Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria
|
|
|
- Dortha Greer
- 8 years ago
- Views:
Transcription
1 PHYSIOLOGIA PLANTARUM 119: Copyright # Physiologia Plantarum 2003 Printed in Denmark all rights reserved Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria Christine H. Foyer a, * and Graham Noctor b a Crop Performance and Improvement Division, Rothamsted Research, Harpenden, Hertfordshire AL5 2JQ, UK b Institut de Biotechnologie des Plantes, Baˆtiment 630, Universite Paris XI, Orsay Cedex, France *Corresponding author, christine.foyer@bbsrc.ac.uk Received 4 February 2003; revised 19 May 2003 Chloroplasts and mitochondria are the powerhouses of photosynthetic cells. The oxidation-reduction (redox) cascades of the photosynthetic and respiratory electron transport chains not only provide the driving forces for metabolism but also generate redox signals, which participate in and regulate every aspect of plant biology from gene expression and translation to enzyme chemistry. Plastoquinone, thioredoxin and reactive oxygen have all been shown to have signalling functions. Moreover, the intrinsic involvement of molecular oxygen in electron transport processes with the inherent generation of superoxide, hydrogen peroxide and singlet oxygen provides a repertoire of additional extremely powerful signals. Accumulating evidence implicates the major redox buffers of plant cells, ascorbate and glutathione, in redox signal transduction. The network of redox signals from energy-generating organelles orchestrates metabolism to adjust energy production to utilization, interfacing with hormone signalling to respond to environmental change at every stage of plant development. Introduction All living organisms are oxidation reduction (redox) systems. They use anabolic, reductive processes to store energy and catabolic, oxidative processes to release it. Through photosynthesis, plants set this global wheel in motion. By harnessing light energy to drive biochemistry, photosynthetic organisms have perfected the art of redox control. Redox signals are the most fundamental forms of information monitored by photosynthetic organisms (Noctor and Foyer 1998). These types of signals may well belong to the earliest evolved controls since they prevent uncontrolled boom and bust scenarios in energy availability, utilization and exchange. More complex aspects of redox control of physiology through regulation of gene expression developed with the evolution of higher plants. It is now widely accepted that redox signals are key regulators of plant metabolism, morphology and development, and it may even be that all intermediates and other systems of signal transduction arose around this central core. These signals exert control on nearly every aspect of plant biology from chemistry to development, growth and eventual death. Controlled production of reactive oxygen species (ROS) acts as a second messenger, alongside other mediators such as calcium, not only in plant responses to pathogens but also in hormone signalling (Pei et al. 2000). Recent developments have greatly increased our understanding of how systems in organelles involved in photosynthesis sense redox changes, particularly those linked to reactive oxygen in order to regulate whole cell redox homeostasis. The key role of reactive oxygen species and antioxidants in plant redox homeostasis Photosynthetic and respiratory electron transport chains are the primary energy-transducing processes in eukaryotic organisms. The evolution of oxygenic photosynthesis, Abbreviations ABA, abscissic acid; APX, ascorbate peroxidase; DHA, dehydroascorbate; GPX, glutathione peroxidase; GRX, glutaredoxin; GSH, reduced glutathione; GSSG, glutathione disulphide; GST, glutathione S-transferase; LHC, light-harvesting complex; M-POX, Mehler-peroxidase; (i)nos, (inducible) nitric oxide synthase; PQ, plastoquinone; PRX, peroxiredoxin; PSI, photosystem I; PSII, photosystem II; ROS, reactive oxygen species; TRX, thioredoxin. Physiol. Plant. 119,
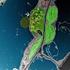
2 an event that occurred two billion years ago, provided abundant oxygen and facilitated the elaboration of reactions involving O 2, particularly aerobic respiration. Almost all life is based on the essential energy exchange reactions of photosynthesis and respiration. The evolution of photosystem II first allowed use of the very high electrochemical potential (Em 7 ¼ mv) of the O 2 /H 2 O redox couple. Oxygenic photosynthesis and aerobic respiration today deal with concerted, four-electron exchange between water and oxygen, without release of reactive, partially reduced intermediates. However, many processes in plants (and in other organisms) catalyse only partial reduction of oxygen, and so generate superoxide, H 2 O 2, and hydroxyl radicals (Noctor and Foyer 1998, Mittler 2002). In addition, a reaction potentially common in the thylakoid pigment beds involves photodynamic energy transfer to ground-state triplet O 2 and leads to the formation of highly reactive singlet O 2. Redox signals are involved in all aspects of plant biology. They are particularly important in defence responses and cross-tolerance phenomena, enabling a general acclimation of plants to stressful conditions. H 2 O 2 has long been recognized as a signal-transducing molecule in the activation of defence responses in plants. It mediates intra- and extra-cellular communication during plant reactions to pathogens and several studies have suggested a role in systemic acquired resistance. The hypersensitive response (HR) is a widespread phenomenon that is responsible for the activation and establishment of plant immunity to disease. HR is an example of plant programmed cell death as it leads to rapid, localized cell death at infection sites. This contributes to the limitation of the growth and spread of the invading pathogen. One of the earliest events in the HR is the rapid accumulation of ROS through the activation of enzyme systems, some of which are similar to neutrophil NADPH oxidase (Keller et al. 1998). The oxidative burst is a central component of an integrated HR signalling system, whose function is rapid amplification of the signal. Other signalling components involved in HR are salicylic acid (SA) and cytosolic Ca 21.H 2 O 2 has a strong regulatory influence on fluxes through Ca 21 channels and on Ca 21 concentrations in different cellular compartments. During HR, H 2 O 2 is required to trigger localized host cell death but it seems that NO is also needed to induce an efficient cell death response (Delledonne et al. 1998). Further evidence that the H 2 O 2 cascade interacts closely with other signalling systems comes from studies on hormone-mediated stomatal movement (Pei et al. 2000), cell growth (Rodriguez et al. 2002) and tropic responses (Joo et al. 2001). Such studies show that H 2 O 2 is a common secondary messenger in hormone-mediated events. ensure control of the cellular redox state rather than to facilitate the complete elimination of H 2 O 2 (Foyer and Noctor 2000). The antioxidative system determines the lifetime of H 2 O 2 in planta. Plant cells are strongly redox buffered and contain very large quantities of the soluble hydrophilic antioxidants, ascorbate ( mm) and glutathione ( mm) (Noctor and Foyer 1998, Hartmann et al. 2003). Most of their intracellular compartments hence have the capacity to deal with even very high fluxes of H 2 O 2 production. Rapid compartmentspecific differences in redox state (and hence signalling) that influence the operation of many fundamental processes in plants, can be achieved by modifying ROS (particularly H 2 O 2 ) production or by repression or activation of antioxidant defences. Recent evidence suggests that glutathione and ascorbate are key components of redox signalling in plants (Baier et al. 2000, Noctor et al. 2000, Horling et al. 2003). Specific compartmentbased signalling and regulation of gene expression can be achieved via differential compartment-based changes in either the absolute concentrations of ascorbate and glutathione or the ascorbate/dehydroascorbate and GSH/ GSSG ratios, which are very high and stable in the absence of stress (Noctor et al. 2000). Three factors are particularly significant in governing the importance of H 2 O 2 in signalling redox status in a given compartment. The first is the absolute rate of H 2 O 2 production. Figure 1 shows rates of H 2 O 2 production in different compartments during photosynthesis at moderately high light and optimal temperatures. Photosynthesis produces superoxide as result of direct electron transfer to oxygen, from which H 2 O 2 is produced and metabolized through the Mehler-peroxidase (M-POX) reaction. Assuming, however, that the M-POX reaction is not a sink for more than 10% of photosynthetic electron flow, H 2 O 2 is probably produced even faster in the peroxisomes, at least in C3 plants, by virtue of the glycollate oxidase reaction. The second important factor is the rate of scavenging by detoxifying systems. These two factors are likely to be the main determinants of H 2 O 2 concentration. Third, the probability of reaction of H 2 O 2 with signalling components is probably a key determinant of signalling intensity. As we discuss below, early components in redox signalling are likely to include antioxidants and/or antioxidative enzymes. The influence of a given compartment in redox signalling may differ as a function of how well primed each compartment is with sensing components, the nature of the signal that these components sense (e.g. H 2 O 2 or other ROS, electron transport components, organic peroxides), and to what extent sensing components are coupled to downstream transduction factors such as kinase cascades (Kovtun et al. 2000). Rates of H 2 O 2 production in different compartments during C 3 photosynthesis Plants are more tolerant of H 2 O 2 than animals, and their antioxidant systems appear to have been designed to Redox signalling from the chloroplast The chloroplast is an excellent model for understanding redox-regulated plant gene expression (Pfannschmidt et al. 1999). The light-driven chemistry of photosynthesis 356 Physiol. Plant. 119, 2003
3 Chloroplast Peroxisome Mitochondrion 4030 nmol.m 2.s 1 H 2 O 2 Photorespiration CO 2 fixation Reductant Glycolate Glyoxylate H 2 O nmol.m 2.s 1 Shuttle NADH TCA cycle Electron transport chain H 2 O 2 DARK <182 nmol.m 2.s 1 Electron transport Serine Glycine NADH Electron transport chain H 2 O 2 NADH LIGHT <216 nmol.m 2.s 1 Fig. 1. Typical rates of H 2 O 2 production in the organelles of mesophyll cells during C 3 photosynthesis. Numerous other ROS-generating reactions are not considered the above processes are presumed to be the fastest ROS-generating reactions in photosynthetic cells, at least in the absence of an oxidative burst at the plasmalemma. Values are necessarily approximate and are supposed to compare likely typical rates of H 2 O 2 production in the different compartments. These are derived using established models of C 3 photosynthesis (eg. Noctor et al. (2002)), in which TCA cycle activity in the light is taken to be 50% of the rate in the dark and 50% of the NADH produced by glycine decarboxylase is oxidized by the mitochondrial electron transport chain. Rates of net photosynthetic CO 2 assimilation (19 mmol m 2 leaf surface s 1 ) are taken to be about 10 times faster than total respiratory O 2 uptake in the dark (2 mmol m 2 leaf surface s 1 ). Chloroplastic and mitochondrial H 2 O 2 production are calculated assuming likely upper limits of electron leakage to superoxide in optimal conditions (10% of total photosynthetic electron flow and 5% of total respiratory electron flow for further discussion, see Foyer and Noctor (2000)). Peroxisomal H 2 O 2 production through reactions other than glycollate oxidation is considered to be relatively minor in C 3 leaves in the light, and is not considered. consists of a series of redox steps involving structural components or functionally coupled pools of redoxactive compounds, such as thioredoxin (TRX), ascorbate and glutathione. Changes in the redox state of these components regulate the expression of both plastomeand nuclear-encoded chloroplast proteins. This redox information co-ordinates expression in both compartments (Allen and Pfannschmidt 2000). To date, genetic and biochemical analysis has identified plastoquinone (PQ), ROS, ascorbate and the ferredoxin/trx system as redox-active signalling components (Danon and Mayfield 1994, Escoubas et al. 1995, Karpinski et al. 1997, Pfannschmidt et al. 1999, Schu rmann and Jacquot 2000, Kiddle et al. 2003). There is little doubt that other redox signalling molecules remain to be discovered. For example, the membrane-bound peroxiredoxins (PRX), whose main role may be protection against lipid peroxidation, have all the necessary molecular characteristics of thiol signal-transducing components. Thus far, redox signals have been shown to control post-translational modification of proteins via phosphorylation, redox modulation of assimilatory reactions and control of gene transcription and translation (Somanchi and Mayfield 1999, Link 1999, Pfannschmidt et al. 1999). Photosynthetic control of gene expression can now be described for the genes located in chloroplasts themselves, both at transcriptional and post-transcriptional levels (Pfannschmidt et al. 1999). Redox signals also leave the chloroplast to provide a decisive input into transcriptional control in the cell nucleus. Allen (1993) suggested that redox signalling is a key function of the cytoplasmic genomes found in the chloroplast and mitochondria, with the exchange of redox information between these organelles and the nucleus functioning within the global network regulating plastid and mitochondrial metabolism and development. Physiol. Plant. 119,
4 Coupling of photosynthetic electron transport and redox poise to the expression of genes encoding chloroplast proteins enables plants to respond to diverse environmental conditions. Of the latter, light quality and quantity have been most intensely studied. The expression of plastid-encoded photosynthetic proteins is precisely co-ordinated with that of their nuclear-encoded partners (Kettunen et al. 1997, Surpin and Chory 1997). The light-activated increases in chloroplast protein synthesis are governed by redox-modulated transcriptional and translational controls (Rochaix 1996). For example, the activity of the psba-rna binding protein complex is subject to redox-regulation, probably via TRX (Danon and Mayfield 1994). In this system, the flux of electrons through the electron transport chain induces a change in the sulfhydryl status of TRX and hence the RNP complex, thereby allowing binding to the psba mrna and formation of a translation initiation complex. A second light-modulated system, which has been described in detail, involves the reversible redox activation of membrane protein kinases. The kinases phosphorylate two distinct groups of proteins. The first are the light-harvesting chlorophyll a/b antenna system of PSII. The reversible activation of the light-harvesting antenna complex II (LHCII) kinase(s) is related to the regulation and optimization of energy transfer between PSII and PSI. Activation of the LHCII protein kinase occurs via dynamic conformational changes in the cytochrome b 6 f complex taking place during plastoquinol oxidation. De-activation of the kinase involves its re-association with an oxidized cytochrome complex. Maximal phosphorylation of components such as Lhcb1 and Lhcb2 proteins is only observed at irradiances far below those experienced by the leaves during growth. Such observations are explained by the presence of a redox (probably TRX)-dependent regulatory loop that inhibits the activation of the kinase at high light via reduction of protein disulphide groups (Gal et al. 1997). Recently, a thylakoid protein kinase with these properties has been cloned from Chlamydomonas (Depège et al. 2003). The second group of phosphorylated thylakoid proteins are the PSII core proteins (D1 and D2 reaction centre proteins, CP43 internal antenna protein and the 9 kda psbh gene product). However, it is noteworthy that the thylakoid protein kinase that phosphorylates CP43 (but not LHCII) shows both redox-dependent and redox-independent regulation (Vink et al. 2000). With midpoint redox potentials as low as 0.9 V (primary acceptors of photosystem I), the photosynthetic electron transport chain generates reductants with potentials far lower than those involved in the mitochondrial respiratory chain. This and other aspects of the essential photoelectrochemistry of photosynthesis first evolved in an anaerobic world, where there was probably little selective pressure against such low potential components (Allen 1993). Electron flow from these highly reducing components is thermodynamically favourable for reduction of oxygen to superoxide (Em 7 ¼ 0.33 V). Evolution has led to the integration of signals from such inevitable side reactions into cellular control mechanisms involving profound changes in gene function (Allen and Raven 1996, Foyer and Noctor 2000, Noctor et al. 2000). Because of the potentially high capacity of photosynthesis for the production of superoxide, hydrogen peroxide and singlet oxygen, the intracellular levels of these oxidants are tightly buffered and controlled by the detoxifying antioxidant system, comprising a network of enzymatic and non-enzymatic components (Noctor and Foyer 1998, Mittler 2002). Major detoxification reactions associated with photosynthesis are mediated, first, by catalase (in the peroxisomes) and, second (in all compartments), by reductive processes involving the major redox buffers of plant cells, ascorbate and glutathione pools (Noctor and Foyer 1998). Two notable, fairly common features of the oxidative stress response are that (1) the most strongly induced genes are not necessarily those on the front line of the antioxidant defence system: genes markedly induced are those encoding, for example, pathogenesis-related proteins and heat-shock proteins, rather than classical antioxidants; and (2) antioxidant genes that are induced often tend to encode cytosolic rather than chloroplastic antioxidative proteins, even in conditions in which the major stress is predicted to be located in the chloroplast. For example, cytosolic APX transcripts, as well as the promoter activity of this nuclear gene, were shown to be influenced by the redox state of the photosynthetic electron transport chain (Karpinski et al. 1997). Peroxisomes and reactive oxygen and nitrogen species Peroxisomes are known to release signals that regulate nuclear gene expression. Signals arising from peroxisomes regulate photomorphogenesis, plant development peroxisomal biogenesis, light signalling and stress responses (Hu et al. 2002). Redox signals are very likely important in this process since peroxisomes produce H 2 O 2 at high rates through several reactions, including b-oxidation of long chain fatty acids and glycollate oxidase. The latter reaction means that during moderate to high rates of photosynthesis, the peroxisomes of C 3 plants are the site of massive light-dependent generation of H 2 O 2 (Fig. 1). To cope with this, these organelles have a very high antioxidant capacity, notably including catalase but also APX and other enzymes of the ascorbate/ glutathione system (Jiménez et al. 1997). Catalase is clearly necessary for photorespiratory H 2 O 2 processing (Smith et al. 1984, Willekens et al. 1997) and insufficient activity of this enzyme causes perturbation of cellular redox state when photorespiration is rapid, notably associated with a marked accumulation of oxidized glutathione. Photorespiration-linked perturbation of the redox states of both ascorbate and glutathione can occur transiently in plants with a full complement of catalase (Noctor et al. 2002), and the glycollate oxidase reaction could possibly be one way in which signals 358 Physiol. Plant. 119, 2003
5 linked to photosynthesis are exported from the chloroplast. Such a mechanism may be important in certain conditions such as drought, other stresses that decrease stomatal conductance, or increased temperature. Under other conditions, such as low temperature, peroxisomal H 2 O 2 formation will become less important whereas the probability (although perhaps not the absolute rate) of mitochondrial and chloroplastic production may tend to increase in these conditions. It should also be noted that peroxisomes can generate significant amounts of superoxide from which H 2 O 2 is produced by a peroxisomal superoxide dismutase (for review, see del Rı o et al. 2002). It is possible that peroxisomes are a major site of nitric oxide (NO) synthesis in plants (Corpas et al. 2001). There is some evidence to suggest that NO synthase (NOS), a key enzyme involved in mammalian macrophage action, is conserved in plants (Ninnemann and Maier 1996, Beligni and Lamattina 2001). An NADPHand Ca 21 -dependent NOS activity was found in pea peroxisomes (Barroso et al. 1999). Moreover, a 130-kDa peroxisomal pea protein was recognized by a polyclonal antibody raised against 14 residues from the C-terminus of inos (Barroso et al. 1999). Whereas the sequencing of the complete Arabidopsis genome did not reveal the presence of putative NOS genes, a complementary DNA sequence with high homology to a protein NOS inhibitor, has been described in plants (Jaffrey and Snyder 1996). Redox signalling associated with mitochondrial respiration Like chloroplasts, mitochondria also originated as bacterial endosymbionts, and they retain a specialized genome. Redox signalling as the function of the mitochondrial genome has wide implications. The free radical and mitochondrial theories of ageing are central tenets to animal and human biology but have not been explored in plants (Allen 1993). Mitochondria have not traditionally been regarded as a major source of ROS in leaves, although it has been known for many years that reactions associated with complexes I and III produce superoxide (for review, see Møller 2001). Indeed, various mitochondrial enzymes, such as aconitase and enzymes containing lipoic acid, are susceptible to oxidative inactivation. From a whole leaf point of view, however, at least in C 3 plants at moderate to high light intensities, peroxisomal and chloroplastic H 2 O 2 production may be up to times faster than formation of H 2 O 2 in the mitochondria (Fig. 1). Interestingly, calculations suggest that mitochondrial ROS production is not likely to be greatly different in the light and dark, since total O 2 consumption is less affected by light than tricarboxylic acid cycle activity. However, the probability of superoxide production by the respiratory chain could be changed on illumination, notably if light affects alternative oxidase (AOX) capacity (Dutilleul et al. 2003a). This enzyme influences ROS generation (Maxwell et al. 1999) and is involved in the determination of cell survival in oxidative conditions (Robson and Vanlerberghe 2002, Vanlerberghe et al. 2002). It should be noted that the relative rates shown in Fig. 1 will probably change differentially as a function of the type of stress applied, and in certain conditions the mitochondrial contribution may be significant, even in the light. Even under conditions in which the mitochondria contribute only a fraction of total cellular ROS production, the mitochondrial oxidative load could be crucial in influencing and setting the cellular redox-state, either because of the presence of specific signalling components or because detoxification capacity is relatively low in comparison with the chloroplast and peroxisome. Like these other organelles, however, mitochondria house both enzymic and non-enzymic antioxidants (Rasmusson and Møller 1990, Jime nez et al. 1997), including a TRX system (Laloi et al. 2001), and are the site of ascorbic acid biosynthesis. The final step of ascorbic acid biosynthesis is catalysed by a galactonog-lactone dehydrogenase located in the inner mitochondrial membrane (Bartoli et al. 2000). Evidence has recently been obtained that this enzyme is an intrinsic component of complex I, and that respiratory electron flow may exert significant control on ascorbate synthesis (Millar et al. 2003). Evidence that perturbation of the mitochondria redox state has important consequences for whole cell redox homeostasis has been provided by studies on a Nicotiana sylvestris mutant, CMSII, which lacks functional complex I and has hence lost a major NADH sink. Substantial re-adjustments in antioxidant defence and related changes in stress tolerance are observed in the mutant (Dutilleul et al. 2003a). When complex I function is perturbed, signalling is initiated which resets the antoxidative capacity throughout the cell. This includes enhanced expression of mitochondrial antioxidants (AOX and Mn-SOD) and also of chloroplastic Fe-SOD, peroxisomal catalase, and cytosolic APX. Mitochondrial signalling hence acts to lower cellular H 2 O 2 and allows soluble cellular antioxidants (ascorbate, glutathione) to retain a high reduction state. This redox re-adjustment is associated with the inability of the mutant to use its photosynthetic capacity as efficiently as the wild type (Dutilleul et al. 2003b), probably due to perturbations in NAD(P)H shuttling between intracellular compartments (for reviews, see Gardestro m et al. 2002, Scheibe 2003). These shuttles represent another example of the important interactions between chloroplasts and mitochondria, and could be crucial in adjusting the rate of ROS production in both compartments. Reductant export from the chloroplast to the mitochondria might act to lower ROS production in the chloroplast by relieving electron pressure, while increasing the probability of ROS formation in the respiratory chain. At the origin of mitochondrial redox signalling may be changes in the redox state of ubiquinone or components of the cytochrome bc 1 complex, by analogy to the signalling linked to the chloroplast plastoquinone pool. Such changes could Physiol. Plant. 119,
6 be linked to increased ROS generation through electron leakage to superoxide. ROS and redox signal perception and transduction To date no ROS receptor has been unambiguously identified in plants, and thus a key question is: how is increased ROS production sensed? One simple possibility is direct modification of transcription factors with redoxsensitive groups (Tron et al. 2002), but there are very likely much more complex signal transduction routes. This must be the case when redox changes in organelles are signalled to the nucleus. Important sensing components may be found in the antioxidative system itself. This system is a strong buffer against ROS, maintaining relatively low oxidant concentrations under most conditions. Although localized increases in ROS can occur in certain circumstances, such as during the oxidative burst at the plasmalemma, in many cases redox balance can be markedly perturbed without large changes in H 2 O 2 concentration. This is, perhaps, not suprising given the plethora of components capable of scavenging H 2 O 2.In catalase mutants placed in conditions where the leaf can no longer cope with photorespiratory H 2 O 2 production, H 2 O 2 concentration is not greatly increased relative to the wild type but the glutathione pool is massively perturbed (Noctor et al. 2002). Increased availability of ROS may therefore be sensed by the cell as increased oxidative flux through key components, rather than (or as well as) marked increases in ROS concentration (Fig. 2). This view suggests a dynamic system constructed to allow acclimatory changes through components of the antioxidative system that are plugged into signalling networks. It would allow appropriate responses to occur as a result of increased flux to ROS, even in the absence of marked changes in ROS concentration. Furthermore, changes in ROS trigger profound modifications in gene expression that extend far beyond the antioxidative system (Kovtun et al. 2000, Desikan et al. 2001, Vranova et al. 2002). This confirms the view that the defence system comprising pathogen responses, defence against xenobiotics, stabilizing components, and antioxidants forms an integrated network with extensive crosstalk that can be triggered by ROS. Moreover, priming of different components can be triggered by low ROS concentrations such that the system responds more rapidly and/or effectively to subsequent assault (Vranova et al. 2002). We suggest that some antioxidative components have a dual function of scavenging and signalling A Optimal conditions Photosynthesis Photorespiration Respiration B Stress conditions Photosynthesis Photorespiration Respiration [ROS] [ROS] Detox-scavenging Sensor-scavenging Detox-scavenging + Sensor-scavenging (Thiol oxidation? Glutathionylation?) Acclimatory pathway Other defences + OR Kinase cascades Cell death pathway Fig. 2. A model for the perception of increased ROS production via the antioxidant system. In optimal conditions (A), ROS are produced by many metabolic reactions and are efficiently removed by detoxification processes ( Detox-scavenging ). Stress conditions (B) cause increased production of ROS or decreased antioxidant activity, which can cause an increase in ROS concentration. This could lead to increased oxidation of specific sensor-scavenging antioxidant components locked into signal transduction pathways. Stimulation of transduction pathways occurs via kinase cascades and other second messengers, and leads to either acclimation (upregulation of ROS detoxification capacity, induction of other defences) or cell death pathways involving defence withdrawal. The decisive factors that determine either acclimatory or cell death responses are not known, but could include location of the initial signal or signal intensity. Control of cell fate by signal intensity would be a molecular application to plants of the aphorism formulated, more than a century ago, by the philosopher Nietszche: That which does not kill me, makes me stronger. 360 Physiol. Plant. 119, 2003
7 (Fig. 2: sensor-scavenging components). These could perhaps be relatively low in antioxidative capacity comparedtoclassicaldetoxification-scavenging(fig. 2: Detoxscavenging ) components such as catalase or chloroplastic APX. What could be the nature of such sensor-scavenging components)? It is perhaps noteworthy that catalases and APX are haem-based whereas many other antioxidant components operate through thiol disulphide exchange reactions. As well as the soluble thiol glutathione, the plant cell contains numerous proteins with redox-active thiol groups, some of which have been shown to have activity against peroxides. These include both chloroplastic and cytosolic glutathione peroxidases (GPX: Eshdat et al. 1997, Mullineaux et al. 1998), chloroplastic and cytosolic PRX (Baier and Dietz 1999, Rouhier et al. 2001, Horling et al. 2003), and glutaredoxins (GRX). Specific TRX are also found in several compartments of the photosynthetic cell (Rivera-Madrid et al. 1995). There is considerable heterogeneity within these families: for PRX, there are monomeric and dimeric enzymes, and differences in the number of Cys involved in catalysis. Similarly, several sequences predicted to encode TRX-like proteins and both dithiol and monothiol GRX-like proteins (Vlamis-Gardikas and Holmgren 2002) are found in the Arabidopsis genome. Although the exact roles of most of these plant thiol proteins remain to be elucidated, it is clear there may be considerable divergence of function within each class. In mammals, where TRX exists as mitochondrial (TRX2) and cytosolic/nuclear (TRX1) forms, expression is influenced by an ARE (antioxidant response element) cis-acting factor, and TRX1 mediates numerous defence processes, including Ref-1 activity and the nuclear versus cytosolic localization of the transcription factor NF-kB (Vlamis-Gardikas and Holmgren 2002). Recently, it has been shown in yeast that the redox transduction sensor, YAP-1, interacts in a complex fashion with both TRX and non-selenium (i.e. plant-type) GPX (Delauney et al. 2002). YAP-1 is a basic zipper-type transcription factor that induces several genes in response to peroxides. Peroxides enhance the nuclear accumulation of YAP-1 by oxidizing two Cys residues to form an intramolecular disulphide bond that appears to act to trap YAP-1 in the nucleus, thereby increasing its activity (Kuge et al. 1997). Oxidation of YAP-1 is not mediated directly, however, but occurs via H 2 O 2 reduction by a GPX-like protein, which is then reduced by YAP-1 (Delauney et al. 2002). Re-reduction of YAP-1 may occur by a TRXdependent pathway (Delauney et al. 2002). Although the Arabidopsis genome does not appear to contain sequences homologous to YAP-1, there may well be functionally similar elements in the initial perception of changes in redox state in plants. PRX are often alternatively termed TRX peroxidase and chloroplastic PRX purified from Chlamydomonas has been shown to reduce peroxides using reductant from TRX (Goyer et al. 2002). This enzyme system is therefore closely linked to the photosynthetic electron transport chain in vivo, probably via a novel, specific chloroplastic TRX isoform (Collin et al. 2003). A PRX localized in poplar phloem can function with either the TRX h/nadph-trx reductase or GRX/ glutathione/gr systems (Rouhier et al. 2001). It is possible that the primary role of at least some of these thiol proteins, particularly those with relatively low capacity, is not to detoxify peroxides but to sense their increased production, that is, their most important function may be as signal or signal-scavenging components (Fig. 2). Net oxidation of the glutathione pool, accompanied by increased total glutathione, is a clearly established response to many stresses or to insufficient detoxification capacity (Smith et al. 1984, Sen Gupta et al. 1991, Willekens et al. 1997, Noctor et al. 2002). A mechanism important in sensing this redox perturbation may be protein glutathionylation, in which glutathione forms a mixed disulphide with a target protein. In yeast and animals, glutathionylation has been shown to modify the activity of enzymes and transcription factors and is considered likely to play an important role in redox signalling and protection of protein structure and function (Klatt and Lamas 2000). For example, gluthathionylation of aldolase and triose phosphate isomerase has recently been reported in Arabidopsis leaves (Ito et al. 2003). Spontaneous formation of mixed disulphides may require decreases in GSH/GSSG that are not thought to be physiologically relevant, at least in most mammalian cells, but in the absence of marked accumulation of GSSG the reaction can be catalysed by GRX (Starke et al. 2003). Reversal of glutathionylation may be carried out by dithiol or monothiol GRX, as well as the dithiol protein, TRX. It is interesting to note that these components belong to a large super-family of proteins, and that proteins related to classical TRX, but with a monocysteinic active site motif, also exist in plants. Considerable work will be required to elucidate the functions of many of these proteins. It is possible that whereas only the dithiol enzymes have activity against protein disulphide bonds, the monothiol GRX may be the most important in deglutathionylating mixed disulphides to protein-sh and GSH (Shenton et al. 2002, Vlamis-Gardikas and Holmgren 2002). Another important group of thiol proteins may be certain subclasses of the GST super-family that are active in reducing peroxides or dehydroascorbate (DHA), or in catalysing thiol transferase (i.e. GRX) activity (Bartling et al. 1993, Dixon et al. 2002). Some GSTs are strongly induced by oxidative stress, including that linked to the pathogen response (Conklin and Last 1995, Desikan et al. 2001, Dixon et al. 2002). Until recently considered as exclusively cytosolic enzymes, GSTs form a large gene family in Arabidopsis, but the physiological functions of many of these genes remain unclear. Lately, it has been shown that in two subclasses of the GST family found in Arabidopsis, the classical GST active site Ser is replaced by Cys (Dixon et al. 2002). This confers both DHA reductase and GRX activity on the first class, and DHA reductase activity on the second: in each class, gene sequences predicted at least one chloroplastic and one cytosolic product (Dixon et al. 2002). Physiol. Plant. 119,
8 Integration of redox signalling and plant development: interactions between ROS and phytohormones While redox sensing in plants may have some similarities with that observed in yeast, downstream signalling is probably more intricate, because the photosynthetic, multicellular nature of plants necessitates integration of a complex array of internal and external signals during development. MAP kinase cascades are implicated in the H 2 O 2 response in plants (Kovtun et al. 2000). In signalling linked to hormones such as ethylene and cytokinins, such cascades are frequently initiated by specific two-componentphosphorelayreceptors(schaller2000,inoue et al. 2001), and it may be that analogous redox-sensitive receptors are important in ROS signal sensing. Available data point to both upstream and downstream interactions between ROS production and the action of hormones such as abscissic acid (ABA) and ethylene (Pei et al. 2000, Desikan et al. 2001, Moeder et al. 2002). Leaf ascorbate content has recently been shown to impact on ABA content and signalling (Pastori et al. 2003). Ascorbate deficiency in the Arabidopsis thaliana vtc1 mutant, associated with slow growth and late flowering, led to the differential expression of 171 genes. Many of these were genes involved in plant defence but transcript changes also indicated that growth and development are constrained in vtc1 by modulation of the balance between ABA and gibberellic acid (GA) signalling (Pastori et al. 2003). Incubating vtc1 leaf discs in ascorbate can reverse key features of the molecular signature of ascorbate deficiency. Feeding ascorbate modified the abundance of 495 transcripts including genes involved in plant defence and photosynthetic metabolism, notably those encoding TRX-regulated chloroplast enzymes (Kiddle et al. 2003). Conclusions and perspectives Plants have created the aerobic world in which we live and use redox reactions and signals, particularly those involving oxygen, in all aspects of their biology. There is no doubt that oxygen is potentially toxic and that all aerobic life forms survive by virtue of efficient antioxidant systems. It is therefore not surprising that the amounts of major antioxidants such as ascorbate are sensed and exert control over plant growth and development. Oxygen is a very useful substrate for energy exchange reactions and has hence been incorporated into both photosynthetic and respiratory energy exchange reactions. The power of its radical (superoxide, hydroxyl radical) and non-radical (hydrogen peroxide) derivatives can be effectively harnessed (because ROS are relatively short-lived) to convey redox information or unleashed, when antioxidant defence is withdrawn, to trigger cell death. These signals are incorporated into the complex redox network that involves the intrinsic electron carriers (plastoquinone, ubiquinone) and electron acceptors (ferredoxin, TRX). It is crucial to note that molecular oxygen is a natural oxidant of plastoquinone, ferredoxin and TRX, and that such auto-oxidation reactions produce superoxide. Moreover, there are numerous interactions between redox and hormonal controls. These situate ROS and antioxidants at the heart of the fine control of plant development and acclimation to external conditions, and suggest that elucidating the details of the signalling networks will be a challenging and fascinating task. References Allen JF (1993) Control of gene expression by redox potential and the requirement for chloroplast and mitochondrial genomes. J Theor Biol 165: Allen JF, Pfannschmidt T (2000) Balancing the two photosystems: photosynthetic electron transfer governs transcription of reaction centre genes in chloroplasts. Phil Trans Roy Lond B 355: Allen JF, Raven JA (1996) Free-radical-induced mutation versus redox regulation: costs and benefits of genes in organelles. J Mol Evol 42: Baier M, Dietz K-J (1999) Alkyl hydroperoxide reductases: the way out of the oxidative breakdown of lipids in chloroplasts. Trends Plant Sci 4: Baier M, Noctor G, Foyer CH, Dietz KJ (2000) Antisense suppression of 2-cys peroxiredoxin in Arabidopsis thaliana specifically enhances the activites and expression of enzymes associated with ascorbate metabolism, but not glutathione metabolism. Plant Physiol 124: Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, Lupianez JA, del Rio LA (1999) Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem 274: Bartling D, Radzio R, Steiner U, Weiler EW (1993) A glutathione- S-transferasewithglutathioneperoxidaseactivityfromArabidopsis thaliana molecular cloning and functional characterization. Eur J Biochem 216: Bartoli C, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123: Beligni MV, Lamattina L (2001) Nitric oxide in plants: the history is just beginning. Plant Cell Environ 24: Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin JM, Knaff DB, Miginiac-Maslow M (2003) The Arabidopsis plastidial thioredoxins: new functions and new insights into specificity. J Biol Chem 278: Conklin PL, Last RL (1995) Differential accumulation of antioxidant mrnas in Arabidopsis thaliana exposed to ozone. Plant Physiol 109: Corpas FJ, Barroso JB, del Rio LA (2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 6: Danon A, Mayfield SP (1994) Light-regulated translation of chloroplast messenger RNAs through redox potential. Science 266: Delauney A, Pflieger D, Barrault MB, Vinh J, Toledano MB (2002) A thiol peroxidase is an H 2 O 2 receptor and redox-transducer in gene activation. Cell 111: 1 11 Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394: Depège N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein Stt7 kinase in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: Desikan R, Mackerness SAH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: Dixon DP, Davis BG, Edwards R (2002) Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J Biol Chem 277: Dutilleul C, Garmier M, Noctor G, Mathieu CD, Chétrit P, Foyer CH, De Paepe R (2003a) Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 15: Physiol. Plant. 119, 2003
9 Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003b) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol 131: Escoubas JM, Lomas M, Laroche J, Falkowski PG (1995) Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA 92: Eshdat Y, Holland D, Faltin Z, Ben-Hayyim G (1997) Plant glutathione peroxidases. Physiol Plant 100: Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146: Gal A, Zer H, Ohad I (1997) Redox-controlled thylakoid protein phosphorylation. Physiol Plant 100: Gardestro m P, Igamberdiev AU, Raghavendra AS (2002) Mitochondrial functions in the light and significance to carbon nitrogen interactions. In: Foyer CH, Noctor G (eds) Photosynthetic Nitrogen Assimilation and Associated Respiratory and Carbon Interactions. Advances in Photosynthesis and Respiration, Vol. 12. Kluwer Academic Publishers, Dordrecht, pp Goyer A, Haslekås C, Miginiac-Maslow M, Klein U, Le Marechal P, Jacquot JP, Decottignies P (2002) Isolation and charcterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. Eur J Biochem 269: Hartmann TN, Fricker MD, Rennenberg H, Meyer AJ (2003) Cellspecific measurement of cytosolic glutathione in poplar leaves. Plant Cell Environ 26: Horling F, Lamkemeyer P, Konig J, Finkemeier I, Kandlbinder A, Baier M, Dietz K-J (2003) Divergent light-, ascorbate- and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiol 131: Hu J, Aguirre M, Peto C, Alonso J, Ecker J, Chory J (2002) A role for peroxisomes in photomorphogenesis and development of Arabidopsis. Science 297: InoueT,HiguchiM,HashimotoY,SekiM,KobayashiM,KatoT, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: Ito H, Iwabuchi M, Ogawa K (2003) The sugar-metabolic enzymes aldolase and triosephospahe isomerase are targets of glutathionylation in Arabidopsis thaliana: detection using biotinylated glutathione. Plant Cell Physiol 44: Jaffrey SR, Snyder SH (1996) PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274: Jime nez A, Hernández JA, del Río L, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114: Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126: Karpinski S, Escobar C, Karprinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91 phox subunit gene encodes a plasma membrane protein with Ca 21 binding motifs. Plant Cell 10: Kettunen R, Pursiheimo S, Rintamaki E, Van Wijk KJ, Aro EM (1997) Transcriptional and translational adjustments of psba gene expression in mature chloroplasts during photoinhibition and subsequent repair of photosystem II. Eur J Biochem 247: Kiddle G, Pastori GM, Bernard S, Pignocchi C, Antoniw J, Verrier PJ, Foyer CH (2003) Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antioxid Redox Signal 5: Klatt P, Lamas S (2000) Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267: Kovtun Y, Chiu W-L, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: Kuge S, Jones N, Nomoto A (1997) Regulation of YAP-1 nuclear localization in response to oxidative stress. EMBO J 16: Laloi C, Rayapuram N, Chartier Y, Grienenberger JM, Bonnard G, Meyer Y (2001) Identification and characterization of a mitochondrial thioredoxin system in plants. Proc Natl Acad Sci USA 95: Link G (1999) Redox regulation of photosynthetic genes. In: Andersson B, Aro E-M (eds) Advances in Photosynthesis. Kluwer Academic Publishers, Dordrecht, pp Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen species production in plant cells. Proc Natl Acad Sci USA 96: Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: Moeder W, Barry CS, Tauriainen AA, Betz C, Tuomainen J, Utriainen M, Grierson D, Sandermann H, Langebartels C, Kangasjärvi J (2002) Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase is required for hydrogen peroxide accumulation and cell death in ozoneexposed tomato. Plant Physiol 130: Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, Foyer CH (2003) Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol, in press Mullineaux PM, Karpinski S, Jimenez A, Cleary SP, Robinson C, Creissen GP (1998) Identification of cdnas encoding plastidtargeted glutathione peroxidase. Plant J 13: Ninnemann H, Maier J (1996) Indications of the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem Photobiol 64: Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in wheat leaves: a predominant role for photorespiration? Ann Bot 89: Noctor G, Veljovic-Jovanovic S, Foyer CH (2000) Peroxide processing in photosynthesis: antixoxidant coupling and redox signalling. Phil Trans Roy Soc B 355: Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: Pfannschmidt T, Nilsson A, Allen JF (1999) Photosynthetic control of chloroplast gene expression. Nature 397: Rasmusson AG, Møller IM (1990) NADP-utilizing enzymes in the matrix of plant mitochondria. Plant Physiol 94: del Río L, Corpas FJ, Sandalio LM, Palma JM, Gómez M, Barroso JB (2002) Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J Exp Bot 53: Rivera-Madrid R, Mestres D, Marinho P, Jacquot JP, Decottignies P, Miginiac-Maslow M, Meyer Y (1995) Evidence for five divergent thioredoxin h sequences in Arabidopsis thaliana. Proc Natl Acad Sci USA 92: Robson CA, Vanlerberghe GC (2002) Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and independent pathways of cell death. Plant Physiol 129: Rochaix JD (1996) Post-transcriptional regulation of chloroplast gene expression in Chlamydomonas reinhardtii. Plant Mol Biol 32: Rodriguez AA, Grunberg K, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf expansion. Plant Physiol 129: Rouhier N, Gelhaye E, Sautiere PE, Brun A, Laurent P, Tagu D, Gerard J, de Fay Meyer Y, Jacquot JP (2001) Isolation and characterization of a new peroxiredoxin from poplar sieve tubes that uses either glutaredoxin or thioredoxin as a proton donor. Plant Physiol 127: Schaller GE (2000) Histidine kinases and the role of twocomponent systems in plants. Adv Bot Res 32: Scheibe R (2003) Malate valves to balance cellular energy supply. Physiol Plant, in press Physiol. Plant. 119,
10 Schürmann P, Jacquot J-P (2000) Plant thioredoxin systems revisited. Annu Rev Plant Physiol Plant Mol Biol 51: Sen Gupta A, Alscher RG, McCune D (1991) Response of photosynthesis and cellular antioxidants to ozone in Populus leaves. Plant Physiol 96: Shenton D, Perrone G, Quinn KA, Dawes IW, Grant CM (2002) Regulation of protein S-thiolation by glutaredoxin 5 in the yeast Saccharomyces cerevisiae. J Biol Chem 277: Smith IK, Kendall AC, Keys AJ, Turner JC, Lea PJ (1984) Increased levels of glutathione in a catalase-deficient mutant of barley (Hordeum vulgare L.). Plant Sci Lett 37: Somanchi A, Mayfield SP (1999) Nuclear-chloroplast signalling. Curr Opin Plant Biol 2: Starke DW, Chock PB, Mieyal JJ (2003) Glutathione thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem 278: Surpin M, Chory J (1997) The co-ordination of nuclear and organellar genome expression in eukaryotic cells. Essays Biochem 32: Tron AE, Bertoncini CW, Chan RL, Gonzalez DH (2002) Redox regulation of plant homeodomain transcription factors. J Biol Chem 277: Vanlerberghe GC, Robson CA, Yip JYH (2002) Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol 129: Vink M, Zer H, Herrmann RG, Andersson B, Ohad I (2000) Regulation of photosystem II core protein phosphorylation at the substrate level: Light induces exposure of the CP43 chlorophyll a protein complex to thylakoid protein kinase(s). Photosynth Res 64: Vlamis-Gardikas A, Holmgren A (2002) Thioredoxin and glutaredoxin isoforms. In: Sies H, Packer L (eds) Methods in Enzymology, Vol. 347 Protein Sensors and Reactive Oxygen Species. A. Selenoproteins and Thioredoxin. Academic Press, San Diego, CA, pp Vranova E, Atichartpongkul S, Villarroel R, Van Montagu M, Inzé D, Van Camp W (2002) Comprehensive analysis of gene expression in Nicotiana tabacum leaves acclimated to oxidative stress. Proc Natl Acad Sci USA 99: Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inze D, Van Camp W (1997) Catalase is a sink for H 2 O 2 and is indispensable for stress defense in C 3 plants. EMBO J 16: Edited by P. Gardestro m 364 Physiol. Plant. 119, 2003
* Is chemical energy potential or kinetic energy? The position of what is storing energy?
 Biology 1406 Exam 2 - Metabolism Chs. 5, 6 and 7 energy - capacity to do work 5.10 kinetic energy - energy of motion : light, electrical, thermal, mechanical potential energy - energy of position or stored
Biology 1406 Exam 2 - Metabolism Chs. 5, 6 and 7 energy - capacity to do work 5.10 kinetic energy - energy of motion : light, electrical, thermal, mechanical potential energy - energy of position or stored
AP Bio Photosynthesis & Respiration
 AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
Electron Transport Generates a Proton Gradient Across the Membrane
 Electron Transport Generates a Proton Gradient Across the Membrane Each of respiratory enzyme complexes couples the energy released by electron transfer across it to an uptake of protons from water in
Electron Transport Generates a Proton Gradient Across the Membrane Each of respiratory enzyme complexes couples the energy released by electron transfer across it to an uptake of protons from water in
Chapter 9 Mitochondrial Structure and Function
 Chapter 9 Mitochondrial Structure and Function 1 2 3 Structure and function Oxidative phosphorylation and ATP Synthesis Peroxisome Overview 2 Mitochondria have characteristic morphologies despite variable
Chapter 9 Mitochondrial Structure and Function 1 2 3 Structure and function Oxidative phosphorylation and ATP Synthesis Peroxisome Overview 2 Mitochondria have characteristic morphologies despite variable
The correct answer is d C. Answer c is incorrect. Reliance on the energy produced by others is a characteristic of heterotrophs.
 1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
Reactive oxygen species in leaves and how to catch these
 Reactive oxygen species in leaves and how to catch these Éva Hideg Molecular Stress- & Photobiology Group, BRC Szeged EPPN Summer School 2013 Outline How to prove that ROS are involved? The central role
Reactive oxygen species in leaves and how to catch these Éva Hideg Molecular Stress- & Photobiology Group, BRC Szeged EPPN Summer School 2013 Outline How to prove that ROS are involved? The central role
Chem 306 Chapter 21 Bioenergetics Lecture Outline III
 Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Visualizing Cell Processes
 Visualizing Cell Processes A Series of Five Programs produced by BioMEDIA ASSOCIATES Content Guide for Program 3 Photosynthesis and Cellular Respiration Copyright 2001, BioMEDIA ASSOCIATES www.ebiomedia.com
Visualizing Cell Processes A Series of Five Programs produced by BioMEDIA ASSOCIATES Content Guide for Program 3 Photosynthesis and Cellular Respiration Copyright 2001, BioMEDIA ASSOCIATES www.ebiomedia.com
Reactive Oxygen Species and Reactive Nitrogen Species in Peroxisomes. Production, Scavenging, and Role in Cell Signaling 1
 Update on Reactive Oxygen and Nitrogen Species in Peroxisomes Reactive Oxygen Species and Reactive Nitrogen Species in Peroxisomes. Production, Scavenging, and Role in Cell Signaling 1 Luis A. del Río*,
Update on Reactive Oxygen and Nitrogen Species in Peroxisomes Reactive Oxygen Species and Reactive Nitrogen Species in Peroxisomes. Production, Scavenging, and Role in Cell Signaling 1 Luis A. del Río*,
Summary of Metabolism. Mechanism of Enzyme Action
 Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
21.8 The Citric Acid Cycle
 21.8 The Citric Acid Cycle The carbon atoms from the first two stages of catabolism are carried into the third stage as acetyl groups bonded to coenzyme A. Like the phosphoryl groups in ATP molecules,
21.8 The Citric Acid Cycle The carbon atoms from the first two stages of catabolism are carried into the third stage as acetyl groups bonded to coenzyme A. Like the phosphoryl groups in ATP molecules,
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman
 SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
Metabolism Poster Questions
 Metabolism Poster Questions Answer the following questions concerning respiration. 1. Consider the mitochondrial electron transport chain. a. How many hydrogen ions can be pumped for every NADH? b. How
Metabolism Poster Questions Answer the following questions concerning respiration. 1. Consider the mitochondrial electron transport chain. a. How many hydrogen ions can be pumped for every NADH? b. How
REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction
 Annu. Rev. Plant Biol. 2004. 55:373 99 doi: 10.1146/annurev.arplant.55.031903.141701 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on January 12,
Annu. Rev. Plant Biol. 2004. 55:373 99 doi: 10.1146/annurev.arplant.55.031903.141701 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on January 12,
The Physiology of Hyperbaric Oxygen Therapy. Free Radicals and Reactive Oxygen Species. I. Introduction Definition, Source, function and Purpose
 The Physiology of Hyperbaric Oxygen Therapy Free Radicals and Reactive Oxygen Species I. Introduction Definition, Source, function and Purpose A. Definition of free radicals and reactive oxygen species
The Physiology of Hyperbaric Oxygen Therapy Free Radicals and Reactive Oxygen Species I. Introduction Definition, Source, function and Purpose A. Definition of free radicals and reactive oxygen species
Photosynthesis (CO 2 + H 2 O C 6 H 12 O 6 + O 2 )
 The vital role of A This is the energy-rich compound that is the source of energy for all living things. It is a nucleotide, comprising a 5C sugar (ribose); an organic base (adenosine); and 3 phosphate
The vital role of A This is the energy-rich compound that is the source of energy for all living things. It is a nucleotide, comprising a 5C sugar (ribose); an organic base (adenosine); and 3 phosphate
Chapter 19a Oxidative Phosphorylation and Photophosphorylation. Multiple Choice Questions
 Chapter 19a Oxidative Phosphorylation and Photophosphorylation Multiple Choice Questions 1. Electron-transfer reactions in mitochondria Page: 707 Difficulty: 1 Ans: E Almost all of the oxygen (O 2 ) one
Chapter 19a Oxidative Phosphorylation and Photophosphorylation Multiple Choice Questions 1. Electron-transfer reactions in mitochondria Page: 707 Difficulty: 1 Ans: E Almost all of the oxygen (O 2 ) one
Student name ID # 2. (4 pts) What is the terminal electron acceptor in respiration? In photosynthesis? O2, NADP+
 1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
Evolution of Metabolism. Introduction. Introduction. Introduction. How Cells Harvest Energy. Chapter 7 & 8
 How ells Harvest Energy hapter 7 & 8 Evolution of Metabolism A hypothetical timeline for the evolution of metabolism - all in prokaryotic cells!: 1. ability to store chemical energy in ATP 2. evolution
How ells Harvest Energy hapter 7 & 8 Evolution of Metabolism A hypothetical timeline for the evolution of metabolism - all in prokaryotic cells!: 1. ability to store chemical energy in ATP 2. evolution
Copyright 2000-2003 Mark Brandt, Ph.D. 54
 Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline
 AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
Chapter 2: Cell Structure and Function pg. 70-107
 UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
Like The Guy From Krypton Photosynthesis: Energy from Sunlight What Is Photosynthesis?
 Like The Guy From Krypton Photosynthesis: Energy from Sunlight What Is Photosynthesis? Photosynthesis: synthesis from light The broad outline: Plants take in CO 2 and release water and O 2 Light is required
Like The Guy From Krypton Photosynthesis: Energy from Sunlight What Is Photosynthesis? Photosynthesis: synthesis from light The broad outline: Plants take in CO 2 and release water and O 2 Light is required
Photosystems I and II
 Photosystems I and II March 17, 2003 Bryant Miles Within the thylakoid membranes of the chloroplast, are two photosystems. Photosystem I optimally absorbs photons of a wavelength of 700 nm. Photosystem
Photosystems I and II March 17, 2003 Bryant Miles Within the thylakoid membranes of the chloroplast, are two photosystems. Photosystem I optimally absorbs photons of a wavelength of 700 nm. Photosystem
Photosynthesis takes place in three stages:
 Photosynthesis takes place in three stages: Light-dependent reactions Light-independent reactions The Calvin cycle 1. Capturing energy from sunlight 2. Using energy to make ATP and NADPH 3. Using ATP and
Photosynthesis takes place in three stages: Light-dependent reactions Light-independent reactions The Calvin cycle 1. Capturing energy from sunlight 2. Using energy to make ATP and NADPH 3. Using ATP and
Figure 5. Energy of activation with and without an enzyme.
 Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
APh/BE161: Physical Biology of the Cell Winter 2009 Recap on Photosynthesis Rob Phillips
 APh/BE161: Physical Biology of the Cell Winter 2009 Recap on Photosynthesis Rob Phillips Big picture: why are we doing this? A) photosynthesis will explain shortly, b) more generally, interaction of light
APh/BE161: Physical Biology of the Cell Winter 2009 Recap on Photosynthesis Rob Phillips Big picture: why are we doing this? A) photosynthesis will explain shortly, b) more generally, interaction of light
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
1. f. Students know usable energy is captured from sunlight by chloroplasts and is stored through the synthesis of sugar from carbon dioxide.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
PHOTOSYNTHESIS AND CELLULAR RESPIRATION
 reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
Part I: Evolution, Complexity and Regulation of Photosynthetic Structures
 Contents Editorial Contents Preface Color Plates v vii xvii CP-1 Part I: Evolution, Complexity and Regulation of Photosynthetic Structures 1 Thylakoid Biogenesis and Dynamics: The Result of a Complex Phylogenetic
Contents Editorial Contents Preface Color Plates v vii xvii CP-1 Part I: Evolution, Complexity and Regulation of Photosynthetic Structures 1 Thylakoid Biogenesis and Dynamics: The Result of a Complex Phylogenetic
Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochemistry Journal. August 1, 2007 405, pp.
 Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies 1 Biochemistry Journal August 1, 2007 405, pp. 559 568 Joseph Friedman, Sarah Kraus, Yirmi Hauptman, Yoni Schiff
Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies 1 Biochemistry Journal August 1, 2007 405, pp. 559 568 Joseph Friedman, Sarah Kraus, Yirmi Hauptman, Yoni Schiff
Chapter 7 Active Reading Guide Cellular Respiration and Fermentation
 Name: AP Biology Mr. Croft Chapter 7 Active Reading Guide Cellular Respiration and Fermentation Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second
Name: AP Biology Mr. Croft Chapter 7 Active Reading Guide Cellular Respiration and Fermentation Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second
-Loss of energy -Loss of hydrogen from carbons. -Gain of energy -Gain of hydrogen to carbons
 Cellular Respiration- Equation C6H12O6 + 6O2 6CO2 +6H20 and energy -The energy is released from the chemical bonds in the complex organic molecules -The catabolic process of releasing energy from food
Cellular Respiration- Equation C6H12O6 + 6O2 6CO2 +6H20 and energy -The energy is released from the chemical bonds in the complex organic molecules -The catabolic process of releasing energy from food
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
008 Chapter 8. Student:
 008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
> C 6 H 12 O 6 + 6O 2
 Photosynthesis- is the process that converts light energy into chemical energy. This chemical energy is usually a carbohydrate. Only photoautrotrops can do photosynthesis. Heterotrophs must obtain their
Photosynthesis- is the process that converts light energy into chemical energy. This chemical energy is usually a carbohydrate. Only photoautrotrops can do photosynthesis. Heterotrophs must obtain their
Chapter 8. Summary and Perspectives
 Chapter 8 Summary and Perspectives 131 Chapter 8 Summary Overexpression of the multidrug resistance protein MRP1 confer multidrug resistance (MDR) to cancer cells. The contents of this thesis describe
Chapter 8 Summary and Perspectives 131 Chapter 8 Summary Overexpression of the multidrug resistance protein MRP1 confer multidrug resistance (MDR) to cancer cells. The contents of this thesis describe
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Cellular Respiration and Fermentation
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
2. PHOTOSYNTHESIS. The general equation describing photosynthesis is light + 6 H 2 O + 6 CO 2 C 6 H 12 O 6 + 6 O 2
 2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
Syllabus Chemistry 431B Biochemistry Winter 2013. Course Prerequisite: Grade of C- or better in Biochemistry I (Chem 431A)
 Syllabus Chemistry 431B Biochemistry Winter 2013 Instructor: Jamil Momand, Ph.D. Class location and time: Salazar Hall, C-265 MWF 11:40-12:30 Office Hours: La Kretz Hall, Room 270 M 10-11, T 10-11 Email:
Syllabus Chemistry 431B Biochemistry Winter 2013 Instructor: Jamil Momand, Ph.D. Class location and time: Salazar Hall, C-265 MWF 11:40-12:30 Office Hours: La Kretz Hall, Room 270 M 10-11, T 10-11 Email:
Photosynthesis and (Aerobic) Respiration. Photosynthesis
 Photosynthesis and (Aerobic) Respiration These two processes have many things in common. 1. occur in organelles that seem to be descended from bacteria (endosymbiont theory): chloroplasts and mitochondria
Photosynthesis and (Aerobic) Respiration These two processes have many things in common. 1. occur in organelles that seem to be descended from bacteria (endosymbiont theory): chloroplasts and mitochondria
Todays Outline. Metabolism. Why do cells need energy? How do cells acquire energy? Metabolism. Concepts & Processes. The cells capacity to:
 and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
Photosynthesis and Cellular Respiration. Stored Energy
 Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
Lecture 7 Outline (Ch. 10)
 Lecture 7 Outline (Ch. 10) I. Photosynthesis overview A. Purpose B. Location II. The light vs. the dark reaction III. Chloroplasts pigments A. Light absorption B. Types IV. Light reactions A. Photosystems
Lecture 7 Outline (Ch. 10) I. Photosynthesis overview A. Purpose B. Location II. The light vs. the dark reaction III. Chloroplasts pigments A. Light absorption B. Types IV. Light reactions A. Photosystems
BCOR 011 Exam 2, 2004
 BCOR 011 Exam 2, 2004 Name: Section: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. According to the first law of thermodynamics, A. the universe
BCOR 011 Exam 2, 2004 Name: Section: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. According to the first law of thermodynamics, A. the universe
Chapter 10: Photosynthesis
 Name Period Chapter 10: Photosynthesis This chapter is as challenging as the one you just finished on cellular respiration. However, conceptually it will be a little easier because the concepts learned
Name Period Chapter 10: Photosynthesis This chapter is as challenging as the one you just finished on cellular respiration. However, conceptually it will be a little easier because the concepts learned
Biological energy cycle BIOMASS
 Photosynthesis Global carbon cycle Biological energy cycle BIOMASS Photosynthetic Reaction equation light CO 2 + 2H 2 O (CH 2 O) + O 2 + H 2 O Beware! This is not what s going on! The crucial equation
Photosynthesis Global carbon cycle Biological energy cycle BIOMASS Photosynthetic Reaction equation light CO 2 + 2H 2 O (CH 2 O) + O 2 + H 2 O Beware! This is not what s going on! The crucial equation
Eukaryotes have organelles
 Energy-transducing Eukaryotes have organelles membrane systems An organelle is a discrete membrane bound cellular structure specialized functions. An organelle is to the cell what an organ is to the body
Energy-transducing Eukaryotes have organelles membrane systems An organelle is a discrete membrane bound cellular structure specialized functions. An organelle is to the cell what an organ is to the body
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT
 CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
(e) i. 22. (a) ii (b) iv (c) v (d) iii
 Chapter 5 Review, pages 246 251 Knowledge 1. a 2. b 3. b 4. d 5. d 6. b 7. b 8. b 9. b 10. a 11. True 12. False. Chlorophyll a, when excited, becomes oxidized as it passes an electron to a primary electron
Chapter 5 Review, pages 246 251 Knowledge 1. a 2. b 3. b 4. d 5. d 6. b 7. b 8. b 9. b 10. a 11. True 12. False. Chlorophyll a, when excited, becomes oxidized as it passes an electron to a primary electron
Electron transport chain, oxidative phosphorylation & mitochondrial transport systems. Joško Ivica
 Electron transport chain, oxidative phosphorylation & mitochondrial transport systems Joško Ivica Electron transport chain & oxidative phosphorylation collects e - & -H Oxidation of foodstuffs oxidizes
Electron transport chain, oxidative phosphorylation & mitochondrial transport systems Joško Ivica Electron transport chain & oxidative phosphorylation collects e - & -H Oxidation of foodstuffs oxidizes
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Biology. Slide 1of 51. End Show. Copyright Pearson Prentice Hall
 Biology 1of 51 8-3 The Reactions of Photosynthesis 2of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3of 51 Inside
Biology 1of 51 8-3 The Reactions of Photosynthesis 2of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3of 51 Inside
Lecture 8. Protein Trafficking/Targeting. Protein targeting is necessary for proteins that are destined to work outside the cytoplasm.
 Protein Trafficking/Targeting (8.1) Lecture 8 Protein Trafficking/Targeting Protein targeting is necessary for proteins that are destined to work outside the cytoplasm. Protein targeting is more complex
Protein Trafficking/Targeting (8.1) Lecture 8 Protein Trafficking/Targeting Protein targeting is necessary for proteins that are destined to work outside the cytoplasm. Protein targeting is more complex
Photosynthesis. Photosynthesis: Converting light energy into chemical energy. Photoautotrophs capture sunlight and convert it to chemical energy
 Photosynthesis: Converting light energy into chemical energy Photosynthesis 6 + 12H 2 O + light energy Summary Formula: C 6 H 12 O 6 + 6O 2 + 6H 2 O 6 + 6H 2 O C 6 H 12 O 6 + 6O 2 Photosythesis provides
Photosynthesis: Converting light energy into chemical energy Photosynthesis 6 + 12H 2 O + light energy Summary Formula: C 6 H 12 O 6 + 6O 2 + 6H 2 O 6 + 6H 2 O C 6 H 12 O 6 + 6O 2 Photosythesis provides
Anabolic and Catabolic Reactions are Linked by ATP in Living Organisms
 Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
8-3 The Reactions of Photosynthesis Slide 1 of 51
 8-3 The of Photosynthesis 1 of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 2 of 51 Inside a Chloroplast Chloroplasts
8-3 The of Photosynthesis 1 of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 2 of 51 Inside a Chloroplast Chloroplasts
Organelles and Their Functions
 Organelles and Their Functions The study of cell organelles and their functions is a fascinating part of biology. The current article provides a brief description of the structure of organelles and their
Organelles and Their Functions The study of cell organelles and their functions is a fascinating part of biology. The current article provides a brief description of the structure of organelles and their
Regulation of the Citric Acid Cycle
 Regulation of the itric Acid ycle I. hanges in Free Energy February 17, 2003 Bryant Miles kj/mol 40 20 0 20 40 60 80 Reaction DGo' DG TA Free Energy hanges 1 2 3 4 5 6 7 8 9 1.) itrate Synthase 2.) Aconitase
Regulation of the itric Acid ycle I. hanges in Free Energy February 17, 2003 Bryant Miles kj/mol 40 20 0 20 40 60 80 Reaction DGo' DG TA Free Energy hanges 1 2 3 4 5 6 7 8 9 1.) itrate Synthase 2.) Aconitase
Photosynthesis. Name. Light reactions Calvin cycle Oxidation Reduction Electronegativity Photosystem Electron carrier NADP+ Concentration gradient
 Vocabulary Terms Photoautotroph Chemoautotroph Electromagnetic spectrum Wavelength Chloroplast Thylakoid Stroma Chlorophyll Absorption spectrum Photosynthesis Light reactions Calvin cycle Oxidation Reduction
Vocabulary Terms Photoautotroph Chemoautotroph Electromagnetic spectrum Wavelength Chloroplast Thylakoid Stroma Chlorophyll Absorption spectrum Photosynthesis Light reactions Calvin cycle Oxidation Reduction
Molecular Biology of the Cell
 Alberts Johnson Lewis Raff Roberts Walter Molecular Biology of the Cell Fifth Edition Chapter 14 1. The Genetic System of Mitochondria and Plastids 2. The Evolution of Electron-Transport Chains Copyright
Alberts Johnson Lewis Raff Roberts Walter Molecular Biology of the Cell Fifth Edition Chapter 14 1. The Genetic System of Mitochondria and Plastids 2. The Evolution of Electron-Transport Chains Copyright
Review Questions Photosynthesis
 Review Questions Photosynthesis 1. Describe a metabolic pathway. In a factory, labor is divided into small individual jobs. A carmaker, for example, will have one worker install the front windshield, another
Review Questions Photosynthesis 1. Describe a metabolic pathway. In a factory, labor is divided into small individual jobs. A carmaker, for example, will have one worker install the front windshield, another
Carbon Hydrogen Oxygen Nitrogen
 Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
1. Explain the difference between fermentation and cellular respiration.
 : Harvesting Chemical Energy Name Period Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second to look at the big picture. Photosynthesis and cellular
: Harvesting Chemical Energy Name Period Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second to look at the big picture. Photosynthesis and cellular
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES. Bio 171 Week 6
 RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 171 Week 6 Procedure Label test tubes well, including group name 1) Add solutions listed to small test tubes 2) For
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 171 Week 6 Procedure Label test tubes well, including group name 1) Add solutions listed to small test tubes 2) For
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Biology I. Chapter 8/9
 Biology I Chapter 8/9 NOTEBOOK #1 Interest Grabber Suppose you earned extra money by having a part-time job. At first, you might be tempted to spend all of the money, but then you decide to open a bank
Biology I Chapter 8/9 NOTEBOOK #1 Interest Grabber Suppose you earned extra money by having a part-time job. At first, you might be tempted to spend all of the money, but then you decide to open a bank
Chapter 14- RESPIRATION IN PLANTS
 Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
Chapter 9 Review Worksheet Cellular Respiration
 1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
Green pigment that absorbs solar energy and is important in photosynthesis
 PHOTOSYNTHESIS REVIEW SHEET FOR TEST Part A: Match the terms below with the correct description Chlorophyll Chloroplast Electromagnetic spectrum Electron transport chain Grana Light-dependant reactions
PHOTOSYNTHESIS REVIEW SHEET FOR TEST Part A: Match the terms below with the correct description Chlorophyll Chloroplast Electromagnetic spectrum Electron transport chain Grana Light-dependant reactions
Plant Growth & Development. Growth Stages. Differences in the Developmental Mechanisms of Plants and Animals. Development
 Plant Growth & Development Plant body is unable to move. To survive and grow, plants must be able to alter its growth, development and physiology. Plants are able to produce complex, yet variable forms
Plant Growth & Development Plant body is unable to move. To survive and grow, plants must be able to alter its growth, development and physiology. Plants are able to produce complex, yet variable forms
REVIEW UNIT 3: METABOLISM (RESPIRATION & PHOTOSYNTHESIS) SAMPLE QUESTIONS
 Period Date REVIEW UNIT 3: METABOLISM (RESPIRATION & PHOTOSYNTHESIS) SAMPLE QUESTIONS A. Sample Multiple Choice Questions Complete the multiple choice questions to review this unit. 1. The carbon that
Period Date REVIEW UNIT 3: METABOLISM (RESPIRATION & PHOTOSYNTHESIS) SAMPLE QUESTIONS A. Sample Multiple Choice Questions Complete the multiple choice questions to review this unit. 1. The carbon that
Electron Transport System. May 16, 2014 Hagop Atamian hatamian@ucdavis.edu
 Electron Transport System May 16, 2014 Hagop Atamian hatamian@ucdavis.edu What did We learn so far? Glucose is converted to pyruvate in glycolysis. The process generates two ATPs. Pyruvate is taken into
Electron Transport System May 16, 2014 Hagop Atamian hatamian@ucdavis.edu What did We learn so far? Glucose is converted to pyruvate in glycolysis. The process generates two ATPs. Pyruvate is taken into
BIOMARKERS AND TOXICITY MECHANISMS 06 Mechanisms Metabolism & Detoxification. Luděk Bláha, PřF MU, RECETOX www.recetox.cz
 BIOMARKERS AND TOXICITY MECHANISMS 06 Mechanisms Metabolism & Detoxification Luděk Bláha, PřF MU, RECETOX www.recetox.cz Metabolism and detoxification Chemicals enter body... mostly via food Pass directly
BIOMARKERS AND TOXICITY MECHANISMS 06 Mechanisms Metabolism & Detoxification Luděk Bláha, PřF MU, RECETOX www.recetox.cz Metabolism and detoxification Chemicals enter body... mostly via food Pass directly
Name Date Class. energy phosphate adenine charged ATP chemical bonds work ribose
 Energy in a Cell Reinforcement and Study Guide Section.1 The Need for Energy In your textbook, read about cell energy. Use each of the terms below just once to complete the passage. energy phosphate adenine
Energy in a Cell Reinforcement and Study Guide Section.1 The Need for Energy In your textbook, read about cell energy. Use each of the terms below just once to complete the passage. energy phosphate adenine
Name Date Period. 2. When a molecule of double-stranded DNA undergoes replication, it results in
 DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
1. Enzymes. Biochemical Reactions. Chapter 5: Microbial Metabolism. 1. Enzymes. 2. ATP Production. 3. Autotrophic Processes
 Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Photosynthesis 6CO 2 + 6H 2 O C 6 H 12 O 6 + 6O 2. An anabolic, endergonic, carbon dioxide (CO 2
 PHOTOSYNTHESIS Photosynthesis An anabolic, endergonic, carbon dioxide (CO 2 ) requiring process that uses light energy (photons) and water (H 2 O) to produce organic macromolecules (glucose). photons SUN
PHOTOSYNTHESIS Photosynthesis An anabolic, endergonic, carbon dioxide (CO 2 ) requiring process that uses light energy (photons) and water (H 2 O) to produce organic macromolecules (glucose). photons SUN
The world of non-coding RNA. Espen Enerly
 The world of non-coding RNA Espen Enerly ncrna in general Different groups Small RNAs Outline mirnas and sirnas Speculations Common for all ncrna Per def.: never translated Not spurious transcripts Always/often
The world of non-coding RNA Espen Enerly ncrna in general Different groups Small RNAs Outline mirnas and sirnas Speculations Common for all ncrna Per def.: never translated Not spurious transcripts Always/often
Oxidative Phosphorylation
 Oxidative Phosphorylation NADH from Glycolysis must be transported into the mitochondrion to be oxidized by the respiratory electron transport chain. Only the electrons from NADH are transported, these
Oxidative Phosphorylation NADH from Glycolysis must be transported into the mitochondrion to be oxidized by the respiratory electron transport chain. Only the electrons from NADH are transported, these
Photosynthesis (Life from Light)
 Photosynthesis Photosynthesis (Life from Light) Energy needs of life All life needs a constant input of energy o Heterotrophs (consumers) Animals, fungi, most bacteria Get their energy from other organisms
Photosynthesis Photosynthesis (Life from Light) Energy needs of life All life needs a constant input of energy o Heterotrophs (consumers) Animals, fungi, most bacteria Get their energy from other organisms
ATP & Photosynthesis Honors Biology
 ATP & Photosynthesis Honors Biology ATP All cells need for life. Some things we use energy for are: Moving Thinking Sleeping Breathing Growing Reproducing ENERGY Labeled Sketch: The principal chemical
ATP & Photosynthesis Honors Biology ATP All cells need for life. Some things we use energy for are: Moving Thinking Sleeping Breathing Growing Reproducing ENERGY Labeled Sketch: The principal chemical
CHAPTER 6: PHOTOSYNTHESIS CAPTURING & CONVERTING ENERGY
 CHAPTER 6: PHOTOSYNTHESIS CAPTURING & CONVERTING ENERGY 2 PROCESSES OF PHOTOSYNTHESIS Photosynthesis is actually 2 processes: light reactions - convert solar energy (sunlight) to chemical energy (ATP &
CHAPTER 6: PHOTOSYNTHESIS CAPTURING & CONVERTING ENERGY 2 PROCESSES OF PHOTOSYNTHESIS Photosynthesis is actually 2 processes: light reactions - convert solar energy (sunlight) to chemical energy (ATP &
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
NO CALCULATORS OR CELL PHONES ALLOWED
 Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Photosynthesis Part I: Overview & The Light-Dependent Reactions
 Photosynthesis Part I: Overview & The Light-Dependent Reactions Photosynthesis: The BIG Picture Photosynthesis is the process by which PHOTOAUTOTROPHS convert the energy in SUNLIGHT into the energy stored
Photosynthesis Part I: Overview & The Light-Dependent Reactions Photosynthesis: The BIG Picture Photosynthesis is the process by which PHOTOAUTOTROPHS convert the energy in SUNLIGHT into the energy stored
A. Incorrect! No, while this statement is correct, it is not the best answer to the question.
 Biochemistry - Problem Drill 18: Photosynthesis No. 1 of 10 1. What is photosynthesis? Select the best answer. (A) Photosynthesis happens in the chloroplasts. (B) Light absorption by chlorophyll induces
Biochemistry - Problem Drill 18: Photosynthesis No. 1 of 10 1. What is photosynthesis? Select the best answer. (A) Photosynthesis happens in the chloroplasts. (B) Light absorption by chlorophyll induces
Microbial Metabolism. Biochemical diversity
 Microbial Metabolism Biochemical diversity Metabolism Define Requirements Energy Enzymes Rate Limiting step Reaction time Types Anabolic Endergonic Dehydration Catabolic Exergonic Hydrolytic Metabolism
Microbial Metabolism Biochemical diversity Metabolism Define Requirements Energy Enzymes Rate Limiting step Reaction time Types Anabolic Endergonic Dehydration Catabolic Exergonic Hydrolytic Metabolism
Photosynthesis and Sucrose Production
 Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps):
 Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps): 1) How many ATP molecules are produced for each glucose molecule used in fermentation?
Biology 20 Cellular Respiration Review NG Know the process of Cellular Respiration (use this picture if it helps): 1) How many ATP molecules are produced for each glucose molecule used in fermentation?
Which regions of the electromagnetic spectrum do plants use to drive photosynthesis?
 Which regions of the electromagnetic spectrum do plants use to drive photosynthesis? Green Light: The Forgotten Region of the Spectrum. In the past, plant physiologists used green light as a safe light
Which regions of the electromagnetic spectrum do plants use to drive photosynthesis? Green Light: The Forgotten Region of the Spectrum. In the past, plant physiologists used green light as a safe light
Cell Structure and Function. Eukaryotic Cell: Neuron
 Cell Structure and Function Eukaryotic Cell: Neuron Cell Structure and Function Eukaryotic Cells: Blood Cells Cell Structure and Function Prokaryotic Cells: Bacteria Cell Structure and Function All living
Cell Structure and Function Eukaryotic Cell: Neuron Cell Structure and Function Eukaryotic Cells: Blood Cells Cell Structure and Function Prokaryotic Cells: Bacteria Cell Structure and Function All living
The Need for a PARP in vivo Pharmacodynamic Assay
 The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
Biology Slide 1 of 51
 Biology 1 of 51 8-3 The Reactions of Photosynthesis 2 of 51 Inside a Chloroplast 1. In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3 of 51 Inside a Chloroplast
Biology 1 of 51 8-3 The Reactions of Photosynthesis 2 of 51 Inside a Chloroplast 1. In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3 of 51 Inside a Chloroplast
Citric Acid Cycle. Cycle Overview. Metabolic Sources of Acetyl-Coenzyme A. Enzymes of the Citric Acid Cycle. Regulation of the Citric Acid Cycle
 Citric Acid Cycle Cycle Overview Metabolic Sources of Acetyl-Coenzyme A Enzymes of the Citric Acid Cycle Regulation of the Citric Acid Cycle The Amphibolic Nature of the Citric Acid Cycle Cycle Overview
Citric Acid Cycle Cycle Overview Metabolic Sources of Acetyl-Coenzyme A Enzymes of the Citric Acid Cycle Regulation of the Citric Acid Cycle The Amphibolic Nature of the Citric Acid Cycle Cycle Overview
