Retinal Input Directs the Recruitment of Inhibitory Interneurons into Thalamic Visual Circuits
|
|
|
- Rosalyn Johnson
- 8 years ago
- Views:
Transcription
1 Article Retinal Input Directs the Recruitment of Inhibitory Interneurons into Thalamic Visual Circuits Bruno Golding, 1 Gabrielle Pouchelon, 1 Camilla Bellone, 1 Sahana Murthy, 1 Ariel A. Di Nardo, 4 Subashika Govindan, 1 Masahuro Ogawa, 5 Tomomi Shimogori, 5 Christian Lüscher, 1,2 Alexandre Dayer, 1,3 and Denis Jabaudon 1,2, * 1 Department of Basic Neurosciences, University of Geneva, 1 Rue Michel Servet, 1211 Geneva, Switzerland 2 Department of Neurology 3 Department of Psychiatry Geneva University Hospital, 4 Rue Gabrielle-Perret-Gentil, 125 Geneva, Switzerland 4 Center for Interdisciplinary Research in Biology, UMR CNRS 7241/INSERM U15, Collège de France, 11 Place Marcelin Berthelot, 755 Paris, France 5 Riken Brain Science Institute, 2-1 Hirosawa Wako City, Saitama , Japan *Correspondence: denis.jabaudon@unige.ch SUMMARY Inhibitory interneurons (INs) critically control the excitability and plasticity of neuronal networks, but whether activity can direct INs into specific circuits during development is unknown. Here, we report that in the dorsal lateral geniculate nucleus (), which relays retinal input to the cortex, circuit activity is required for the migration, molecular differentiation, and functional integration of INs. We first characterize the prenatal origin and molecular identity of INs, revealing their recruitment from an Otx2 + neuronal pool located in the adjacent ventral LGN. Using timelapse and electrophysiological recordings, together with genetic and pharmacological perturbation of retinal waves, we show that retinal activity directs the navigation and circuit incorporation of INs during the first postnatal week, thereby regulating the inhibition of thalamocortical circuits. These findings identify an input-dependent mechanism regulating IN migration and circuit inhibition, which may account for the progressive recruitment of INs into expanding excitatory circuits during evolution. INTRODUCTION Two broad functional classes of cells, excitatory and inhibitory neurons, form the neuronal circuits of the CNS. Circuits with high levels of excitatory activity require a compensatory inhibition to allow activity-dependent plasticity while avoiding excitotoxicity (Spitzer, 26; Akerman and Cline, 27; Miraucourt et al., 212). Inhibitory interneurons (INs) control the dynamics and temporal limits of this plasticity by regulating the spatial and temporal spread of incoming excitatory signals (Isaacson and Scanziani, 211), as exemplified during the development of ocular dominance columns in the visual cortex (Hensch, 25; Hooks and Chen, 27; Sugiyama et al., 28; Runyan et al., 21; Southwell et al., 21). While recent reports have revealed that the migration and differentiation of specific subtypes of neocortical INs relies partly on cell-intrinsic activity, and that their migration is guided by target-derived factors (López-Bendito et al., 28; Bortone and Polleux, 29; De Marco García et al., 211; Lodato et al., 211; Miyoshi and Fishell, 211; Wang et al., 211a), whether the level or pattern of activity within target neuronal circuits acts to recruit migrating INs during development is unknown. Here, we investigate this possibility by studying the development and integration of INs into visual circuits of the dorsolateral geniculate nucleus () of the thalamus, which has a well-characterized input-output connectivity, is comprised of a single type of excitatory projection neuron, and has local INs that form feedforward inhibitory connections. We show that while the initial migration of INs into the does not rely on circuit activity, their targeted navigation and synaptic integration into circuits is controlled by retinal input. Thalamocortical (TC) neurons in the receive monosynaptic input from retinal ganglion cells and send their axonal output to the visual cortex (López-Bendito and Molnár, 23). In contrast to other rodent thalamic relay nuclei, which consist exclusively of excitatory TC neurons, the contains 2% of local INs, a proportion similar to that found in the neocortex (Arcelli et al., 1997). TC neurons and local INs both receive input from the retina, and feedforward inhibition of TC neurons by INs controls the transmission of visual signals to the neocortex (Wang et al., 211b). This inhibition enhances selectivity for stimulus features in space and time and is regulated by cholinergic input from the brainstem, thereby providing a critical precortical filtering of retinal input (Sillito and Kemp, 1983; Berardi and Morrone, 1984; Norton and Godwin, 1992; Hu et al., 2; Blitz and Regehr, 25; Wang et al., 211b, 211c). Consistent with a prominent role in visual processing, the proportion of INs to TC neurons increases dramatically in primates, in which hand-eye coordination and stereoscopic and color vision are behaviorally central (Arcelli et al., 1997). Neuron 81, , March 5, 214 ª214 Elsevier Inc. 157
2 Although INs were first described by Cajal over a century ago and their functional importance has since been established, their molecular identity and ontology remain largely unknown (Cajal, 22; Letinic and Rakic, 21; Jones, 22; Hayes et al., 23; Ortino et al., 23; Bickford et al., 21). Furthermore, while retinal activity plays a critical role in the retinotopy and eye-specific segregation of retinogeniculate axons (Penn et al., 1998; Hooks and Chen, 27; Huberman et al., 28), whether retinal input plays a role in the assembly of local inhibitory circuits in the is unknown. In this study, we show that INs are born from a restricted proliferative niche in the ventral wall of the third ventricle and reside in the, an ancestral retinal target, until birth. Only postnatally are they progressively recruited into circuits. Using microsurgical, genetic, pharmacological, and electrophysiological analyses in vitro and in vivo, we show that retinal activity controls the postnatal migration and functional synaptic integration of INs, thereby acting to regulate TC neuron excitability during a critical period of postnatal development. Together, these findings indicate that retinal activity directs the incorporation of inhibitory neurons into the, establishing the balance of excitation and inhibition in thalamic visual circuits. RESULTS A Molecularly Defined Population of GABAergic INs Migrates into the during the First Postnatal Week In order to determine how the inhibitory circuits of the visual thalamus develop, we used GAD67 GFP transgenic mice (Tamamaki et al., 23) to track the distribution of GABAergic INs in the during development. At birth, while extrathalamic retinal targets such as the ventral lateral geniculate nucleus and intergeniculate leaflet (here collectively referred to as ) already contain large numbers of GFP + INs, the only contains very few INs at postnatal day (P) 1 (Figures 1A and 1B). During the first postnatal week, however, GFP + INs progressively migrate into the (Figures 1B 1D), initially in the most superficial tier of the, just below the optic tract, where they appear to be migrating out of the (Figure 1B, arrowheads), and later into deeper tiers of the nucleus (Figures 1C 1G). This postnatal IN migration is strictly limited to the, since GFP + neurons are not found in the adjacent ventral posterior medial () nucleus (Figures 1A 1E). Therefore, the migration of INs into the is a progressive process, proceeding from superficial to deeper tiers of this nucleus during the first postnatal week. In order to investigate the developmental origins of INs, we next sought to characterize their molecular identity. For this purpose, we used a gene microarray approach in samples collected at P, P3, and P1 from the (which is progressively populated by INs) and the (which lacks INs) (Figure 1H). We performed a cluster analysis of the developmental time course of Gad67 expression to identify putative IN-specific genes. An initial set of 71 genes with GAD67-like expression profiles were identified, which we narrowed down to eight top IN candidate genes after in silico screening and confirmation of expression by in situ hybridization and immunocytochemistry (Figures 1I 1R and S1A S1F available online). Genes specifically expressed by INs, but not by TC neurons, included the transcription factor Otx2, which critically controls IN development and plasticity in the visual cortex (Sugiyama et al., 28), and its binding partner Meis2 (Mrg1) (Agoston and Schulte, 29)(Figures 1I 1L ), as well as a set of three synaptic proteins: complexin 3 (Cplx3), a presynaptic protein that binds SNARE complexes and regulates vesicular release (Reim et al., 29) (Figures 1M 1N ), and two subunits of postsynaptic nicotinic acetylcholine receptors, a6 and b3 (Figures 1O 1R ). Remarkably, in contrast to the and neocortex, where inhibitory INs belong to distinct molecular subtypes (Gelman and Marín, 21; Jeong et al., 211), INs were molecularly homogenous, i.e., this set of genes was expressed by essentially all INs in this nucleus at P2 (Figures 1I 1R and S1G S1N ; Table 1). This molecular homogeneity is consistent with the largely uniform morphology and physiological properties of these neurons (Williams et al., 1996) and suggests that INs belong to a single neuronal lineage. INs Migrate from the and Are Born from a Prethalamic Progenitor Microdomain Expression of the synaptic proteins Cplx3, a6 nachr, b3 nachr, and Meis2 is specific to INs since only a few scattered INs express these genes in the (Figures S1C S1E). In contrast, Otx2 is expressed by both INs and a subpopulation of INs (Figures 1I and S1A), suggesting common origins. As a first step to test this hypothesis, we followed the migration of Otx2 + INs during embryonic development. Examining GAD67 GFP mice, we found that between embryonic day (E) 12.5 and E18.5, Otx2 + INs form a migratory stream between a small region of the ventral wall of the third ventricle (V3) and the ventral part of the (Figures 2A 2B ; data not shown). This stream is specific to Otx2 + INs, since INs, which express Tal1 and Sox14 (Vue et al., 27; Kataoka and Shimogori, 28; Scholpp and Lumsden, 21; Suzuki-Hirano et al., 211; Delogu et al., 212), migrate into the through a distinct, more dorsal pathway (Figures 2C 2D and S2A S2B ). This suggests that Otx2 + INs and Otx2,Tal1 + INs belong to distinct lineages. Supporting this distinction, Otx2 Tal1 + INs remain in the postnatally and are not found in the (Figures S2C and S2D ), while Otx2 + INs are found in both the and (Figures 1I and S1A). To further examine whether and Otx2 + INs have common origins, we fate mapped INs by electroporating an RFP reporter plasmid in utero at E12.5 into the ventral wall of V3, from where the Otx2 + IN stream originates (Figure S2E). When examined at P7, the RFP + progeny of these cells consisted exclusively of and INs (Figures S2F and S2G ), indicating that INs and subsets of INs are born together in the ventral V3 neuroepithelium. Furthermore, TC neurons did not express RFP (Figures S2G S2G ), nor did INs express the panthalamic projection neuron marker SOX2, indicating that the ontogeny of INs is distinct from that of TC neurons (Figures S2H and S2I ). Finally, we used a genetic fate-mapping strategy to precisely identify the site of birth of INs and to confirm the shared lineage relationship of Otx2 + and INs. Using an in silico screen of genes expressed in the V3 wall during 158 Neuron 81, , March 5, 214 ª214 Elsevier Inc.
3 Neuron Figure 1. A Molecularly Defined Population of GABAergic INs Migrates into the during the First Postnatal Week (A) Left: schematic coronal section showing location of the photomicrographs taken in (B) (E). Middle: retinal ganglionic cell axons form the optic tract and project to the and as revealed by anterograde labeling from the retina. Right: coronal section in GAD67GFP mice showing INs in the prethalamus ( and ZI). Within the thalamus, INs are exclusively found in the. (B E) INs are initially found in the superficial part of the, close to the border with the (B) and then progressively distribute within the during the first postnatal week (C E, arrowheads). (C) P3, (D) P7, and (E) P21. (F) Quantification of postnatal migration of INs into the. (G) Developmental distribution of INs within the. Fisher s exact test, n = 96 P1 and n = 44 P7 INs. Number in italic indicates p value. Inset shows schematic delineation of bin 1 (superficial) and 2 (deep), also represented by the dashed red lines in (C) (E). (H R ) Molecular identity of INs. (H) Left: microarray experimental principle: INs (green dots) are present in the at P1 but not P and remain absent from the. Right: microarray fluorescence intensity of the transcripts identified by cluster analysis of probes whose expression covaries with Gad67. (I R ) In situ hybridization in GAD67GFP mice confirms IN specificity of the genes identified in (H). Values in (J ), (L ), (N ), (P ), and (R ) indicate proportion of GFP+ INs expressing each transcript. Abbreviations: CTB, cholera toxin B; hip, hippocampus; thal, thalamus;, dorsolateral geniculate nucleus;, ventrolateral geniculate nucleus;, ventroposterior medial nucleus; ZI, zona incerta. Scale bars represent 25 mm in (A); 1 mm in (B) (E), (I), (K), (M), (O), and (Q); and 5 mm in (J) (J ), (L) (L ), (N) (N ), (P) (P ), and (R) (R ). Values are represented as mean ± SEM. See also Figure S1. Neuron 81, , March 5, 214 ª214 Elsevier Inc. 159
4 Table 1. Molecular Identity of Thalamic and Prethalamic Interneurons Gene RN/ZI 5HT3a Cre Calret ++ Chrna6 (+) +++ Chrnb3 (+) +++ Cplx3 ++ (+) +++ GAD Meis Nkx Nkx5.1 NPY ++ (+) a Otx Parv ++ Rln Sst ++ VIP , >95% INs express gene; ++, 5%; +, 25%; (+), <1%. RN, reticular nucleus; ZI, zona incerta. a Present in the intergeniculate leaflet (IGL) development, we found that Fgf8 + neurons colocalize with Otx2 + INs along the ventral migratory stream during embryogenesis but that, in contrast to Otx2, Fgf8 expression in progenitor is restricted to a small prethalamic third ventricular domain (Kataoka and Shimogori, 28) (Figures 2E and 2E ). Since Fgf8 itself is not expressed in the (data not shown), we fate mapped the neurons born from this niche using an Fgf8 Cre transgenic mouse (Toyoda et al., 21) and found that these progenitors give rise to specific subsets of INs in the, as well as within the (Figures 2F and S2J). Together, these findings indicate that INs are born from a restricted prethalamic progenitor domain and migrate along a dedicated pathway to first reach the, between E12.5 and E15.5. Only postnatally does a specific subset of Otx2 + INs migrate into and distribute within the (Figure 2G). Otx2 Function Is Required Postnatally to Establish Inhibitory Circuits Otx2 + INs initially reach the at E15.5 and reside in the ventral part of the nucleus before migrating toward the border by approximately E18.5 (Figures 3A and 3B), after which a subset of these neurons exit the to migrate into the at P P1 (Figure 3C). We next investigated the molecular mechanisms that allow a subpopulation of Otx2 + INs to migrate out of the into the. Our microarray analyses revealed that Meis2, a binding partner and activator of OTX2 function (Figure 3D; Agoston and Schulte, 29), is specifically expressed by Otx2 + INs in the but not the (Figures 3E and S1B). We therefore hypothesized that OTX2 protein activity might distinguish and Otx2 + INs and examined whether other OTX2-interacting proteins might be differentially expressed in these two populations. Supporting this possibility, we found that TLE4, which binds to and inactivates OTX2 (Figure 3D; Heimbucher et al., 27; Agoston and Schulte, 29), is expressed exclusively by INs (Figure 3F). Therefore, Meis2, an OTX2 activator, and TLE4, an OTX2 inhibitor, are mutually exclusively expressed in and INs. Therefore, OTX2 might be inactive in the and become active in the. To directly test whether OTX2 activation is required for IN function, we thus used an inducible self-knockout strategy (Otx2 CreERT2 / Otx2 flox ) to postnatally ablate Otx2 expression specifically in postmitotic Otx2 + neurons at P (Figure S3; Fossat et al., 26). Such perinatal removal of OTX2 leads to a specific loss of INs, while INs were seemingly unaffected (Figures 3G, 3H, and S3). Therefore, OTX2 function is required for INs to migrate into or survive within the and reveals a critical role for this transcription factor in the assembly of local inhibitory circuits within the visual thalamus. Retinal Input Directs the Migration and Functional Synaptic Integration of INs during a Critical Period of Development Since retinal input is critical for the assembly of retinogeniculate and thalamocortical circuits (Huberman et al., 28) and paracrine neurotransmitter release regulates the migration of neurons in several brain regions (Manent et al., 25, 26; Spitzer, 26), we hypothesized that the postnatal migration and circuit integration of INs might be regulated in an input-dependent manner. As a first step to assess a role for neuronal activity in IN migration, we used in vitro time-lapse imaging of acute coronal brain sections from P1 GAD67 GFP mice. We examined whether IN migration was affected by blocking spontaneous circuit activity using bath application of the voltage-gated sodium channel blocker tetrodotoxin (TTX), which dramatically reduced the migration speed of INs (Figure 4A). Remarkably, this effect was specific to this IN population, since the migration of nearby hippocampal INs remained unaffected (Figure 4A; p =.2 for versus TTX in INs; p =.94 for hippocampal INs; paired t test). These results indicate that neuronal activity specifically affects the migration of INs after birth. Since TTX could affect IN migration both by cell-autonomous and non-cell-autonomous mechanisms, we next specifically manipulated input in vivo. Glutamatergic retinogeniculate axons are a primary source of excitatory input to the, and INs first migrate into this nucleus in close proximity to these axons (Figure 1B), at a time when retinal activity is progressively increasing (Huberman et al., 28). This suggested to us that retinogeniculate input may play a role in the incorporation of INs into circuits. To test this possibility, we performed a bilateral optic nerve section (ONS) at birth in GAD67 GFP mice and found that lack of retinal input profoundly affected the migration of INs at P2. While GAD67 GFP INs normally migrate into the superficial tiers of the and then distribute homogeneously within this nucleus (Figures 1B 1D), in the absence of retinal input they remained predominantly located in superficial tiers of the, where they displayed a seemingly differentiated morphology, and failed to distribute in deeper tiers of the (Figures 4B 4G). Interestingly, IN migration along the lateromedial axis of the was unaffected (Figures 4E and 4F), suggesting that different processes regulate the tangential 16 Neuron 81, , March 5, 214 ª214 Elsevier Inc.
5 Figure 2. INs Migrate from the and Are Born from a Prethalamic Progenitor Microdomain (A B ) At E15.5, Otx2 + INs (A; red arrowheads in B and B ) form a ventral stream of cells in the prethalamus, bridging a microdomain on the V3 wall with the ventral. (C D ) Tal1 + Otx2 INs (C; red arrowheads in D and D ) take a distinct, more dorsal path in the thalamus to reach the dorsal. (B ) and (D ) are overlays of (B) and (B ) and (D) and (D ), respectively, with the ISH pseudocolored in red. Dashed red line indicates borders of the thalamus proper. (E F) Genetic fate mapping in Fgf8 Cre /bgal STOPflox transgenic mice specifically labels neurons in the ventral stream (E; red arrowheads in E ) at E14.5 and INs in the and at P7 (white arrowheads in F). (G) Schematic summary of the findings. Scale bars represent 3 mm in (A), (C), and (E); 1 mm in (B) (B ), (D) (D ), and (E ); and 5 mm in (F). Abbreviations: Cx, cortex; Th, thalamus; V3, third ventricle. See also Figure S2. and radial migration of INs. This abnormal laminar distribution reflected abnormal migration rather than selective cell death, since the total number of INs and expression of the apoptotic marker cleaved caspase-3 were unaffected by ONS (Figure 4H 4I ; : 25 ± 14 GFP + INs/section; ONS 262 ± 1 GFP + INs/section; p =.5, n = 1,167 INs, n = 1,219 ONS INs, Student s t test). Confirming these findings, IN migration was also impaired in Ey1 anophthalmic mice, which have a specific defect in eye formation during embryogenesis (Tucker et al., 21; Charbonneau et al., 212) (Figures 4J 4N). Together, these findings indicate that retinal input is required for the laminar positioning of INs within the. We next examined whether retinal input was also required at a later stage of IN differentiation, i.e., during synaptogenesis. For this purpose, we took advantage of the IN-specific pre- and postsynaptic markers Cplx3 and a6 nachr identified above (Figures 1I 1R ). Eliminating retinal input at birth by ONS or prenatally in Ey1 mice induced a dramatic repression of the transcripts for the presynaptic protein Cplx3 and the vesicular transporter for GABA, VGAT. The a6 subunit of the postsynaptic nicotinic cholinergic receptor (a6 nachr) was also repressed, leading to specific loss of cholinergic responses in INs but not TC neurons (Figures 5A 5F, quantified in 5M 5O, and S4A S4H). In contrast, Otx2 expression was only minimally changed, and expression of other IN-specific genes such as GABA, Reelin, and the b3 subunit of postsynaptic nicotinic cholinergic receptor was unaffected (Figures S4I S4K and data not shown), indicating that retinal input controls specific genetic differentiation programs in INs. To distinguish whether this abnormal molecular synaptic differentiation was the consequence of IN mismigration or was independently regulated by retinal input, we performed an ONS at P8, after completion of neuronal migration (Figure 1D). Supporting an independent regulation of synaptic differentiation by retinal input, we found that the synaptic markers Cplx3 and a6 nachr were strongly downregulated after P8 ONS, despite the normal distribution of INs within the (Figures 5G 5I). Interestingly, Otx2 expression was unaffected, consistent with a primary role of this transcription factor in migration rather than synaptogenesis. In contrast, inhibition of neuronal firing by overexpression of the voltage-gated potassium channel Kir2.1 (De Marco García et al., 211) into INs repressed Cplx3 expression without detectably affecting migration (Figures S4L and S4M ; : 82/82 Cplx3 + INs [Figure 1N ], Kir2.1: 3/23 Cplx3 + INs, n = 2 mice, p <.1, Fisher s exact test). This further suggests that migration and synaptogenesis are differentially regulated and supports a requirement for cell-intrinsic neuronal activity in IN synapse formation, as previously reported for select neocortical IN subtypes (De Marco García et al., 211). Finally, ONS at P12 did not affect molecular synaptic differentiation (Figures 5J 5L), thereby defining the end of a critical period for input-dependent synaptic differentiation of INs. Impaired Migration and Synaptic Differentiation of INs Leads to Disinhibition of TC Neurons We next examined whether abnormal migration (P P8) and/or synaptic differentiation (P8 P12) of INs following ONS results in increased excitability of postsynaptic TC neurons (Figures 6A and 6B). We determined excitability by counting the number of action potentials in P2 TC neurons in response to brief current pulses in slices from mice that underwent ONS at P1, P8, or P12 (Figures 6B and 6C). TC neuron excitability was dramatically increased following P1 and P8 ONS but normal after P12 ONS (Figure 6C), suggesting that TC excitability increases when IN migration or synaptic differentiation is impaired. Neuron 81, , March 5, 214 ª214 Elsevier Inc. 161
6 E15.5 A GAD67 A D active MEIS2 repressed TLE4 OTX2 OTX2 E Meis2 Otx2 P2 E15.5 E18. 5 Otx2 B F TLE4 Otx2 no tamoxifen Gad67 To demonstrate the involvement of INs in controlling TC neuron excitability, we next pharmacologically activated IN presynaptic terminals with the metabotropic glutamate receptor group I (mglur-i) agonist. In agreement with previous studies, DHPG evoked inhibitory responses in TC neurons (Figure 6D; Cox and Sherman, 2; Errington et al., 211; Govindaiah and Cox, 26). In contrast, these responses were not evoked following P1 or P8 ONS but were still present following P12 ONS, identifying a critical period for TC neuron inhibition by INs (Figure 6D). Direct stimulation of IN-TC synapses using optogenetic activation of channelrhodopsin-2 (ChR2)- expressing INs (see Experimental Procedures) further confirmed reduced inhibitory input onto TC neurons following P1 ONS (Figure 6E). Finally, we directly examined whether lack of INs leads to hyperexcitability of TC neurons. For this purpose, we took advantage of the Otx2 CreERT2 /Otx2 flox transgenic mice described above, in which INs are specifically eliminated by P tamoxifen injection (see Figures 3G and 3H). In contrast to noninjected controls, which displayed normal input-output responses, TC neuron excitability was increased in tamoxifeninjected transgenic mice (Figure 6F), directly demonstrating a causal link between decreased inhibition and TC neuron hyperexcitability. Taken together, these data indicate that retinal input is required for the functional integration of INs, thus controlling the excitability of TC circuits. Retinal Waves Direct the Migration and Synaptic Integration of INs Although both microsurgical and genetic ablation of the eye reveal that retinal input is necessary for IN migration and CreERT2 flox Otx2 G Otx2 C H P1 + P tamoxifen Gad67 Figure 3. INs Require Otx2 Function Postnatally (A) Coronal section from an E15.5 GAD67 GFP brain showing delineation between the, which is rich in INs, and the, which is devoid of these neurons. (A C) At E15.5, Otx2 + INs are located in the ventral part of the (A, same slice as A) and progressively migrate toward the - border (E18.5; B) before entering into the at P1 (C, arrowheads). (D) MEIS2 is an OTX2 activator, while TLE4 is an OTX2 repressor. (E and F) Meis2 is expressed by INs but not by INs (E, arrowheads), while TLE4 has a complementary pattern of expression (F, arrowheads). (G and H) Loss of OTX2 function in Otx2 + INs (arrowhead in G) by P injection of tamoxifen in Otx2 CreERT2 /Otx2 flox mice leads to a specific loss of INs in the (empty arrowhead in H). Scale bars represent 1 mm. See also Figure S3. P2 synaptic differentiation, whether specific patterns of retinal activity are required for these inhibitory circuits to form remains unaddressed. In mice, eyes do not open before P15; accordingly, dark rearing from birth did not affect IN migration or synaptic differentiation (Figure S5A). During the first postnatal week, however, retinal waves (Meister et al., 1991), rather than light-evoked activity, are the main source of retinogeniculate activity. These coordinated depolarizations of retinal ganglion cells are critical to the eye-specific segregation of retinogeniculate and TC axons (Penn et al., 1998; Huberman et al., 28; Stafford et al., 29; Ackman et al., 212), but whether they are also required for inhibitory circuit assembly is unknown. To investigate this possibility, we took advantage of Chrnb2 / mice, a whole-body knockout line in which stage 2 retinal waves are abolished during the first postnatal week (Bansal et al., 2; Muir-Robinson et al., 22; McLaughlin et al., 23; Sun et al., 28; Stafford et al., 29). Supporting a critical role for retinal waves in the assembly of inhibitory circuits, the distribution of INs was abnormal in GAD67 GFP -Chrnb2 / mice. INs, which do not express Chrnb2 (Figure S5B), were predominantly located in the superficial half of this nucleus (Figures 7A 7E, S5C, and S5D), similar to what was observed after optic nerve section. Loss of retinal waves also affected the synaptic integration of INs, as indicated by a dramatic repression of the transcripts for the presynaptic proteins Cplx3 and VGAT, and the postsynaptic protein a6 nachr (Figures 7F, 7G, S5E, and S5F). IN mismigration and synaptic misdifferentiation could also be elicited by daily P P1 intraocular injections of epibatidine, a high-affinity cholinergic agonist that blocks retinal waves in vitro and in vivo (Ackman et al., 212) (Figures S5G S5O). Interestingly, IN migration was less disrupted by perturbation of retinal waves than by physical loss of retinal input (i.e., ONS and Ey1 mice) (Figure S5P), suggesting that retinal wave-independent mechanisms contribute to the migration of INs within the. Finally, consistent with abnormal functional assembly of inhibitory circuits, TC neuron excitability was dramatically increased in Chrnb2 / mice, while DHPG-evoked inhibitory responses were strongly reduced (Figures 7H and 7I). Taken together, these results indicate that retinal waves are required during the first postnatal week for the assembly of inhibitory circuits. DISCUSSION The two main findings of this study are that INs of the visual thalamus are recruited into retinogeniculate circuits from the, an evolutionarily older retinal target, and that the laminar and synaptic integration of these INs into visual circuits is controlled by patterns of retinal activity. These findings reveal an inputdependent mechanism for circuit construction in the CNS, in which the level and pattern of activity within a target circuit controls the incorporation of migrating inhibitory neurons. Such usedependent homeostatic recruitment is well suited to balance excitatory and inhibitory activity during development and may have driven the experience-dependent incorporation of inhibitory units into increasingly complex excitatory circuits during vertebrate evolution. 162 Neuron 81, , March 5, 214 ª214 Elsevier Inc.
7 Figure 4. Retinal Input Directs the Migration of INs (A) Time-lapse image of the in GAD67 GFP mice at P1. Individual INs are visible in the (white arrowheads) and hippocampus (empty arrowhead). Application of TTX specifically affects the migration of INs, while hippocampal INs are unaffected. Paired t test. (B D) P1 optic nerve section (ONS) causes abnormal distribution of INs (arrowheads in B), which remain at more superficial locations than in control littermates (arrowheads in C), where they morphologically differentiate (biocytin labeling in D). Dashed red line indicates separation between bin 1 (superficial) and 2 (deep). (E G) Summary of the results. (E and F) Top: cumulative scatter plot showing location of individual INs in control (E) and ONS (F) mice. Outlines of the are indicated in gray and normalized for maximum width and height. The location of the neurons shown in (D) is indicated. The same scatter plot is shown on the right with x axis scaled down to highlight lack of INs at deep locations (red arrowheads). Bottom: IN density heatmap (left, grid; right, grid), showing accumulation of INs at superficial locations after ONS. (G) Quantification of the data shown in (E) and (F) using cumulative distribution (left) and fraction of bin 2-located INs (right). n = 1,167 INs, n = 1,219 ONS INs, 5 mice per condition. Cumulative distribution: **p <.1, Kolmogorov-Smirnov test. Bin 2 fraction: Fisher s exact test. (H I ) ONS does not lead to IN cell death, as indicated by total counts of INs (H) and absence of cleaved Casp3 + neurons in the (I and I ). (J and K) INs fail to migrate to deeper tiers of the in Ey1 anophthalmic mice (K) compared to control mice (J). (L N) Cumulative scatter plots (L and M, see E and F for details) and quantifications (N) show accumulation of INs in bin 1. n = 413 INs, n = 315 ONS INs, 3 mice per condition. Cumulative distribution: **p <.1, Kolmogorov-Smirnov test. Bin 2 fraction: Fisher s exact test. Abbreviations:, control; Hip, hippocampus; ONS, optic nerve section; TTX, tetrodotoxin. Scale bars represent 1 mm in (A) (C), (I) (K); 1 mm in (D); and 5 mm (I) and (I ). Values are represented as mean ± SEM. Regulation of Circuit Homeostasis by Modulation of Inhibition Inhibitory input is central to homeostatic synaptic plasticity in the adult somatosensory and visual cortices, where decreases in circuit activity after visual deprivation (Maffei et al., 24), or increases after whisker stimulation (Knott et al., 22), lead to compensatory changes in inhibitory synapses (Pozo and Goda, 21; Wenner, 211). Similarly, circuit activity can regulate the synthesis of inhibitory neurotransmitters: during spinal cord and tectum development in Xenopus laevi, synthesis of GABA Neuron 81, , March 5, 214 ª214 Elsevier Inc. 163
8 P1 ONS A Otx2 Cplx3 6 nachr P2 B C D E F G H I Figure 5. Retinal Input Directs the Synaptic Differentiation of INs during a Critical Period of Development (A F) The synaptic differentiation of INs is impaired by P1 ONS: compared to control mice (A C), Otx2 expression is mildly decreased by P2 (D), while Cplx3 (E) and a6 nachr (F) are fully repressed after ONS. (G L) When ONS is performed at P8 (G I), there are no migratory abnormalities (G), but the expression of the synaptic proteins Cplx3 (H) and a6 nachr (I) is strongly decreased, which is not the case when ONS is performed at P12 (J L). (M O) Quantification of expression illustrated in (A) (L); a signal intensity of 1 corresponds to background levels. *p =.1, **p <.5, Student s t test, n R 39 INs per condition. Abbreviation: ND, not detectable. Scale bar represents 1 mm. Values are represented as mean ± SEM. See also Figure S4. ONS P12 ONS P8 ONS J P2 Otx2 M N O P1 P8 P12 cellular signal 1.5 intensity 2 changes with circuit activity (Borodinsky et al., 24; Miraucourt et al., 212) and, in adult primates, visual deprivation reversibly decreases GABA expression in the visual cortex (Hendry and Jones, 1986, 1988). To the best of our knowledge, our findings provide the first instance in which circuit homeostasis is achieved by regulating the incorporation of migrating inhibitory neurons during development. Inhibitory Neurons Are Recruited into Visual Circuits from the, an Evolutionarily Older Retinal Target Our findings indicate that INs belong to a specialized lineage of neurons, which are born during midembryogenesis from a restricted prethalamic Fgf8 + progenitor domain. These INs reside in the until birth, become molecularly distinct from neighboring INs, and progressively incorporate retinogeniculate K P2 Cplx3 * ** ** N.D. ** L P2 6 nachr circuits during the first postnatal week. The is an evolutionary ancient retinal target already present in fish, reptiles, and birds, which projects to the suprachiasmatic nucleus of the hypothalamus and is involved in nonimage-forming tasks such as generation of circadian rhythms (Harrington, 1997; Delogu et al., 212). In contrast, the functional importance of the has progressively increased with expansion of retinogeniculate connections, culminating in primates (Butler, 28; Mueller, 212). Interestingly, the proportion of INs increases in parallel with the evolutionary development of retinogeniculate connections (Arcelli et al., 1997), with extensive IN incorporation in the of mammals with color vision and broad binocular fields. In these species, the itself dramatically expands and becomes corticalized, i.e., has distinct layers and a variety of IN subtypes, which in humans, but not in nonhuman primates, also originate from subpallial sources (Letinic and Rakic, 21). Integrating our data with these findings, we propose that the increasing importance of vision in behavior and underlying expansion of retinogeniculate circuits may have driven the use-dependent recruitment of INs into the. Supporting a broader use of such a circuit construction strategy, INs progressively populate all thalamic nuclei in higher mammals, in line with the increasing complexity and expansion of TC circuits (Arcelli et al., 1997). Interestingly, Cplx3, Otx2, and Reelin are also expressed by subsets of neurons in the small New World monkey Callithrix jacchus (Mashiko et al., 212; data not shown), suggesting that at least some of the IN-specific developmental programs identified here are conserved in primates. 164 Neuron 81, , March 5, 214 ª214 Elsevier Inc.
9 A After ONS B from retina TC TC to cortex to cortex IN IN birth P1 P8 P12 migration synaptic diff. P18-22 ONS ONS ONS ephy C ONS P1 P8 P12 2 ms 4 mv n spikes / pulse ~P2 Iclamp Input (pa) P8 ONS *.2 P1 ONS *.2 n spikes / pulse n.s. * P12 ONS 1 n.s..78 ONS day P1 P8 P12 IN migration OK OK OK IN synaptic diff. OK - - OK D ~P2 Vclamp DHPG 1 pa 2 min ONS P1 P8 P12 DHPG n.s. * P1 P8 P DHPG-evoked ipscs freq. (x baseline) E ~P2 Vclamp ChR2 ChR2-GFP P2 2 m GAD65 Cre + Ptx ONS P1 4 ms 2 pa n = 9 / 1 *.4 n = 3 / ONS P1 Inhibited TC neurons (% total) F Otx2 CreERT2 / flox no tam P tam ~P2 Iclamp TC + P tam n spikes / pulse 4 mv 2 ms P tam <.1 * no tam Input (pa) Figure 6. Impaired Migration and Synaptic Differentiation of INs Leads to Disinhibition of TC Neurons (A) Schematic representation of the findings: decreased inhibitory input following ONS leads to hyperexcitability of TC neurons. (B) Schematic summary of the experiments performed in (C) and (D). (C) Perturbing IN migration (P1 ONS) or IN synaptic differentiation (P8 ONS) leads to hyperexcitability of TC neurons, while ONS at P12 (i.e., after synaptic differentiation) has no effect on excitability. Left: sample traces showing responses to a 3 pa current pulse. Middle: spike numbers in response to increasing current steps. Right: quantification of the response to a 3 pa pulse. : n = 6, P1 ONS: n = 6, P8 ONS: n = 5, P12 ONS: n = 5. Slope regression comparison with (middle), Mann-Whitney test (right). (D) Activation of group I mglurs by DHPG evokes strong inhibitory responses in TC neurons in control conditions that are not evoked after P1 or P8 ONS but still present following P12 ONS. Mann-Whitney test, : n = 5, P1 ONS: n = 5, P8 ONS: n = 5, P12 ONS: n = 8. (E). Optogenetic stimulation of INs evokes picrotoxin-sensitive inhibitory responses in most TC neurons, but not following P1 ONS (Fisher s exact test). Photomicrograph shows ChR2 + INs in an acute 3-mm-thick GAD65 Cre section. (F) TC neurons are hyperexcitable following induced ablation of INs by tamoxifen administration in Otx2 CRE-ERT2 /Otx2 flox mice (see Figures 3G and 3H). No tam: n = 6, tam: n = 8. Slope regression comparison. Abbreviations: n.s., not significant; ephy, electrophysiological assessment; Ptx, picrotoxin; tam, tamoxifen. Values are represented as mean ± SEM. Mechanisms of Circuit Integration and Implications for Visual Plasticity While the migration of INs from the into the is independent of retinal input, appropriate laminar positioning (i.e., superficial versus deep location) of these INs requires retinogeniculate activity. This is reminiscent of the laminar migratory abnormalities found in specific subtypes of cortical INs when cell-intrinsic electrical activity is blocked, suggesting convergent mechanisms of action (De Marco García et al., 211). Our Otx2 loss-of-function experiments suggest a permissive role for OTX2 activation in IN differentiation or migration; supporting the latter function, this transcription factor has recently been shown to enhance neuronal migration in the hindbrain (Wortham et al., 212). We identify a critical period before P12 during which retinal input controls the migration (P P8) and synaptic differentiation (P8 P12) of INs, and our observations in Chrnb2 / mice, in which loss of retinal waves is limited to the first postnatal week (Bansal et al., 2; Muir-Robinson et al., 22; McLaughlin et al., 23), reveal a critical role for cholinergic retinal waves in setting the excitatory/inhibitory balance of circuits. Because the Chrnb2 mutation is nonconditional, the respective contributions of retinogeniculate and TC neuron function cannot, however, fully be distinguished. Although retinotopic refinement defects reported in Chrnb2 / mice are largely thought to be of retinal origin (see e.g., McLaughlin et al., 23; Triplett et al., 29), and pharmacological blockade of b2-containing nicotinic receptors did not increase TC neuron excitability in vitro (data not shown), loss of Chrnb2 function in TC neurons may have also contributed to abnormal assembly of inhibitory circuits, perhaps via changes in their firing properties. Nonsynaptic activation of glutamatergic receptors controls developmental neuron migration in the hippocampus and cerebellum (Komuro and Rakic, 1993; Manent et al., 25, 26). Therefore, activity-dependent release of glutamate may directly act to regulate IN migration within the ; supporting this possibility, extracellular glutamate is reduced in the after optic nerve section (Sakurai and Okada, 1992). Similarly, synchronous depolarization of retinal ganglion cells during retinal waves probably increases extracellular glutamate levels in the and could thereby provide an instructive signal for migration by transiently activating low-affinity glutamate receptors on migrating INs. INs may contribute to retinogeniculate and thalamocortical mapping of visual input during development, since abnormal Neuron 81, , March 5, 214 ª214 Elsevier Inc. 165
10 Neuron B Figure 7. Retinal Waves Direct the Migration and Synaptic Integration of INs max height (%) 75 bin 1 bin D P2 bin 1 1 GAD67 bin 2 A M D max height (%) 75 bin 1 1 GAD67 bin 1 bin 2 bin D M 5 1 max width(%) 1 IN density (x average) 1 75 ** 5 I * I.1 5 Chrnb2-/2-/- * < P1 ONS ms Input (pa) n = 5 2 n spikes (x ) 4 n = 6 n spikes (x ) 3 2 mv TC DHPG DHPG Chrnb2-/DHPG plug. C57/Bl6 mice were provided by Charles River Laboratories. GAD67GFP mice were generated by Tamamaki and colleagues (Tamamaki et al., 23), Fgf8Cre mice by Toyoda and N.S. * colleagues (Toyoda et al., 21), Chrnb knockout mice were obtained from Marla Feller, and Otx2CreERT2 and Otx2flox mice were generated by Fossat and colleagues (Fossat et al., 26). Transgenic mice were compared to littermate controls. GAD67GFP knockin mice were bred as heterozygotes. 1 1 pa H 5 1 % total IN G 25 bin 2 Cplx3 **.3 6 nachr F IN in bin 2 (% total) bin 1 E 3 2 min migration and synaptic integration of these neurons leads to disinhibition of TC neurons. Recent reports have established the instructive role of retinal activity in the retinotopic mapping and interocular competition of retinogeniculate axons (Zhang et al., 212; Xu et al., 211) and the role of activity in the maturation of cortical INs (Di Cristo et al., 27; De Marco Garcı a et al., 211). It will be interesting to examine whether imbalances in the eye-specific incorporation of INs contributes to the outcome of interocular competition. More broadly, it will be important to assess whether activity-driven circuit integration of INs occurs in other brain regions, e.g., via oscillatory activity such as cortical waves, and whether distinct neuronal circuits within a given area can compete to integrate specific subtypes of incoming neurons. EXPERIMENTAL PROCEDURES Animals All experimental procedures were approved by the Geneva Cantonal Veterinary Authority. Embryonic day (E).5 was established as the day of vaginal 166 Neuron 81, , March 5, 214 ª214 Elsevier Inc. IPSC frequency (x baseline average) C (A D) Loss of retinal waves in Chrnb2 / mice causes abnormal distribution of INs, which remain at more superficial locations than in control littermates. (A and C) Photomicrographs of IN distribution in GAD67GFP (A) and Chrnb2 / -GAD67GFP (C) mice. (B and D) Left: cumulative scatter plot showing location of individual INs in control (B) and Chrnb2 / (D) mice. Right: IN density heatmap showing accumulation of INs at superficial locations in Chrnb2 /. See Figure 4 for details. (E) Quantification of the data shown in (B) and (D) using cumulative distribution (left) and fraction of bin 2-located INs (right). n = 79 INs, n = 993 Chrnb2 /, 5 mice per condition. Cumulative distribution: **p <.1, Kolmogorov-Smirnov test. Bin 2 fraction: Fisher s exact test. (F and G) The synaptic differentiation of INs in Chrnb2 / mice is impaired, as shown by repressed expression Cplx3 (F) and a6 nachr (G). (H) TC neurons are hyperexcitable in Chrnb2 / mice, as found following P1 ONS (dashed red circles, see Figure 6C). Graph on the right represents response to a 25 pa pulse, illustrated by the sample traces on the left. Slope regression comparison (middle) and Mann-Whitney test (right) are shown. (I) DHPG fails to evoke IPSCs in TC neurons in Chrnb2 / mice. Paired t test. Scale bars represent 1 mm in (A), (C), (F), and (G). Values are represented as mean ± SEM. See also Figure S5. In Situ Hybridization and Image Acquisition Tissue Collection Mice were perfused with.9% NaCl followed by 4% paraformaldehyde. Brains were removed and postfixed overnight in 4% PFA at 4 C and cut at 5 mm on a vibrating microtome. ISH In situ hybridization on slides was performed according to methods described previously (De la Rossa et al., 213). Briefly, hybridization was carried out overnight at 6 C with DIG-labeled RNA probes (Table S1). Following hybridization, sections were washed and incubated with alkaline phosphatase-conjugated antidigoxigenin antibody (Roche 1:2,) overnight at 4 C. Sections were then washed and the color reaction was carried out overnight at 4 C in an NBT/BCIP solution (Roche). After color revelation, sections were washed, postfixed for 3 min in 4% PFA, and mounted with Fluoromount (Sigma). Image Acquisition and Quantification Photomicrographs were taken with an Eclipse 9i (Nikon) fluorescence microscope or a Zeiss LSM7 confocal microscope. For quantification of IN distribution, the was divided into two bins by tracing a curved line midway between the pial surface and - boundary. Five to eight
11 sequential rostrocaudal sections (Paxinos atlas coordinates from Bregma: between 1.94 and 2.7) were used for quantification of distribution in control, ONS, Ey1, Chrnb2 /, and epibatidine/vehicle-injected mice. We observed a homogenous 1% decrease in the dorsoventral (12%) and lateromedial (9%) size of the following ONS. LGN size was adjusted to maximal length and height for IN scatterplots and density heatmaps across sections (Krahe et al., 211). Quantification of ISH intensity was performed blindly using the equalize function of Adobe Photoshop CS6 to normalize background intensity and measuring the threshold at which 5% of the pixels in individual labeled neurons were visible. Tissue Microdissection and Microarray Pups collected from a single litter were used for each of three biological replicates of and samples at P, P3, and P1. Fresh coronal brain sections (14 mm) were cut on a vibrating microtome and and nuclei were visually identified and microdissected in ice-cold oxygenated artificial cerebrospinal fluid under RNase-free conditions. Samples were stored in RNAlater at 8 C. RNA was extracted using the Ambion mirvana Isolation kit followed by two-cycle amplification and labeling was performed using MEGAscript T7 kit and MessageAmpIIaRNA amplification kit from Ambion. Labeled crna was applied to Affymetrix mouse microarray chips. Microarray CEL files were normalized with MAS5 Affymetrix algorithm and Robust Multichip Analysis (RMA) Identification of transcripts that covaried with GAD67 was performed using the Principal Components Analysis tool of the Partek Genomics Suite software. Candidate genes were secondarily screened using the online expression databases Allen Brain Atlas and BGEM. Surgical Procedures Injection of a reporter plasmid under the control of Ubiquitin promoter with a fluorochrome reporter (red, Tomato) (1.6 mg/ml) or Kir 2.1 (2. mg/ml) (Mire et al., 212) into the third ventricle of E12.5 timed pregnant C57/BL6 dams was performed under ultrasound guidance, and brief voltage pulses (5 mv, 5 ms) were applied using external paddles as described previously (De la Rossa et al., 213). Pups were collected at P7. Optic nerve section (ONS) was carried out on P1 pups; animals were deeply anesthetized on ice. A small incision was made in the eyelid with a scalpel and the eye was separated from the optic nerve with scissors and removed from the orbit with forceps. Pups were briefly warmed on a heating pad and were returned to their mother. The optic tract was labeled by intravitreal injection of 1 ml of cholera toxin subunit B (.2%) (Invitrogen) at P18 and brains were collected 48 hr later. Tamoxifen (Sigma, stocked at 2 mg/ml in corn oil,) was injected intraperitoneally at P (.2 mg stock solution/g of body weight). In Vitro Time-Lapse Imaging P1 coronal sections (2 mm thick) from GAD67 GFP mice were cut on a vibrating microtome and placed in neurobasal medium (Invitrogen) supplemented with 2% B27 (GIBCO, Invitrogen), 2 mm glutamine, 1 mm sodium pyruvate, 2 mm N-acetyl-cysteine, and 1% penicillin-streptomycin. Timelapse imaging was performed 6 hr after slice preparation for over a minimum of 2 hr in a thermoregulated chamber maintained at 37 C and at 5% CO 2. Time-lapse movies were acquired using a fluorescent microscope (Eclipse TE2; Nikon) using a Nikon Plan 23/.3 objective or a Zeiss Nipkow spinning-disk confocal microscope. Images were acquired using the Open-lab software (version 5.) every 5 min for at least 2 hr. Time-lapse stacks were generated and single-cell tracking of labeled neurons (n = 18 hippocampal INs, n = 24 INs, n = 3 mice) was performed using the Metamorph software (version 7.4). Migration speed was calculated as the total distance traveled by the cell divided by total imaging time. Values were normalized to the average migration speed of each population (hip INs and INs) in control conditions. Electrophysiology Coronal 3-mm-thick sections were prepared from P18 P22 mice. Slices were kept in artificial cerebrospinal fluid (3 32 C, 2 3 ml/min, submerged slices) containing 119 mm NaCl, 2.5 mm KCl, 1.3 mm MgCl, 2.5 mm CaCl 2, 1. mm Na 2 HPO 4, 26.2 mm NaHCO 3, and 11 mm glucose bubbled with 95% O 2 and 5% CO 2. TC neurons were identified by their large round soma and their relatively hyperpolarized V m compared to INs, as previously described (Williams et al., 1996). These criteria were confirmed in experiments in which GAD67 GFP mice were used and following biocytin filling of INs. Slices were visualized with a 43 objective lens. Neurons were recorded by means of whole-cell voltage clamp at 6 mv except for excitability measurements where current clamp was used. Access resistances were monitored by a hyperpolarizing step of 4 mv at the onset of every sweep and the experiment was discarded if the access resistance changed by more than 2%. The internal solution contained 14 mm K + -gluconate, 5 mm KCl, 1 mm HEPES,.2 mm EGTA, 2 mm MgCl 2, 4 mm Na 2 ATP,.3 mm Na 3 GTP, and 1 mm creatine-phosphate except for sipsc recordings where the solution was 3 mm K-gluconate, 1 mm KCl, 4 mm Mg 2 Cl 2, 1 mm creatine-phosphate, 3.4 mm Na 2 ATP,.1 mm Na 3 GTP, 1.1 mm EGTA, and 5 mm HEPES (reversal potential for Cl : 5 mv and K + : 14 mv). Kynurenic acid (2 mm) was added to block glutamatergic transmission in experiments when measuring DHPG (6 mm)-evoked inhibitory postsynaptic currents (IPSCs). Currents were amplified (Multiclamp 7B, Axon Instruments), filtered at 5 khz, and digitized at 2 khz (National Instruments Board PCI-MIO-16E4, Igor, WaveMetrics). The liquid junction potential was not corrected. The firing patterns of individual neurons were determined by step current injections of 5 ms. For optogenetic stimulation, stereotaxic injections of an AAV5-floxed-ChR2 virus (16 nl, Addgene, UNC Vectore Core) were performed at P6 in GAD65 Cre mice (Kätzel et al., 211) into the (coordinates from Lambda: AP mm, ML + 2. mm, depth 2.6 mm), resulting in infection of 5% of INs. ChR2 was stimulated by flashing a 47 nm blue light (.1 2 ms) at.1 Hz through the light path of the microscope (43 lens) using an LED under computer control. Recorded TC neurons were located within 2 mm of ChR2-expressing INs. Light-evoked IPSCs were recorded in the presence of kynurenic acid (2 mm). Picrotoxin (1 mm) was used to confirm the GABAergic nature of the photo-evoked currents. For cell filling, INs were visually identified in GAD67 GFP mice and filled with 3 mg/ml biocytin for at least 3 min. Sections were fixed for 2 hr and biocytin immunocytochemistry was performed using a streptavidin-conjugated Cy3 antibody (Jackson ImmunoResearch). SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at /j.neuron AUTHOR CONTRIBUTIONS G.P. and C.B. contributed equally to this work. ACKNOWLEDGMENTS We are thankful to Florian Smets and Audrey Benoit for technical assistance and to the members of our laboratories and Eiman Azim for helpful comments and contributing to improve the manuscript. We thank Dr. T. Lamonerie for the gift of Otx2 Cre ERT2 andotx2 flox mice, Dr. M. Feller for providing Chrnb2 / brains and mice, Dr. D. Boire for providing the Ey1 brains, and Dr. G. Lopez- Bendito for providing the Kir2.1-GFP construct. C.B. is supported by an Ambizione grant from the Swiss National Science Foundation; C.L. is supported by the Swiss National Science Foundation; A.D. is supported by the Swiss National Science Foundation and the NCCR Synapsy; work in the Jabaudon laboratory is supported by the Swiss National Science Foundation, the Velux Foundation, the Leenaards Foundation, and the NARSAD Foundation. Accepted: January 13, 214 Published: March 5, 214 REFERENCES Ackman, J.B., Burbridge, T.J., and Crair, M.C. (212). Retinal waves coordinate patterned activity throughout the developing visual system. Nature 49, Neuron 81, , March 5, 214 ª214 Elsevier Inc. 167
12 Agoston, Z., and Schulte, D. (29). Meis2 competes with the Groucho corepressor Tle4 for binding to Otx2 and specifies tectal fate without induction of a secondary midbrain-hindbrain boundary organizer. Development 136, Akerman, C.J., and Cline, H.T. (27). Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 3, Arcelli, P., Frassoni, C., Regondi, M.C., De Biasi, S., and Spreafico, R. (1997). GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res. Bull. 42, Bansal, A., Singer, J.H., Hwang, B.J., Xu, W., Beaudet, A., and Feller, M.B. (2). Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J. Neurosci. 2, Berardi, N., and Morrone, M.C. (1984). The role of gamma-aminobutyric acid mediated inhibition in the response properties of cat lateral geniculate nucleus neurones. J. Physiol. 357, Bickford, M.E., Slusarczyk, A., Dilger, E.K., Krahe, T.E., Kucuk, C., and Guido, W. (21). Synaptic development of the mouse dorsal lateral geniculate nucleus. J. Comp. Neurol. 518, Blitz, D.M., and Regehr, W.G. (25). Timing and specificity of feed-forward inhibition within the LGN. Neuron 45, Borodinsky, L.N., Root, C.M., Cronin, J.A., Sann, S.B., Gu, X., and Spitzer, N.C. (24). Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429, Bortone, D., and Polleux, F. (29). KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron 62, Butler, A.B. (28). Evolution of the thalamus: a morphological and functional review. Thalamus Relat. Syst. 4, Cajal, S.R.Y. (22). Texture of the Nervous System of Man and the Vertebrates, Volume III. (New York: Springer-Verlag). Charbonneau, V., Laramée, M.-E., Boucher, V., Bronchti, G., and Boire, D. (212). Cortical and subcortical projections to primary visual cortex in anophthalmic, enucleated and sighted mice. Eur. J. Neurosci. 36, Cox, C.L., and Sherman, S.M. (2). of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron 27, De la Rossa, A., Bellone, C., Golding, B., Vitali, I., Moss, J., Toni, N., Lüscher, C., and Jabaudon, D. (213). In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nat. Neurosci. 16, De Marco García, N.V., Karayannis, T., and Fishell, G. (211). Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 472, Delogu, A., Sellers, K., Zagoraiou, L., Bocianowska-Zbrog, A., Mandal, S., Guimera, J., Rubenstein, J.L.R., Sugden, D., Jessell, T., and Lumsden, A. (212). Subcortical visual shell nuclei targeted by iprgcs develop from a Sox14 + -GABAergic progenitor and require Sox14 to regulate daily activity rhythms. Neuron 75, Di Cristo, G., Chattopadhyaya, B., Kuhlman, S.J., Fu, Y., Bélanger, M.-C., Wu, C.Z., Rutishauser, U., Maffei, L., and Huang, Z.J. (27). Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 1, Errington, A.C., Di Giovanni, G., Crunelli, V., and Cope, D.W. (211). mglur control of interneuron output regulates feedforward tonic GABAA inhibition in the visual thalamus. J. Neurosci. 31, Fossat, N., Chatelain, G., Brun, G., and Lamonerie, T. (26). Temporal and spatial delineation of mouse Otx2 functions by conditional self-knockout. EMBO Rep. 7, Gelman, D.M., and Marín, O. (21). Generation of interneuron diversity in the mouse cerebral cortex. Eur. J. Neurosci. 31, Govindaiah, G., and Cox, C.L. (26). Metabotropic glutamate receptors differentially regulate GABAergic inhibition in thalamus. J. Neurosci. 26, Harrington, M.E. (1997). The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci. Biobehav. Rev. 21, Hayes, S.G., Murray, K.D., and Jones, E.G. (23). Two epochs in the development of gamma-aminobutyric acidergic neurons in the ferret thalamus. J. Comp. Neurol. 463, Heimbucher, T., Murko, C., Bajoghli, B., Aghaallaei, N., Huber, A., Stebegg, R., Eberhard, D., Fink, M., Simeone, A., and Czerny, T. (27). Gbx2 and Otx2 interact with the WD4 domain of Groucho/Tle corepressors. Mol. Cell. Biol. 27, Hendry, S.H., and Jones, E.G. (1986). Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature 32, Hendry, S.H., and Jones, E.G. (1988). Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron 1, Hensch, T.K. (25). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, Hooks, B.M., and Chen, C. (27). Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron 56, Hu, B., Li, X., Zhou, Y., and Shou, T. (2). Effects of bicuculline on directionsensitive relay cells in the dorsal lateral geniculate nucleus (LGNd) of cats. Brain Res. 885, Huberman, A.D., Feller, M.B., and Chapman, B. (28). Mechanisms underlying development of visual maps and receptive fields. Annu. Rev. Neurosci. 31, Isaacson, J.S., and Scanziani, M. (211). How inhibition shapes cortical activity. Neuron 72, Jeong, Y., Dolson, D.K., Waclaw, R.R., Matise, M.P., Sussel, L., Campbell, K., Kaestner, K.H., and Epstein, D.J. (211). Spatial and temporal requirements for sonic hedgehog in the regulation of thalamic interneuron identity. Development 138, Jones, E.G. (22). Dichronous appearance and unusual origins of GABA neurons during development of the mammalian thalamus. Thalamus Relat. Syst. 1, Kataoka, A., and Shimogori, T. (28). Fgf8 controls regional identity in the developing thalamus. Development 135, Kätzel, D., Zemelman, B.V., Buetfering, C., Wölfel, M., and Miesenböck, G. (211). The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat. Neurosci. 14, Knott, G.W., Quairiaux, C., Genoud, C., and Welker, E. (22). Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, Komuro, H., and Rakic, P. (1993). Modulation of neuronal migration by NMDA receptors. Science 26, Krahe, T.E., El-Danaf, R.N., Dilger, E.K., Henderson, S.C., and Guido, W. (211). Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J. Neurosci. 31, Letinic, K., and Rakic, P. (21). Telencephalic origin of human thalamic GABAergic neurons. Nat. Neurosci. 4, Lodato, S., Rouaux, C., Quast, K.B., Jantrachotechatchawan, C., Studer, M., Hensch, T.K., and Arlotta, P. (211). Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron 69, López-Bendito, G., and Molnár, Z. (23). Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 4, López-Bendito, G., Sánchez-Alcañiz, J.A., Pla, R., Borrell, V., Picó, E., Valdeolmillos, M., and Marín, O. (28). Chemokine signaling controls 168 Neuron 81, , March 5, 214 ª214 Elsevier Inc.
13 intracortical migration and final distribution of GABAergic interneurons. J. Neurosci. 28, Maffei, A., Nelson, S.B., and Turrigiano, G.G. (24). Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat. Neurosci. 7, Manent, J.-B., Demarque, M., Jorquera, I., Pellegrino, C., Ben-Ari, Y., Aniksztejn, L., and Represa, A. (25). A noncanonical release of GABA and glutamate modulates neuronal migration. J. Neurosci. 25, Manent, J.-B., Jorquera, I., Ben-Ari, Y., Aniksztejn, L., and Represa, A. (26). Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J. Neurosci. 26, Mashiko, H., Yoshida, A.C., Kikuchi, S.S., Niimi, K., Takahashi, E., Aruga, J., Okano, H., and Shimogori, T. (212). Comparative anatomy of marmoset and mouse cortex from genomic expression. J. Neurosci. 32, Meister, M., Wong, R.O., Baylor, D.A., and Shatz, C.J. (1991). Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252, McLaughlin, T., Torborg, C.L., Feller, M.B., and O Leary, D.D.M. (23). Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 4, Miraucourt, L.S., Silva, J.S., Burgos, K., Li, J., Abe, H., Ruthazer, E.S., and Cline, H.T. (212). GABA expression and regulation by sensory experience in the developing visual system. PLoS ONE 7, e2986. Mire, E., Mezzera, C., Leyva-Díaz, E., Paternain, A.V., Squarzoni, P., Bluy, L., Castillo-Paterna, M., López, M.J., Peregrín, S., Tessier-Lavigne, M., et al. (212). Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nat. Neurosci. 15, Miyoshi, G., and Fishell, G. (211). GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb. Cortex 21, Mueller, T. (212). What is the thalamus in zebrafish? Front. Neurosci. 6, 64. Muir-Robinson, G., Hwang, B.J., and Feller, M.B. (22). Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 22, Norton, T.T., and Godwin, D.W. (1992). Inhibitory GABAergic control of visual signals at the lateral geniculate nucleus. Prog. Brain Res. 9, Ortino, B., Inverardi, F., Morante-Oria, J., Fairén, A., and Frassoni, C. (23). Substrates and routes of migration of early generated neurons in the developing rat thalamus. Eur. J. Neurosci. 18, Penn, A.A., Riquelme, P.A., Feller, M.B., and Shatz, C.J. (1998). Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, Pozo, K., and Goda, Y. (21). Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66, Reim, K., Regus-Leidig, H., Ammermüller, J., El-Kordi, A., Radyushkin, K., Ehrenreich, H., Brandstätter, J.H., and Brose, N. (29). Aberrant function and structure of retinal ribbon synapses in the absence of complexin 3 and complexin 4. J. Cell Sci. 122, Runyan, C.A., Schummers, J., Van Wart, A., Kuhlman, S.J., Wilson, N.R., Huang, Z.J., and Sur, M. (21). Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron 67, Sakurai, T., and Okada, Y. (1992). Selective reduction of glutamate in the rat superior colliculus and dorsal lateral geniculate nucleus after contralateral enucleation. Brain Res. 573, Scholpp, S., and Lumsden, A. (21). Building a bridal chamber: development of the thalamus. Trends Neurosci. 33, Sillito, A.M., and Kemp, J.A. (1983). The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (). Brain Res. 277, Southwell, D.G., Froemke, R.C., Alvarez-Buylla, A., Stryker, M.P., and Gandhi, S.P. (21). Cortical plasticity induced by inhibitory neuron transplantation. Science 327, Spitzer, N.C. (26). Electrical activity in early neuronal development. Nature 444, Stafford, B.K., Sher, A., Litke, A.M., and Feldheim, D.A. (29). Spatial-temporal patterns of retinal waves underlying activity-dependent refinement of retinofugal projections. Neuron 64, Sugiyama, S., Di Nardo, A.A., Aizawa, S., Matsuo, I., Volovitch, M., Prochiantz, A., and Hensch, T.K. (28). Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, Sun, C., Warland, D.K., Ballesteros, J.M., van der List, D., and Chalupa, L.M. (28). Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 15, Suzuki-Hirano, A., Ogawa, M., Kataoka, A., Yoshida, A.C., Itoh, D., Ueno, M., Blackshaw, S., and Shimogori, T. (211). Dynamic spatiotemporal gene expression in embryonic mouse thalamus. J. Comp. Neurol. 519, Tamamaki, N., Yanagawa, Y., Tomioka, R., Miyazaki, J., Obata, K., and Kaneko, T. (23). Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467, Toyoda, R., Assimacopoulos, S., Wilcoxon, J., Taylor, A., Feldman, P., Suzuki- Hirano, A., Shimogori, T., and Grove, E.A. (21). FGF8 acts as a classic diffusible morphogen to pattern the neocortex. Development 137, Triplett, J.W., Owens, M.T., Yamada, J., Lemke, G., Cang, J., Stryker, M.P., and Feldheim, D.A. (29). Retinal input instructs alignment of visual topographic maps. Cell 139, Tucker, P., Laemle, L., Munson, A., Kanekar, S., Oliver, E.R., Brown, N., Schlecht, H., Vetter, M., and Glaser, T. (21). The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis 31, Vue, T.Y., Aaker, J., Taniguchi, A., Kazemzadeh, C., Skidmore, J.M., Martin, D.M., Martin, J.F., Treier, M., and Nakagawa, Y. (27). Characterization of progenitor domains in the developing mouse thalamus. J. Comp. Neurol. 55, Wang, Y., Li, G., Stanco, A., Long, J.E., Crawford, D., Potter, G.B., Pleasure, S.J., Behrens, T., and Rubenstein, J.L.R. (211a). CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron 69, Wang, X., Sommer, F.T., and Hirsch, J.A. (211b). Inhibitory circuits for visual processing in thalamus. Curr. Opin. Neurobiol. 21, Wang, X., Vaingankar, V., Sanchez, C.S., Sommer, F.T., and Hirsch, J.A. (211c). Thalamic interneurons and relay cells use complementary synaptic mechanisms for visual processing. Nat. Neurosci. 14, Wenner, P. (211). Mechanisms of GABAergic homeostatic plasticity. Neural Plast. 211, Williams, S.R., Turner, J.P., Anderson, C.M., and Crunelli, V. (1996). Electrophysiological and morphological properties of interneurones in the rat dorsal lateral geniculate nucleus in vitro. J. Physiol. 49, Wortham, M., Jin, G., Sun, J.L., Bigner, D.D., He, Y., and Yan, H. (212). Aberrant Otx2 expression enhances migration and induces ectopic proliferation of hindbrain neuronal progenitor cells. PLoS ONE 7, e Xu, H.-P., Furman, M., Mineur, Y.S., Chen, H., King, S.L., Zenisek, D., Zhou, Z.J., Butts, D.A., Tian, N., Picciotto, M.R., and Crair, M.C. (211). An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 7, Zhang, J., Ackman, J.B., Xu, H.-P., and Crair, M.C. (212). Visual map development depends on the temporal pattern of binocular activity in mice. Nat. Neurosci. 15, Neuron 81, , March 5, 214 ª214 Elsevier Inc. 169
Eye-specific retinogeniculate segregation proceeds normally following disruption of patterned spontaneous retinal activity
 Speer et al. Neural Development 214, 9:25 RESEARCH ARTICLE Eye-specific retinogeniculate segregation proceeds normally following disruption of patterned spontaneous retinal activity Open Access Colenso
Speer et al. Neural Development 214, 9:25 RESEARCH ARTICLE Eye-specific retinogeniculate segregation proceeds normally following disruption of patterned spontaneous retinal activity Open Access Colenso
CHAPTER 6 PRINCIPLES OF NEURAL CIRCUITS.
 CHAPTER 6 PRINCIPLES OF NEURAL CIRCUITS. 6.1. CONNECTIONS AMONG NEURONS Neurons are interconnected with one another to form circuits, much as electronic components are wired together to form a functional
CHAPTER 6 PRINCIPLES OF NEURAL CIRCUITS. 6.1. CONNECTIONS AMONG NEURONS Neurons are interconnected with one another to form circuits, much as electronic components are wired together to form a functional
Auditory neuroanatomy: the Spanish heritage. Santiago Ramón y Cajal, 1852 1934
 Auditory neuroanatomy: the Spanish heritage Santiago Ramón y Cajal, 1852 1934 Rafael Lorente de Nó, 1902 1990 3 The nervous system is made up of cells. Estimates of the number of cells vary from
Auditory neuroanatomy: the Spanish heritage Santiago Ramón y Cajal, 1852 1934 Rafael Lorente de Nó, 1902 1990 3 The nervous system is made up of cells. Estimates of the number of cells vary from
The Visual Cortex 0 http://www.tutis.ca/neuromd/index.htm 20 February 2013
 T he Visual Cortex 0 Chapter contents Contents Chapter 2... 0 T he Visual Cortex... 0 Chapter Contents... 1 Introduction... 2 Optic Chiasm... 2 Where do the eye's ganglion cells project to?... 3 To where
T he Visual Cortex 0 Chapter contents Contents Chapter 2... 0 T he Visual Cortex... 0 Chapter Contents... 1 Introduction... 2 Optic Chiasm... 2 Where do the eye's ganglion cells project to?... 3 To where
Bi 360: Midterm Review
 Bi 360: Midterm Review Basic Neurobiology 1) Many axons are surrounded by a fatty insulating sheath called myelin, which is interrupted at regular intervals at the Nodes of Ranvier, where the action potential
Bi 360: Midterm Review Basic Neurobiology 1) Many axons are surrounded by a fatty insulating sheath called myelin, which is interrupted at regular intervals at the Nodes of Ranvier, where the action potential
ALLEN Mouse Brain Atlas
 TECHNICAL WHITE PAPER: QUALITY CONTROL STANDARDS FOR HIGH-THROUGHPUT RNA IN SITU HYBRIDIZATION DATA GENERATION Consistent data quality and internal reproducibility are critical concerns for high-throughput
TECHNICAL WHITE PAPER: QUALITY CONTROL STANDARDS FOR HIGH-THROUGHPUT RNA IN SITU HYBRIDIZATION DATA GENERATION Consistent data quality and internal reproducibility are critical concerns for high-throughput
Name: Teacher: Olsen Hour:
 Name: Teacher: Olsen Hour: The Nervous System: Part 1 Textbook p216-225 41 In all exercises, quizzes and tests in this class, always answer in your own words. That is the only way that you can show that
Name: Teacher: Olsen Hour: The Nervous System: Part 1 Textbook p216-225 41 In all exercises, quizzes and tests in this class, always answer in your own words. That is the only way that you can show that
Neurophysiology. 2.1 Equilibrium Potential
 2 Neurophysiology 2.1 Equilibrium Potential An understanding of the concepts of electrical and chemical forces that act on ions, electrochemical equilibrium, and equilibrium potential is a powerful tool
2 Neurophysiology 2.1 Equilibrium Potential An understanding of the concepts of electrical and chemical forces that act on ions, electrochemical equilibrium, and equilibrium potential is a powerful tool
Synaptophysin Regulates the Kinetics of Synaptic Vesicle Endocytosis in Central Neurons
 Neuron, Volume 70 Supplemental Information Synaptophysin Regulates the Kinetics of Synaptic Vesicle Endocytosis in Central Neurons Sung E. Kwon and Edwin R. Chapman Supplemental Figure Legends Figure
Neuron, Volume 70 Supplemental Information Synaptophysin Regulates the Kinetics of Synaptic Vesicle Endocytosis in Central Neurons Sung E. Kwon and Edwin R. Chapman Supplemental Figure Legends Figure
Supplemental Fig. S1. The schematic diagrams of the expression constructs used in this study.
 1 Supplemental data Supplemental Fig. S1. The schematic diagrams of the expression constructs used in this study. Supplemental Fig. S2. Ingenuity Pathway Analysis (IPA) of the 56 putative caspase substrates
1 Supplemental data Supplemental Fig. S1. The schematic diagrams of the expression constructs used in this study. Supplemental Fig. S2. Ingenuity Pathway Analysis (IPA) of the 56 putative caspase substrates
Mechanosensitive Neurons on the Internal Reproductive Tract Contribute to Egg-Laying- Induced Acetic Acid Attraction in Drosophila
 Cell Reports, Volume 9 Supplemental Information Mechanosensitive Neurons on the Internal Reproductive Tract Contribute to Egg-Laying- Induced Acetic Acid Attraction in Drosophila Bin Gou, Ying Liu, Ananya
Cell Reports, Volume 9 Supplemental Information Mechanosensitive Neurons on the Internal Reproductive Tract Contribute to Egg-Laying- Induced Acetic Acid Attraction in Drosophila Bin Gou, Ying Liu, Ananya
Resting membrane potential ~ -70mV - Membrane is polarized
 Resting membrane potential ~ -70mV - Membrane is polarized (ie) Electrical charge on the outside of the membrane is positive while the electrical charge on the inside of the membrane is negative Changes
Resting membrane potential ~ -70mV - Membrane is polarized (ie) Electrical charge on the outside of the membrane is positive while the electrical charge on the inside of the membrane is negative Changes
CHAPTER 5 SIGNALLING IN NEURONS
 5.1. SYNAPTIC TRANSMISSION CHAPTER 5 SIGNALLING IN NEURONS One of the main functions of neurons is to communicate with other neurons. An individual neuron may receive information from many different sources.
5.1. SYNAPTIC TRANSMISSION CHAPTER 5 SIGNALLING IN NEURONS One of the main functions of neurons is to communicate with other neurons. An individual neuron may receive information from many different sources.
Masters research projects. 1. Adapting Granger causality for use on EEG data.
 Masters research projects 1. Adapting Granger causality for use on EEG data. Background. Granger causality is a concept introduced in the field of economy to determine which variables influence, or cause,
Masters research projects 1. Adapting Granger causality for use on EEG data. Background. Granger causality is a concept introduced in the field of economy to determine which variables influence, or cause,
Processing the Image or Can you Believe what you see? Light and Color for Nonscientists PHYS 1230
 Processing the Image or Can you Believe what you see? Light and Color for Nonscientists PHYS 1230 Optical Illusions http://www.michaelbach.de/ot/mot_mib/index.html Vision We construct images unconsciously
Processing the Image or Can you Believe what you see? Light and Color for Nonscientists PHYS 1230 Optical Illusions http://www.michaelbach.de/ot/mot_mib/index.html Vision We construct images unconsciously
THE HUMAN BRAIN. observations and foundations
 THE HUMAN BRAIN observations and foundations brains versus computers a typical brain contains something like 100 billion miniscule cells called neurons estimates go from about 50 billion to as many as
THE HUMAN BRAIN observations and foundations brains versus computers a typical brain contains something like 100 billion miniscule cells called neurons estimates go from about 50 billion to as many as
PART I: Neurons and the Nerve Impulse
 PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
http://abcnews.go.com/politics/video/obama-says-brain-initiative-will-be-transformative-18861944
 http://abcnews.go.com/politics/video/obama-says-brain-initiative-will-be-transformative-18861944 What are the nervous system s functions? The nervous system organizes and controls an individual s appropriate
http://abcnews.go.com/politics/video/obama-says-brain-initiative-will-be-transformative-18861944 What are the nervous system s functions? The nervous system organizes and controls an individual s appropriate
Slide 1: Introduction Introduce the purpose of your presentation. Indicate that you will explain how the brain basically works and how and where
 Slide 1: Introduction Introduce the purpose of your presentation. Indicate that you will explain how the brain basically works and how and where drugs such as heroin and cocaine work in the brain. Tell
Slide 1: Introduction Introduce the purpose of your presentation. Indicate that you will explain how the brain basically works and how and where drugs such as heroin and cocaine work in the brain. Tell
Supplementary Figure 1 Characterization of 5xFAD derived hippocampal cultures. A-B) Average total numbers of cells per hippocampus as well as average
 Supplementary Figure 1 Characterization of 5xFAD derived hippocampal cultures. A-B) Average total numbers of cells per hippocampus as well as average total numbers of cells per field of view were similar
Supplementary Figure 1 Characterization of 5xFAD derived hippocampal cultures. A-B) Average total numbers of cells per hippocampus as well as average total numbers of cells per field of view were similar
HOMEOSTATIC PLASTICITY IN THE DEVELOPING NERVOUS SYSTEM
 HOMEOSTATIC PLASTICITY IN THE DEVELOPING NERVOUS SYSTEM Gina G. Turrigiano and Sacha B. Nelson Activity has an important role in refining synaptic connectivity during development, in part through Hebbian
HOMEOSTATIC PLASTICITY IN THE DEVELOPING NERVOUS SYSTEM Gina G. Turrigiano and Sacha B. Nelson Activity has an important role in refining synaptic connectivity during development, in part through Hebbian
ANIMATED NEUROSCIENCE
 ANIMATED NEUROSCIENCE and the Action of Nicotine, Cocaine, and Marijuana in the Brain Te a c h e r s G u i d e Films for the Humanities & Sciences Background Information This program, made entirely of
ANIMATED NEUROSCIENCE and the Action of Nicotine, Cocaine, and Marijuana in the Brain Te a c h e r s G u i d e Films for the Humanities & Sciences Background Information This program, made entirely of
In developmental genomic regulatory interactions among genes, encoding transcription factors
 JOURNAL OF COMPUTATIONAL BIOLOGY Volume 20, Number 6, 2013 # Mary Ann Liebert, Inc. Pp. 419 423 DOI: 10.1089/cmb.2012.0297 Research Articles A New Software Package for Predictive Gene Regulatory Network
JOURNAL OF COMPUTATIONAL BIOLOGY Volume 20, Number 6, 2013 # Mary Ann Liebert, Inc. Pp. 419 423 DOI: 10.1089/cmb.2012.0297 Research Articles A New Software Package for Predictive Gene Regulatory Network
Electrophysiological Recording Techniques
 Electrophysiological Recording Techniques Wen-Jun Gao, PH.D. Drexel University College of Medicine Goal of Physiological Recording To detect the communication signals between neurons in real time (μs to
Electrophysiological Recording Techniques Wen-Jun Gao, PH.D. Drexel University College of Medicine Goal of Physiological Recording To detect the communication signals between neurons in real time (μs to
Introduction to Neurophysiological Psychology (PSY 451)
 Portland State University Physiological Psychology Page 1 Introduction to Neurophysiological Psychology (PSY 451) Bill Griesar, Ph.D., Adjunct Instructor, bgriesar@pacifier.com OFFICE HOURS: Mondays, 11:30
Portland State University Physiological Psychology Page 1 Introduction to Neurophysiological Psychology (PSY 451) Bill Griesar, Ph.D., Adjunct Instructor, bgriesar@pacifier.com OFFICE HOURS: Mondays, 11:30
BIOPHYSICS OF NERVE CELLS & NETWORKS
 UNIVERSITY OF LONDON MSci EXAMINATION May 2007 for Internal Students of Imperial College of Science, Technology and Medicine This paper is also taken for the relevant Examination for the Associateship
UNIVERSITY OF LONDON MSci EXAMINATION May 2007 for Internal Students of Imperial College of Science, Technology and Medicine This paper is also taken for the relevant Examination for the Associateship
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.aac6472/dc1 Supplementary Materials for A GABAergic projection from the zona incerta to cortex promotes cortical neuron development Jiadong Chen* and Arnold
www.sciencemag.org/cgi/content/full/science.aac6472/dc1 Supplementary Materials for A GABAergic projection from the zona incerta to cortex promotes cortical neuron development Jiadong Chen* and Arnold
Neuro imaging: looking with lasers in the brain
 Neuro imaging: looking with lasers in the brain Aim: To image life cells, label free, with cellular resolution in deep tissue Marloes Groot Vrije Universiteit Amsterdam Faculteit Exacte Wetenschappen Natuurkunde
Neuro imaging: looking with lasers in the brain Aim: To image life cells, label free, with cellular resolution in deep tissue Marloes Groot Vrije Universiteit Amsterdam Faculteit Exacte Wetenschappen Natuurkunde
Analysis of gene expression data. Ulf Leser and Philippe Thomas
 Analysis of gene expression data Ulf Leser and Philippe Thomas This Lecture Protein synthesis Microarray Idea Technologies Applications Problems Quality control Normalization Analysis next week! Ulf Leser:
Analysis of gene expression data Ulf Leser and Philippe Thomas This Lecture Protein synthesis Microarray Idea Technologies Applications Problems Quality control Normalization Analysis next week! Ulf Leser:
Andrew Rosen - Chapter 3: The Brain and Nervous System Intro:
 Intro: Brain is made up of numerous, complex parts Frontal lobes by forehead are the brain s executive center Parietal lobes wave sensory information together (maps feeling on body) Temporal lobes interpret
Intro: Brain is made up of numerous, complex parts Frontal lobes by forehead are the brain s executive center Parietal lobes wave sensory information together (maps feeling on body) Temporal lobes interpret
CELLS IN THE NERVOUS SYSTEM
 NEURONS AND GLIA CELLS IN THE NERVOUS SYSTEM Glia Insulates, supports, and nourishes neurons Neurons Process information Sense environmental changes Communicate changes to other neurons Command body response
NEURONS AND GLIA CELLS IN THE NERVOUS SYSTEM Glia Insulates, supports, and nourishes neurons Neurons Process information Sense environmental changes Communicate changes to other neurons Command body response
How To Understand The Distributed Potential Of A Dendritic Tree
 Systems Biology II: Neural Systems (580.422) Lecture 8, Linear cable theory Eric Young 5-3164 eyoung@jhu.edu Reading: D. Johnston and S.M. Wu Foundations of Cellular Neurophysiology (MIT Press, 1995).
Systems Biology II: Neural Systems (580.422) Lecture 8, Linear cable theory Eric Young 5-3164 eyoung@jhu.edu Reading: D. Johnston and S.M. Wu Foundations of Cellular Neurophysiology (MIT Press, 1995).
Barbara St. Marie, PhD Candidate Nurse Practitioner Supervisor Pain and Palliative Care Fairview Ridges Hospital Minneapolis, MN
 Barbara St. Marie, PhD Candidate Nurse Practitioner Supervisor Pain and Palliative Care Fairview Ridges Hospital Minneapolis, MN Pain Physiology Objectives: Explain how pain is transmitted through the
Barbara St. Marie, PhD Candidate Nurse Practitioner Supervisor Pain and Palliative Care Fairview Ridges Hospital Minneapolis, MN Pain Physiology Objectives: Explain how pain is transmitted through the
Chapter 7: The Nervous System
 Chapter 7: The Nervous System Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways involved in a withdraw reflex Define
Chapter 7: The Nervous System Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways involved in a withdraw reflex Define
Origin of Electrical Membrane Potential
 Origin of Electrical Membrane Potential parti This book is about the physiological characteristics of nerve and muscle cells. As we shall see, the ability of these cells to generate and conduct electricity
Origin of Electrical Membrane Potential parti This book is about the physiological characteristics of nerve and muscle cells. As we shall see, the ability of these cells to generate and conduct electricity
Nervous System: Spinal Cord and Spinal Nerves (Chapter 13) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College
 Nervous System: Spinal Cord and Spinal Nerves (Chapter 13) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Sources for figures and content: Eastern Campus Marieb,
Nervous System: Spinal Cord and Spinal Nerves (Chapter 13) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Sources for figures and content: Eastern Campus Marieb,
Influence of GSM and UMTS on the Blood Brain Barrier in vitro additional results
 Influence of GSM and UMTS on the Blood Brain Barrier in vitro additional results Intl. Workshop on long term effects, München, 11.-12. Okt. 2007 Dr. rer. nat. Helmut Franke Klinik und Poliklinik für Neurologie
Influence of GSM and UMTS on the Blood Brain Barrier in vitro additional results Intl. Workshop on long term effects, München, 11.-12. Okt. 2007 Dr. rer. nat. Helmut Franke Klinik und Poliklinik für Neurologie
life&brain Electrophysiology Services
 life&brain Electrophysiology Services Life & Brain GmbH Electrophysiology Services NEUROPLASTICITY LIFE & BRAIN Gmbh Sigmund-Freud-Str. 25 53127 Bonn Tel.: 49-228-6885 215 Fax.: 49-228-6885 296 E-mail:
life&brain Electrophysiology Services Life & Brain GmbH Electrophysiology Services NEUROPLASTICITY LIFE & BRAIN Gmbh Sigmund-Freud-Str. 25 53127 Bonn Tel.: 49-228-6885 215 Fax.: 49-228-6885 296 E-mail:
Vocabulary & General Concepts of Brain Organization
 Vocabulary & General Concepts of Brain Organization Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine Course Outline Lecture 1: Vocabulary & General Concepts of Brain
Vocabulary & General Concepts of Brain Organization Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine Course Outline Lecture 1: Vocabulary & General Concepts of Brain
Activity 4 Long-Term Effects of Drug Addiction
 Activity 4 Long-Term Effects of Drug Addiction Core Concept: Addictive drugs may lead to long-term changes in brain function. Class time required: Approximately 60-80 minutes Teacher Provides: Copy of
Activity 4 Long-Term Effects of Drug Addiction Core Concept: Addictive drugs may lead to long-term changes in brain function. Class time required: Approximately 60-80 minutes Teacher Provides: Copy of
Parts of the Nerve Cell and Their Functions
 Parts of the Nerve Cell and Their Functions Silvia Helena Cardoso, PhD [ 1. Cell body] [2. Neuronal membrane] [3. Dendrites] [4. Axon] [5. Nerve ending] 1. Cell body The cell body (soma) is the factory
Parts of the Nerve Cell and Their Functions Silvia Helena Cardoso, PhD [ 1. Cell body] [2. Neuronal membrane] [3. Dendrites] [4. Axon] [5. Nerve ending] 1. Cell body The cell body (soma) is the factory
Questions on The Nervous System and Gas Exchange
 Name: Questions on The Nervous System and Gas Exchange Directions: The following questions are taken from previous IB Final Papers on Topics 6.4 (Gas Exchange) and 6.5 (Nerves, hormones and homeostasis).
Name: Questions on The Nervous System and Gas Exchange Directions: The following questions are taken from previous IB Final Papers on Topics 6.4 (Gas Exchange) and 6.5 (Nerves, hormones and homeostasis).
1 Cornea 6 Macula 2 Lens 7 Vitreous humor 3 Iris 8 Optic disc 4 Conjunctiva 9 Ciliary muscles 5 Sclera 10 Choroid
 Anatomy and Physiology Quiz 1 Sample Question Answers Use the following table to answer Questions 1 2. 1 Cornea 6 Macula 2 Lens 7 Vitreous humor 3 Iris 8 Optic disc 4 Conjunctiva 9 Ciliary muscles 5 Sclera
Anatomy and Physiology Quiz 1 Sample Question Answers Use the following table to answer Questions 1 2. 1 Cornea 6 Macula 2 Lens 7 Vitreous humor 3 Iris 8 Optic disc 4 Conjunctiva 9 Ciliary muscles 5 Sclera
The Making of the Fittest: Evolving Switches, Evolving Bodies
 OVERVIEW MODELING THE REGULATORY SWITCHES OF THE PITX1 GENE IN STICKLEBACK FISH This hands-on activity supports the short film, The Making of the Fittest:, and aims to help students understand eukaryotic
OVERVIEW MODELING THE REGULATORY SWITCHES OF THE PITX1 GENE IN STICKLEBACK FISH This hands-on activity supports the short film, The Making of the Fittest:, and aims to help students understand eukaryotic
Biological Neurons and Neural Networks, Artificial Neurons
 Biological Neurons and Neural Networks, Artificial Neurons Neural Computation : Lecture 2 John A. Bullinaria, 2015 1. Organization of the Nervous System and Brain 2. Brains versus Computers: Some Numbers
Biological Neurons and Neural Networks, Artificial Neurons Neural Computation : Lecture 2 John A. Bullinaria, 2015 1. Organization of the Nervous System and Brain 2. Brains versus Computers: Some Numbers
12. Nervous System: Nervous Tissue
 12. Nervous System: Nervous Tissue I. Introduction to the Nervous System General functions of the nervous system The nervous system has three basic functions: 1. Gather sensory input from the environment
12. Nervous System: Nervous Tissue I. Introduction to the Nervous System General functions of the nervous system The nervous system has three basic functions: 1. Gather sensory input from the environment
AP Biology I. Nervous System Notes
 AP Biology I. Nervous System Notes 1. General information: passage of information occurs in two ways: Nerves - process and send information fast (eg. stepping on a tack) Hormones - process and send information
AP Biology I. Nervous System Notes 1. General information: passage of information occurs in two ways: Nerves - process and send information fast (eg. stepping on a tack) Hormones - process and send information
What role does the nucleolus have in cell functioning? Glial cells
 Nervous System Lab The nervous system of vertebrates can be divided into the central nervous system, which consists of the brain and spinal cord, and the peripheral nervous system, which contains nerves,
Nervous System Lab The nervous system of vertebrates can be divided into the central nervous system, which consists of the brain and spinal cord, and the peripheral nervous system, which contains nerves,
Brain Computer Interfaces (BCI) Communication Training of brain activity
 Brain Computer Interfaces (BCI) Communication Training of brain activity Brain Computer Interfaces (BCI) picture rights: Gerwin Schalk, Wadsworth Center, NY Components of a Brain Computer Interface Applications
Brain Computer Interfaces (BCI) Communication Training of brain activity Brain Computer Interfaces (BCI) picture rights: Gerwin Schalk, Wadsworth Center, NY Components of a Brain Computer Interface Applications
The Neuron and the Synapse. The Neuron. Parts of the Neuron. Functions of the neuron:
 The Neuron and the Synapse The Neuron Functions of the neuron: Transmit information from one point in the body to another. Process the information in various ways (that is, compute). The neuron has a specialized
The Neuron and the Synapse The Neuron Functions of the neuron: Transmit information from one point in the body to another. Process the information in various ways (that is, compute). The neuron has a specialized
Supplemental Information. Notch Inhibition Induces Cochlear Hair Cell. Regeneration and Recovery of Hearing. after Acoustic Trauma.
 Neuron, Volume 77 Supplemental Information Notch Inhibition Induces Cochlear Hair Cell Regeneration and Recovery of Hearing after Acoustic Trauma Kunio Mizutari, Masato Fujioka, Makoto Hosoya, Naomi Bramhall,
Neuron, Volume 77 Supplemental Information Notch Inhibition Induces Cochlear Hair Cell Regeneration and Recovery of Hearing after Acoustic Trauma Kunio Mizutari, Masato Fujioka, Makoto Hosoya, Naomi Bramhall,
PHYSIOLOGICAL PSYCHOLOGY
 UNIVERSITY OF CALICUT SCHOOL OF DISTANCE EDUCATION B Sc COUNSELLING PSYCHOLOGY (2011 Admission Onwards) I Semester Complementary Course PHYSIOLOGICAL PSYCHOLOGY QUESTION BANK 1. are the basic units of
UNIVERSITY OF CALICUT SCHOOL OF DISTANCE EDUCATION B Sc COUNSELLING PSYCHOLOGY (2011 Admission Onwards) I Semester Complementary Course PHYSIOLOGICAL PSYCHOLOGY QUESTION BANK 1. are the basic units of
The Physiology of the Senses Lecture 1 - The Eye www.tutis.ca/senses/
 The Physiology of the Senses Lecture 1 - The Eye www.tutis.ca/senses/ Contents Objectives... 2 Introduction... 2 Accommodation... 3 The Iris... 4 The Cells in the Retina... 5 Receptive Fields... 8 The
The Physiology of the Senses Lecture 1 - The Eye www.tutis.ca/senses/ Contents Objectives... 2 Introduction... 2 Accommodation... 3 The Iris... 4 The Cells in the Retina... 5 Receptive Fields... 8 The
LIST OF FIGURES. Figure 1: Diagrammatic representation of electromagnetic wave. Figure 2: A representation of the electromagnetic spectrum.
 LIST OF FIGURES Figure 1: Diagrammatic representation of electromagnetic wave. Figure 2: A representation of the electromagnetic spectrum. Figure 3: Picture depicting the internal circuit of mobile phone
LIST OF FIGURES Figure 1: Diagrammatic representation of electromagnetic wave. Figure 2: A representation of the electromagnetic spectrum. Figure 3: Picture depicting the internal circuit of mobile phone
Human Physiology Study Questions-2
 Human Physiology Study Questions-2 Action potentials: Handout-8, Chapter 8 1. Explain the positive feedback component of an action potential that is, how the opening of one voltage-gated sodium (or calcium)
Human Physiology Study Questions-2 Action potentials: Handout-8, Chapter 8 1. Explain the positive feedback component of an action potential that is, how the opening of one voltage-gated sodium (or calcium)
Neurotrophic factors and Their receptors
 Neurotrophic factors and Their receptors Huang Shu-Hong Institute of neurobiology 1 For decades, scientists believed that brain cells of the central nervous system could not regrow following damage due
Neurotrophic factors and Their receptors Huang Shu-Hong Institute of neurobiology 1 For decades, scientists believed that brain cells of the central nervous system could not regrow following damage due
Visual area MT responds to local motion. Visual area MST responds to optic flow. Visual area STS responds to biological motion. Macaque visual areas
 Visual area responds to local motion MST a Visual area MST responds to optic flow MST a Visual area STS responds to biological motion STS Macaque visual areas Flattening the brain What is a visual area?
Visual area responds to local motion MST a Visual area MST responds to optic flow MST a Visual area STS responds to biological motion STS Macaque visual areas Flattening the brain What is a visual area?
Nerves and Nerve Impulse
 Nerves and Nerve Impulse Terms Absolute refractory period: Period following stimulation during which no additional action potential can be evoked. Acetylcholine: Chemical transmitter substance released
Nerves and Nerve Impulse Terms Absolute refractory period: Period following stimulation during which no additional action potential can be evoked. Acetylcholine: Chemical transmitter substance released
Introduction to flow cytometry
 Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
Unit 2 - Subcortical systems, neurochemistry and brain function
 Unit 2 - Subcortical systems, neurochemistry and brain function Subcortical anatomy: Most of the five major subdivisions of the brain are subcortical. I. Telencephalon (cortical - part of forebrain) -
Unit 2 - Subcortical systems, neurochemistry and brain function Subcortical anatomy: Most of the five major subdivisions of the brain are subcortical. I. Telencephalon (cortical - part of forebrain) -
Standards Alignment Minnesota Science Standards Alignment Matrix www.brainu.org/resources/mnstds
 Lesson Summary: Neurons transfer information by releasing neurotransmitters across the synapse or space between neurons. Students model the chemical communication between pre-synaptic and post-synaptic
Lesson Summary: Neurons transfer information by releasing neurotransmitters across the synapse or space between neurons. Students model the chemical communication between pre-synaptic and post-synaptic
Neural Coding by Two Classes of Principal Cells in the Mouse Piriform Cortex
 11938 The Journal of Neuroscience, November 15, 2006 26(46):11938 11947 Cellular/Molecular Neural Coding by Two Classes of Principal Cells in the Mouse Piriform Cortex Norimitsu Suzuki and John M. Bekkers
11938 The Journal of Neuroscience, November 15, 2006 26(46):11938 11947 Cellular/Molecular Neural Coding by Two Classes of Principal Cells in the Mouse Piriform Cortex Norimitsu Suzuki and John M. Bekkers
Biological Sciences Initiative. Human Genome
 Biological Sciences Initiative HHMI Human Genome Introduction In 2000, researchers from around the world published a draft sequence of the entire genome. 20 labs from 6 countries worked on the sequence.
Biological Sciences Initiative HHMI Human Genome Introduction In 2000, researchers from around the world published a draft sequence of the entire genome. 20 labs from 6 countries worked on the sequence.
CHAPTER- 6. Okadaic acid induced neurotoxicity leads to central cholinergic dysfunction in rats. 1. Introduction. 2. Methods
 CHAPTER- 6 Okadaic acid induced neurotoxicity leads to central cholinergic dysfunction in rats 1. Introduction Neurodegenerative disorders, such as AD are often characterized by the degeneration of the
CHAPTER- 6 Okadaic acid induced neurotoxicity leads to central cholinergic dysfunction in rats 1. Introduction Neurodegenerative disorders, such as AD are often characterized by the degeneration of the
Activity 5: The Action Potential: Measuring Its Absolute and Relative Refractory Periods. 250 20 Yes. 125 20 Yes. 60 20 No. 60 25 No.
 3: Neurophysiology of Nerve Impulses (Part 2) Activity 5: The Action Potential: Measuring Its Absolute and Relative Refractory Periods Interval between stimuli Stimulus voltage (mv) Second action potential?
3: Neurophysiology of Nerve Impulses (Part 2) Activity 5: The Action Potential: Measuring Its Absolute and Relative Refractory Periods Interval between stimuli Stimulus voltage (mv) Second action potential?
Biology/ANNB 261 Exam 1 Spring, 2006
 Biology/ANNB 261 Exam 1 Spring, 2006 Name * = correct answer Multiple Choice: 1. Axons and dendrites are two types of a) Neurites * b) Organelles c) Synapses d) Receptors e) Golgi cell components 2. The
Biology/ANNB 261 Exam 1 Spring, 2006 Name * = correct answer Multiple Choice: 1. Axons and dendrites are two types of a) Neurites * b) Organelles c) Synapses d) Receptors e) Golgi cell components 2. The
Synaptic pathways and inhibitory gates in the spinal cord dorsal horn
 Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Neurons and Networks in the Spinal Cord Synaptic pathways and inhibitory gates in the spinal cord dorsal horn Tomonori
Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Neurons and Networks in the Spinal Cord Synaptic pathways and inhibitory gates in the spinal cord dorsal horn Tomonori
Systemic Pesticides as a Causal Factor of Developmental Brain Disorders (ADHD, autism, etc.)
 2012 9 2 浸 透 性 農 薬 フォーラム 脳 の 発 達 障 害 (ADHD 自 閉 症 など) の 原 因 としての 浸 透 性 農 薬 : ネオニコチノイド Systemic Pesticides as a Causal Factor of Developmental Brain Disorders (ADHD, autism, etc.) 環 境 脳 神 経 科 学 情 報 センター
2012 9 2 浸 透 性 農 薬 フォーラム 脳 の 発 達 障 害 (ADHD 自 閉 症 など) の 原 因 としての 浸 透 性 農 薬 : ネオニコチノイド Systemic Pesticides as a Causal Factor of Developmental Brain Disorders (ADHD, autism, etc.) 環 境 脳 神 経 科 学 情 報 センター
Ion Channels. Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com)
 Ion Channels Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) ** There are a number of ion channels introducted in this topic which you
Ion Channels Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) ** There are a number of ion channels introducted in this topic which you
Hierarchical Clustering Analysis
 Hierarchical Clustering Analysis What is Hierarchical Clustering? Hierarchical clustering is used to group similar objects into clusters. In the beginning, each row and/or column is considered a cluster.
Hierarchical Clustering Analysis What is Hierarchical Clustering? Hierarchical clustering is used to group similar objects into clusters. In the beginning, each row and/or column is considered a cluster.
Neurobiology of Depression in Relation to ECT. PJ Cowen Department of Psychiatry, University of Oxford
 Neurobiology of Depression in Relation to ECT PJ Cowen Department of Psychiatry, University of Oxford Causes of Depression Genetic Childhood experience Life Events (particularly losses) Life Difficulties
Neurobiology of Depression in Relation to ECT PJ Cowen Department of Psychiatry, University of Oxford Causes of Depression Genetic Childhood experience Life Events (particularly losses) Life Difficulties
Chapter 9 Nervous System
 Chapter 9 Nervous System Nervous System function: The nervous system is composed of neurons and neuroglia. at the ends of peripheral nerves gather information and convert it into nerve impulses. When sensory
Chapter 9 Nervous System Nervous System function: The nervous system is composed of neurons and neuroglia. at the ends of peripheral nerves gather information and convert it into nerve impulses. When sensory
Computational Neuroscience. Models of Synaptic Transmission and Plasticity. Prof. Dr. Michele GIUGLIANO 2036FBDBMW
 Computational Neuroscience 2036FBDBMW Master of Science in Computer Science (Scientific Computing) Master of Science in Biomedical Sciences (Neurosciences) Master of Science in Physics Prof. Dr. Michele
Computational Neuroscience 2036FBDBMW Master of Science in Computer Science (Scientific Computing) Master of Science in Biomedical Sciences (Neurosciences) Master of Science in Physics Prof. Dr. Michele
CSE511 Brain & Memory Modeling. Lect04: Brain & Spine Neuroanatomy
 CSE511 Brain & Memory Modeling CSE511 Brain & Memory Modeling Lect02: BOSS Discrete Event Simulator Lect04: Brain & Spine Neuroanatomy Appendix of Purves et al., 4e Larry Wittie Computer Science, StonyBrook
CSE511 Brain & Memory Modeling CSE511 Brain & Memory Modeling Lect02: BOSS Discrete Event Simulator Lect04: Brain & Spine Neuroanatomy Appendix of Purves et al., 4e Larry Wittie Computer Science, StonyBrook
Thermo Scientific DyNAmo cdna Synthesis Kit for qrt-pcr Technical Manual
 Thermo Scientific DyNAmo cdna Synthesis Kit for qrt-pcr Technical Manual F- 470S 20 cdna synthesis reactions (20 µl each) F- 470L 100 cdna synthesis reactions (20 µl each) Table of contents 1. Description...
Thermo Scientific DyNAmo cdna Synthesis Kit for qrt-pcr Technical Manual F- 470S 20 cdna synthesis reactions (20 µl each) F- 470L 100 cdna synthesis reactions (20 µl each) Table of contents 1. Description...
Human Neuroanatomy. Grades 9-12. Driving Question: How did the evolution of the human brain impact the structure and function it has today?
 Human Neuroanatomy Grades 9-12 Driving Question: How did the evolution of the human brain impact the structure and function it has today? Objectives: Students will be able to Describe the basic parts and
Human Neuroanatomy Grades 9-12 Driving Question: How did the evolution of the human brain impact the structure and function it has today? Objectives: Students will be able to Describe the basic parts and
Random Response Fluctuations Lead to Spurious Paired-Pulse Facilitation
 The Journal of Neuroscience, December 15, 2001, 21(24):9608 9618 Random Response Fluctuations Lead to Spurious Paired-Pulse Facilitation Jimok Kim 1 and Bradley E. Alger 1,2 1 Program in Neuroscience and
The Journal of Neuroscience, December 15, 2001, 21(24):9608 9618 Random Response Fluctuations Lead to Spurious Paired-Pulse Facilitation Jimok Kim 1 and Bradley E. Alger 1,2 1 Program in Neuroscience and
The intermedius nucleus of the medulla: A potential site for the integration of cervical information and the generation of autonomic responses
 The intermedius nucleus of the medulla: A potential site for the integration of cervical information and the generation of autonomic responses 1 Journal of Chemical Neuroanatomy November 2009, 38, pp.
The intermedius nucleus of the medulla: A potential site for the integration of cervical information and the generation of autonomic responses 1 Journal of Chemical Neuroanatomy November 2009, 38, pp.
Biology/ANNB 261 Exam 1 Name Fall, 2006
 Biology/ANNB 261 Exam 1 Name Fall, 2006 * = correct answer. 1. The Greek philosopher Aristotle hypothesized that the brain was a) A radiator for cooling the blood.* b) The seat of the soul. c) The organ
Biology/ANNB 261 Exam 1 Name Fall, 2006 * = correct answer. 1. The Greek philosopher Aristotle hypothesized that the brain was a) A radiator for cooling the blood.* b) The seat of the soul. c) The organ
REVIEW SHEET EXERCISE 3 Neurophysiology of Nerve Impulses Name Lab Time/Date. The Resting Membrane Potential
 REVIEW SHEET EXERCISE 3 Neurophysiology of Nerve Impulses Name Lab Time/Date ACTIVITY 1 The Resting Membrane Potential 1. Explain why increasing extracellular K + reduces the net diffusion of K + out of
REVIEW SHEET EXERCISE 3 Neurophysiology of Nerve Impulses Name Lab Time/Date ACTIVITY 1 The Resting Membrane Potential 1. Explain why increasing extracellular K + reduces the net diffusion of K + out of
Introduction to Flow Cytometry
 Introduction to Flow Cytometry presented by: Flow Cytometry y Core Facility Biomedical Instrumentation Center Uniformed Services University Topics Covered in this Lecture What is flow cytometry? Flow cytometer
Introduction to Flow Cytometry presented by: Flow Cytometry y Core Facility Biomedical Instrumentation Center Uniformed Services University Topics Covered in this Lecture What is flow cytometry? Flow cytometer
THE SPINAL CORD AND THE INFLUENCE OF ITS DAMAGE ON THE HUMAN BODY
 THE SPINAL CORD AND THE INFLUENCE OF ITS DAMAGE ON THE HUMAN BODY THE SPINAL CORD. A part of the Central Nervous System The nervous system is a vast network of cells, which carry information in the form
THE SPINAL CORD AND THE INFLUENCE OF ITS DAMAGE ON THE HUMAN BODY THE SPINAL CORD. A part of the Central Nervous System The nervous system is a vast network of cells, which carry information in the form
2006 7.012 Problem Set 6 KEY
 2006 7.012 Problem Set 6 KEY ** Due before 5 PM on WEDNESDAY, November 22, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You create an artificial
2006 7.012 Problem Set 6 KEY ** Due before 5 PM on WEDNESDAY, November 22, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You create an artificial
PROTOCOL. Immunocytochemistry (ICC) MATERIALS AND EQUIPMENT REQUIRED
 PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
Pyramidal neurons: dendritic structure and synaptic integration
 Pyramidal neurons: dendritic structure and synaptic integration Nelson Spruston Abstract Pyramidal neurons are characterized by their distinct apical and basal dendritic trees and the pyramidal shape of
Pyramidal neurons: dendritic structure and synaptic integration Nelson Spruston Abstract Pyramidal neurons are characterized by their distinct apical and basal dendritic trees and the pyramidal shape of
SmartFlare RNA Detection Probes: Principles, protocols and troubleshooting
 Technical Guide SmartFlare RNA Detection Probes: Principles, protocols and troubleshooting Principles of SmartFlare technology RNA detection traditionally requires transfection, laborious sample prep,
Technical Guide SmartFlare RNA Detection Probes: Principles, protocols and troubleshooting Principles of SmartFlare technology RNA detection traditionally requires transfection, laborious sample prep,
AP Biology 2015 Free-Response Questions
 AP Biology 2015 Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online home
AP Biology 2015 Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online home
Protein Protein Interaction Networks
 Functional Pattern Mining from Genome Scale Protein Protein Interaction Networks Young-Rae Cho, Ph.D. Assistant Professor Department of Computer Science Baylor University it My Definition of Bioinformatics
Functional Pattern Mining from Genome Scale Protein Protein Interaction Networks Young-Rae Cho, Ph.D. Assistant Professor Department of Computer Science Baylor University it My Definition of Bioinformatics
Lab #6: Neurophysiology Simulation
 Lab #6: Neurophysiology Simulation Background Neurons (Fig 6.1) are cells in the nervous system that are used conduct signals at high speed from one part of the body to another. This enables rapid, precise
Lab #6: Neurophysiology Simulation Background Neurons (Fig 6.1) are cells in the nervous system that are used conduct signals at high speed from one part of the body to another. This enables rapid, precise
Nerves and Conduction of Nerve Impulses
 A. Introduction 1. Innovation in Cnidaria - Nerve net a. We need to talk more about nerves b. Cnidaria have simple nerve net - 2 way conduction c. Basis for more complex system in Vertebrates B. Vertebrate
A. Introduction 1. Innovation in Cnidaria - Nerve net a. We need to talk more about nerves b. Cnidaria have simple nerve net - 2 way conduction c. Basis for more complex system in Vertebrates B. Vertebrate
Diagrams and Graphs of Statistical Data
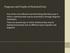 Diagrams and Graphs of Statistical Data One of the most effective and interesting alternative way in which a statistical data may be presented is through diagrams and graphs. There are several ways in
Diagrams and Graphs of Statistical Data One of the most effective and interesting alternative way in which a statistical data may be presented is through diagrams and graphs. There are several ways in
Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service
 Introduction into Flow Cytometry Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service How does a FACS look like? FACSCalibur FACScan What is Flow Cytometry?
Introduction into Flow Cytometry Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service How does a FACS look like? FACSCalibur FACScan What is Flow Cytometry?
DISSECTION OF THE SHEEP'S BRAIN
 DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach you one of the great methods of studying
DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach you one of the great methods of studying
An Overview of Cells and Cell Research
 An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
NEURON AND NEURAL TRAMSMISSION: ANATOMY OF A NEURON. created by Dr. Joanne Hsu
 NEURON AND NEURAL TRAMSMISSION: ANATOMY OF A NEURON NEURON AND NEURAL TRAMSMISSION: MICROSCOPIC VIEW OF NEURONS A photograph taken through a light microscope (500x) of neurons in the spinal cord. NEURON
NEURON AND NEURAL TRAMSMISSION: ANATOMY OF A NEURON NEURON AND NEURAL TRAMSMISSION: MICROSCOPIC VIEW OF NEURONS A photograph taken through a light microscope (500x) of neurons in the spinal cord. NEURON
Tinnitus and the Brain
 Tinnitus and the Brain Dirk De Ridder & Berthold Langguth Moving animals have developed a brain in order to reduce the inherent uncertainty present in an ever changing environment. The auditory system
Tinnitus and the Brain Dirk De Ridder & Berthold Langguth Moving animals have developed a brain in order to reduce the inherent uncertainty present in an ever changing environment. The auditory system
NERVOUS SYSTEM B 1. Which of the following is controlled by the somatic nervous system? A. rate of heartbeat B. contraction of skeletal muscles C.
 NERVOUS SYSTEM B 1. Which of the following is controlled by the somatic nervous system? A. rate of heartbeat B. contraction of skeletal muscles C. increased blood flow to muscle tissue D. movement of food
NERVOUS SYSTEM B 1. Which of the following is controlled by the somatic nervous system? A. rate of heartbeat B. contraction of skeletal muscles C. increased blood flow to muscle tissue D. movement of food
How To Understand The Hypothalamus
 883 Hypothalamus HYPOTHALAMUS Introduction The hypothalamus is a very small, but extremely important part of the diencephalon that is involved in the mediation of endocrine, autonomic and behavioral functions.
883 Hypothalamus HYPOTHALAMUS Introduction The hypothalamus is a very small, but extremely important part of the diencephalon that is involved in the mediation of endocrine, autonomic and behavioral functions.
Philemon S. Yang, Badr A. Alseikhan, Hakim Hiel, Masayuki X. Mori, Wanjun Yang, Paul A. Fuchs, and David T. Yue
 Supplemental Data Switching of Ca 2+ -dependent inactivation of Ca V 1.3 channels by calcium binding proteins of auditory hair cells Yang et al (2006) J Neurosci 26:10677-10689 Philemon S. Yang, Badr A.
Supplemental Data Switching of Ca 2+ -dependent inactivation of Ca V 1.3 channels by calcium binding proteins of auditory hair cells Yang et al (2006) J Neurosci 26:10677-10689 Philemon S. Yang, Badr A.
huwentoxin-iv; TTX, tetrodotoxin; DRG, dorsal root ganglia; HWTX-I, huwentoxin-i; HEK293, human embryonic kidney 293; WT, wild type.
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 40, pp. 27300 27313, October 3, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Tarantula Huwentoxin-IV
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 40, pp. 27300 27313, October 3, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Tarantula Huwentoxin-IV
