Chemistry A: Periodic Table Packet Name: Hour: Page 1. Chemistry A Periodic Table
|
|
|
- Morgan Simpson
- 7 years ago
- Views:
Transcription
1 Chemistry A: Periodic Table Packet Name: Hour: Page 1 Chemistry A Periodic Table
2 Chemistry A: Periodic Table Packet Name: Hour: Page 2 Worksheet #1: Periodic Table Inquiry Activity Directions: I know that we have not talked about these ideas yet, but I want to see how much you can figure out on your own. Sometimes you might feel like you are guessing at the answers, but if you read carefully and look at the pictures and diagrams all the information you need to answer the questions is on this sheet. A little background The periodic table is, in many ways, the world s greatest cheat sheet. It was created a long time ago by a guy named Dmitri Mendeleev who, probably like you, did not want to memorize tons of information. The periodic table lists all of the elements (simple substances that make up more complex materials) like gold, silver, tin, lead and mercury. It also provides lots of information about these elements. The good news is that you will be able to use the periodic table on all of your chemistry exams. The bad news is that first you have to learn all the symbols Mendeleev used for this information. Questions: 1. Who created the first periodic table? 2. What is an element? 3. Each box represents a different element. How many elements are on this periodic table? What makes up each element? The parts that make up an element are called sub-atomic particles. There are three basic sub-atomic particles that we will talk about in chemistry, they are called protons, neutrons and electrons. Each proton has one positive charge of electricity (+1). Each electron has one negative charge of electricity (-1). Neutrons are neutral, which means they do not have a charge. 4. What is a sub-atomic particle? 5. What is the difference between a proton, a neutron and an electron?
3 Chemistry A: Periodic Table Packet Name: Hour: Page 3 Here is a close-up of the periodic table symbol for carbon, an element that is very common and we will study about this trimester: Atomic Number Mass Number Here is a close-up of the element carbon if we could see it under a very powerful microscope: KEY p = proton n = neutron = electron Nucleus 6. Are the protons and neutrons The electron found inside cloud is or outside the nucleus? made of shells that hold the electrons. Carbon has 2 shells and is in the 2 nd 7. Are the electrons found inside or outside the nucleus? row of the periodic table. 8. How many electrons does carbon have? 9. How many protons does carbon have? 10. How many neutrons does carbon have? 11. What is the total positive charge of carbon? 12. What is the total negative charge of carbon? These + and charges cancel out making a neutral carbon atom. 13. Match the following a. Atomic Number i. the number of protons in the nucleus of an atom b. Atomic Mass ii. the number of protons + number of neutrons (approximately)
4 Worksheet #2: Atoms All matter (air, water, soil, people) is made the elements on the Periodic Table. The smallest unit of one element that can exist on its own is called an atom. Atoms are made of a central nucleus, which contains protons and neutrons. Protons are positively charged and neutrons have no charge. Surrounding these nuclei are little negatively charged particles called electrons. The space around the nucleus that contains the electrons is called the electron cloud or shell. # of shells= row of periodic table Electron Cloud (Shell) Neutron no charge (neutral) Electron negative charge Proton positive charge Each atom is different from every other atom by the number of protons it has in its nucleus. Hydrogen has one proton; helium has two. Calcium has 20 and gold has 79. The number of protons in an atom is equal to the atomic number of the element. Mass Number = # protons + number of neutrons bigger Atomic Number = # of protons. smaller The proton has a positive charge and a mass of approximately one amu (atomic mass unit). A neutron is a neutral particle with a mass of approximately one amu. To determine the number of neutrons in an atom you subtract the atomic number from the mass number of the atom. The electrons are negatively charged particles located in energy levels outside the nucleus. The mass of an electron is extremely small we describe it as negligible since it would take nearly two thousand electrons to have the mass of a single proton. The number of electrons in an atom is equal to the atomic number and, since the charge on an electron is equal in size but opposite in sign to that of the proton, an atom is neutral. Complete the following table: (+) charge + (-) charge = 0 Particle Name Charge Mass Location in Atom What tells you how many are in an atom? PROTON ELECTRON NEUTRON 4
5 Worksheet #3: Atomic and Mass Number Practice Draw a picture of the symbol of carbon from the periodic table. Label its atomic number and mass number: 1. Carbon has 6 protons. How many electrons does it have? 2. Lead has an atomic number of 82. How many protons? Electrons? 3. How many protons does Silicon have? Electrons? 4. An atom has a mass number of 42 and an atomic number of 39. How many neutrons does it have? 5. What is the mass number of calcium? 6. How many neutrons does calcium have? 5
6 Worksheet #4: Organization of the Periodic Table In addition to being different from each other because of the number of protons they have, atoms also differ in their behavior. Potassium and sodium are extremely explosive in the presence of water, while a chunk of copper or silver would just sink effortlessly to the bottom of a swimming pool. Dmitry Mendeleev ( ) was a Russian chemist who proposed a method of arranging atoms according to their mass as well as their behavior. He noticed that certain elements behaved similarly to others, and he arranged these on his table so that they were in the same vertical row. For example, if you look at a periodic table of elements, you will notice that H, Li, Na, K, Rb, Cs, and Fr are all in the same vertical column. These elements all share common behaviors and also share a similar electron arrangement. Mendeleev's work is important to us today because he was able to successfully classify the chemical elements in order to give scientists a better understanding of how atoms interact with each other and of the properties they hold. The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups or families. By setting up this periodic table, he was even successful in predicting the existence of at least three more elements that had yet to be discovered (gallium, scandium, and germanium). Arranging elements by mass resulted in several elements being placed in groups of elements with different properties. Henry Mosely (1913) discovered that atoms of each element contain a unique number of protons in their nuclei (plural of nucleus), the number of protons being equal to the atomic number. Arranging the periodic table by atomic number, instead of mass, eliminated the problems with the Mendeleev s periodic table. 1) What are the vertical (up and down) columns of the periodic table called? 2) What are the horizontal (back and forth) rows of the periodic table called? 3) Which elements have similar properties, those in the same period or in the same family? 4) How did Mosley improve the organization of the periodic table? Have your table out so we can label it together. The Modern Periodic Table: There are three main classifications for the elements- metals, nonmetals, and metalloids. Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. Most metals are ductile and malleable, meaning that they can be pounded into thin sheets and drawn into wires. Most group A elements and all group B elements are metals. If you look at boron (B), you see a heavy stair-step line that zigzags down to astatine (At) at the bottom of group 7A. This stair step line serves as a visual divider between the metals and the nonmetals on the table. Except for hydrogen, all of the elements on the left side of the table are metals. The group 1A elements (except for hydrogen) are known as the alkali metals. The group 2A elements are known as the alkaline earth metals. Both the alkali metals and the alkaline earth metals are chemically reactive, with the alkali metals being more reactive of the two groups. The elements in the center of the periodic table are called transition metals. As with all metals, the transition elements are both ductile and malleable, and conduct electricity and heat. We will learn later in the trimester what makes transition metals unique in their behavior. The 2 sets of inner transition metals, known as the lanthanide and actinide series, are located along the bottom of the periodic table. Nonmetals are elements that are generally gases or brittle dull-looking solids found in the upper right side of the periodic table. They are poor conductors of heat and electricity. The highly reactive group 7A elements are known as halogens, and the extremely unreactive group 8A elements are commonly called the noble gases. 6
7 Worksheet #4 Continued: Organization of the Periodic Table The elements bordering the stair-step line are called metalloids. Metalloids are elements with physical and chemical properties of both metals and nonmetals. Silicon and germanium are 2 of the most important metalloids, as they are extensively used in computer chips and solar cells. Directions: Fill in the blanks on the right with the information in the chart below. Word List actinide series alkali metal alkaline earth metal atomic mass atomic number family group halogen lanthanide series metal metalloid Moseley noble gas nonmetal period periodic law periodic table transition element Dmitri Mendeleev developed a chart-like arrangement of the elements 7. called the (1). He stated that if the elements were listed in order of increasing 8. (2), their properties repeated in a regular manner. He called this the (3) 9. of the elements. The arrangement used today, devised by (4), differs from 10. that of Mendeleev in that the elements are arranged in order of increasing (5). 11. Each horizontal row of elements is called a(n) (6). Each vertical column is called a(n) (7), or, because of the resemblance between elements in the same 12. column, a(n) (8). 13. In rows 4 through 7, there is a wide central section containing elements, 14. each of which is called a(n) (9). Rows 6 and 7 also contain two other sets of 15. elements that are listed below the main chart. These are called the (10) and 16. the (11), respectively. Each of these elements, as well as those in the first two columns at the left end of the chart, is classified as a(n) (12). Each of the 17. elements at the right side of the chart is classified as a(n) (13). Each of the 18. elements between these two main types of elements, having some properties in common with each, is called a(n) (14). Each of the elements in Group 1A is called a(n) (15). Each of the elements in the Group 2A is called a(n) (16). Each of the elements in Group 7A is called a(n) (17). Each of the elements in Group 8A is called a(n) (18). 7
8 Worksheet #5: Color Coding the Periodic Table REVIEW: The Periodic Table is a list of all the known elements. It is organized by increasing atomic number. There are two main groups on the periodic table: metals and nonmetals. The left side of the table contains elements with the greatest metallic properties. As you move from the left to the right, the elements become less metallic with the far right side of the table consisting of nonmetals. The elements in the middle of the table are called transition elements because they are changed from metallic properties to nonmetallic properties. A small group whose members touch the zigzag line are called metalloids because they have both metallic and nonmetallic properties. NEW: The table is also arranged in vertical columns called groups or families and horizontal rows called periods. Each arrangement is significant. Groups (vertical, up and down ) same number of valence electrons (electrons in outermost shell of atom) which gives elements of the same group similar properties. Example: All group 1 elements have one electron in the outer shells and all group 2 elements have two electrons in their outer shells. Periods (horizontal, back and forth ) same number of electron shells around nucleus Example: The elements in the first period all have one shell, the elements in the second period all have two shells etc. There are a number of major groups with similar properties. They are as follows: Hydrogen: This element does not match the properties of any other group so it stands alone. It is placed above group 1 but it is not part of that group. It is a very reactive, colorless, odorless gas at room temperature. (1 valence electron) Group 1: Alkali Metals These metals are extremely reactive and are never found in nature in their pure form. They are silver colored and shiny. They are soft enough to be cut with a knife. (1 valence electron) Group 2: Alkaline-earth Metals Slightly less reactive than alkali metals. They are silver colored and more dense than alkali metals. (2 valence electrons) Groups 3 12: Transition Metals These metals have a wide range of properties. In general, they are shiny and good conductors of heat and electricity. They also have higher densities and melting points than groups 1 & 2. (1 or 2 valence electrons) Lanthanides and Actinides: These are also transition metals that were taken out and placed at the bottom of the table so the table wouldn t be so wide. The elements in each of these two periods share many properties. The lanthanides are shiny and reactive. Elements 95 through 103 do not exist in nature but have been manufactured in the lab-thus are man-made. Group 13: Boron Group Contains one metalloid (B) and 4 metals. Reactive. Aluminum is in this group. It is also the most common metal in the earth s crust. (3 valence electrons) Group 14: Carbon Group Contains one nonmetal, two metalloids (Si & Ge), and two metals. (4 valence electrons) Group 15: Nitrogen Group Contains two nonmetals, two metalloids (As & Sb), and one metal.(5 valence electrons) Group 16: Oxygen Group Contains three nonmetals and two metalloids (Te & Po).(6 valence electrons) Groups 17: Halogens All nonmetals. Very reactive. (7 valence electrons) Groups 18: Noble Gases Unreactive nonmetals. All are colorless, odorless gases at room temperature. All found in earth s atmosphere in small amounts. (8 valence electrons) 8
Class Notes Standards Addressed: 8.3.11
 Name: Period #: Class Notes Standards Addressed: 8.3.11 History of the Periodic Table: Demitri Mendeleev = Russian chemist who discovered a pattern to the in 1869. o How did he discovery a pattern to the
Name: Period #: Class Notes Standards Addressed: 8.3.11 History of the Periodic Table: Demitri Mendeleev = Russian chemist who discovered a pattern to the in 1869. o How did he discovery a pattern to the
Unit 3.2: The Periodic Table and Periodic Trends Notes
 Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Section 1: Arranging the Elements Pages 106-112
 Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
PERIODIC TABLE. reflect
 reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Unit 2 Periodic Behavior and Ionic Bonding
 Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations.
 The Periodic Table Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations. Vertical Rows are called Families or Groups.
The Periodic Table Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations. Vertical Rows are called Families or Groups.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
5.4 Trends in the Periodic Table
 5.4 Trends in the Periodic Table Think about all the things that change over time or in a predictable way. For example, the size of the computer has continually decreased over time. You may become more
5.4 Trends in the Periodic Table Think about all the things that change over time or in a predictable way. For example, the size of the computer has continually decreased over time. You may become more
Chemistry: The Periodic Table and Periodicity
 Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
THE PERIODIC TABLE O F T H E E L E M E N T S. The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27)
 THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Trends of the Periodic Table Basics
 Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Chapter 5 Periodic Table. Dmitri Mendeleev: Russian Chemist credited with the discovery of the periodic table.
 Chapter 5 Periodic Table Dmitri Mendeleev: Russian Chemist credited with the discovery of the periodic table. How did he organize the elements? According to similarities in their chemical and physical
Chapter 5 Periodic Table Dmitri Mendeleev: Russian Chemist credited with the discovery of the periodic table. How did he organize the elements? According to similarities in their chemical and physical
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
TRENDS IN THE PERIODIC TABLE
 Noble gases Period alogens Alkaline earth metals Alkali metals TRENDS IN TE PERIDI TABLE Usual charge +1 + +3-3 - -1 Number of Valence e - s 1 3 4 5 6 7 Electron dot diagram X X X X X X X X X 8 Group 1
Noble gases Period alogens Alkaline earth metals Alkali metals TRENDS IN TE PERIDI TABLE Usual charge +1 + +3-3 - -1 Number of Valence e - s 1 3 4 5 6 7 Electron dot diagram X X X X X X X X X 8 Group 1
Atomic Theory: History of the Atom
 Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Chapter Test. Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a.
 Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Chemistry - Elements Electron Configurations The Periodic Table. Ron Robertson
 Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Periodic Table Trends in Element Properties Ron Robertson
 Periodic Table Trends in Element Properties Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\ch9trans2.doc The Periodic Table Quick Historical Review Mendeleev in 1850 put together
Periodic Table Trends in Element Properties Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\ch9trans2.doc The Periodic Table Quick Historical Review Mendeleev in 1850 put together
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Chemistry Worksheet: Matter #1
 Chemistry Worksheet: Matter #1 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up
Chemistry Worksheet: Matter #1 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Periodic Table. 1. In the modern Periodic Table, the elements are arranged in order of increasing. A. atomic number B. mass number
 Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
Tro's "Introductory Chemistry", Chapter 4
 1 Introductory Chemistry, 3 rd Edition Nivaldo Tro Atoms and Elements Opening figure showing a shore scene with molecules of O 2, N 2, triethyl amine (CH 3 CH 2 ) 3 N, and rocks made of silicates containing
1 Introductory Chemistry, 3 rd Edition Nivaldo Tro Atoms and Elements Opening figure showing a shore scene with molecules of O 2, N 2, triethyl amine (CH 3 CH 2 ) 3 N, and rocks made of silicates containing
Look at a periodic table to answer the following questions:
 Look at a periodic table to answer the following questions: 1. What is the name of group 1? 2. What is the name of group 2? 3. What is the name of group 17? 4. What is the name of group 18? 5. What is
Look at a periodic table to answer the following questions: 1. What is the name of group 1? 2. What is the name of group 2? 3. What is the name of group 17? 4. What is the name of group 18? 5. What is
CHAPTER REVIEW. 3. What category do most of the elements of the periodic table fall under?
 CHAPTER REVIEW EVIEW ANSWERS 1. alkaline-earth metals 2. halogens 3. metals. electron affinity 5. actinides 6. answers should involve the transmutation of one element to another by a change in the number
CHAPTER REVIEW EVIEW ANSWERS 1. alkaline-earth metals 2. halogens 3. metals. electron affinity 5. actinides 6. answers should involve the transmutation of one element to another by a change in the number
CHAPTER 4: ATOMS AND ELEMENTS
 CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
Chapter 3, Elements, Atoms, Ions, and the Periodic Table
 1. Which two scientists in 1869 arranged the elements in order of increasing atomic masses to form a precursor of the modern periodic table of elements? Ans. Mendeleev and Meyer 2. Who stated that the
1. Which two scientists in 1869 arranged the elements in order of increasing atomic masses to form a precursor of the modern periodic table of elements? Ans. Mendeleev and Meyer 2. Who stated that the
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
Name Date Class CHAPTER 1 REVIEW. Answer the following questions in the space provided.
 CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Properties of Atoms and the Periodic Table
 Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Metals are located on the left side of the periodic table and are generally shiny, malleable, ductile, and good conductors.
 Section 1: are located on the left side of the periodic table and are generally shiny, malleable, ductile, and good conductors. K What I Know W What I Want to Find Out L What I Learned Essential Questions
Section 1: are located on the left side of the periodic table and are generally shiny, malleable, ductile, and good conductors. K What I Know W What I Want to Find Out L What I Learned Essential Questions
Chapter 7. Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 7 John D. Bookstaver St. Charles Community College Cottleville, MO Development of Table
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 7 John D. Bookstaver St. Charles Community College Cottleville, MO Development of Table
Atoms, Elements, and the Periodic Table (Chapter 2)
 Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More
 KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More The Modern Periodic Table The Periodic Law - when elements are arranged according
KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More The Modern Periodic Table The Periodic Law - when elements are arranged according
Chapter 7 Periodic Properties of the Elements
 Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
Student Exploration: Electron Configuration
 Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
P. Table & E Configuration Practice TEST
 P. Table & E Configuration Practice TEST Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A line spectrum is produced when an electron moves from one energy
P. Table & E Configuration Practice TEST Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A line spectrum is produced when an electron moves from one energy
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
Electron Configurations, Isoelectronic Elements, & Ionization Reactions. Chemistry 11
 Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Structure and Properties of Atoms
 PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
 Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004. Name (print) SSN
 CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
Chapter 3. Elements, Atoms, Ions, and the Periodic Table
 Chapter 3. Elements, Atoms, Ions, and the Periodic Table The Periodic Law and the Periodic Table In the early 1800's many elements had been discovered and found to have different properties. In 1817 Döbreiner's
Chapter 3. Elements, Atoms, Ions, and the Periodic Table The Periodic Law and the Periodic Table In the early 1800's many elements had been discovered and found to have different properties. In 1817 Döbreiner's
EXAMPLE EXERCISE 4.1 Change of Physical State
 EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Truth is ever to be found in the simplicity, and not in the multiplicity and confusion of things.
 UNIT 6 Periodic Trends What are the Patterns in the Chemical and Physical Properties of Elements? Truth is ever to be found in the simplicity, and not in the multiplicity and confusion of things. Isaac
UNIT 6 Periodic Trends What are the Patterns in the Chemical and Physical Properties of Elements? Truth is ever to be found in the simplicity, and not in the multiplicity and confusion of things. Isaac
Ionic and Metallic Bonding
 Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
PERIODIC TABLE OF THE ELEMENTS
 PERIODIC TABLE OF THE ELEMENTS Periodic Table: an arrangement of elements in horizontal rows (Periods) and vertical columns (Groups) exhibits periodic repetition of properties First Periodic Table: discovered
PERIODIC TABLE OF THE ELEMENTS Periodic Table: an arrangement of elements in horizontal rows (Periods) and vertical columns (Groups) exhibits periodic repetition of properties First Periodic Table: discovered
Bohr s Model of the Atom
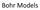 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
Chem term # 1 review sheet C. 12 A. 1
 hem term # 1 review sheet Name: ate: 1. n isotope of which element has an atomic number of 6 and a mass number of 14?. carbon. magnesium. nitrogen. silicon 6. Which atoms represent different isotopes of
hem term # 1 review sheet Name: ate: 1. n isotope of which element has an atomic number of 6 and a mass number of 14?. carbon. magnesium. nitrogen. silicon 6. Which atoms represent different isotopes of
Find a pair of elements in the periodic table with atomic numbers less than 20 that are an exception to the original periodic law.
 Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Chapter 4, Lesson 2: The Periodic Table
 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
CHEM 150 Exam 1 KEY Name Multiple Choice
 CEM 150 Exam 1 KEY Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. _B 1. Which of the following is synonymous with "fact"? a. a hypothesis
CEM 150 Exam 1 KEY Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. _B 1. Which of the following is synonymous with "fact"? a. a hypothesis
Chapter Test A. Elements, Compounds, and Mixtures MULTIPLE CHOICE. chemically combined? MIXs2 a. element b. compound c. mixture d.
 Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
The Advanced Placement Examination in Chemistry. Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010
 The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Student Exploration: Electron Configuration
 www.explorelearning.com Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund
www.explorelearning.com Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund
Models of the Atom and periodic Trends Exam Study Guide
 Name 1. What is the term for the weighted average mass of all the naturally occurring isotopes of an element? ans: atomic mass 2. Which is exactly equal to 1/12 the mass of a carbon -12 atom? ans: atomic
Name 1. What is the term for the weighted average mass of all the naturally occurring isotopes of an element? ans: atomic mass 2. Which is exactly equal to 1/12 the mass of a carbon -12 atom? ans: atomic
Bonding Practice Problems
 NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
