Heat Transfer. Phys101 Lectures 35, 36. Key points: Heat as Energy Transfer Specific Heat Heat Transfer: Conduction, Convection, Radiation
|
|
|
- Cuthbert Robbins
- 7 years ago
- Views:
Transcription
1 Phys101 Lectures 35, 36 Heat Transfer Key points: Heat as Energy Transfer Specific Heat Heat Transfer: Conduction, Convection, Radiation Ref: 16-1,3,4,10. Page 1
2 19-1 Heat as Energy Transfer We often speak of heat as though it were a material that flows from one object to another; it is not. Rather, it is a form of energy. Unit of heat: calorie (cal) 1 cal is the amount of heat necessary to raise the temperature of 1 g of water by 1 Celsius degree. Don t be fooled the calories on our food labels are really kilocalories (kcal or Calories), the heat necessary to raise 1 kg of water by 1 Celsius degree.
3 19-1 Heat as Energy Transfer If heat is a form of energy, it ought to be possible to equate it to other forms. The experiment below found the mechanical equivalent of heat by using the falling weight to heat the water: J = 1 cal kj = 1 kcal
4 19-1 Heat as Energy Transfer Definition of heat: Heat is energy transferred from one object to another because of a difference in temperature. Remember that the temperature of a gas is a measure of the kinetic energy of its molecules.
5 Example 19-1: Working off the extra calories. Suppose you throw caution to the wind and eat too much ice cream and cake on the order of 500 Calories. To compensate, you want to do an equivalent amount of work climbing stairs or a mountain. How much total height must you climb?
6 19-3 Specific Heat The amount of heat required to change the temperature of a material is proportional to the mass and to the temperature change: The specific heat, c, is characteristic of the material. Some values are listed at left.
7 Example 19-2: How heat transferred depends on specific heat. (a) How much heat input is needed to raise the temperature of an empty 20-kg vat made of iron from 10 C to 90 C? (b) What if the vat is filled with 20 kg of water?
8 19-4 Calorimetry Solving Problems Closed system: no mass enters or leaves, but energy may be exchanged Open system: mass may transfer as well Isolated system: closed system in which no energy in any form is transferred For an isolated system, energy out of one part = energy into another part
9 19-4 Calorimetry Solving Problems Example 19-3: The cup cools the tea. If 200 cm 3 of tea at 95 C is poured into a 150-g glass cup initially at 25 C, what will be the common final temperature T of the tea and cup when equilibrium is reached, assuming no heat flows to the surroundings?
10 19-4 Calorimetry Solving Problems The instrument to the left is a calorimeter, which makes quantitative measurements of heat exchange. A sample is heated to a well-measured high temperature and plunged into the water, and the equilibrium temperature is measured. This gives the specific heat of the sample.
11 Example 19-4: Unknown specific heat determined by calorimetry. An engineer wishes to determine the specific heat of a new metal alloy. A kg sample of the alloy is heated to 540 C. It is then quickly placed in kg of water at 10.0 C, which is contained in a kg aluminum calorimeter cup. (We do not need to know the mass of the insulating jacket since we assume the air space between it and the cup insulates it well, so that its temperature does not change significantly.) The final temperature of the system is 30.5 C. Calculate the specific heat of the alloy.
12 19-10 Heat Transfer: Conduction, Convection, Radiation Heat conduction can be visualized as occurring through molecular collisions. The heat flow per unit time is given by:
13 19-10 Heat Transfer: Conduction, Convection, Radiation The constant k is called the thermal conductivity. Materials with large k are called conductors; those with small k are called insulators.
14 Example 19-13: Heat loss through windows. A major source of heat loss from a house is through the windows. Calculate the rate of heat flow through a glass window 2.0 m x 1.5 m in area and 3.2 mm thick, if the temperatures at the inner and outer surfaces are 15.0 C and 14.0 C, respectively.
15 19-10 Heat Transfer: Conduction, Convection, Radiation Building materials are measured using R- values rather than thermal conductivity: Here, is the thickness of the material.
16 19-10 Heat Transfer: Conduction, Convection, Radiation Convection occurs when heat flows by the mass movement of molecules from one place to another. It may be natural or forced; both these examples are natural convection.
17 19-10 Heat Transfer: Conduction, Convection, Radiation Radiation is the form of energy transfer we receive from the Sun; if you stand close to a fire, most of the heat you feel is radiated as well. The energy radiated has been found to be proportional to the fourth power of the temperature:
18 19-10 Heat Transfer: Conduction, Convection, Radiation The constant σ is called the Stefan-Boltzmann constant: The emissivity ε is a number between 0 and 1 characterizing the surface; black objects have an emissivity near 1, while shiny ones have an emissivity near 0. It is the same for absorption; a good emitter is also a good absorber.
19 Example 19-14: Cooling by radiation. An athlete is sitting unclothed in a locker room whose dark walls are at a temperature of 15 C. Estimate his rate of heat loss by radiation, assuming a skin temperature of 34 C and ε = Take the surface area of the body not in contact with the chair to be 1.5 m 2.
20 19-10 Heat Transfer: Conduction, Convection, Radiation If you are in the sunlight, the Sun s radiation will warm you. In general, you will not be perfectly perpendicular to the Sun s rays, and will absorb energy at the rate:
21 19-10 Heat Transfer: Conduction, Convection, Radiation This cos θ effect is also responsible for the seasons.
22 19-10 Heat Transfer: Conduction, Convection, Radiation Thermography the detailed measurement of radiation from the body can be used in medical imaging. Warmer areas may be a sign of tumors or infection; cooler areas on the skin may be a sign of poor circulation.
23 Example 19-15: Star radius. The giant star Betelgeuse emits radiant energy at a rate 10 4 times greater than our Sun, whereas its surface temperature is only half (2900 K) that of our Sun. Estimate the radius of Betelgeuse, assuming ε = 1 for both. The Sun s radius is r S = 7 x 10 8 m.
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics. Problems: 8, 11, 13, 17, 21, 27, 29, 37, 39, 41, 47, 51, 57
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Problems: 8, 11, 13, 17, 21, 27, 29, 37, 39, 41, 47, 51, 57 Thermodynamics study and application of thermal energy temperature quantity
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Problems: 8, 11, 13, 17, 21, 27, 29, 37, 39, 41, 47, 51, 57 Thermodynamics study and application of thermal energy temperature quantity
The Three Heat Transfer Modes in Reflow Soldering
 Section 5: Reflow Oven Heat Transfer The Three Heat Transfer Modes in Reflow Soldering There are three different heating modes involved with most SMT reflow processes: conduction, convection, and infrared
Section 5: Reflow Oven Heat Transfer The Three Heat Transfer Modes in Reflow Soldering There are three different heating modes involved with most SMT reflow processes: conduction, convection, and infrared
Forms of Energy. Freshman Seminar
 Forms of Energy Freshman Seminar Energy Energy The ability & capacity to do work Energy can take many different forms Energy can be quantified Law of Conservation of energy In any change from one form
Forms of Energy Freshman Seminar Energy Energy The ability & capacity to do work Energy can take many different forms Energy can be quantified Law of Conservation of energy In any change from one form
Chapter 4: Transfer of Thermal Energy
 Chapter 4: Transfer of Thermal Energy Goals of Period 4 Section 4.1: To define temperature and thermal energy Section 4.2: To discuss three methods of thermal energy transfer. Section 4.3: To describe
Chapter 4: Transfer of Thermal Energy Goals of Period 4 Section 4.1: To define temperature and thermal energy Section 4.2: To discuss three methods of thermal energy transfer. Section 4.3: To describe
Energy and Energy Transformations Test Review
 Energy and Energy Transformations Test Review Completion: 1. Mass 13. Kinetic 2. Four 14. thermal 3. Kinetic 15. Thermal energy (heat) 4. Electromagnetic/Radiant 16. Thermal energy (heat) 5. Thermal 17.
Energy and Energy Transformations Test Review Completion: 1. Mass 13. Kinetic 2. Four 14. thermal 3. Kinetic 15. Thermal energy (heat) 4. Electromagnetic/Radiant 16. Thermal energy (heat) 5. Thermal 17.
Every mathematician knows it is impossible to understand an elementary course in thermodynamics. ~V.I. Arnold
 Every mathematician knows it is impossible to understand an elementary course in thermodynamics. ~V.I. Arnold Radiation Radiation: Heat energy transmitted by electromagnetic waves Q t = εσat 4 emissivity
Every mathematician knows it is impossible to understand an elementary course in thermodynamics. ~V.I. Arnold Radiation Radiation: Heat energy transmitted by electromagnetic waves Q t = εσat 4 emissivity
Name: Class: Date: 10. Some substances, when exposed to visible light, absorb more energy as heat than other substances absorb.
 Name: Class: Date: ID: A PS Chapter 13 Review Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. 1. In all cooling
Name: Class: Date: ID: A PS Chapter 13 Review Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. 1. In all cooling
Chapter 10: Temperature and Heat
 Chapter 10: Temperature and Heat 1. The temperature of a substance is A. proportional to the average kinetic energy of the molecules in a substance. B. equal to the kinetic energy of the fastest moving
Chapter 10: Temperature and Heat 1. The temperature of a substance is A. proportional to the average kinetic energy of the molecules in a substance. B. equal to the kinetic energy of the fastest moving
UNIT 6a TEST REVIEW. 1. A weather instrument is shown below.
 UNIT 6a TEST REVIEW 1. A weather instrument is shown below. Which weather variable is measured by this instrument? 1) wind speed 3) cloud cover 2) precipitation 4) air pressure 2. Which weather station
UNIT 6a TEST REVIEW 1. A weather instrument is shown below. Which weather variable is measured by this instrument? 1) wind speed 3) cloud cover 2) precipitation 4) air pressure 2. Which weather station
What Is Heat? What Is Heat?
 What Is Heat? Paul shivered inside the wood cabin. It was cold outside, and inside the cabin it wasn t much warmer. Paul could hear the rain beating down on the roof. Every few minutes there would be a
What Is Heat? Paul shivered inside the wood cabin. It was cold outside, and inside the cabin it wasn t much warmer. Paul could hear the rain beating down on the roof. Every few minutes there would be a
TEACHER BACKGROUND INFORMATION THERMAL ENERGY
 TEACHER BACKGROUND INFORMATION THERMAL ENERGY In general, when an object performs work on another object, it does not transfer all of its energy to that object. Some of the energy is lost as heat due to
TEACHER BACKGROUND INFORMATION THERMAL ENERGY In general, when an object performs work on another object, it does not transfer all of its energy to that object. Some of the energy is lost as heat due to
The First Law of Thermodynamics
 The First aw of Thermodynamics Q and W are process (path)-dependent. (Q W) = E int is independent of the process. E int = E int,f E int,i = Q W (first law) Q: + heat into the system; heat lost from the
The First aw of Thermodynamics Q and W are process (path)-dependent. (Q W) = E int is independent of the process. E int = E int,f E int,i = Q W (first law) Q: + heat into the system; heat lost from the
Convection, Conduction & Radiation
 Convection, Conduction & Radiation There are three basic ways in which heat is transferred: convection, conduction and radiation. In gases and liquids, heat is usually transferred by convection, in which
Convection, Conduction & Radiation There are three basic ways in which heat is transferred: convection, conduction and radiation. In gases and liquids, heat is usually transferred by convection, in which
Thermodynamics AP Physics B. Multiple Choice Questions
 Thermodynamics AP Physics B Name Multiple Choice Questions 1. What is the name of the following statement: When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium
Thermodynamics AP Physics B Name Multiple Choice Questions 1. What is the name of the following statement: When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium
Worksheet #17. 2. How much heat is released when 143 g of ice is cooled from 14 C to 75 C, if the specific heat capacity of ice is 2.087 J/(g C).
 Worksheet #17 Calculating Heat 1. How much heat is needed to bring 12.0 g of water from 28.3 C to 43.87 C, if the specific heat capacity of water is 4.184 /(g? 2. How much heat is released when 143 g of
Worksheet #17 Calculating Heat 1. How much heat is needed to bring 12.0 g of water from 28.3 C to 43.87 C, if the specific heat capacity of water is 4.184 /(g? 2. How much heat is released when 143 g of
Heat Energy FORMS OF ENERGY LESSON PLAN 2.7. Public School System Teaching Standards Covered
 FORMS OF ENERGY LESSON PLAN 2.7 Heat Energy This lesson is designed for 3rd 5th grade students in a variety of school settings (public, private, STEM schools, and home schools) in the seven states served
FORMS OF ENERGY LESSON PLAN 2.7 Heat Energy This lesson is designed for 3rd 5th grade students in a variety of school settings (public, private, STEM schools, and home schools) in the seven states served
2. Room temperature: C. Kelvin. 2. Room temperature:
 Temperature I. Temperature is the quantity that tells how hot or cold something is compared with a standard A. Temperature is directly proportional to the average kinetic energy of molecular translational
Temperature I. Temperature is the quantity that tells how hot or cold something is compared with a standard A. Temperature is directly proportional to the average kinetic energy of molecular translational
Introduction to Chapter 27
 9 Heating and Cooling Introduction to Chapter 27 What process does a hot cup of coffee undergo as it cools? How does your bedroom become warm during the winter? How does the cooling system of a car work?
9 Heating and Cooling Introduction to Chapter 27 What process does a hot cup of coffee undergo as it cools? How does your bedroom become warm during the winter? How does the cooling system of a car work?
ES 106 Laboratory # 2 HEAT AND TEMPERATURE
 ES 106 Laboratory # 2 HEAT AND TEMPERATURE Introduction Heat transfer is the movement of heat energy from one place to another. Heat energy can be transferred by three different mechanisms: convection,
ES 106 Laboratory # 2 HEAT AND TEMPERATURE Introduction Heat transfer is the movement of heat energy from one place to another. Heat energy can be transferred by three different mechanisms: convection,
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Sample Mid-Term 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) If you double the frequency of a vibrating object, its period A) is quartered.
Sample Mid-Term 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) If you double the frequency of a vibrating object, its period A) is quartered.
Phys222 W11 Quiz 1: Chapters 19-21 Keys. Name:
 Name:. In order for two objects to have the same temperature, they must a. be in thermal equilibrium.
Name:. In order for two objects to have the same temperature, they must a. be in thermal equilibrium.
What is Energy? What is the relationship between energy and work?
 What is Energy? What is the relationship between energy and work? Compare kinetic and potential energy What are the different types of energy? What is energy? Energy is the ability to do work. Great, but
What is Energy? What is the relationship between energy and work? Compare kinetic and potential energy What are the different types of energy? What is energy? Energy is the ability to do work. Great, but
Heat Transfer and Energy
 What is Heat? Heat Transfer and Energy Heat is Energy in Transit. Recall the First law from Thermodynamics. U = Q - W What did we mean by all the terms? What is U? What is Q? What is W? What is Heat Transfer?
What is Heat? Heat Transfer and Energy Heat is Energy in Transit. Recall the First law from Thermodynamics. U = Q - W What did we mean by all the terms? What is U? What is Q? What is W? What is Heat Transfer?
The Ideal Gas Law. Gas Constant. Applications of the Gas law. P = ρ R T. Lecture 2: Atmospheric Thermodynamics
 Lecture 2: Atmospheric Thermodynamics Ideal Gas Law (Equation of State) Hydrostatic Balance Heat and Temperature Conduction, Convection, Radiation Latent Heating Adiabatic Process Lapse Rate and Stability
Lecture 2: Atmospheric Thermodynamics Ideal Gas Law (Equation of State) Hydrostatic Balance Heat and Temperature Conduction, Convection, Radiation Latent Heating Adiabatic Process Lapse Rate and Stability
Partnerships Implementing Engineering Education Worcester Polytechnic Institute Worcester Public Schools Supported by: National Science Foundation
 Temperature: 6.D.3 Temperature and Heat Transfer Grade Level 6 Sessions Seasonality Instructional Mode(s) Team Size WPS Benchmarks MA Frameworks Key Words 1 Approximately 1.5 hours (10 minutes for cleanup)
Temperature: 6.D.3 Temperature and Heat Transfer Grade Level 6 Sessions Seasonality Instructional Mode(s) Team Size WPS Benchmarks MA Frameworks Key Words 1 Approximately 1.5 hours (10 minutes for cleanup)
UNIT 1 GCSE PHYSICS 1.1.1 Infrared Radiation 2011 FXA
 1 All objects emit and absorb thermal radiation. The hotter an object is the infrared radiation it radiates in a given time. It is continually being transferred to and from all objects. The hotter the
1 All objects emit and absorb thermal radiation. The hotter an object is the infrared radiation it radiates in a given time. It is continually being transferred to and from all objects. The hotter the
Transferring Solar Energy
 activity 14 Transferring Solar Energy BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 4 Quarter 2 Activity 14 SC.B.1.2.2 The student recognizes various forms of energy (e.g., heat, light, and electricity).
activity 14 Transferring Solar Energy BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 4 Quarter 2 Activity 14 SC.B.1.2.2 The student recognizes various forms of energy (e.g., heat, light, and electricity).
1. At which temperature would a source radiate the least amount of electromagnetic energy? 1) 273 K 3) 32 K 2) 212 K 4) 5 K
 1. At which temperature would a source radiate the least amount of electromagnetic energy? 1) 273 K 3) 32 K 2) 212 K 4) 5 K 2. How does the amount of heat energy reflected by a smooth, dark-colored concrete
1. At which temperature would a source radiate the least amount of electromagnetic energy? 1) 273 K 3) 32 K 2) 212 K 4) 5 K 2. How does the amount of heat energy reflected by a smooth, dark-colored concrete
CHAPTER 2 Energy and Earth
 CHAPTER 2 Energy and Earth This chapter is concerned with the nature of energy and how it interacts with Earth. At this stage we are looking at energy in an abstract form though relate it to how it affect
CHAPTER 2 Energy and Earth This chapter is concerned with the nature of energy and how it interacts with Earth. At this stage we are looking at energy in an abstract form though relate it to how it affect
Energy and Chemical Reactions. Characterizing Energy:
 Energy and Chemical Reactions Energy: Critical for virtually all aspects of chemistry Defined as: We focus on energy transfer. We observe energy changes in: Heat Transfer: How much energy can a material
Energy and Chemical Reactions Energy: Critical for virtually all aspects of chemistry Defined as: We focus on energy transfer. We observe energy changes in: Heat Transfer: How much energy can a material
Exam on Heat and Energy
 Exam on Heat and Energy True/False Indicate whether the statement is true or false. 1. Energy is the ability to cause change. 2. Energy is measured in joules. 3. When you ride a playground swing, your
Exam on Heat and Energy True/False Indicate whether the statement is true or false. 1. Energy is the ability to cause change. 2. Energy is measured in joules. 3. When you ride a playground swing, your
Answer, Key Homework 6 David McIntyre 1
 Answer, Key Homework 6 David McIntyre 1 This print-out should have 0 questions, check that it is complete. Multiple-choice questions may continue on the next column or page: find all choices before making
Answer, Key Homework 6 David McIntyre 1 This print-out should have 0 questions, check that it is complete. Multiple-choice questions may continue on the next column or page: find all choices before making
Q1. (a) The graph shows the temperature inside a flat between 5 pm and 9 pm. The central heating was on at 5 pm.
 Q. (a) The graph shows the temperature inside a flat between 5 pm and 9 pm. The central heating was on at 5 pm. (i) What time did the central heating switch off? () (ii) Closing the curtains reduces heat
Q. (a) The graph shows the temperature inside a flat between 5 pm and 9 pm. The central heating was on at 5 pm. (i) What time did the central heating switch off? () (ii) Closing the curtains reduces heat
SAM Teachers Guide Heat and Temperature
 SAM Teachers Guide Heat and Temperature Overview Students learn that temperature measures average kinetic energy, and heat is the transfer of energy from hot systems to cold systems. They consider what
SAM Teachers Guide Heat and Temperature Overview Students learn that temperature measures average kinetic energy, and heat is the transfer of energy from hot systems to cold systems. They consider what
OBJECTIVES THE STUDENTS WILL: Participate in cooperative problem solving in a group setting.
 ICE CAPADES THE POWER OF INSULATION GRADE LEVEL: Upper Elementary/Middle School (High School with extensions) SUBJECT AREA: Sciences, Mathematics DURATION: Preparation time 30 minutes Activity time: One
ICE CAPADES THE POWER OF INSULATION GRADE LEVEL: Upper Elementary/Middle School (High School with extensions) SUBJECT AREA: Sciences, Mathematics DURATION: Preparation time 30 minutes Activity time: One
Provided by TryEngineering - www.tryengineering.org
 Provided by TryEngineering - Lesson Focus Lesson focuses on the engineering behind keeping food and other items cool. Students work in teams to develop a system to make an insulated liquid container that
Provided by TryEngineering - Lesson Focus Lesson focuses on the engineering behind keeping food and other items cool. Students work in teams to develop a system to make an insulated liquid container that
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat What are temperature and heat? Are they the same? What causes heat? What Is Temperature? How do we measure temperature? What are we actually measuring? Temperature and Its
Chapter 10 Temperature and Heat What are temperature and heat? Are they the same? What causes heat? What Is Temperature? How do we measure temperature? What are we actually measuring? Temperature and Its
Preview of Period 5: Thermal Energy, the Microscopic Picture
 Preview of Period 5: Thermal Energy, the Microscopic Picture 5.1 Temperature and Molecular Motion What is evaporative cooling? 5.2 Temperature and Phase Changes How much energy is required for a phase
Preview of Period 5: Thermal Energy, the Microscopic Picture 5.1 Temperature and Molecular Motion What is evaporative cooling? 5.2 Temperature and Phase Changes How much energy is required for a phase
Lecture 30 - Chapter 6 Thermal & Energy Systems (Examples) 1
 Potential Energy ME 101: Thermal and Energy Systems Chapter 7 - Examples Gravitational Potential Energy U = mgδh Relative to a reference height Increase in elevation increases U Decrease in elevation decreases
Potential Energy ME 101: Thermal and Energy Systems Chapter 7 - Examples Gravitational Potential Energy U = mgδh Relative to a reference height Increase in elevation increases U Decrease in elevation decreases
Chapter 4 Practice Quiz
 Chapter 4 Practice Quiz 1. Label each box with the appropriate state of matter. A) I: Gas II: Liquid III: Solid B) I: Liquid II: Solid III: Gas C) I: Solid II: Liquid III: Gas D) I: Gas II: Solid III:
Chapter 4 Practice Quiz 1. Label each box with the appropriate state of matter. A) I: Gas II: Liquid III: Solid B) I: Liquid II: Solid III: Gas C) I: Solid II: Liquid III: Gas D) I: Gas II: Solid III:
8.5 Comparing Canadian Climates (Lab)
 These 3 climate graphs and tables of data show average temperatures and precipitation for each month in Victoria, Winnipeg and Whitehorse: Figure 1.1 Month J F M A M J J A S O N D Year Precipitation 139
These 3 climate graphs and tables of data show average temperatures and precipitation for each month in Victoria, Winnipeg and Whitehorse: Figure 1.1 Month J F M A M J J A S O N D Year Precipitation 139
Practice Test. 4) The planet Earth loses heat mainly by A) conduction. B) convection. C) radiation. D) all of these Answer: C
 Practice Test 1) Increase the pressure in a container of oxygen gas while keeping the temperature constant and you increase the A) molecular speed. B) molecular kinetic energy. C) Choice A and choice B
Practice Test 1) Increase the pressure in a container of oxygen gas while keeping the temperature constant and you increase the A) molecular speed. B) molecular kinetic energy. C) Choice A and choice B
Physics PH1FP. (Jun15PH1FP01) General Certificate of Secondary Education Foundation Tier June 2015. Unit Physics P1. Unit Physics P1 TOTAL
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Science A Unit Physics P1 Physics Unit Physics P1 Friday 12 June 2015 General
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Science A Unit Physics P1 Physics Unit Physics P1 Friday 12 June 2015 General
How Do Oceans Affect Weather and Climate?
 How Do Oceans Affect Weather and Climate? In Learning Set 2, you explored how water heats up more slowly than land and also cools off more slowly than land. Weather is caused by events in the atmosphere.
How Do Oceans Affect Weather and Climate? In Learning Set 2, you explored how water heats up more slowly than land and also cools off more slowly than land. Weather is caused by events in the atmosphere.
Energy Transport. Focus on heat transfer. Heat Transfer Mechanisms: Conduction Radiation Convection (mass movement of fluids)
 Energy Transport Focus on heat transfer Heat Transfer Mechanisms: Conduction Radiation Convection (mass movement of fluids) Conduction Conduction heat transfer occurs only when there is physical contact
Energy Transport Focus on heat transfer Heat Transfer Mechanisms: Conduction Radiation Convection (mass movement of fluids) Conduction Conduction heat transfer occurs only when there is physical contact
REASONING AND SOLUTION
 39. REASONING AND SOLUTION The heat released by the blood is given by Q cm T, in which the specific heat capacity c of the blood (water) is given in Table 12.2. Then Therefore, T Q cm 2000 J 0.8 C [4186
39. REASONING AND SOLUTION The heat released by the blood is given by Q cm T, in which the specific heat capacity c of the blood (water) is given in Table 12.2. Then Therefore, T Q cm 2000 J 0.8 C [4186
THERMAL RADIATION (THERM)
 UNIVERSITY OF SURREY DEPARTMENT OF PHYSICS Level 2 Classical Laboratory Experiment THERMAL RADIATION (THERM) Objectives In this experiment you will explore the basic characteristics of thermal radiation,
UNIVERSITY OF SURREY DEPARTMENT OF PHYSICS Level 2 Classical Laboratory Experiment THERMAL RADIATION (THERM) Objectives In this experiment you will explore the basic characteristics of thermal radiation,
6 th Grade Science Assessment: Weather & Water Select the best answer on the answer sheet. Please do not make any marks on this test.
 Select the be answer on the answer sheet. Please do not make any marks on this te. 1. Weather is be defined as the A. changes that occur in cloud formations from day to day. B. amount of rain or snow that
Select the be answer on the answer sheet. Please do not make any marks on this te. 1. Weather is be defined as the A. changes that occur in cloud formations from day to day. B. amount of rain or snow that
1/9/2013. Terminology Calculating Heat Transfer Code Requirements Design Examples and Sustainability
 1/9/13 By Brett T. Fagan, P.E. Presented by Marie Horan, P.E. Building Diagnostics, Inc. Building Diagnostics Terminology Calculating Heat Transfer Code Requirements Design Examples and Sustainability
1/9/13 By Brett T. Fagan, P.E. Presented by Marie Horan, P.E. Building Diagnostics, Inc. Building Diagnostics Terminology Calculating Heat Transfer Code Requirements Design Examples and Sustainability
Topic Page Contents Page
 Heat energy (11-16) Contents Topic Page Contents Page Heat energy and temperature 3 Latent heat energy 15 Interesting temperatures 4 Conduction of heat energy 16 A cooling curve 5 Convection 17 Expansion
Heat energy (11-16) Contents Topic Page Contents Page Heat energy and temperature 3 Latent heat energy 15 Interesting temperatures 4 Conduction of heat energy 16 A cooling curve 5 Convection 17 Expansion
HEAT AND MASS TRANSFER
 MEL242 HEAT AND MASS TRANSFER Prabal Talukdar Associate Professor Department of Mechanical Engineering g IIT Delhi prabal@mech.iitd.ac.in MECH/IITD Course Coordinator: Dr. Prabal Talukdar Room No: III,
MEL242 HEAT AND MASS TRANSFER Prabal Talukdar Associate Professor Department of Mechanical Engineering g IIT Delhi prabal@mech.iitd.ac.in MECH/IITD Course Coordinator: Dr. Prabal Talukdar Room No: III,
Cloud Radiation and the Law of Attraction
 Convec,on, cloud and radia,on Convection redistributes the thermal energy yielding (globally-averaged), a mean lapse rate of ~ -6.5 o C/km. Radiative processes tend to produce a more negative temperature
Convec,on, cloud and radia,on Convection redistributes the thermal energy yielding (globally-averaged), a mean lapse rate of ~ -6.5 o C/km. Radiative processes tend to produce a more negative temperature
Heat Transfer: Conduction, Convection, and Radiation
 Heat Transfer: Conduction, Convection, and Radiation Introduction We have learned that heat is the energy that makes molecules move. Molecules with more heat energy move faster, and molecules with less
Heat Transfer: Conduction, Convection, and Radiation Introduction We have learned that heat is the energy that makes molecules move. Molecules with more heat energy move faster, and molecules with less
Note: You will receive no credit for late submissions. To learn more, read your instructor's Grading Policy
 1/7 2009/11/14 上 午 11:10 Manage this Assignment: Chapter 16 Due: 12:00am on Saturday, July 3, 2010 Note: You will receive no credit for late submissions. To learn more, read your instructor's Grading Policy
1/7 2009/11/14 上 午 11:10 Manage this Assignment: Chapter 16 Due: 12:00am on Saturday, July 3, 2010 Note: You will receive no credit for late submissions. To learn more, read your instructor's Grading Policy
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms, and use it an
Chapter 10 Temperature and Heat GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms, and use it an
Learning outcomes. Students will be able to:
 Learning structure of the lesson The big picture This lesson is designed to exemplify an argumentation approach to practical work, using a predict-observe-explain framework. Students often think that some
Learning structure of the lesson The big picture This lesson is designed to exemplify an argumentation approach to practical work, using a predict-observe-explain framework. Students often think that some
Project 1.3.4 Renewable Insulation Example Teacher Notes
 Project 1.3.4 Renewable Insulation Example Teacher Notes Sample Data and Teacher Notes This guide is designed to provide sample calculations, background, and tips for the teachers performing this project
Project 1.3.4 Renewable Insulation Example Teacher Notes Sample Data and Teacher Notes This guide is designed to provide sample calculations, background, and tips for the teachers performing this project
CPI Links Content Guide & Five Items Resource
 CPI Links Content Guide & Five Items Resource Introduction The following information should be used as a companion to the CPI Links. It provides clarifications concerning the content and skills contained
CPI Links Content Guide & Five Items Resource Introduction The following information should be used as a companion to the CPI Links. It provides clarifications concerning the content and skills contained
5 Answers and Solutions to Text Problems
 Energy and States of Matter 5 Answers and Solutions to Text Problems 5.1 At the top of the hill, all of the energy of the car is in the form of potential energy. As it descends down the hill, potential
Energy and States of Matter 5 Answers and Solutions to Text Problems 5.1 At the top of the hill, all of the energy of the car is in the form of potential energy. As it descends down the hill, potential
Melting ice Student sheet
 Melting ice Student sheet Predict Which ice cube will melt first? Observe Describe what you saw happen. Why? (Give a scientific explanation) Questions to think about: Why does ice melt? Why might one ice
Melting ice Student sheet Predict Which ice cube will melt first? Observe Describe what you saw happen. Why? (Give a scientific explanation) Questions to think about: Why does ice melt? Why might one ice
Greenhouse Glazing Effects on Heat Transfer for Winter Heating and Summer Cooling
 Greenhouse Glazing Effects on Heat Transfer for Winter Heating and Summer Cooling David R. Mears, Ph.D. Bioresource Engineering Department of Plant Biology and Pathology Rutgers University 20 Ag Extension
Greenhouse Glazing Effects on Heat Transfer for Winter Heating and Summer Cooling David R. Mears, Ph.D. Bioresource Engineering Department of Plant Biology and Pathology Rutgers University 20 Ag Extension
Energy - Heat, Light, and Sound
 Science Benchmark: 06:06 Heat, light, and sound are all forms of energy. Heat can be transferred by radiation, conduction and convection. Visible light can be produced, reflected, refracted, and separated
Science Benchmark: 06:06 Heat, light, and sound are all forms of energy. Heat can be transferred by radiation, conduction and convection. Visible light can be produced, reflected, refracted, and separated
Radiation Transfer in Environmental Science
 Radiation Transfer in Environmental Science with emphasis on aquatic and vegetation canopy media Autumn 2008 Prof. Emmanuel Boss, Dr. Eyal Rotenberg Introduction Radiation in Environmental sciences Most
Radiation Transfer in Environmental Science with emphasis on aquatic and vegetation canopy media Autumn 2008 Prof. Emmanuel Boss, Dr. Eyal Rotenberg Introduction Radiation in Environmental sciences Most
Temperature. PJ Brucat
 PJ Brucat Temperature - the measure of average kinetic energy (KE) of a gas, liquid, or solid. KE is energy of motion. KE = ½ mv 2 where m=mass and v=velocity (speed) 1 All molecules have KE whether solid,
PJ Brucat Temperature - the measure of average kinetic energy (KE) of a gas, liquid, or solid. KE is energy of motion. KE = ½ mv 2 where m=mass and v=velocity (speed) 1 All molecules have KE whether solid,
Chemistry Unit 3 Reading Assignment Energy and Kinetic Molecular Theory
 Chemistry Unit 3 Reading Assignment Energy and Kinetic Molecular Theory The story behind the difficulty we have with energy is fascinating to those of us who struggle with trying to teach energy in a coherent
Chemistry Unit 3 Reading Assignment Energy and Kinetic Molecular Theory The story behind the difficulty we have with energy is fascinating to those of us who struggle with trying to teach energy in a coherent
Calculating Heat Loss by Mark Crombie, Chromalox
 Calculating Heat Loss by Mark Crombie, Chromalox Posted: January 30, 2006 This article deals with the basic principles of heat transfer and the calculations used for pipes and vessels. By understanding
Calculating Heat Loss by Mark Crombie, Chromalox Posted: January 30, 2006 This article deals with the basic principles of heat transfer and the calculations used for pipes and vessels. By understanding
14 HEAT AND HEAT TRANSFER METHODS
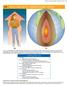 CHAPTER 14 HEAT AND HEAT TRANSFER METHODS 469 14 HEAT AND HEAT TRANSFER METHODS Figure 14.1 (a) The chilling effect of a clear breezy night is produced by the wind and by radiative heat transfer to cold
CHAPTER 14 HEAT AND HEAT TRANSFER METHODS 469 14 HEAT AND HEAT TRANSFER METHODS Figure 14.1 (a) The chilling effect of a clear breezy night is produced by the wind and by radiative heat transfer to cold
Heat Transfer: Introduction
 Heat Transfer: Introduction As warm-blooded animals, we all care about heat and temperature! Our survival, not to mention comfort, depends on keeping our bodies at a constant temperature, despite huge
Heat Transfer: Introduction As warm-blooded animals, we all care about heat and temperature! Our survival, not to mention comfort, depends on keeping our bodies at a constant temperature, despite huge
Lecture 9, Thermal Notes, 3.054
 Lecture 9, Thermal Notes, 3.054 Thermal Properties of Foams Closed cell foams widely used for thermal insulation Only materials with lower conductivity are aerogels (tend to be brittle and weak) and vacuum
Lecture 9, Thermal Notes, 3.054 Thermal Properties of Foams Closed cell foams widely used for thermal insulation Only materials with lower conductivity are aerogels (tend to be brittle and weak) and vacuum
PREPARATION FOR CHEMISTRY LAB: COMBUSTION
 1 Name: Lab Instructor: PREPARATION FOR CHEMISTRY LAB: COMBUSTION 1. What is a hydrocarbon? 2. What products form in the complete combustion of a hydrocarbon? 3. Combustion is an exothermic reaction. What
1 Name: Lab Instructor: PREPARATION FOR CHEMISTRY LAB: COMBUSTION 1. What is a hydrocarbon? 2. What products form in the complete combustion of a hydrocarbon? 3. Combustion is an exothermic reaction. What
CFD SIMULATION OF SDHW STORAGE TANK WITH AND WITHOUT HEATER
 International Journal of Advancements in Research & Technology, Volume 1, Issue2, July-2012 1 CFD SIMULATION OF SDHW STORAGE TANK WITH AND WITHOUT HEATER ABSTRACT (1) Mr. Mainak Bhaumik M.E. (Thermal Engg.)
International Journal of Advancements in Research & Technology, Volume 1, Issue2, July-2012 1 CFD SIMULATION OF SDHW STORAGE TANK WITH AND WITHOUT HEATER ABSTRACT (1) Mr. Mainak Bhaumik M.E. (Thermal Engg.)
Forms of Energy Explain
 Forms of Energy Explain DIRECTIONS 1. For the Explain portion of the section, work through each slide 2. For each form there are three slides: 1. Introduce the form of energy 2. Give examples of the form
Forms of Energy Explain DIRECTIONS 1. For the Explain portion of the section, work through each slide 2. For each form there are three slides: 1. Introduce the form of energy 2. Give examples of the form
Energy Pathways in Earth s Atmosphere
 BRSP - 10 Page 1 Solar radiation reaching Earth s atmosphere includes a wide spectrum of wavelengths. In addition to visible light there is radiation of higher energy and shorter wavelength called ultraviolet
BRSP - 10 Page 1 Solar radiation reaching Earth s atmosphere includes a wide spectrum of wavelengths. In addition to visible light there is radiation of higher energy and shorter wavelength called ultraviolet
Chillin Out: Designing an Insulator
 SHPE Jr. Chapter May 2015 STEM Activity Instructor Resource Chillin Out: Designing an Insulator Students learn about the three ways heat can be transferred from one object to another. They also learn what
SHPE Jr. Chapter May 2015 STEM Activity Instructor Resource Chillin Out: Designing an Insulator Students learn about the three ways heat can be transferred from one object to another. They also learn what
SunMor SM-V30 Solar Water Heater Installation Manual
 SunMor SM-V30 Solar Water Heater Installation Manual Rev. 1.02 Congratulations on the purchase of your new SunMor SM-V30 from Nature s Comfort LLC! You must read this entire instruction manual before beginning
SunMor SM-V30 Solar Water Heater Installation Manual Rev. 1.02 Congratulations on the purchase of your new SunMor SM-V30 from Nature s Comfort LLC! You must read this entire instruction manual before beginning
Energy transfers (Particle theory, conduction, convection, IR, evaporation)
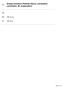 Energy transfers (Particle theory, conduction, convection, IR, evaporation) 88 minutes 88 marks Page of 72 Q. (a) The diagrams, X, Y and Z, show how the particles are arranged in the three states of matter.
Energy transfers (Particle theory, conduction, convection, IR, evaporation) 88 minutes 88 marks Page of 72 Q. (a) The diagrams, X, Y and Z, show how the particles are arranged in the three states of matter.
Shaking Water. What Happens When You Add Energy? Overview. Objectives. Time Requirements. Materials
 CON EDISON WEB-BASED MIDDLE SCHOOL ACTIVITY Shaking Water What Happens When You Add Energy? Overview This activity demonstrates several fundamental science and energy concepts. Students vigorously shake
CON EDISON WEB-BASED MIDDLE SCHOOL ACTIVITY Shaking Water What Happens When You Add Energy? Overview This activity demonstrates several fundamental science and energy concepts. Students vigorously shake
Department of Engineering Enzo Ferrari University of Modena and Reggio Emilia
 Department of Engineering Enzo Ferrari University of Modena and Reggio Emilia Object: Measurement of solar reflectance, thermal emittance and Solar Reflectance Index Report Reference person: Alberto Muscio
Department of Engineering Enzo Ferrari University of Modena and Reggio Emilia Object: Measurement of solar reflectance, thermal emittance and Solar Reflectance Index Report Reference person: Alberto Muscio
CHAPTER 3. The sun and the seasons. Locating the position of the sun
 zenith 90 summer solstice 75 equinox 52 winter solstice 29 altitude angles observer Figure 3.1: Solar noon altitude angles for Melbourne SOUTH winter midday shadow WEST summer midday shadow summer EAST
zenith 90 summer solstice 75 equinox 52 winter solstice 29 altitude angles observer Figure 3.1: Solar noon altitude angles for Melbourne SOUTH winter midday shadow WEST summer midday shadow summer EAST
Kinetic Theory. Energy. Transfers and Efficiency. The National Grid
 AQA P1 Revision Infrared Radiation Heating and Insulating Buildings Kinetic Theory Energy Transfers and Efficiency Energy Transfer by Heating Transferring Electrical Energy Generating Electricity The National
AQA P1 Revision Infrared Radiation Heating and Insulating Buildings Kinetic Theory Energy Transfers and Efficiency Energy Transfer by Heating Transferring Electrical Energy Generating Electricity The National
In today s world of wireless communications
 TUTORIAL RF CERAMIC CHIP CAPACITORS IN HIGH RF POWER APPLICATIONS Reprinted with permission of MICROWAVE JOURNAL from the April 2000 issue. 2000 Horizon House Publications, Inc. In today s world of wireless
TUTORIAL RF CERAMIC CHIP CAPACITORS IN HIGH RF POWER APPLICATIONS Reprinted with permission of MICROWAVE JOURNAL from the April 2000 issue. 2000 Horizon House Publications, Inc. In today s world of wireless
Peltier Application Note
 Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Physics 5D - Nov 18, 2013
 Physics 5D - Nov 18, 2013 30 Midterm Scores B } Number of Scores 25 20 15 10 5 F D C } A- A A + 0 0-59.9 60-64.9 65-69.9 70-74.9 75-79.9 80-84.9 Percent Range (%) The two problems with the fewest correct
Physics 5D - Nov 18, 2013 30 Midterm Scores B } Number of Scores 25 20 15 10 5 F D C } A- A A + 0 0-59.9 60-64.9 65-69.9 70-74.9 75-79.9 80-84.9 Percent Range (%) The two problems with the fewest correct
A. Kinetic Molecular Theory (KMT) = the idea that particles of matter are always in motion and that this motion has consequences.
 I. MOLECULES IN MOTION: A. Kinetic Molecular Theory (KMT) = the idea that particles of matter are always in motion and that this motion has consequences. 1) theory developed in the late 19 th century to
I. MOLECULES IN MOTION: A. Kinetic Molecular Theory (KMT) = the idea that particles of matter are always in motion and that this motion has consequences. 1) theory developed in the late 19 th century to
Chapter 2: Solar Radiation and Seasons
 Chapter 2: Solar Radiation and Seasons Spectrum of Radiation Intensity and Peak Wavelength of Radiation Solar (shortwave) Radiation Terrestrial (longwave) Radiations How to Change Air Temperature? Add
Chapter 2: Solar Radiation and Seasons Spectrum of Radiation Intensity and Peak Wavelength of Radiation Solar (shortwave) Radiation Terrestrial (longwave) Radiations How to Change Air Temperature? Add
Temperature. Temperature
 Chapter 8 Temperature Temperature a number that corresponds to the warmth or coldness of an object measured by a thermometer is a per-particle property no upper limit definite limit on lower end Temperature
Chapter 8 Temperature Temperature a number that corresponds to the warmth or coldness of an object measured by a thermometer is a per-particle property no upper limit definite limit on lower end Temperature
Practical Applications of Freezing by Boiling Process
 Practical Applications of Freezing by Boiling Process Kenny Gotlieb, Sasha Mitchell and Daniel Walsh Physics Department, Harvard-Westlake School 37 Coldwater Canyon, N. Hollywood, CA 9164 Introduction
Practical Applications of Freezing by Boiling Process Kenny Gotlieb, Sasha Mitchell and Daniel Walsh Physics Department, Harvard-Westlake School 37 Coldwater Canyon, N. Hollywood, CA 9164 Introduction
Overview. What is EMR? Electromagnetic Radiation (EMR) LA502 Special Studies Remote Sensing
 LA502 Special Studies Remote Sensing Electromagnetic Radiation (EMR) Dr. Ragab Khalil Department of Landscape Architecture Faculty of Environmental Design King AbdulAziz University Room 103 Overview What
LA502 Special Studies Remote Sensing Electromagnetic Radiation (EMR) Dr. Ragab Khalil Department of Landscape Architecture Faculty of Environmental Design King AbdulAziz University Room 103 Overview What
Heat Transfer. Energy from the Sun. Introduction
 Introduction The sun rises in the east and sets in the west, but its exact path changes over the course of the year, which causes the seasons. In order to use the sun s energy in a building, we need to
Introduction The sun rises in the east and sets in the west, but its exact path changes over the course of the year, which causes the seasons. In order to use the sun s energy in a building, we need to
Practice final for Basic Physics spring 2005 answers on the last page Name: Date:
 Practice final for Basic Physics spring 2005 answers on the last page Name: Date: 1. A 12 ohm resistor and a 24 ohm resistor are connected in series in a circuit with a 6.0 volt battery. Assuming negligible
Practice final for Basic Physics spring 2005 answers on the last page Name: Date: 1. A 12 ohm resistor and a 24 ohm resistor are connected in series in a circuit with a 6.0 volt battery. Assuming negligible
How To Build A Solar Energised Power Plant
 Project Title Solar Energised Power Plant Project Description In recent years energy crisis has become one of the most talked about issues around the world. Especially in the developing countries, the
Project Title Solar Energised Power Plant Project Description In recent years energy crisis has become one of the most talked about issues around the world. Especially in the developing countries, the
Green Heating. Pupil Research Brief. Teachers Notes. Syllabus Coverage Subject Knowledge and Understanding. Route through the Brief UPIL ESEARCHER
 R P UPIL ESEARCHER Green Heating I NITIATIVE Pupil Research Brief Teachers Notes Syllabus Coverage Subject Knowledge and Understanding all types of electromagnetic radiation form a continuous spectrum
R P UPIL ESEARCHER Green Heating I NITIATIVE Pupil Research Brief Teachers Notes Syllabus Coverage Subject Knowledge and Understanding all types of electromagnetic radiation form a continuous spectrum
CHAPTER 5 Lectures 10 & 11 Air Temperature and Air Temperature Cycles
 CHAPTER 5 Lectures 10 & 11 Air Temperature and Air Temperature Cycles I. Air Temperature: Five important factors influence air temperature: A. Insolation B. Latitude C. Surface types D. Coastal vs. interior
CHAPTER 5 Lectures 10 & 11 Air Temperature and Air Temperature Cycles I. Air Temperature: Five important factors influence air temperature: A. Insolation B. Latitude C. Surface types D. Coastal vs. interior
Year 10 Investigation. What Makes Ice Melt Fastest? By Rebecca Hogan
 Investigation What Makes Ice Melt Fastest? MY WEBSITE: http://whatsubstancemeltsicefastest.weebly.com/ Nature of Investigation: What keeps us cool on hot days? What is used in our cool, refreshing beverages?
Investigation What Makes Ice Melt Fastest? MY WEBSITE: http://whatsubstancemeltsicefastest.weebly.com/ Nature of Investigation: What keeps us cool on hot days? What is used in our cool, refreshing beverages?
POROUS BURNER - A New Approach to Infrared
 Page: 1 POROUS BURNER - A New Approach to Infrared 1. Preface There are several possibilities to produce infrared radiation in the technical sense. Regarding the source of energy you can distinguish between
Page: 1 POROUS BURNER - A New Approach to Infrared 1. Preface There are several possibilities to produce infrared radiation in the technical sense. Regarding the source of energy you can distinguish between
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Tidal forces in general are the result of A) unequal forces acting on different parts
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Tidal forces in general are the result of A) unequal forces acting on different parts
Thermochemistry. r2 d:\files\courses\1110-20\99heat&thermorans.doc. Ron Robertson
 Thermochemistry r2 d:\files\courses\1110-20\99heat&thermorans.doc Ron Robertson I. What is Energy? A. Energy is a property of matter that allows work to be done B. Potential and Kinetic Potential energy
Thermochemistry r2 d:\files\courses\1110-20\99heat&thermorans.doc Ron Robertson I. What is Energy? A. Energy is a property of matter that allows work to be done B. Potential and Kinetic Potential energy
5 Things. You Must Know. Before Buying. Radiant Barrier
 Consumer Report: 5 Things You Must Know Before Buying Radiant Barrier 1 INDEX I: How Do Radiant Barriers Really Work pg 3 A: Understanding Heat Transfer pg 3 B: How Radiant Barriers Stop the Heat pg 4
Consumer Report: 5 Things You Must Know Before Buying Radiant Barrier 1 INDEX I: How Do Radiant Barriers Really Work pg 3 A: Understanding Heat Transfer pg 3 B: How Radiant Barriers Stop the Heat pg 4
RESPONSE TIME INDEX OF SPRINKLERS
 , Number l, p.1-6, 29 RESPONSE TIME INDEX OF SPRINKLERS C.K. Sze Department of Building Services Engineering, The Hong Kong Polytechnic University, Hong Kong, China ABSTRACT The Plunge test would be carried
, Number l, p.1-6, 29 RESPONSE TIME INDEX OF SPRINKLERS C.K. Sze Department of Building Services Engineering, The Hong Kong Polytechnic University, Hong Kong, China ABSTRACT The Plunge test would be carried
8001782 Owner s Manual
 8001782 Digital Infrared Thermometer Owner s Manual Introduction This instrument is a portable, easy to use compact-size digital thermometer with laser sighting designed for one hand operation. The meter
8001782 Digital Infrared Thermometer Owner s Manual Introduction This instrument is a portable, easy to use compact-size digital thermometer with laser sighting designed for one hand operation. The meter
