Structure of materials
|
|
|
- Sheryl Charles
- 9 years ago
- Views:
Transcription
1 Structure of materials The atomic number is the number of protons for each element. Atoms of the same element have the same number of protons in the nucleus but may differ by one or more neutrons forming isotopes of the element. Atoms can also gain or lose one or more electrons, forming charged ions. Except for the simplest hydrogen atom with a single proton as its entire nucleus, all atoms contain neutrons in addition to protons. The sum of the protons and neutrons is the mass number of an atom. M520 Page 1
2 Quantum theory describes electrons. It predicts discrete allowed energy levels and gives probability distributions for electrons, which describe where electrons are more or less likely to be found. The probability distributions are commonly referred to as electron orbitals. The Bohr model represents an early attempt to describe electrons in terms of both position (electron orbitals) and energy (quantized energy levels). M520 Page 2
3 The electron configuration of an atom represents the manner in which the quantum states are occupied. In the conventional notation the number of electrons in each subshell is indicated by a superscript after the shell subshell designation. When all the electrons occupy the lowest possible energy levels, an atom is said to be in its ground state. M520 Page 3
4 Valence electrons are those that occupy the outermost shell. These electrons are extremely important because they participate in the bonding between atoms to form atomic and molecular aggregates. Furthermore, many of the physical and chemical properties of solids are based on these valence electrons. Some atoms have what are termed as stable electron configurations, that is, the states within the outermost or valence electron shell are completely filled. M520 Page 4
5 All the elements have been classified according to electron configuration in the periodic table. The elements are situated, with increasing atomic number, in seven horizontal rows called periods. The arrangement is such that all elements arrayed in a given column or group have similar valence electron structures, as well as chemical and physical properties. Ionic bonding is always found in compounds that are composed of both metallic and nonmetallic elements, which are situated at the horizontal extremities of the periodic table. Atoms of a metallic element easily give up their valence electrons to the nonmetallic atoms. In the process all the atoms acquire stable configurations and gain an electrical charge, thus becoming ions. Ionic materials are characteristically hard and brittle and, furthermore, electrically and thermally insulative. M520 Page 5
6 In covalent bonding, stable electron configurations are assumed by the sharing of electrons between adjacent atoms. Two atoms that are covalently bonded will each contribute at least one electron to the bond, and the shared electrons may be considered to belong to both atoms. Covalent bonding is schematically illustrated for a molecule of methane (CH 4 ). The carbon atom has four valence electrons, whereas each of the four hydrogen atoms has a single valence electron. Each hydrogen atom can acquire a helium electron configuration (two 1s valence electrons) when the carbon atom shares with it one electron. The carbon now has four additional shared electrons, one from each hydrogen, for a total of eight valence electrons. Many nonmetallic elemental molecules as well as molecules containing dissimilar atoms are covalently bonded. This type of bonding is found in elemental solids such as diamond (carbon), silicon, germanium and other solid compounds composed of elements that are located on the right-hand side of the periodic table. M520 Page 6
7 The number of covalent bonds that is possible for a particular atom is determined by the number of valence electrons. For Y valence electrons, an atom can covalently bond with at most 8 - Y other atoms. For example, for carbon, and each carbon atom has 4 valence electrons to share, so it can covalently bond with at most 4 other atoms. Metallic bonding is found in metals and their alloys. Metallic materials have one, two, or at most, three valence electrons. These valence electrons are not bound to any particular atom in the solid and are more or less free to drift throughout the entire metal. They may be thought of as belonging to the metal as a whole, or forming a sea of electrons or an electron cloud. The remaining nonvalence electrons and atomic nuclei form what are called ion cores, which possess a net positive charge equal in magnitude to the total valence electron charge per atom. The free electrons act as a glue to hold the ion cores together. M520 Page 7
8 Solid materials may be classified according to the regularity with which atoms or ions are arranged with respect to one another. A crystalline material is one in which the atoms are situated in a repeating or periodic array; that is, long-range order exists, such that upon solidification, the atoms will position themselves in a repetitive three-dimensional pattern, in which each atom is bonded to its nearest-neighbor atoms. All metals, many ceramic materials, and certain polymers form crystalline structures under normal solidification conditions. Some of the properties of crystalline solids depend on the crystal structure of the material, the manner in which atoms, ions, or molecules are spatially arranged. There is an extremely large number of different crystal structures all having long range atomic order; these vary from relatively simple structures for metals to exceedingly complex ones, as displayed by some of the ceramic and polymeric materials. M520 Page 8
9 Some metals, as well as nonmetals, may have more than one crystal structure, a phenomenon known as polymorphism. When found in elemental solids, the condition is often termed allotropy. The prevailing crystal structure depends on both the temperature and the external pressure. One familiar example is found in carbon: graphite is the stable polymorph at ambient conditions, whereas diamond is formed at extremely high pressures. Most often a modification of the density and other physical properties accompanies a polymorphic transformation. cooling The unit cell geometry is completely defined in terms of six parameters: the three edge lengths a, b, and c, and the three interaxial angles. They are also known as the lattice parameters of a crystal structure. On this basis there are seven different possible combinations and each of which represents a distinct crystal system: cubic, hexagonal, tetragonal, rhombohedral, orthorhombic, monoclinic and triclinic. M520 Page 9
10 M520 Page 10
11 For a crystalline solid, when the periodic and repeated arrangement of atoms is perfect or extends throughout the entirety of the specimen without interruption, the result is a single crystal. All unit cells interlock in the same way and have the same orientation. Single crystals exist in nature, but they may also be produced artificially. They are ordinarily difficult to grow, because the environment must be carefully controlled. If the extremities of a single crystal are permitted to grow without any external constraint, the crystal will assume a regular geometric shape having flat faces, as with some of the gem stones. The shape is indicative of the crystal structure. Within the past few years, single crystals have become extremely important to many modern technologies, in particular microcircuits, which use single crystals of silicon and other semiconductors. Most crystalline solids are composed of a collection of many small crystals or grains; such materials are termed polycrystalline. Initially, small crystals form at various positions. These have random crystallographic orientations. The small grains grow by the successive addition from the surrounding liquid of atoms to the structure of each. The extremities of adjacent grains impinge on one another as the solidification process approaches completion. The crystallographic orientation varies from grain to grain. Also, there exists some atomic mismatch within the region where two grains meet. M520 Page 11
12 The physical properties of single crystals of some substances depend on the crystallographic direction in which measurements are taken. For example, the elastic modulus, the electrical conductivity and the index of refraction may have different values depending on the crystallographic directions. This directionality of properties is termed anisotropy, and it is associated with the variance of atomic or ionic spacing with crystallographic direction. Substances in which measured properties are independent of the direction of measurement are isotropic. The extent and magnitude of anisotropic effects in crystalline materials are functions of the symmetry of the crystal structure. The degree of anisotropy increases with decreasing structural symmetry triclinic structures normally are highly anisotropic. For many polycrystalline materials, the crystallographic orientations of the individual grains are totally random. Under these circumstances, even though each grain may be anisotropic, a specimen composed of the grain aggregate behaves isotropically. Also, the magnitude of a measured property represents some average of the directional values. M520 Page 12
13 Noncrystalline solids lack a systematic and regular arrangement of atoms over relatively large atomic distances. Sometimes such materials are also called amorphous (meaning literally without form), or supercooled liquids, inasmuch as their atomic structure resembles that of a liquid. An amorphous condition may be illustrated by comparison of the crystalline and noncrystalline structures of the ceramic compound silicon dioxide, which may exist in both states. Even though each silicon ion bonds to three oxygen ions for both states, beyond this, the structure is much more disordered and irregular for the noncrystalline structure. Whether a crystalline or amorphous solid forms depends on the ease with which a random atomic structure in the liquid can transform to an ordered state during solidification. Amorphous materials, therefore, are characterized by atomic or molecular structures that are relatively complex and become ordered only with some difficulty. Furthermore, rapidly cooling through the freezing temperature favors the formation of a noncrystalline solid, since little time is allowed for the ordering process. Metals normally form crystalline solids. Polymers may be completely noncrystalline and semicrystalline consisting of varying degrees of crystallinity. M520 Page 13
14 Many of the properties of materials are profoundly sensitive to deviations from crystalline perfection; the influence is not always adverse, and often specific characteristics are deliberately fashioned by the introduction of controlled amounts or numbers of particular defects. The simplest of the point defects is a vacancy, or vacant lattice site, one normally occupied from which an atom is missing. All crystalline solids contain vacancies and, in fact, it is not possible to create such a material that is free of these defects. The presence of vacancies increases the entropy (i.e., the randomness) of the crystal. The equilibrium number of vacancies for a given quantity of material depends on and increases with temperature. A self-interstitial is an atom from the crystal that is crowded into an interstitial site, a small void space that under ordinary circumstances is not occupied. In metals, a self-interstitial introduces relatively large distortions in the surrounding lattice because the atom is substantially larger than the interstitial position in which it is situated. Consequently, the formation of this defect is not highly probable, and it exists in very small concentrations, which are significantly lower than for vacancies. M520 Page 14
15 A pure metal consisting of only one type of atom just isn t possible; impurity or foreign atoms will always be present, and some will exist as crystalline point defects. Even with relatively sophisticated techniques, it is difficult to refine metals to a purity in excess of %. Most familiar metals are not highly pure; rather, they are alloys, in which impurity atoms have been added intentionally to impart specific characteristics to the material. Ordinarily, alloying is used in metals to improve mechanical strength and corrosion resistance. For example, sterling silver is 92.5% silver and 7.5% copper alloy. In normal ambient environments, pure silver is highly corrosion resistant, but also very soft. Alloying with copper significantly enhances the mechanical strength without depreciating the corrosion resistance appreciably. The addition of impurity atoms to a metal will result in the formation of a solid solution, depending on the kinds of impurity, their concentrations, and the temperature of the alloy. With regard to alloys, solvent represents the element or compound that is present in the greatest amount and solute is used to denote an element or compound present in a minor concentration. M520 Page 15
KINETIC MOLECULAR THEORY OF MATTER
 KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
Chapter Outline. How do atoms arrange themselves to form solids?
 Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Simple cubic Face-centered cubic Body-centered cubic Hexagonal close-packed
Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Simple cubic Face-centered cubic Body-centered cubic Hexagonal close-packed
Unit 2 Periodic Behavior and Ionic Bonding
 Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Ch. 4: Imperfections in Solids Part 1. Dr. Feras Fraige
 Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
CHAPTER 6 Chemical Bonding
 CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
Ionic and Metallic Bonding
 Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Defects Introduction. Bonding + Structure + Defects. Properties
 Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Type of Chemical Bonds
 Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 2. Atomic Structure and Interatomic Bonding
 Chapter 2. Atomic Structure and Interatomic Bonding Interatomic Bonding Bonding forces and energies Primary interatomic bonds Secondary bonding Molecules Bonding Forces and Energies Considering the interaction
Chapter 2. Atomic Structure and Interatomic Bonding Interatomic Bonding Bonding forces and energies Primary interatomic bonds Secondary bonding Molecules Bonding Forces and Energies Considering the interaction
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Unit 12 Practice Test
 Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s)
 BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
(1) e.g. H hydrogen that has lost 1 electron c. anion - negatively charged atoms that gain electrons 16-2. (1) e.g. HCO 3 bicarbonate anion
 GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds.
 Problem 1 Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds. Ionic Bonds Two neutral atoms close to each can undergo an ionization process in order
Problem 1 Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds. Ionic Bonds Two neutral atoms close to each can undergo an ionization process in order
Theme 3: Bonding and Molecular Structure. (Chapter 8)
 Theme 3: Bonding and Molecular Structure. (Chapter 8) End of Chapter questions: 5, 7, 9, 12, 15, 18, 23, 27, 28, 32, 33, 39, 43, 46, 67, 77 Chemical reaction valence electrons of atoms rearranged (lost,
Theme 3: Bonding and Molecular Structure. (Chapter 8) End of Chapter questions: 5, 7, 9, 12, 15, 18, 23, 27, 28, 32, 33, 39, 43, 46, 67, 77 Chemical reaction valence electrons of atoms rearranged (lost,
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Chapter 8 Basic Concepts of the Chemical Bonding
 Chapter 8 Basic Concepts of the Chemical Bonding 1. There are paired and unpaired electrons in the Lewis symbol for a phosphorus atom. (a). 4, 2 (b). 2, 4 (c). 4, 3 (d). 2, 3 Explanation: Read the question
Chapter 8 Basic Concepts of the Chemical Bonding 1. There are paired and unpaired electrons in the Lewis symbol for a phosphorus atom. (a). 4, 2 (b). 2, 4 (c). 4, 3 (d). 2, 3 Explanation: Read the question
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
CHAPTER 6 REVIEW. Chemical Bonding. Answer the following questions in the space provided.
 Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Chapter 4: Structure and Properties of Ionic and Covalent Compounds
 Chapter 4: Structure and Properties of Ionic and Covalent Compounds 4.1 Chemical Bonding o Chemical Bond - the force of attraction between any two atoms in a compound. o Interactions involving valence
Chapter 4: Structure and Properties of Ionic and Covalent Compounds 4.1 Chemical Bonding o Chemical Bond - the force of attraction between any two atoms in a compound. o Interactions involving valence
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Crystalline solids. A solid crystal consists of different atoms arranged in a periodic structure.
 Crystalline solids A solid crystal consists of different atoms arranged in a periodic structure. Crystals can be formed via various bonding mechanisms: Ionic bonding Covalent bonding Metallic bonding Van
Crystalline solids A solid crystal consists of different atoms arranged in a periodic structure. Crystals can be formed via various bonding mechanisms: Ionic bonding Covalent bonding Metallic bonding Van
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance
 Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
All about Chemical Bonding Ionic
 Program Support Notes by: Peter Gribben BA BSc (Hons) PGCE Produced by: VEA Pty Ltd Commissioning Editor: Darren Gray Cert IV Training & Assessment Executive Producer: Simon Garner B.Ed, Dip Management
Program Support Notes by: Peter Gribben BA BSc (Hons) PGCE Produced by: VEA Pty Ltd Commissioning Editor: Darren Gray Cert IV Training & Assessment Executive Producer: Simon Garner B.Ed, Dip Management
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Bonding Practice Problems
 NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 6 Assessment. Name: Class: Date: ID: A. Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Chapter 12 - Liquids and Solids
 Chapter 12 - Liquids and Solids 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Chapter 12 - Liquids and Solids 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.
 CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together is called a(n)
 Chemistry I ATOMIC BONDING PRACTICE QUIZ Mr. Scott Select the best answer. 1) A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together is
Chemistry I ATOMIC BONDING PRACTICE QUIZ Mr. Scott Select the best answer. 1) A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together is
BOND TYPES: THE CLASSIFICATION OF SUBSTANCES
 BOND TYPES: THE CLASSIFICATION OF SUBSTANCES Every (pure) substance has a unique set of intrinsic properties which distinguishes it from all other substances. What inferences, if any can be made from a
BOND TYPES: THE CLASSIFICATION OF SUBSTANCES Every (pure) substance has a unique set of intrinsic properties which distinguishes it from all other substances. What inferences, if any can be made from a
Atoms and Elements. Atoms: Learning Goals. Chapter 3. Atoms and Elements; Isotopes and Ions; Minerals and Rocks. Clicker 1. Chemistry Background?
 Chapter 3 Atoms Atoms and Elements; Isotopes and Ions; Minerals and Rocks A Review of Chemistry: What geochemistry tells us Clicker 1 Chemistry Background? A. No HS or College Chemistry B. High School
Chapter 3 Atoms Atoms and Elements; Isotopes and Ions; Minerals and Rocks A Review of Chemistry: What geochemistry tells us Clicker 1 Chemistry Background? A. No HS or College Chemistry B. High School
A pure covalent bond is an equal sharing of shared electron pair(s) in a bond. A polar covalent bond is an unequal sharing.
 CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras
 Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
Name Class Date. In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Matter, Materials, Crystal Structure and Bonding. Chris J. Pickard
 Matter, Materials, Crystal Structure and Bonding Chris J. Pickard Why should a theorist care? Where the atoms are determines what they do Where the atoms can be determines what we can do Overview of Structure
Matter, Materials, Crystal Structure and Bonding Chris J. Pickard Why should a theorist care? Where the atoms are determines what they do Where the atoms can be determines what we can do Overview of Structure
Chapter Outline. Review of Atomic Structure Electrons, Protons, Neutrons, Quantum mechanics of atoms, Electron states, The Periodic Table
 Review of Atomic Structure Electrons, Protons, Neutrons, Quantum mechanics of atoms, Electron states, The Periodic Table Atomic Bonding in Solids Bonding Energies and Forces Periodic Table Chapter Outline
Review of Atomic Structure Electrons, Protons, Neutrons, Quantum mechanics of atoms, Electron states, The Periodic Table Atomic Bonding in Solids Bonding Energies and Forces Periodic Table Chapter Outline
Bonds. Bond Length. Forces that hold groups of atoms together and make them function as a unit. Bond Energy. Chapter 8. Bonding: General Concepts
 Bonds hapter 8 Bonding: General oncepts Forces that hold groups of atoms together and make them function as a unit. Bond Energy Bond Length It is the energy required to break a bond. The distance where
Bonds hapter 8 Bonding: General oncepts Forces that hold groups of atoms together and make them function as a unit. Bond Energy Bond Length It is the energy required to break a bond. The distance where
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
Molecular Models & Lewis Dot Structures
 Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chemistry - Elements Electron Configurations The Periodic Table. Ron Robertson
 Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
Chapter 1 Structure and Bonding. Modified by Dr. Daniela Radu
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Chapter 3: Structure of Metals and Ceramics. Chapter 3: Structure of Metals and Ceramics. Learning Objective
 Chapter 3: Structure of Metals and Ceramics Chapter 3: Structure of Metals and Ceramics Goals Define basic terms and give examples of each: Lattice Basis Atoms (Decorations or Motifs) Crystal Structure
Chapter 3: Structure of Metals and Ceramics Chapter 3: Structure of Metals and Ceramics Goals Define basic terms and give examples of each: Lattice Basis Atoms (Decorations or Motifs) Crystal Structure
Science Standard Articulated by Grade Level Strand 5: Physical Science
 Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Chemistry Diagnostic Questions
 Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
Understanding the p-n Junction by Dr. Alistair Sproul Senior Lecturer in Photovoltaics The Key Centre for Photovoltaic Engineering, UNSW
 Understanding the p-n Junction by Dr. Alistair Sproul Senior Lecturer in Photovoltaics The Key Centre for Photovoltaic Engineering, UNSW The p-n junction is the fundamental building block of the electronic
Understanding the p-n Junction by Dr. Alistair Sproul Senior Lecturer in Photovoltaics The Key Centre for Photovoltaic Engineering, UNSW The p-n junction is the fundamental building block of the electronic
In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and oxygen. [Include any charges or partial charges.
 Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Multi-electron atoms
 Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
ANSWER KEY. Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take!
 ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
M n = (DP)m = (25,000)(104.14 g/mol) = 2.60! 10 6 g/mol
 14.4 (a) Compute the repeat unit molecular weight of polystyrene. (b) Compute the number-average molecular weight for a polystyrene for which the degree of polymerization is 25,000. (a) The repeat unit
14.4 (a) Compute the repeat unit molecular weight of polystyrene. (b) Compute the number-average molecular weight for a polystyrene for which the degree of polymerization is 25,000. (a) The repeat unit
2 ATOMIC SYSTEMATICS AND NUCLEAR STRUCTURE
 2 ATOMIC SYSTEMATICS AND NUCLEAR STRUCTURE In this chapter the principles and systematics of atomic and nuclear physics are summarised briefly, in order to introduce the existence and characteristics of
2 ATOMIC SYSTEMATICS AND NUCLEAR STRUCTURE In this chapter the principles and systematics of atomic and nuclear physics are summarised briefly, in order to introduce the existence and characteristics of
12.524 2003 Lec 17: Dislocation Geometry and Fabric Production 1. Crystal Geometry
 12.524 2003 Lec 17: Dislocation Geometry and Fabric Production 1. Bibliography: Crystal Geometry Assigned Reading: [Poirier, 1985]Chapter 2, 4. General References: [Kelly and Groves, 1970] Chapter 1. [Hirth
12.524 2003 Lec 17: Dislocation Geometry and Fabric Production 1. Bibliography: Crystal Geometry Assigned Reading: [Poirier, 1985]Chapter 2, 4. General References: [Kelly and Groves, 1970] Chapter 1. [Hirth
The elements of the second row fulfill the octet rule by sharing eight electrons, thus acquiring the electronic configuration of neon, the noble gas o
 2. VALENT BNDING, TET RULE, PLARITY, AND BASI TYPES F FRMULAS LEARNING BJETIVES To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its
2. VALENT BNDING, TET RULE, PLARITY, AND BASI TYPES F FRMULAS LEARNING BJETIVES To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its
Molecular Geometry and VSEPR We gratefully acknowledge Portland Community College for the use of this experiment.
 Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Section 3: Crystal Binding
 Physics 97 Interatomic forces Section 3: rystal Binding Solids are stable structures, and therefore there exist interactions holding atoms in a crystal together. For example a crystal of sodium chloride
Physics 97 Interatomic forces Section 3: rystal Binding Solids are stable structures, and therefore there exist interactions holding atoms in a crystal together. For example a crystal of sodium chloride
Chapter 2: Atomic Structure and Chemical Bonding
 Chapter 2: Atomic Structure and Chemical Bonding Materials Molecules Atoms Atoms = protons (p) + neutrons (n) + electrons (e) Protons and neutrons are made of quarks Quantitative measurements need units:
Chapter 2: Atomic Structure and Chemical Bonding Materials Molecules Atoms Atoms = protons (p) + neutrons (n) + electrons (e) Protons and neutrons are made of quarks Quantitative measurements need units:
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
3 Atomic Structure 15
 3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
Wafer Manufacturing. Reading Assignments: Plummer, Chap 3.1~3.4
 Wafer Manufacturing Reading Assignments: Plummer, Chap 3.1~3.4 1 Periodic Table Roman letters give valence of the Elements 2 Why Silicon? First transistor, Shockley, Bardeen, Brattain1947 Made by Germanium
Wafer Manufacturing Reading Assignments: Plummer, Chap 3.1~3.4 1 Periodic Table Roman letters give valence of the Elements 2 Why Silicon? First transistor, Shockley, Bardeen, Brattain1947 Made by Germanium
Sample Exercise 12.1 Calculating Packing Efficiency
 Sample Exercise 12.1 Calculating Packing Efficiency It is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is the fraction of space in a crystal
Sample Exercise 12.1 Calculating Packing Efficiency It is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is the fraction of space in a crystal
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Chapter Outline Dislocations and Strengthening Mechanisms
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Covalent Bonding and Molecular Geometry
 Name Section # Date of Experiment Covalent Bonding and Molecular Geometry When atoms combine to form molecules (this also includes complex ions) by forming covalent bonds, the relative positions of the
Name Section # Date of Experiment Covalent Bonding and Molecular Geometry When atoms combine to form molecules (this also includes complex ions) by forming covalent bonds, the relative positions of the
Bohr s Model of the Atom
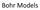 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Ionic and Covalent Bonds
 Ionic and Covalent Bonds Ionic Bonds Transfer of Electrons When metals bond with nonmetals, electrons are from the metal to the nonmetal The becomes a cation and the becomes an anion. The between the cation
Ionic and Covalent Bonds Ionic Bonds Transfer of Electrons When metals bond with nonmetals, electrons are from the metal to the nonmetal The becomes a cation and the becomes an anion. The between the cation
