1 Development of the Atomic Theory
|
|
|
- Cori Wilkins
- 9 years ago
- Views:
Transcription
1 CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has the atomic theory changed over time? How Does New Information Change Scientific Ideas? Have you ever watched a mystery movie and thought you knew who the criminal was? You may have changed your mind once you saw a new fact or clue. This is what happens in science. Sometimes an idea or a model is changed when new information is collected. One example of this is the atomic model. Our ideas about atoms have changed over time as we gather new information. What Is an Atom? Imagine cutting something in half, then cutting again and again. Could you keep cutting forever? Around 440 BCE, a Greek philosopher named Democritus studied this question. He thought that you would eventually reach a piece of matter that could not be cut. He called this particle an atom. It was a long time before there was scientific evidence that Democritus was on the right track. We now know that all matter is made of tiny particles called atoms. An atom is the smallest particle into which an element can be divided and still keep its properties. The figure shows images of aluminum atoms taken with a scanning tunneling electron microscope. Early scientists had ideas about atoms and models of atoms even though they did not have pictures of them. STUDY TIP Connect Concepts In your notebook, create a Concept Map about the scientists who studied atoms and what they learned. 1. Identify What is an atom? Aluminum cans are made of atoms. Aluminum atoms can be seen in the image from a scanning tunneling electron microscope (STM). Notice the regular, repeating pattern of the aluminum atoms. 2. Describe How are the atoms of aluminum arranged? Interactive Textbook 199 Introduction to Atoms
2 3. Discuss When would scientists need to change a theory? What Was the First Scientific Theory of Atoms? The first scientific theory about atoms was published by John Dalton in Unlike Democritus, Dalton based his ideas on experiments. His theory helped explain observations that he and other scientists had made about elements and compounds. Dalton s theory stated that: All substances are made of atoms. Atoms are small particles that cannot be created, destroyed, or divided. All atoms of one element are exactly alike, and atoms of different elements are different. Atoms can join with other atoms to make new substances. Many scientists agreed that Dalton s theory explained much of what they saw. However, scientists later found new information that did not fit Dalton s theory. The atomic theory was changed to more correctly describe the atom. How Were Electrons Discovered? In 1897, J. J. Thomson, a British scientist, discovered that atoms are not the smallest particles. There are even smaller particles inside the atom. Thomson made this discovery when he was experimenting with invisible beams called cathode rays. Cathode rays were made by connecting a special glass tube to a source of electricity. To find out more about cathode rays, Thomson placed two metal plates inside the tube. One plate had a positive electrical charge and the other had a negative charge. Thomson discovered that cathode rays are attracted to the plate with the positive charge. 4. Identify What is the electrical charge on the plate that causes the beam to bend toward that plate? Interactive Textbook 200 Introduction to Atoms
3 THOMSON S PLUM PUDDING MODEL Thomson concluded that cathode rays must be made of tiny particles that come from atoms. Since the particles are attracted to a positively charged metal plate, the particles must have a negative charge. Remember that opposite charges attract each other. These negatively charged particles are called electrons. Thomson s experiment showed that atoms contain electrons, but it did not show where electrons are located within an atom. Thomson suggested that electrons might be scattered throughout the atom. This new model of the atom was called the plum pudding model. It was named after a popular dessert at the time. Today, we would probably call this a chocolate chip ice cream model of the atom. How Did Rutherford Study the Atom? In 1909, one of Thomson s students wanted to test the theory that electrons are scattered throughout the atom. Ernest Rutherford decided to shoot a beam of tiny, positively charged particles at a thin sheet of gold foil. Rutherford guessed that atoms are soft blobs of matter with electrons and positively charged particles scattered throughout. He thought that most of the particles would pass right through the gold atoms. Particles that hit other particles would stop or bounce to the side. When Rutherford performed the experiment, he found that most of the positively charged particles did pass through the gold foil. Some were deflected sideways, just as he expected. What surprised him was that some particles bounced straight back. 5. Identify What type of electrical charge does an electron have? 6. Identify What did Rutherford shoot at the gold foil in his experiment? Rutherford s Gold-Foil Experiment a Tiny particles with a positive charge came from an element such as radium. e Strangely, a few particles bounced straight back. b A beam of particles was aimed at a thin sheet of gold foil. d Some of the particles bounced off to the side. c Most of the particles passed straight through the gold foil. 7. Compare What happened to most of the particles that were shot at the gold foil? Interactive Textbook 201 Introduction to Atoms
4 8. Describe What is the nuclear model of the atom? What Was Rutherford s Atomic Model? The plum pudding model did not explain what Rutherford saw. He reasoned that there was only one way that the positively charged particles could bounce straight back. That was if they hit a very dense part of the atom that had a positive charge. Remember that identical charges repel. He concluded that most of the matter in the atom must be in a very small part of the atom. Based on the results of his experiment, Rutherford proposed a new model of the atom, called the nuclear model. In his model, the center of the atom is a tiny, dense, positively charged area called the nucleus. The electrons move outside the nucleus in mostly empty space. Nucleus Electron In Rutherford s model, the atom contains a nucleus. Electrons move around the nucleus. Math Focus 9. Make Comparisons If an atom had a nucleus 1 ft in diameter, what would be the diameter of the atom, in miles? Show your work. Round to the nearest mile. (1 mi 5,280 ft) 10. Describe According to Bohr s theory, how do electrons move around the nucleus? Rutherford concluded that the nucleus must be very small but very dense in order to deflect the fast-moving particles. He used his observations to calculate the diameter of an atom. It was about 100,000 times greater than the diameter of its nucleus. The atom is mostly empty space. What Did Bohr Discover about the Atom? In 1913, Niels Bohr, a Danish scientist, studied the way that atoms react to light. He made a slight change to Rutherford s model, based on his observations. Bohr proposed that electrons move around the nucleus in definite paths, or orbits, called energy levels. In Bohr s model, electrons could not exist between these levels. Think of the levels as rungs on a ladder. You can stand on the rungs of a ladder, but not between the rungs. However, the electrons could jump from one level to another as they gained or lost energy. Once again, the atomic theory was changed to account for new data. Interactive Textbook 202 Introduction to Atoms
5 Bohr Model of the Atom In the Bohr model, electrons move around the nucleus like planets around the sun. 11. Identify Label the nucleus and the electrons in the figure. What Is the Modern Atomic Theory? Atomic theory has changed over the past 100 years. Scientists such as Erwin Schrödinger from Austria and Werner Heisenberg from Germany have done important work. They have made observations that show that the Bohr model is not quite right. Scientists still think that electrons are moving constantly around the nucleus. However, they now know that electrons do not orbit the nucleus like planets orbit the sun. In fact, no one can predict the exact path an electron will follow as it moves around the nucleus. However, scientists can predict where electrons are likely to be found. In the modern atomic model, the locations of electrons are described with electron clouds. Electron clouds are regions where electrons are most likely to be found. The figure below shows this model. 12. Define What are electron clouds? In the current model of the atom, electrons move around the nucleus in electron clouds. No one can say exactly where a particular electron is at any particular time. Interactive Textbook 203 Introduction to Atoms
6 Section 1 Review SECTION VOCABULARY atom the smallest unit of an element that maintains the properties of that element electron a subatomic particle that has a negative charge electron cloud a region around the nucleus of an atom where electrons are likely to be found nucleus in physical science, an atom s central region, which is made up of protons and neutrons 1. Describe How does the diameter of an atom compare with the diameter of the nucleus? 2. Recall Finish the table below to summarize some of the advances in the development of atomic theory and those responsible for them. Scientist Idea that was added to the atomic theory Each element is made of a different type of atom. Thomson Rutherford Electrons are found in specific energy levels. Modern scientists 3. Apply How did the discovery of electrons show that there are also positively charged parts of the atom? 4. Evaluate What would cause scientists to change or replace the modern atomic theory? 5. Explain Describe Thomson s plum pudding model of the atom. Interactive Textbook 204 Introduction to Atoms
Development of the Atomic Theory
 Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
5.1 Evolution of the Atomic Model
 5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
The Models of the Atom
 The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS
 ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
Atomic Calculations. 2.1 Composition of the Atom. number of protons + number of neutrons = mass number
 2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
Atoms and Elements [6th grade]
![Atoms and Elements [6th grade] Atoms and Elements [6th grade]](/thumbs/40/21183873.jpg) Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
NOTES ON The Structure of the Atom
 NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
For convenience, we may consider an atom in two parts: the nucleus and the electrons.
 Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atomic Theory Part 1
 Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
The Structure of the Atom
 The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
Unit 1 Practice Test. Matching
 Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
NYC K-8 Science Scope and Sequence: PS Standard 4 - Properties of Matter: 3.1a, 3.3a-d MST Standard 1 Inquiry Skills MST Standard 4 Process Skills
 Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Ernest Rutherford Atomic Model 1911. Plum Pudding Model J.J. Thomson 1897
 1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Cathode Rays Figure 1: Figure 2:
 Cathode Rays The first ideas about electrons came from experiments with cathode-ray tubes. A forerunner of neon signs, fluorescent lights, and TV picture tubes, a typical cathode-ray tube is a partially
Cathode Rays The first ideas about electrons came from experiments with cathode-ray tubes. A forerunner of neon signs, fluorescent lights, and TV picture tubes, a typical cathode-ray tube is a partially
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
An Atom Apart by Leslie Cargile
 Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Theory: History of the Atom
 Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
EARLY ATOMIC THEORY AND STRUCTURE
 CHAPTER 5 EARLY ATOMIC THEORY AND STRUCTURE SOLUTIONS TO REVIEW QUESTIONS 1. Elements are composed of indivisable particles called atoms. Atoms of the same element have the same properties; atoms of different
CHAPTER 5 EARLY ATOMIC THEORY AND STRUCTURE SOLUTIONS TO REVIEW QUESTIONS 1. Elements are composed of indivisable particles called atoms. Atoms of the same element have the same properties; atoms of different
CHAPTER 4: ATOMS AND ELEMENTS
 CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Tro's "Introductory Chemistry", Chapter 4
 1 Introductory Chemistry, 3 rd Edition Nivaldo Tro Atoms and Elements Opening figure showing a shore scene with molecules of O 2, N 2, triethyl amine (CH 3 CH 2 ) 3 N, and rocks made of silicates containing
1 Introductory Chemistry, 3 rd Edition Nivaldo Tro Atoms and Elements Opening figure showing a shore scene with molecules of O 2, N 2, triethyl amine (CH 3 CH 2 ) 3 N, and rocks made of silicates containing
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004. Name (print) SSN
 CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
Atomic structure. Resources and methods for learning about these subjects (list a few here, in preparation for your research):
 Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Answers to Review Questions for Atomic Theory Quiz #1
 Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
Credits Copyright, Utah State Office of Education, 2015.
 Credits Copyright, Utah State Office of Education, 2015. Unless otherwise noted, the contents of this book are licensed under the Creative Commons Attribution NonCommercial ShareAlike license. Detailed
Credits Copyright, Utah State Office of Education, 2015. Unless otherwise noted, the contents of this book are licensed under the Creative Commons Attribution NonCommercial ShareAlike license. Detailed
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
Properties of Atoms and the Periodic Table
 Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Bohr s Model of the Atom
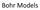 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Atomic Structure Ron Robertson
 Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Introduction to Nuclear Physics
 Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Name Date Class ELECTRONS IN ATOMS. Standard Curriculum Core content Extension topics
 13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
Nuclear Structure. particle relative charge relative mass proton +1 1 atomic mass unit neutron 0 1 atomic mass unit electron -1 negligible mass
 Protons, neutrons and electrons Nuclear Structure particle relative charge relative mass proton 1 1 atomic mass unit neutron 0 1 atomic mass unit electron -1 negligible mass Protons and neutrons make up
Protons, neutrons and electrons Nuclear Structure particle relative charge relative mass proton 1 1 atomic mass unit neutron 0 1 atomic mass unit electron -1 negligible mass Protons and neutrons make up
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Introduction to Chemistry Exam 2 Practice Problems 1 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1.Atoms consist principally of what three
Introduction to Chemistry Exam 2 Practice Problems 1 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1.Atoms consist principally of what three
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Radioactivity & Particles
 Radioactivity & Particles Introduction... 2 Atomic structure... 2 How are these particles arranged?... 2 Atomic notation... 4 Isotopes... 4 What is radioactivity?... 5 Types of Radiation: alpha, beta and
Radioactivity & Particles Introduction... 2 Atomic structure... 2 How are these particles arranged?... 2 Atomic notation... 4 Isotopes... 4 What is radioactivity?... 5 Types of Radiation: alpha, beta and
Session 2 The Particle Nature of Matter: Solids, Liquids, and Gases
 Session 2 The Particle Nature of Matter: Solids, Liquids, and Gases What explanation might account for the differences between the states of matter, as well as explain its different properties? Session
Session 2 The Particle Nature of Matter: Solids, Liquids, and Gases What explanation might account for the differences between the states of matter, as well as explain its different properties? Session
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
The microscope is an important tool.
 KEY CONCEPT Microscopes allow us to see inside the cell. BEFORE, you learned Some organisms are unicellular and some are multicellular A microscope is necessary to study most cells The cell theory describes
KEY CONCEPT Microscopes allow us to see inside the cell. BEFORE, you learned Some organisms are unicellular and some are multicellular A microscope is necessary to study most cells The cell theory describes
GETTING CURRENT: Generating Electricity Using a Magnet
 GETTING CURRENT: Generating Electricity Using a Magnet PLANNING OVERVIEW SUBJECT AREAS: Physical Science, Math, Language Arts TIMING: Preparation: 30 minutes Activity: 1-2 45-minute class periods Summary
GETTING CURRENT: Generating Electricity Using a Magnet PLANNING OVERVIEW SUBJECT AREAS: Physical Science, Math, Language Arts TIMING: Preparation: 30 minutes Activity: 1-2 45-minute class periods Summary
Practice TEST 2. Explain your reasoning
 Practice TEST 2 1. Imagine taking an elevator ride from the1 st floor to the 10 th floor of a building. While moving between the 1 st and 2 nd floors the elevator speeds up, but then moves at a constant
Practice TEST 2 1. Imagine taking an elevator ride from the1 st floor to the 10 th floor of a building. While moving between the 1 st and 2 nd floors the elevator speeds up, but then moves at a constant
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Sarasota County Schools
 UNIT 6 Atoms: Nuclear Interactions WHAT discoveries led to a modern understanding of the composition of atoms? SECTION A The Nature of Atoms (page 480) WHY does human exposure to some types of radiation
UNIT 6 Atoms: Nuclear Interactions WHAT discoveries led to a modern understanding of the composition of atoms? SECTION A The Nature of Atoms (page 480) WHY does human exposure to some types of radiation
Electricity. Electricity: The Mysterious Force. 32 Intermediate Energy Infobook CARBON ATOM SEVERAL COMMON ELEMENTS
 Electricity: The Mysterious Force What exactly is the mysterious force we call electricity? It is simply moving electrons. And what exactly are electrons? They are tiny particles found in atoms. Everything
Electricity: The Mysterious Force What exactly is the mysterious force we call electricity? It is simply moving electrons. And what exactly are electrons? They are tiny particles found in atoms. Everything
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electricity. Atoms. Protons, Neutrons, and Electrons. Electricity is Moving Electrons. Atom
 Electricity is a mysterious force. We can t see it like we see the sun. We can t hold it like we hold coal. We know when it is working, but it is hard to know exactly what it is. Before we can understand
Electricity is a mysterious force. We can t see it like we see the sun. We can t hold it like we hold coal. We know when it is working, but it is hard to know exactly what it is. Before we can understand
Review for Atomic Theory Quiz #1
 Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Lesson 43: Alpha, Beta, & Gamma Decay
 Lesson 43: Alpha, Beta, & Gamma Decay The late 18s and early 19s were a period of intense research into the new nuclear realm of physics. In 1896 Henri Becquerel found that a sample of uranium he was doing
Lesson 43: Alpha, Beta, & Gamma Decay The late 18s and early 19s were a period of intense research into the new nuclear realm of physics. In 1896 Henri Becquerel found that a sample of uranium he was doing
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
Session 1 What Is Matter? Properties and Classification of Matter
 Session 1 What Is Matter? Properties and Classification of Matter What is matter? This question at first seems simple matter is all around us. Yet how do we define it? What does a block of cheese have
Session 1 What Is Matter? Properties and Classification of Matter What is matter? This question at first seems simple matter is all around us. Yet how do we define it? What does a block of cheese have
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total
 Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
WAVES AND ELECTROMAGNETIC RADIATION
 WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
KINDERGARTEN PHYSICS
 KINDERGARTEN PHYSICS 3 WEEKS LESSON PLANS AND ACTIVITIES APPLIED SCIENCE OVERVIEW OF KINDERGARTEN SCIENCE AND MATH WEEK 1. PRE: Describing and comparing nests, birds, and eggs. LAB: Describing different
KINDERGARTEN PHYSICS 3 WEEKS LESSON PLANS AND ACTIVITIES APPLIED SCIENCE OVERVIEW OF KINDERGARTEN SCIENCE AND MATH WEEK 1. PRE: Describing and comparing nests, birds, and eggs. LAB: Describing different
E/M Experiment: Electrons in a Magnetic Field.
 E/M Experiment: Electrons in a Magnetic Field. PRE-LAB You will be doing this experiment before we cover the relevant material in class. But there are only two fundamental concepts that you need to understand.
E/M Experiment: Electrons in a Magnetic Field. PRE-LAB You will be doing this experiment before we cover the relevant material in class. But there are only two fundamental concepts that you need to understand.
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Light as a Wave. The Nature of Light. EM Radiation Spectrum. EM Radiation Spectrum. Electromagnetic Radiation
 The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
Regents Review Atom & PT Part 2 Worksheet Mr. Beauchamp
 Regents Review Atom & PT Part 2 Worksheet Mr. Beauchamp Base your answers to questions 1 through 3 on the information below. The accepted values for the atomic mass and percent natural abundance of each
Regents Review Atom & PT Part 2 Worksheet Mr. Beauchamp Base your answers to questions 1 through 3 on the information below. The accepted values for the atomic mass and percent natural abundance of each
Chapter 2: The Atom. Structure of the atom
 18 Chapter 2: The Atom An atom is the smallest particle of an element, having the same chemical properties as the bulk element. The first accurate theory explaining the nature of matter was Dalton s Atomic
18 Chapter 2: The Atom An atom is the smallest particle of an element, having the same chemical properties as the bulk element. The first accurate theory explaining the nature of matter was Dalton s Atomic
Structure and Properties of Atoms
 PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
Static Electricity Page 1. Static Electricity. Introduction: Structure of Atoms 2 Sample Curriculum, Materials Needed
 Static Electricity Page 1 Static Electricity Introduction: Structure of Atoms 2 Sample Curriculum, Materials Needed Experiment #1: Creating Static Charges 3 Experiment #2: Like Charges Repel and Unlike
Static Electricity Page 1 Static Electricity Introduction: Structure of Atoms 2 Sample Curriculum, Materials Needed Experiment #1: Creating Static Charges 3 Experiment #2: Like Charges Repel and Unlike
Lesson 26: Reflection & Mirror Diagrams
 Lesson 26: Reflection & Mirror Diagrams The Law of Reflection There is nothing really mysterious about reflection, but some people try to make it more difficult than it really is. All EMR will reflect
Lesson 26: Reflection & Mirror Diagrams The Law of Reflection There is nothing really mysterious about reflection, but some people try to make it more difficult than it really is. All EMR will reflect
4 HOW OUR SOLAR SYSTEM FORMED 750L
 4 HOW OUR SOLAR SYSTEM FORMED 750L HOW OUR SOLAR SYSTEM FORMED A CLOSE LOOK AT THE PLANETS ORBITING OUR SUN By Cynthia Stokes Brown, adapted by Newsela Planets come from the clouds of gas and dust that
4 HOW OUR SOLAR SYSTEM FORMED 750L HOW OUR SOLAR SYSTEM FORMED A CLOSE LOOK AT THE PLANETS ORBITING OUR SUN By Cynthia Stokes Brown, adapted by Newsela Planets come from the clouds of gas and dust that
Chapter 4, Lesson 2: The Periodic Table
 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Aristotelian Physics. Aristotle's physics agrees with most people's common sense, but modern scientists discard it. So what went wrong?
 Aristotelian Physics Aristotle's physics agrees with most people's common sense, but modern scientists discard it. So what went wrong? Here's what Aristotle said: Aristotelian Physics Aristotle s classification
Aristotelian Physics Aristotle's physics agrees with most people's common sense, but modern scientists discard it. So what went wrong? Here's what Aristotle said: Aristotelian Physics Aristotle s classification
ELECTRICAL FUNDAMENTALS
 General Electricity is a form of energy called electrical energy. It is sometimes called an "unseen" force because the energy itself cannot be seen, heard, touched, or smelled. However, the effects of
General Electricity is a form of energy called electrical energy. It is sometimes called an "unseen" force because the energy itself cannot be seen, heard, touched, or smelled. However, the effects of
Basic Nuclear Concepts
 Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
Chapter 2 Atoms and Molecules
 Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
