT STE K SAN DIEGO, CA 92111 858-565 - 4227 NANOCOMPOSIX.COM Note to the Reader: We at nanocomposix have published this document
Compressible Fluids. Faith A. Morrison Associate Professor of Chemical Engineering Michigan Technological University November 4, 2004
 94 c 2004 Faith A. Morrison, all rights reserved. Compressible Fluids Faith A. Morrison Associate Professor of Chemical Engineering Michigan Technological University November 4, 2004 Chemical engineering
94 c 2004 Faith A. Morrison, all rights reserved. Compressible Fluids Faith A. Morrison Associate Professor of Chemical Engineering Michigan Technological University November 4, 2004 Chemical engineering
Introduction OLEDs OTFTs OPVC Summary. Organic Electronics. Felix Buth. Walter Schottky Institut, TU München. Joint Advanced Student School 2008
 Felix Buth Joint Advanced Student School 2008 Outline 1 Introduction Difference organic/inorganic semiconductors From molecular orbitals to the molecular crystal 2 Organic Light Emitting Diodes Basic Principals
Felix Buth Joint Advanced Student School 2008 Outline 1 Introduction Difference organic/inorganic semiconductors From molecular orbitals to the molecular crystal 2 Organic Light Emitting Diodes Basic Principals
Thickeners + Rheology Guide
 Thickeners + Rheology Guide 2 Thickeners + Rheology Guide 3 Rheology Rheology is defined as the study of the deformation and flow of materials. When a force is applied to a liquid, the liquid will flow
Thickeners + Rheology Guide 2 Thickeners + Rheology Guide 3 Rheology Rheology is defined as the study of the deformation and flow of materials. When a force is applied to a liquid, the liquid will flow
8.62 Viscometers Application and Selection
 8.62 ViscometersApplication and Selection C. H. KIM (1969, 1982) B. G. LIPTÁK (1995, 2003) Definition of Viscosity: Viscosity Units: Types of Viscous Behavior: Absolute viscosity is the ratio of applied
8.62 ViscometersApplication and Selection C. H. KIM (1969, 1982) B. G. LIPTÁK (1995, 2003) Definition of Viscosity: Viscosity Units: Types of Viscous Behavior: Absolute viscosity is the ratio of applied
Basic Equations, Boundary Conditions and Dimensionless Parameters
 Chapter 2 Basic Equations, Boundary Conditions and Dimensionless Parameters In the foregoing chapter, many basic concepts related to the present investigation and the associated literature survey were
Chapter 2 Basic Equations, Boundary Conditions and Dimensionless Parameters In the foregoing chapter, many basic concepts related to the present investigation and the associated literature survey were
1 The basic equations of fluid dynamics
 1 The basic equations of fluid dynamics The main task in fluid dynamics is to find the velocity field describing the flow in a given domain. To do this, one uses the basic equations of fluid flow, which
1 The basic equations of fluid dynamics The main task in fluid dynamics is to find the velocity field describing the flow in a given domain. To do this, one uses the basic equations of fluid flow, which
Dimensional Analysis
 Dimensional Analysis An Important Example from Fluid Mechanics: Viscous Shear Forces V d t / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Ƭ = F/A = μ V/d More generally, the viscous
Dimensional Analysis An Important Example from Fluid Mechanics: Viscous Shear Forces V d t / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Ƭ = F/A = μ V/d More generally, the viscous
M n = (DP)m = (25,000)(104.14 g/mol) = 2.60! 10 6 g/mol
 14.4 (a) Compute the repeat unit molecular weight of polystyrene. (b) Compute the number-average molecular weight for a polystyrene for which the degree of polymerization is 25,000. (a) The repeat unit
14.4 (a) Compute the repeat unit molecular weight of polystyrene. (b) Compute the number-average molecular weight for a polystyrene for which the degree of polymerization is 25,000. (a) The repeat unit
Vatten(byggnad) VVR145 Vatten. 2. Vätskors egenskaper (1.1, 4.1 och 2.8) (Föreläsningsanteckningar)
 Vatten(byggnad) Vätskors egenskaper (1) Hydrostatik (3) Grundläggande ekvationer (5) Rörströmning (4) 2. Vätskors egenskaper (1.1, 4.1 och 2.8) (Föreläsningsanteckningar) Vätska som kontinuerligt medium
Vatten(byggnad) Vätskors egenskaper (1) Hydrostatik (3) Grundläggande ekvationer (5) Rörströmning (4) 2. Vätskors egenskaper (1.1, 4.1 och 2.8) (Föreläsningsanteckningar) Vätska som kontinuerligt medium
Flow characteristics of microchannel melts during injection molding of microstructure medical components
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(5):112-117 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Flow characteristics of microchannel melts during
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(5):112-117 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Flow characteristics of microchannel melts during
Supporting Information
 Supporting Information Samanta et al. 1.173/pnas.121966611 SI Text Sample Preparation and Characterization The fluid was prepared by adding the required quantity of polymers to 3 L of distilled water.
Supporting Information Samanta et al. 1.173/pnas.121966611 SI Text Sample Preparation and Characterization The fluid was prepared by adding the required quantity of polymers to 3 L of distilled water.
Lecture Chromo-3: Gas Chromatography. CHEM 5181 Fall 2004 Mass Spectrometry & Chromatography. Jessica Gilman and Prof. Jose-Luis Jimenez CU-Boulder
 Lecture Chromo-3: Gas Chromatography CHEM 5181 Fall 2004 Mass Spectrometry & Chromatography Jessica Gilman and Prof. Jose-Luis Jimenez CU-Boulder Outline Introduction Instrument overview Carrier gas Sample
Lecture Chromo-3: Gas Chromatography CHEM 5181 Fall 2004 Mass Spectrometry & Chromatography Jessica Gilman and Prof. Jose-Luis Jimenez CU-Boulder Outline Introduction Instrument overview Carrier gas Sample
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras
 Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
A Beginner s Guide DMA
 FREQUENTLY ASKED QUESTIONS Dynamic Mechanical Analysis (DMA) A Beginner s Guide This booklet provides an introduction to the concepts of Dynamic Mechanical Analysis (DMA). It is written for the materials
FREQUENTLY ASKED QUESTIONS Dynamic Mechanical Analysis (DMA) A Beginner s Guide This booklet provides an introduction to the concepts of Dynamic Mechanical Analysis (DMA). It is written for the materials
VAPORIZATION IN MORE DETAIL. Energy needed to escape into gas phase GAS LIQUID. Kinetic energy. Average kinetic energy
 30 VAPORIZATION IN MORE DETAIL GAS Energy needed to escape into gas phase LIQUID Kinetic energy Average kinetic energy - For a molecule to move from the liquid phase to the gas phase, it must acquire enough
30 VAPORIZATION IN MORE DETAIL GAS Energy needed to escape into gas phase LIQUID Kinetic energy Average kinetic energy - For a molecule to move from the liquid phase to the gas phase, it must acquire enough
INFLUENCE OF MOLD PROPERTIES ON THE QUALITY OF MOLDED PARTS
 U.P.B. Sci. Bull., Series D, Vol. 69, No. 3, 2007 ISSN 1454-2358 INFLUENCE OF MOLD PROPERTIES ON THE QUALITY OF MOLDED PARTS Sorin ASPROIU 1, Eugen STRĂJESCU 2 O mare parte din materialele plastice sunt
U.P.B. Sci. Bull., Series D, Vol. 69, No. 3, 2007 ISSN 1454-2358 INFLUENCE OF MOLD PROPERTIES ON THE QUALITY OF MOLDED PARTS Sorin ASPROIU 1, Eugen STRĂJESCU 2 O mare parte din materialele plastice sunt
Why? Intermolecular Forces. Intermolecular Forces. Chapter 12 IM Forces and Liquids. Covalent Bonding Forces for Comparison of Magnitude
 1 Why? Chapter 1 Intermolecular Forces and Liquids Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature for such a small molecule? Why does ice float on water?
1 Why? Chapter 1 Intermolecular Forces and Liquids Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature for such a small molecule? Why does ice float on water?
Viscous flow in pipe
 Viscous flow in pipe Henryk Kudela Contents 1 Laminar or turbulent flow 1 2 Balance of Momentum - Navier-Stokes Equation 2 3 Laminar flow in pipe 2 3.1 Friction factor for laminar flow...........................
Viscous flow in pipe Henryk Kudela Contents 1 Laminar or turbulent flow 1 2 Balance of Momentum - Navier-Stokes Equation 2 3 Laminar flow in pipe 2 3.1 Friction factor for laminar flow...........................
Optical anisotropy of flexible polyimide thin films
 Journal of MATERIALS RESEARCH Welcome Comments Help Optical anisotropy of flexible polyimide thin films Baozhong Li, Tianbai He, a) and Mengxian Ding Polymer Physics Laboratory, Changchun Institute of
Journal of MATERIALS RESEARCH Welcome Comments Help Optical anisotropy of flexible polyimide thin films Baozhong Li, Tianbai He, a) and Mengxian Ding Polymer Physics Laboratory, Changchun Institute of
Study the following diagrams of the States of Matter. Label the names of the Changes of State between the different states.
 Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Unit 6 Plane Stress and Plane Strain
 Unit 6 Plane Stress and Plane Strain Readings: T & G 8, 9, 10, 11, 12, 14, 15, 16 Paul A. Lagace, Ph.D. Professor of Aeronautics & Astronautics and Engineering Systems There are many structural configurations
Unit 6 Plane Stress and Plane Strain Readings: T & G 8, 9, 10, 11, 12, 14, 15, 16 Paul A. Lagace, Ph.D. Professor of Aeronautics & Astronautics and Engineering Systems There are many structural configurations
The ratio of inertial to viscous forces is commonly used to scale fluid flow, and is called the Reynolds number, given as:
 12.001 LAB 3C: STOKES FLOW DUE: WEDNESDAY, MARCH 9 Lab Overview and Background The viscosity of a fluid describes its resistance to deformation. Water has a very low viscosity; the force of gravity causes
12.001 LAB 3C: STOKES FLOW DUE: WEDNESDAY, MARCH 9 Lab Overview and Background The viscosity of a fluid describes its resistance to deformation. Water has a very low viscosity; the force of gravity causes
Chemistry 13: States of Matter
 Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
Chapter 10 Liquids & Solids
 1 Chapter 10 Liquids & Solids * 10.1 Polar Covalent Bonds & Dipole Moments - van der Waals constant for water (a = 5.28 L 2 atm/mol 2 ) vs O 2 (a = 1.36 L 2 atm/mol 2 ) -- water is polar (draw diagram)
1 Chapter 10 Liquids & Solids * 10.1 Polar Covalent Bonds & Dipole Moments - van der Waals constant for water (a = 5.28 L 2 atm/mol 2 ) vs O 2 (a = 1.36 L 2 atm/mol 2 ) -- water is polar (draw diagram)
Micellar structures and Whisky
 Micellar structures and Whisky Table of content 1 Table of content Table of content...1 Introduction...2 Micellar structures in Whisky...4 Methods...4 Results...5 Discussion... 10 Disclaimer... 11 Introduction
Micellar structures and Whisky Table of content 1 Table of content Table of content...1 Introduction...2 Micellar structures in Whisky...4 Methods...4 Results...5 Discussion... 10 Disclaimer... 11 Introduction
Statistical Mechanics, Kinetic Theory Ideal Gas. 8.01t Nov 22, 2004
 Statistical Mechanics, Kinetic Theory Ideal Gas 8.01t Nov 22, 2004 Statistical Mechanics and Thermodynamics Thermodynamics Old & Fundamental Understanding of Heat (I.e. Steam) Engines Part of Physics Einstein
Statistical Mechanics, Kinetic Theory Ideal Gas 8.01t Nov 22, 2004 Statistical Mechanics and Thermodynamics Thermodynamics Old & Fundamental Understanding of Heat (I.e. Steam) Engines Part of Physics Einstein
