Lecture 29 High Temperature Geochemistry Formation of the solar system
|
|
|
- Matilda Brown
- 7 years ago
- Views:
Transcription
1 Lecture 29 High Temperature Geochemistry Formation of the solar system Reading this week: White Ch (dig ) and Ch 10.1 to (dig ) Today 1. Age and Origin of the Solar System 2. Condensation Sequence Next time 3. Planetesimal Accretion and Early Solar System Evolution Age and origin of the niverse and our solar system There are 3 main lines of evidence for how old the universe is: ~1. Astronomical approaches use measurements of globular clusters, old white dwarf stars, and the rate of expansion of the niverse (Hubble constant = velocity/distance, using the red shift of distant galaxies or variations in the cosmic microwave background temperature). Recent estimates by different approaches yield ages from about Ga. Cosmic microwave measurements suggest an age of Ga. However, there are uncertainties in most of these methods related to poorly quantified amounts of dark matter in the universe (among other things). Note: Ga = giga annum = 10 9 yrs 1
2 Age and origin of the niverse and our solar system Geochemical estimates can be made from ~2. the rate of consumption (depletion) of nuclear fuel in the most distant stars within our galaxy, or from ~3. the radioactive decay of long lived r-process nulcides. Most of this work has been done with 232 Th, 238, and 235. These methods give ages that are both ~15Ga. Let's examine the last estimate a bit. 232 Th t 1/2 = 1.4 x yr longer time 238 t 1/2 = 4.5 x 10 9 yr medium time 235 t 1/2 = 7.1 x 10 8 yr shorter time We assume, for simplicity, that supernovas have been distributed uniformly throughout the niverse, so that a single mean r-process production rate applies everywhere for each of these three isotopes. There are actually a range of estimates for the r-process production ratios of the long-lived Th and isotopes based on various theoretical and observational lines of evidence. Our approximation assumes a typical value and yields this proportion right after the production ceases: Th Proportions are relative to atoms: 2
3 We ll see next time that a group of meteorites known as the C1 chondrites have ratios of non-gaseous elements that nearly match those of the sun, so they yield an excellent estimate of our primordial solar nebula s composition. The ages of these meteorites (determined from radiometricdating by the Rb-Sr, -Pb, and Pb-Pb dating methods) are 4.55x to 4.6 x 10 9 yr. C1 Chondrites today have: Th i.e., going from most decayed to > least decayed since formation 4.6 Ga ago (1Ga= 10 9 yr) Correcting for radioactive decay in the last 4.6 Ga since solar system formation using a slightly modified form of the decay equation: N 1 = N 2 e ΔT/ t where t is the mean life of 1/l, ΔT is the time interval, N 1 = the r-process production values N 2 = C1 Chondrite 4.6 Ga of each of the three isotopes, we estimate that 4.6 Ga C1 Chondrites (and Earth) had: Th We can then solve to find that ΔT = 2.1 x 10 9 yrs before Earth formed, or 6.7 x 10 9 yrs old, which is 6.7 Ga. 3
4 This is a very low estimate (too low) relative to astronomical estimates. Furthermore, it s badly inconsistent with the presence in our solar system of the decay products of extinct radioactive nuclides that were still alive at 4.6 Ga, when the earliest solids formed, such as: 26 Al 26 Mg T 1/2 = 700 ka 129 I 129 Xe T 1/2 = 17 ma That such highly radioactive elements existed at 4.6 Ga in our solar nebula means that there must have been at least one supernova (and thus r-process event) in our neighborhood of the galaxy shortly before our solar nebula formed (i.e., within a few million years). This has the effect of making our simple estimate of the age of Th and in the solar system too low (young), more so if more than one supernova contributed elements to our solar nebula. We can't really know when or how often earlier supernovas occurred, but if we assume that they occur regularly, at a steady-state in frequency and size, we can use historical observations over the last 2000 years to estimate that there are roughly 10 8 supernovas per 10 Gyr within our galaxy. If the galaxy is well-mixed on very large scales, there will be a time for each isotope when production by the r-process is balanced by decay (i.e., steady state). At steady state, it can be estimated that the isotopic proportions are: Th The time needed for each isotope to reach steady state is indicated in this figure. Clearly, only 235 has reached this condition so far. 4
5 noting that our own nebular values would have been "locked in" at 4.6 Ga (more recent novas have not affected the ratio in our solar system), we can again use the 4.6 Ga values in C1 chondrites (repeated from above) Th to find that 238 and 232 Th were at 84% (= 345/410) and 33% (= 818/2460) of the way to steady state at 4.6 Ga. These ratios are used to estimate galactic ages of 12 Ga from 238 and 9 Ga from 232 Th (average = 10.5) [see red box in image at right] Estimated age of universe: Ga = 15 Ga (before today) A more recent estimate for just the Milky Way galaxy uses similar reasoning by comparing the (1) temporal change in Th/ in very small low metal stars in our galaxy (the LHMS curve) and (2) a mean r-process production rate for stars throughout the galaxy, making various assumptions about how metals transfer among interstellar gas clouds, nebulae, and stars between supernovas to produce a chondritic Th/ ratio (GCE curve). The intersection of the curves is the age of the Galaxy ( , -2.2 Ga) 5
6 Intra- and Extra Galactic Timescales Summarized: Last nearby supernova Next time we will discuss constraints on what I ve labeled the last nearby supernova. Condensation of our Solar Nebula We have learned that the elemental material that makes up our solar system is older than it. We turn now to processes that occurred once our nebula formed from the galactic pool of elemental gas. Elements can be categorized by behavior at assumed values of P and T throughout the solar system. This table notes gross distinctions that are useful for considering planetary surfaces and atmospheres in our solar system. 6
7 Condensation of our Solar Nebula To do this more quantitatively, we must consider what solids will condense from a hot vapor of elements in gaseous form. Step 1. We can get at this question by determining what the main hightemperature solid phases are for each element. The next slide shows examples of gas-solid phase diagrams for Si, Ca, and Al as a function of T at a very low pressure of 10-4 atm, similar to what we think pertained in much of the early pre-solar nebula. Silicon 7
8 We then consider the P-T stability relationships for the high temperature phases, recalling that P TOT will essentially be set by P H2 so long as we are in a gas with solar abundances of the chemical elements (see notes from last lecture). Cooling trajectory The Solar Condensation Sequence Step 2. We then estimate ΔG f and K eq for each compound from the native elements as a function of T and P to determine its condensation temperature at a given pressure. i.e., Al 2 O 3 (s) 2Al (g) +3O (g) K eq = (P Al2 )(P O3 ) So Partial pressure of elemental O, cubed log K = 2logP Al + 3logP O 8
9 The Solar Condensation Sequence Step 3. Next, we need to estimate the concentration of each element in the gas phase nebula with two assumptions. 1. the nebula was an ideal gas: P total V = n total RT 2. P total ~ P H2 We make this second assumption because at early nebular conditions these elemental abundances prevailed: Abund H ~ 94% abund all elemental material For instance, abund He = 6% abund H ; abund all other elements <<0.1% abund H P H2 > 500 P other H species, so that n H2 = ½n H total P total ~ 10-4 to 10-3 atmospheres, depending on location in the gas cloud. Step 4, some algebra The ideal gas law tells us that in a unit volume of solar nebula n H2 = P H2 /RT and so... ½n H total = P H2 /RT using the mole fraction of each other element X i = n i /n total which is X i ~ n i /n H we get an expression for the moles of i in a unit volume: n i = X i (2P H2 /RT) In other words, we can estimate the equilibrium molar concentration of any element per unit volume, given the gas pressure and temperature of the nebula, and the element s abundance ratio to H 2. For stable elements, we can get the original abundance ratio directly from the present-day composition of the sun (which contains 99.8% of the mass of the solar system). 9
10 Step 5. Finally, we estimate the T of condensation for each element in its highest temperate phase by comparing K eq as a function of T with Q (the reaction coefficient) based on actual nebula abundances. 2logPAl + 3logPO Keq Q Cooling trajectory condensation Q > Keq T Results, summarized: key parts of the condensation sequence estimated by this method 10
11 The condensation sequence also takes into account the fact that some hightemperature solids react to form others as the temperature continues to drop. Solid materials in this sequence can be divided into 3 subcategories: a early condensate a silicates and a metals volatiles are separated into higher-t ( K) and lower-t (<600 K) groups. The lower-t volatile group can be subdivided further into ices and gases based on condensation point, if desired. 11
12 the condensation sequence: the sequence of phases we might expect if condensation occurred in a closed system at chemical equilibrium. Temperature, K Perovskite CaTiO3 Diopside CaMgSi 2 O 6 Anorthite CaAl 2 Si 2 O 8 Plagioclase (Na,Ca)(Al,Si) 4 O 8 Sulfates Carbonates Melitite Ca(Al,Mg)(Si,Al) 2O7 Forsterite Mg 2 SiO 4 Corundum Al 2 O 3 Spinel MgAl 2 O 4 Metallic Fe Enstatite MgSiO 3 Olivine, pyroxene of intermediate Fe content (Mg,Fe) 2 SiO 4 (Mg,Fe)SiO 3 Phyllosilicates (Mg,Al,Fe) 6 (Al,Si) 6 O 10 (OH) 8 Ice of H 2 O NH 3 CH 4 Troilite FeS Magnetite Fe 3 O 4 Carbonaceous compounds Me mercury Ve venus E earth Ma mars A asteroids 0 Figure Simplified mineralogical condensation sequence. modified from White, Geochemistry J jupiter 1. Refractory elements primarily associated with corundum, Al 2 O 3, and perovskite CaTiO 3, (Ti, Al, Ca, Os, Zr, Th,, REE, platinum group metals) 2. Fe + Ni as a metal alloy and the first silicates (Mg, Ca-Mg) 3. Alkalis/alkaline earths as feldspars 4. Sulfide minerals, oxides like magnetite, oxidized Fe into olivine, pyroxene. 5. Hydrated phases (silicate minerals) below about 550 K; sulfates, carbonates below this 6. Ices below about 200 K We see remnants of this high T condensation sequence in C1 Chondrites. These meteorites are basically sedimentary rocks or metamorphosed sediments formed of the phases predicted by the condensation sequence. However in detail multiple aspects of the simple equilibrium condensation sequence have been called into question over the last ~20 years, primarily due to evidence in meteorites such as: 1. multiple pulses of heating during cooling of the nebula 2. chemical reactions and kinetic control on processes within some of the materials 3. inheritance of some pre-solar grains in C1 chondrites Nevertheless the notion of a condensation sequence and its general proof in observed materials remains one of the remarkable accomplishments of geochemistry/cosmochemistry. 12
13 What was the setting in which condensation occurred? Imagine dust-size particles forming as the pre-solar nebular gas cools. Models indicate that the cooling cloud collapses into a lens shaped body rather quickly. During equilibrium condensation, the first-formed refractory phases become LOW TEMPERATRE CONDENSATE nucleation sites for lower-t condensates. INTERMEDIATE TEMPERATRE CONDENSATE HIGH T CONDENSATE PROTO SN K Figure 6.4 Diagram showing notational temperature distribution in cocoon nebula surrounding sun, and prior to collapse into discoidal configuration. Small first-generation planetismals form within the shell and sink towards the ecliptic plane in general direction indicated by white arrows (note that actual paths are more complex than this). An important result is that at any given distance from the sun in the ecliptic plane, the solids that collect may have been subjected to a wide range of temperature and pressure conditions. Dust-size particles fall toward the disk, collide & aggregate. They do this in the context of a temperature gradient from the center to the rim of the nebula, giving rise to a range of timetemperature-pressure paths for the initial agglomerations of condensed matter. At non-equilibrium conditions, condensation sequence as applied to the accretion of planetary bodies considers that the primary rock phases would partially separate (fractionate) from each other at various steps during cooling of the nebula, and that each class of material can interact with volatiles at lower temperatures. note: The final low T step, remelting/ accretion, is a later topic. 13
The Main Point. Lecture #34: Solar System Origin II. Chemical Condensation ( Lewis ) Model. How did the solar system form? Reading: Chapter 8.
 Lecture #34: Solar System Origin II How did the solar system form? Chemical Condensation ("Lewis") Model. Formation of the Terrestrial Planets. Formation of the Giant Planets. Planetary Evolution. Reading:
Lecture #34: Solar System Origin II How did the solar system form? Chemical Condensation ("Lewis") Model. Formation of the Terrestrial Planets. Formation of the Giant Planets. Planetary Evolution. Reading:
Element Partitioning and Earth's Core Composition. Bernie J. Wood. Summary by: Dave Stegman
 Element Partitioning and Earth's Core Composition Bernie J. Wood Summary by: Dave Stegman Determining the composition of the Earth's Core is essential for understanding the internal structure, evolution,
Element Partitioning and Earth's Core Composition Bernie J. Wood Summary by: Dave Stegman Determining the composition of the Earth's Core is essential for understanding the internal structure, evolution,
Introduction and Origin of the Earth
 Page 1 of 5 EENS 1110 Tulane University Physical Geology Prof. Stephen A. Nelson Introduction and Origin of the Earth This page last updated on 30-Jul-2015 Geology, What is it? Geology is the study of
Page 1 of 5 EENS 1110 Tulane University Physical Geology Prof. Stephen A. Nelson Introduction and Origin of the Earth This page last updated on 30-Jul-2015 Geology, What is it? Geology is the study of
SGL 101 MATERIALS OF THE EARTH Lecture 1 C.M.NYAMAI LECTURE 1. 1.0 ORIGIN, STRUCTURE AND COMPOSITION OF THE EARTH
 LECTURE 1. 1.0 ORIGIN, STRUCTURE AND COMPOSITION OF THE EARTH 1.1 INTRODUCTION. Welcome to Lecture 1 of this unit. To start with, stop and look around you wherever you are. Take a look at all the things
LECTURE 1. 1.0 ORIGIN, STRUCTURE AND COMPOSITION OF THE EARTH 1.1 INTRODUCTION. Welcome to Lecture 1 of this unit. To start with, stop and look around you wherever you are. Take a look at all the things
Lecture 23: Terrestrial Worlds in Comparison. This lecture compares and contrasts the properties and evolution of the 5 main terrestrial bodies.
 Lecture 23: Terrestrial Worlds in Comparison Astronomy 141 Winter 2012 This lecture compares and contrasts the properties and evolution of the 5 main terrestrial bodies. The small terrestrial planets have
Lecture 23: Terrestrial Worlds in Comparison Astronomy 141 Winter 2012 This lecture compares and contrasts the properties and evolution of the 5 main terrestrial bodies. The small terrestrial planets have
Summary: Four Major Features of our Solar System
 Summary: Four Major Features of our Solar System How did the solar system form? According to the nebular theory, our solar system formed from the gravitational collapse of a giant cloud of interstellar
Summary: Four Major Features of our Solar System How did the solar system form? According to the nebular theory, our solar system formed from the gravitational collapse of a giant cloud of interstellar
Solar System Formation
 Solar System Formation Solar System Formation Question: How did our solar system and other planetary systems form? Comparative planetology has helped us understand Compare the differences and similarities
Solar System Formation Solar System Formation Question: How did our solar system and other planetary systems form? Comparative planetology has helped us understand Compare the differences and similarities
Welcome to Class 4: Our Solar System (and a bit of cosmology at the start) Remember: sit only in the first 10 rows of the room
 Welcome to Class 4: Our Solar System (and a bit of cosmology at the start) Remember: sit only in the first 10 rows of the room What is the difference between dark ENERGY and dark MATTER? Is Earth unique,
Welcome to Class 4: Our Solar System (and a bit of cosmology at the start) Remember: sit only in the first 10 rows of the room What is the difference between dark ENERGY and dark MATTER? Is Earth unique,
Chapter 8 Formation of the Solar System Agenda
 Chapter 8 Formation of the Solar System Agenda Announce: Mercury Transit Part 2 of Projects due next Thursday Ch. 8 Formation of the Solar System Philip on The Physics of Star Trek Radiometric Dating Lab
Chapter 8 Formation of the Solar System Agenda Announce: Mercury Transit Part 2 of Projects due next Thursday Ch. 8 Formation of the Solar System Philip on The Physics of Star Trek Radiometric Dating Lab
The Universe Inside of You: Where do the atoms in your body come from?
 The Universe Inside of You: Where do the atoms in your body come from? Matthew Mumpower University of Notre Dame Thursday June 27th 2013 Nucleosynthesis nu cle o syn the sis The formation of new atomic
The Universe Inside of You: Where do the atoms in your body come from? Matthew Mumpower University of Notre Dame Thursday June 27th 2013 Nucleosynthesis nu cle o syn the sis The formation of new atomic
Chapter 8 Formation of the Solar System. What theory best explains the features of our solar system? Close Encounter Hypothesis
 Chapter 8 Formation of the Solar System What properties of our solar system must a formation theory explain? 1. Patterns of motion of the large bodies Orbit in same direction and plane 2. Existence of
Chapter 8 Formation of the Solar System What properties of our solar system must a formation theory explain? 1. Patterns of motion of the large bodies Orbit in same direction and plane 2. Existence of
Solar Nebula Theory. Basic properties of the Solar System that need to be explained:
 Solar Nebula Theory Basic properties of the Solar System that need to be explained: 1. All planets orbit the Sun in the same direction as the Sun s rotation 2. All planetary orbits are confined to the
Solar Nebula Theory Basic properties of the Solar System that need to be explained: 1. All planets orbit the Sun in the same direction as the Sun s rotation 2. All planetary orbits are confined to the
Astro 102 Test 5 Review Spring 2016. See Old Test 4 #16-23, Test 5 #1-3, Old Final #1-14
 Astro 102 Test 5 Review Spring 2016 See Old Test 4 #16-23, Test 5 #1-3, Old Final #1-14 Sec 14.5 Expanding Universe Know: Doppler shift, redshift, Hubble s Law, cosmic distance ladder, standard candles,
Astro 102 Test 5 Review Spring 2016 See Old Test 4 #16-23, Test 5 #1-3, Old Final #1-14 Sec 14.5 Expanding Universe Know: Doppler shift, redshift, Hubble s Law, cosmic distance ladder, standard candles,
Atoms and Elements. Atoms: Learning Goals. Chapter 3. Atoms and Elements; Isotopes and Ions; Minerals and Rocks. Clicker 1. Chemistry Background?
 Chapter 3 Atoms Atoms and Elements; Isotopes and Ions; Minerals and Rocks A Review of Chemistry: What geochemistry tells us Clicker 1 Chemistry Background? A. No HS or College Chemistry B. High School
Chapter 3 Atoms Atoms and Elements; Isotopes and Ions; Minerals and Rocks A Review of Chemistry: What geochemistry tells us Clicker 1 Chemistry Background? A. No HS or College Chemistry B. High School
1.3 Radioactivity and the age of the solar system
 1.3. RADIOACTIVITY AND THE AGE OF THE SOLAR SYSTEM 57 1.3 Radioactivity and the age of the solar system Most of you are familiar with the phenomenon of radioactive decay: Certain elements have isotopes
1.3. RADIOACTIVITY AND THE AGE OF THE SOLAR SYSTEM 57 1.3 Radioactivity and the age of the solar system Most of you are familiar with the phenomenon of radioactive decay: Certain elements have isotopes
L3: The formation of the Solar System
 credit: NASA L3: The formation of the Solar System UCL Certificate of astronomy Dr. Ingo Waldmann A stable home The presence of life forms elsewhere in the Universe requires a stable environment where
credit: NASA L3: The formation of the Solar System UCL Certificate of astronomy Dr. Ingo Waldmann A stable home The presence of life forms elsewhere in the Universe requires a stable environment where
Science Standard 4 Earth in Space Grade Level Expectations
 Science Standard 4 Earth in Space Grade Level Expectations Science Standard 4 Earth in Space Our Solar System is a collection of gravitationally interacting bodies that include Earth and the Moon. Universal
Science Standard 4 Earth in Space Grade Level Expectations Science Standard 4 Earth in Space Our Solar System is a collection of gravitationally interacting bodies that include Earth and the Moon. Universal
7. In which part of the electromagnetic spectrum are molecules most easily detected? A. visible light B. radio waves C. X rays D.
 1. Most interstellar matter is too cold to be observed optically. Its radiation can be detected in which part of the electromagnetic spectrum? A. gamma ray B. ultraviolet C. infrared D. X ray 2. The space
1. Most interstellar matter is too cold to be observed optically. Its radiation can be detected in which part of the electromagnetic spectrum? A. gamma ray B. ultraviolet C. infrared D. X ray 2. The space
Study Guide: Solar System
 Study Guide: Solar System 1. How many planets are there in the solar system? 2. What is the correct order of all the planets in the solar system? 3. Where can a comet be located in the solar system? 4.
Study Guide: Solar System 1. How many planets are there in the solar system? 2. What is the correct order of all the planets in the solar system? 3. Where can a comet be located in the solar system? 4.
1 A Solar System Is Born
 CHAPTER 3 1 A Solar System Is Born SECTION Formation of the Solar System BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a nebula? How did our solar system
CHAPTER 3 1 A Solar System Is Born SECTION Formation of the Solar System BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a nebula? How did our solar system
Origins of the Cosmos Summer 2016. Pre-course assessment
 Origins of the Cosmos Summer 2016 Pre-course assessment In order to grant two graduate credits for the workshop, we do require you to spend some hours before arriving at Penn State. We encourage all of
Origins of the Cosmos Summer 2016 Pre-course assessment In order to grant two graduate credits for the workshop, we do require you to spend some hours before arriving at Penn State. We encourage all of
Chapter 8 Welcome to the Solar System
 Chapter 8 Welcome to the Solar System 8.1 The Search for Origins What properties of our solar system must a formation theory explain? What theory best explains the features of our solar system? What properties
Chapter 8 Welcome to the Solar System 8.1 The Search for Origins What properties of our solar system must a formation theory explain? What theory best explains the features of our solar system? What properties
Class 2 Solar System Characteristics Formation Exosolar Planets
 Class 1 Introduction, Background History of Modern Astronomy The Night Sky, Eclipses and the Seasons Kepler's Laws Newtonian Gravity General Relativity Matter and Light Telescopes Class 2 Solar System
Class 1 Introduction, Background History of Modern Astronomy The Night Sky, Eclipses and the Seasons Kepler's Laws Newtonian Gravity General Relativity Matter and Light Telescopes Class 2 Solar System
Chapter 6 Formation of Planetary Systems Our Solar System and Beyond
 Chapter 6 Formation of Planetary Systems Our Solar System and Beyond The solar system exhibits clear patterns of composition and motion. Sun Over 99.9% of solar system s mass Made mostly of H/He gas (plasma)
Chapter 6 Formation of Planetary Systems Our Solar System and Beyond The solar system exhibits clear patterns of composition and motion. Sun Over 99.9% of solar system s mass Made mostly of H/He gas (plasma)
UNIT V. Earth and Space. Earth and the Solar System
 UNIT V Earth and Space Chapter 9 Earth and the Solar System EARTH AND OTHER PLANETS A solar system contains planets, moons, and other objects that orbit around a star or the star system. The solar system
UNIT V Earth and Space Chapter 9 Earth and the Solar System EARTH AND OTHER PLANETS A solar system contains planets, moons, and other objects that orbit around a star or the star system. The solar system
Lecture 19: Planet Formation I. Clues from the Solar System
 Lecture 19: Planet Formation I. Clues from the Solar System 1 Outline The Solar System:! Terrestrial planets! Jovian planets! Asteroid belt, Kuiper belt, Oort cloud Condensation and growth of solid bodies
Lecture 19: Planet Formation I. Clues from the Solar System 1 Outline The Solar System:! Terrestrial planets! Jovian planets! Asteroid belt, Kuiper belt, Oort cloud Condensation and growth of solid bodies
Presolar Oxide Grains in Meteorites
 from Astrophysical Implications of the Laboratory Study of Presolar Materials, edited by T. J. Bernatowicz and E. Zinner, AIP CP402, 1997, pp.59 82 Presolar Oxide Grains in Meteorites Larry R. Nittler
from Astrophysical Implications of the Laboratory Study of Presolar Materials, edited by T. J. Bernatowicz and E. Zinner, AIP CP402, 1997, pp.59 82 Presolar Oxide Grains in Meteorites Larry R. Nittler
Lecture 7 Formation of the Solar System. Nebular Theory. Origin of the Solar System. Origin of the Solar System. The Solar Nebula
 Origin of the Solar System Lecture 7 Formation of the Solar System Reading: Chapter 9 Quiz#2 Today: Lecture 60 minutes, then quiz 20 minutes. Homework#1 will be returned on Thursday. Our theory must explain
Origin of the Solar System Lecture 7 Formation of the Solar System Reading: Chapter 9 Quiz#2 Today: Lecture 60 minutes, then quiz 20 minutes. Homework#1 will be returned on Thursday. Our theory must explain
The Birth of the Universe Newcomer Academy High School Visualization One
 The Birth of the Universe Newcomer Academy High School Visualization One Chapter Topic Key Points of Discussion Notes & Vocabulary 1 Birth of The Big Bang Theory Activity 4A the How and when did the universe
The Birth of the Universe Newcomer Academy High School Visualization One Chapter Topic Key Points of Discussion Notes & Vocabulary 1 Birth of The Big Bang Theory Activity 4A the How and when did the universe
Lecture 10 Formation of the Solar System January 6c, 2014
 1 Lecture 10 Formation of the Solar System January 6c, 2014 2 Orbits of the Planets 3 Clues for the Formation of the SS All planets orbit in roughly the same plane about the Sun. All planets orbit in the
1 Lecture 10 Formation of the Solar System January 6c, 2014 2 Orbits of the Planets 3 Clues for the Formation of the SS All planets orbit in roughly the same plane about the Sun. All planets orbit in the
Using Photometric Data to Derive an HR Diagram for a Star Cluster
 Using Photometric Data to Derive an HR Diagram for a Star Cluster In In this Activity, we will investigate: 1. How to use photometric data for an open cluster to derive an H-R Diagram for the stars and
Using Photometric Data to Derive an HR Diagram for a Star Cluster In In this Activity, we will investigate: 1. How to use photometric data for an open cluster to derive an H-R Diagram for the stars and
The spectacular eruption of a volcano, the magnificent scenery of a
 Section 1.1 1.1 What Is Earth Science 1 FOCUS Section Objectives 1.1 Define Earth science. 1.2 Describe the formation of Earth and the solar system. Build Vocabulary Word Parts Ask students to use a dictionary
Section 1.1 1.1 What Is Earth Science 1 FOCUS Section Objectives 1.1 Define Earth science. 1.2 Describe the formation of Earth and the solar system. Build Vocabulary Word Parts Ask students to use a dictionary
Composition of the Atmosphere. Outline Atmospheric Composition Nitrogen and Oxygen Lightning Homework
 Molecules of the Atmosphere The present atmosphere consists mainly of molecular nitrogen (N2) and molecular oxygen (O2) but it has dramatically changed in composition from the beginning of the solar system.
Molecules of the Atmosphere The present atmosphere consists mainly of molecular nitrogen (N2) and molecular oxygen (O2) but it has dramatically changed in composition from the beginning of the solar system.
Ernst Zinner Washington University St. Louis
 QuickTime and a decompressor are needed to see this picture. QuickTime and a decompressor are needed to see this picture. QuickTime and a decompressor are needed to see this picture. Constraints on SN
QuickTime and a decompressor are needed to see this picture. QuickTime and a decompressor are needed to see this picture. QuickTime and a decompressor are needed to see this picture. Constraints on SN
Notes 3: Formation of the solar system
 Notes 3: Formation of the solar system 3.1 Starting Ingredients The first thing we need to do is look at the material out there what is available for the formation of the solar system and where did it
Notes 3: Formation of the solar system 3.1 Starting Ingredients The first thing we need to do is look at the material out there what is available for the formation of the solar system and where did it
165 points. Name Date Period. Column B a. Cepheid variables b. luminosity c. RR Lyrae variables d. Sagittarius e. variable stars
 Name Date Period 30 GALAXIES AND THE UNIVERSE SECTION 30.1 The Milky Way Galaxy In your textbook, read about discovering the Milky Way. (20 points) For each item in Column A, write the letter of the matching
Name Date Period 30 GALAXIES AND THE UNIVERSE SECTION 30.1 The Milky Way Galaxy In your textbook, read about discovering the Milky Way. (20 points) For each item in Column A, write the letter of the matching
The Layout of the Solar System
 The Layout of the Solar System Planets fall into two main categories Terrestrial (i.e. Earth-like) Jovian (i.e. Jupiter-like or gaseous) [~5000 kg/m 3 ] [~1300 kg/m 3 ] What is density? Average density
The Layout of the Solar System Planets fall into two main categories Terrestrial (i.e. Earth-like) Jovian (i.e. Jupiter-like or gaseous) [~5000 kg/m 3 ] [~1300 kg/m 3 ] What is density? Average density
California Standards Grades 9 12 Boardworks 2009 Science Contents Standards Mapping
 California Standards Grades 912 Boardworks 2009 Science Contents Standards Mapping Earth Sciences Earth s Place in the Universe 1. Astronomy and planetary exploration reveal the solar system s structure,
California Standards Grades 912 Boardworks 2009 Science Contents Standards Mapping Earth Sciences Earth s Place in the Universe 1. Astronomy and planetary exploration reveal the solar system s structure,
Test 5 Review questions. 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will
 Name: Thursday, December 13, 2007 Test 5 Review questions 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same 2. The graph below
Name: Thursday, December 13, 2007 Test 5 Review questions 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same 2. The graph below
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMICAL EQUILIBRIUM (ICE METHOD)
 CHEMICAL EQUILIBRIUM (ICE METHOD) Introduction Chemical equilibrium occurs when opposing reactions are proceeding at equal rates. The rate at which the products are formed from the reactants equals the
CHEMICAL EQUILIBRIUM (ICE METHOD) Introduction Chemical equilibrium occurs when opposing reactions are proceeding at equal rates. The rate at which the products are formed from the reactants equals the
Chapter Test B. Chapter: Measurements and Calculations
 Assessment Chapter Test B Chapter: Measurements and Calculations PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.
Assessment Chapter Test B Chapter: Measurements and Calculations PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.
1.1 A Modern View of the Universe" Our goals for learning: What is our place in the universe?"
 Chapter 1 Our Place in the Universe 1.1 A Modern View of the Universe What is our place in the universe? What is our place in the universe? How did we come to be? How can we know what the universe was
Chapter 1 Our Place in the Universe 1.1 A Modern View of the Universe What is our place in the universe? What is our place in the universe? How did we come to be? How can we know what the universe was
Igneous Geochemistry. What is magma? What is polymerization? Average compositions (% by weight) and liquidus temperatures of different magmas
 1 Igneous Geochemistry What is magma phases, compositions, properties Major igneous processes Making magma how and where Major-element variations Classification using a whole-rock analysis Fractional crystallization
1 Igneous Geochemistry What is magma phases, compositions, properties Major igneous processes Making magma how and where Major-element variations Classification using a whole-rock analysis Fractional crystallization
13.1 The Nature of Gases. What is Kinetic Theory? Kinetic Theory and a Model for Gases. Chapter 13: States of Matter. Principles of Kinetic Theory
 Chapter 13: States of Matter The Nature of Gases The Nature of Gases kinetic molecular theory (KMT), gas pressure (pascal, atmosphere, mm Hg), kinetic energy The Nature of Liquids vaporization, evaporation,
Chapter 13: States of Matter The Nature of Gases The Nature of Gases kinetic molecular theory (KMT), gas pressure (pascal, atmosphere, mm Hg), kinetic energy The Nature of Liquids vaporization, evaporation,
Potassium-Argon (K-Ar) Dating
 Potassium-Argon (K-Ar) Dating K-Ar Dating In 10,000 K atoms: 9326 39 K 673 41 K 1 40 K Potassium Decay Potassium Decay Potassium Decay Argon About 1% of atmosphere is argon Three stable isotopes of argon
Potassium-Argon (K-Ar) Dating K-Ar Dating In 10,000 K atoms: 9326 39 K 673 41 K 1 40 K Potassium Decay Potassium Decay Potassium Decay Argon About 1% of atmosphere is argon Three stable isotopes of argon
Chemical Equations & Stoichiometry
 Chemical Equations & Stoichiometry Chapter Goals Balance equations for simple chemical reactions. Perform stoichiometry calculations using balanced chemical equations. Understand the meaning of the term
Chemical Equations & Stoichiometry Chapter Goals Balance equations for simple chemical reactions. Perform stoichiometry calculations using balanced chemical equations. Understand the meaning of the term
CHEMICAL SIGNATURES OF ASTEROID IMPACTS
 CHEMICAL SIGNATURES OF ASTEROID IMPACTS INTRODUCTION The film The Day the Mesozoic Died identifies the presence of high quantities of iridium (Ir) in the clay layer at the boundary between the Cretaceous
CHEMICAL SIGNATURES OF ASTEROID IMPACTS INTRODUCTION The film The Day the Mesozoic Died identifies the presence of high quantities of iridium (Ir) in the clay layer at the boundary between the Cretaceous
The Chemical Composition of a Molecular Cloud at the Outer Edge of the Galaxy
 Carnegie Observatories Astrophysics Series, Vol. 4: Origin and Evolution of the Elements, 2003 ed. A. McWilliam and M. Rauch (Pasadena: Carnegie Observatories, http://www.ociw.edu/ociw/symposia/series/symposium4/proceedings.html)
Carnegie Observatories Astrophysics Series, Vol. 4: Origin and Evolution of the Elements, 2003 ed. A. McWilliam and M. Rauch (Pasadena: Carnegie Observatories, http://www.ociw.edu/ociw/symposia/series/symposium4/proceedings.html)
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
The Expanding Universe
 Stars, Galaxies, Guided Reading and Study This section explains how astronomers think the universe and the solar system formed. Use Target Reading Skills As you read about the evidence that supports the
Stars, Galaxies, Guided Reading and Study This section explains how astronomers think the universe and the solar system formed. Use Target Reading Skills As you read about the evidence that supports the
Vagabonds of the Solar System. Chapter 17
 Vagabonds of the Solar System Chapter 17 ASTR 111 003 Fall 2006 Lecture 13 Nov. 27, 2006 Introduction To Modern Astronomy I Introducing Astronomy (chap. 1-6) Planets and Moons (chap. 7-17) Ch7: Comparative
Vagabonds of the Solar System Chapter 17 ASTR 111 003 Fall 2006 Lecture 13 Nov. 27, 2006 Introduction To Modern Astronomy I Introducing Astronomy (chap. 1-6) Planets and Moons (chap. 7-17) Ch7: Comparative
Related Standards and Background Information
 Related Standards and Background Information Earth Patterns, Cycles and Changes This strand focuses on student understanding of patterns in nature, natural cycles, and changes that occur both quickly and
Related Standards and Background Information Earth Patterns, Cycles and Changes This strand focuses on student understanding of patterns in nature, natural cycles, and changes that occur both quickly and
4 HOW OUR SOLAR SYSTEM FORMED 750L
 4 HOW OUR SOLAR SYSTEM FORMED 750L HOW OUR SOLAR SYSTEM FORMED A CLOSE LOOK AT THE PLANETS ORBITING OUR SUN By Cynthia Stokes Brown, adapted by Newsela Planets come from the clouds of gas and dust that
4 HOW OUR SOLAR SYSTEM FORMED 750L HOW OUR SOLAR SYSTEM FORMED A CLOSE LOOK AT THE PLANETS ORBITING OUR SUN By Cynthia Stokes Brown, adapted by Newsela Planets come from the clouds of gas and dust that
Study the following diagrams of the States of Matter. Label the names of the Changes of State between the different states.
 Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
WHERE DID ALL THE ELEMENTS COME FROM??
 WHERE DID ALL THE ELEMENTS COME FROM?? In the very beginning, both space and time were created in the Big Bang. It happened 13.7 billion years ago. Afterwards, the universe was a very hot, expanding soup
WHERE DID ALL THE ELEMENTS COME FROM?? In the very beginning, both space and time were created in the Big Bang. It happened 13.7 billion years ago. Afterwards, the universe was a very hot, expanding soup
Introduction to the Solar System
 Introduction to the Solar System Lesson Objectives Describe some early ideas about our solar system. Name the planets, and describe their motion around the Sun. Explain how the solar system formed. Introduction
Introduction to the Solar System Lesson Objectives Describe some early ideas about our solar system. Name the planets, and describe their motion around the Sun. Explain how the solar system formed. Introduction
Name Date Class STATES OF MATTER. SECTION 13.1 THE NATURE OF GASES (pages 385 389)
 13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains how temperature
13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains how temperature
Stellar Evolution: a Journey through the H-R Diagram
 Stellar Evolution: a Journey through the H-R Diagram Mike Montgomery 21 Apr, 2001 0-0 The Herztsprung-Russell Diagram (HRD) was independently invented by Herztsprung (1911) and Russell (1913) They plotted
Stellar Evolution: a Journey through the H-R Diagram Mike Montgomery 21 Apr, 2001 0-0 The Herztsprung-Russell Diagram (HRD) was independently invented by Herztsprung (1911) and Russell (1913) They plotted
Chemistry 13: States of Matter
 Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
Lecture 5. elements (Figure 1). In addition, there are many ways of classifying trace elements.
 Lecture 5 Nomenclature for Trace Element Classification We have already grouped elements into two classes, major elements and trace elements (Figure 1). In addition, there are many ways of classifying
Lecture 5 Nomenclature for Trace Element Classification We have already grouped elements into two classes, major elements and trace elements (Figure 1). In addition, there are many ways of classifying
Chem 1A Exam 2 Review Problems
 Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
Answers for the Student Worksheet for the Hubble Space Telescope Scavenger Hunt
 Instructions: Answers are typed in blue. Answers for the Student Worksheet for the Hubble Space Telescope Scavenger Hunt Crab Nebula What is embedded in the center of the nebula? Neutron star Who first
Instructions: Answers are typed in blue. Answers for the Student Worksheet for the Hubble Space Telescope Scavenger Hunt Crab Nebula What is embedded in the center of the nebula? Neutron star Who first
Chemistry - A Quantitative Science
 56 Ba 137.33 1 H 1.0079 2 He 4.0026 5 B 10.811 6 C 12.011 7 N 14.007 8 O 15.999 9 F 18.998 10 Ne 20.180 13 Al 26.982 14 Si 28.086 15 P 30.974 16 S 32.066 17 Cl 35.453 18 Ar 39.948 3 Li 6.941 4 Be 9.0122
56 Ba 137.33 1 H 1.0079 2 He 4.0026 5 B 10.811 6 C 12.011 7 N 14.007 8 O 15.999 9 F 18.998 10 Ne 20.180 13 Al 26.982 14 Si 28.086 15 P 30.974 16 S 32.066 17 Cl 35.453 18 Ar 39.948 3 Li 6.941 4 Be 9.0122
Radiometric Dating. Dating Methods for Igneous Rocks
 Radiometric Dating why radiometric? although several different dating techniques are employed, all but radiometric dating is able to estimate ages in timescales relevant to astronomers. How it works Radiometric
Radiometric Dating why radiometric? although several different dating techniques are employed, all but radiometric dating is able to estimate ages in timescales relevant to astronomers. How it works Radiometric
Faber-Jackson relation: Fundamental Plane: Faber-Jackson Relation
 Faber-Jackson relation: Faber-Jackson Relation In 1976, Faber & Jackson found that: Roughly, L! " 4 More luminous galaxies have deeper potentials Can show that this follows from the Virial Theorem Why
Faber-Jackson relation: Faber-Jackson Relation In 1976, Faber & Jackson found that: Roughly, L! " 4 More luminous galaxies have deeper potentials Can show that this follows from the Virial Theorem Why
8.1 Radio Emission from Solar System objects
 8.1 Radio Emission from Solar System objects 8.1.1 Moon and Terrestrial planets At visible wavelengths all the emission seen from these objects is due to light reflected from the sun. However at radio
8.1 Radio Emission from Solar System objects 8.1.1 Moon and Terrestrial planets At visible wavelengths all the emission seen from these objects is due to light reflected from the sun. However at radio
A i A i. µ(ion) = Z i X i
 Lecture 2 Review: calculation of mean atomic weight of an ionized gas (µ) Given a mass fraction X i (or abundance) for an ionic (or atomic) species with atomic weight A i, we can can calculate µ by: For
Lecture 2 Review: calculation of mean atomic weight of an ionized gas (µ) Given a mass fraction X i (or abundance) for an ionic (or atomic) species with atomic weight A i, we can can calculate µ by: For
Chapter 1 The Atomic Nature of Matter
 Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
CHEM 105 HOUR EXAM III 28-OCT-99. = -163 kj/mole determine H f 0 for Ni(CO) 4 (g) = -260 kj/mole determine H f 0 for Cr(CO) 6 (g)
 CHEM 15 HOUR EXAM III 28-OCT-99 NAME (please print) 1. a. given: Ni (s) + 4 CO (g) = Ni(CO) 4 (g) H Rxn = -163 k/mole determine H f for Ni(CO) 4 (g) b. given: Cr (s) + 6 CO (g) = Cr(CO) 6 (g) H Rxn = -26
CHEM 15 HOUR EXAM III 28-OCT-99 NAME (please print) 1. a. given: Ni (s) + 4 CO (g) = Ni(CO) 4 (g) H Rxn = -163 k/mole determine H f for Ni(CO) 4 (g) b. given: Cr (s) + 6 CO (g) = Cr(CO) 6 (g) H Rxn = -26
General Chemistry I (FC, 09-10) Lab #3: The Empirical Formula of a Compound. Introduction
 General Chemistry I (FC, 09-10) Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant, does not
General Chemistry I (FC, 09-10) Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant, does not
The Hidden Lives of Galaxies. Jim Lochner, USRA & NASA/GSFC
 The Hidden Lives of Galaxies Jim Lochner, USRA & NASA/GSFC What is a Galaxy? Solar System Distance from Earth to Sun = 93,000,000 miles = 8 light-minutes Size of Solar System = 5.5 light-hours What is
The Hidden Lives of Galaxies Jim Lochner, USRA & NASA/GSFC What is a Galaxy? Solar System Distance from Earth to Sun = 93,000,000 miles = 8 light-minutes Size of Solar System = 5.5 light-hours What is
CHEMISTRY. Matter and Change. Section 13.1 Section 13.2 Section 13.3. The Gas Laws The Ideal Gas Law Gas Stoichiometry
 CHEMISTRY Matter and Change 13 Table Of Contents Chapter 13: Gases Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry State the relationships among pressure, temperature,
CHEMISTRY Matter and Change 13 Table Of Contents Chapter 13: Gases Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry State the relationships among pressure, temperature,
The Milky Way Galaxy is Heading for a Major Cosmic Collision
 The Milky Way Galaxy is Heading for a Major Cosmic Collision Roeland van der Marel (STScI) [based on work with a team of collaborators reported in the Astrophysical Journal July 2012] Hubble Science Briefing
The Milky Way Galaxy is Heading for a Major Cosmic Collision Roeland van der Marel (STScI) [based on work with a team of collaborators reported in the Astrophysical Journal July 2012] Hubble Science Briefing
Name: Class: Date: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Class: Date: Minerals Study Guide Modified True/False Indicate whether the sentence or statement is true or false. If false, change the identified word or phrase to make the sentence or statement true.
Class: Date: Minerals Study Guide Modified True/False Indicate whether the sentence or statement is true or false. If false, change the identified word or phrase to make the sentence or statement true.
Mole Notes.notebook. October 29, 2014
 1 2 How do chemists count atoms/formula units/molecules? How do we go from the atomic scale to the scale of everyday measurements (macroscopic scale)? The gateway is the mole! But before we get to the
1 2 How do chemists count atoms/formula units/molecules? How do we go from the atomic scale to the scale of everyday measurements (macroscopic scale)? The gateway is the mole! But before we get to the
Lecture 7: Formation of the Solar System
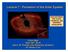 Lecture 7: Formation of the Solar System Dust and debris disk around Fomalhaut, with embedded young planet! Claire Max April 24 th, 2014 Astro 18: Planets and Planetary Systems UC Santa Cruz Solar System
Lecture 7: Formation of the Solar System Dust and debris disk around Fomalhaut, with embedded young planet! Claire Max April 24 th, 2014 Astro 18: Planets and Planetary Systems UC Santa Cruz Solar System
CHAPTER 6 THE TERRESTRIAL PLANETS
 CHAPTER 6 THE TERRESTRIAL PLANETS MULTIPLE CHOICE 1. Which of the following is NOT one of the four stages in the development of a terrestrial planet? 2. That Earth, evidence that Earth differentiated.
CHAPTER 6 THE TERRESTRIAL PLANETS MULTIPLE CHOICE 1. Which of the following is NOT one of the four stages in the development of a terrestrial planet? 2. That Earth, evidence that Earth differentiated.
The Empirical Formula of a Compound
 The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
A: Planets. Q: Which of the following objects would NOT be described as a small body: asteroids, meteoroids, comets, planets?
 Q: Which of the following objects would NOT be described as a small body: asteroids, meteoroids, comets, planets? A: Planets Q: What can we learn by studying small bodies of the solar system? A: We can
Q: Which of the following objects would NOT be described as a small body: asteroids, meteoroids, comets, planets? A: Planets Q: What can we learn by studying small bodies of the solar system? A: We can
Phase. Gibbs Phase rule
 Phase diagrams Phase A phase can be defined as a physically distinct and chemically homogeneous portion of a system that has a particular chemical composition and structure. Water in liquid or vapor state
Phase diagrams Phase A phase can be defined as a physically distinct and chemically homogeneous portion of a system that has a particular chemical composition and structure. Water in liquid or vapor state
Lecture 35: Atmosphere in Furnaces
 Lecture 35: Atmosphere in Furnaces Contents: Selection of atmosphere: Gases and their behavior: Prepared atmospheres Protective atmospheres applications Atmosphere volume requirements Atmosphere sensors
Lecture 35: Atmosphere in Furnaces Contents: Selection of atmosphere: Gases and their behavior: Prepared atmospheres Protective atmospheres applications Atmosphere volume requirements Atmosphere sensors
Name Date Class CHEMICAL QUANTITIES. SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296)
 Name Date Class 10 CHEMICAL QUANTITIES SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296) This section defines the mole and explains how the mole is used to measure matter. It also teaches
Name Date Class 10 CHEMICAL QUANTITIES SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296) This section defines the mole and explains how the mole is used to measure matter. It also teaches
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Chemistry 122 Mines, Spring 2014
 Chemistry 122 Mines, Spring 2014 Answer Key, Problem Set 9 1. 18.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 18.46 (do this for all cells in 18.44
Chemistry 122 Mines, Spring 2014 Answer Key, Problem Set 9 1. 18.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 18.46 (do this for all cells in 18.44
The Mole Concept. The Mole. Masses of molecules
 The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
Chapter 8. Phase Diagrams
 Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1. A chemical equation. (C-4.4)
 Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
CHEMISTRY II FINAL EXAM REVIEW
 Name Period CHEMISTRY II FINAL EXAM REVIEW Final Exam: approximately 75 multiple choice questions Ch 12: Stoichiometry Ch 5 & 6: Electron Configurations & Periodic Properties Ch 7 & 8: Bonding Ch 14: Gas
Name Period CHEMISTRY II FINAL EXAM REVIEW Final Exam: approximately 75 multiple choice questions Ch 12: Stoichiometry Ch 5 & 6: Electron Configurations & Periodic Properties Ch 7 & 8: Bonding Ch 14: Gas
This paper is also taken for the relevant Examination for the Associateship. For Second Year Physics Students Wednesday, 4th June 2008: 14:00 to 16:00
 Imperial College London BSc/MSci EXAMINATION June 2008 This paper is also taken for the relevant Examination for the Associateship SUN, STARS, PLANETS For Second Year Physics Students Wednesday, 4th June
Imperial College London BSc/MSci EXAMINATION June 2008 This paper is also taken for the relevant Examination for the Associateship SUN, STARS, PLANETS For Second Year Physics Students Wednesday, 4th June
neutrons are present?
 AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
Chemical Calculations: The Mole Concept and Chemical Formulas. AW Atomic weight (mass of the atom of an element) was determined by relative weights.
 1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
Introduction to Nuclear Physics
 Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
The Big Bang A Community in the Classroom Presentation for Grade 5
 The Big Bang A Community in the Classroom Presentation for Grade 5 Richard Cupp Engineer STANARDS CONNECTION Grade 5 Physical Science: Elements and their combinations account for all the varied types of
The Big Bang A Community in the Classroom Presentation for Grade 5 Richard Cupp Engineer STANARDS CONNECTION Grade 5 Physical Science: Elements and their combinations account for all the varied types of
Chapter 8: Chemical Equations and Reactions
 Chapter 8: Chemical Equations and Reactions I. Describing Chemical Reactions A. A chemical reaction is the process by which one or more substances are changed into one or more different substances. A chemical
Chapter 8: Chemical Equations and Reactions I. Describing Chemical Reactions A. A chemical reaction is the process by which one or more substances are changed into one or more different substances. A chemical
Unit 8 Lesson 2 Gravity and the Solar System
 Unit 8 Lesson 2 Gravity and the Solar System Gravity What is gravity? Gravity is a force of attraction between objects that is due to their masses and the distances between them. Every object in the universe
Unit 8 Lesson 2 Gravity and the Solar System Gravity What is gravity? Gravity is a force of attraction between objects that is due to their masses and the distances between them. Every object in the universe
World of Particles Big Bang Thomas Gajdosik. Big Bang (model)
 Big Bang (model) What can be seen / measured? basically only light (and a few particles: e ±, p, p, ν x ) in different wave lengths: microwave to γ-rays in different intensities (measured in magnitudes)
Big Bang (model) What can be seen / measured? basically only light (and a few particles: e ±, p, p, ν x ) in different wave lengths: microwave to γ-rays in different intensities (measured in magnitudes)
Top 10 Discoveries by ESO Telescopes
 Top 10 Discoveries by ESO Telescopes European Southern Observatory reaching new heights in astronomy Exploring the Universe from the Atacama Desert, in Chile since 1964 ESO is the most productive astronomical
Top 10 Discoveries by ESO Telescopes European Southern Observatory reaching new heights in astronomy Exploring the Universe from the Atacama Desert, in Chile since 1964 ESO is the most productive astronomical
7. 1.00 atm = 760 torr = 760 mm Hg = 101.325 kpa = 14.70 psi. = 0.446 atm. = 0.993 atm. = 107 kpa 760 torr 1 atm 760 mm Hg = 790.
 CHATER 3. The atmosphere is a homogeneous mixture (a solution) of gases.. Solids and liquids have essentially fixed volumes and are not able to be compressed easily. have volumes that depend on their conditions,
CHATER 3. The atmosphere is a homogeneous mixture (a solution) of gases.. Solids and liquids have essentially fixed volumes and are not able to be compressed easily. have volumes that depend on their conditions,
