PA Standards Covered: Unifying Themes: Describe patterns of change in nature, physical, and man-made systems.
|
|
|
- Hector Osborne
- 7 years ago
- Views:
Transcription
1 Christopher Warner Title: Element Project Educational Filters: The following factors determine the implementation of this unit. o School District of Philadelphia Core Curriculum o PA State Science Standards o National Science Standards o Former Student Feedback Regarding Applicability in High School Courses Goals/Objectives: - Explain the repeating pattern of chemical properties by using the repeating patterns of atomic structure within the periodic table. - Explain how structure determines function. - Describe atoms as composed of even smaller sub-atomic structures. - Name the parts of an atom. - Describe the relationship between numbers of protons and neutrons and atomic number. - Explain how elements are arranged in the periodic table. - Calculate atomic masses. - Convert units. - Use algebraic formulas to determine number of atoms in a sample. PA Standards Covered: Unifying Themes: Describe patterns of change in nature, physical, and man-made systems Inquiry and Design: Apply knowledge and understanding about the nature of scientific and technological knowledge Physical Science : Explain concepts about the structure and properties of matter. National Science Education Standards Covered Science Teaching Standard A o Work together as colleagues within and across disciplines. Science Inquiry Content Standard A o Develop abilities to do scientific inquiry o Understand scientific inquiry Physical Science Content Standard B o Develop an understanding of properties and changes of properties in matter
2 Background Information Early ideas of the atom began with Greek philosophy, but it was not until the early 19 th century that evidence of the existence of atoms began to be provided. Charles Dalton proposed a hypothesis about the existence of atoms that was based on experiments by the French chemist, Antoine Lavoisier. Lavoisier tested the idea that during chemical reactions, matter cannot be created nor destroyed. This idea became the law of conservation of mass. Another French chemist that Dalton considered was Joseph Proust, who observed the idea that some substances (later called compounds) always have elements in the same ratio by mass. This idea is now known as the law of definite proportions (Brady, 1988). Dalton explained these findings by hypothesizing that all matter is composed of atoms, all atoms of the same element are identical, atoms of different atoms are different, and that atoms unite in definite ratios to form compounds. These findings are all true except the idea that all atoms of the same element are identical. Atoms have different varieties called isotopes because different atoms of the same element can have a different number of parts called neutrons (Brady, 1988). Dalton also formed a law that suggests that the ratio of masses of one element that combine with a constant mass of another element can be expressed in small whole numbers. This law was not based on experimental data until another French chemist J.L. Gay-Lussac worked with gas reactions. He observed that volumes of reacting gases were in the ratio of small whole numbers. Amadeo Avogadro, an Italian physicist, supported Gay-Lussac s work with Dalton s theory. Avogadro proposed equal volumes of gases, under the same conditions, have the same number of molecules (Brady, 1988).
3 In the late 19 th century the English scientist Michael Faraday found that chemical changes occur when an electrical current is passed through certain chemical solutions. These experiments were made using gas discharge tubes, glass tubes with a gas at low pressure. J.J. Thomson discovered electrons experimenting with similar tubes called cathode ray tubes, which are streams of electrons. He measured the bending of the path of cathode rays and was then able to determine the ratio of the electron s charge to its mass. The proton was also discovered experimenting with cathode ray tubes. Rays traveled in the direction opposite to that traveled by the cathode rays. These rays had an opposite charge and had a mass 1800 times that of electrons (Smoot, 1987). The English physicist Ernest Rutherford predicted the existence of a third type of particle found in atoms. Walter Bothe and James Chadwick, who repeated Bothe s work, found high energy particles with no charge and a similar mass as the proton. This particle is now known as neutrons (Smoot, 1987). J.J. Thomson also noticed two kinds of neon atoms that were exactly alike chemically, but different in mass. These different types of atoms of the same element are called isotopes. Another English scientist, Henry Mosely, discovered the wavelength of X-rays produced in an X-ray tube depends on the number of protons in the nucleus of the atom. The number of protons was always the same for a specific element. This number of protons, the atomic number, is the element identifier. The mass difference of isotopes caused by the different numbers of neutrons in the nucleus, which determines the particular isotope of the element (Smoot, 1987). Ernest Rutherford and a younger Danish scientist, Niels Bohr, teamed up to construct a model that incorporated their ideas. They made observations from an
4 experiment conducted by Hans Geiger and Ernest Marsden that involved sending subatomic particles through a thin sheet of gold foil. Rutheford concluded that atoms were composed of mostly empty space, a small, dense structure in the center called a nucleus, and negatively charged particles called electrons traveling around the nucleus. The Rutherford-Bohr model is commonly referred to as the planetary model because the electrons orbit around the nucleus in the way that planets revolve around the sun (Smoot, 1987). Through experiments involving substances that are exposed to energy, known as spectroscopy, Bohr discovered that electrons travel around the nucleus in energy levels. When electrons went to lower energy levels, light was emitted and could be studied. Also, when electrons went to higher energy levels, light was absorbed in the same areas in the spectrum and could be studied (Smoot, 1987). Modern atomic theory states that electrons travel around the nucleus in electron clouds where their location and speed cannot be determined. Only the probability of where these electrons are can be calculated (Smoot, 1987). Initial Activity for History Perspective Students will be broken into groups. Each group will be assigned one of the following atomic models: Dalton s Model, Thomson s Model, Rutherford s Model, Bohr s Model, or the Modern Atomic Model. They will each give power point presentations that answer the following questions: 1. What did they find out about atoms? 2. What experiments did they use to support their ideas? 3. What ideas were correct or incorrect?
5 Activity A Instructor should go through notes provided and incorporate exercises so that students become comfortable with atomic number, mass number, atomic mass, and the parts of the atom. Exercises and examples can be found at the end of this outline. NOTES: OBJECTIVE: Describe the size of an atom. Name the parts of the atom. 1) Size of atoms a) How small are they? i) Cannot be seen by normal microscope (smaller than microscopic) ii) Diameter = 3 X 10-8 cm (of an aluminum atom) iii) Size compared to object we know (tin foil example, p.88) 2) Parts of an atom a) Mostly empty space b) Nucleus i) Found at the center of the atom ii) Very small size of a pinhead, if an atom was the size of a football stadium iii) Very high density lot of mass in a very small space (grape analogy p. 89, If a nucleus was the size of a grape, it would weigh 18 billion pounds) iv) Most of an atom s mass is found in the nucleus; v) Made of particles called protons and neutrons (1) Protons (a) Positively charged particles (b) P for positive (c) Protons have a charge of positive 1, charge of one proton = +1 (i) Charge of 5 protons = +5 (d) Have a mass of 1 amu (atomic mass unit) (e) Why are amu s used instead of grams? They re easier to work with mathematically. (i) Ex. Mass of 5 protons= 5 amu s (atomic mass units) (f) Sign of protons: p+ (2) Neutrons (a) Particles with no charge, neutral (b) N for neutral (c) Have a mass of 1 amu (d) Mass of 300 neutrons? 300 amu s c) Outside the Nucleus i) Electrons (1) Negatively charged particles (2) Each electron has a negative charge of negative1 (charge of one electron = -1) (a) Charge of 5 electrons = -5 (3) Very low mass, negligible in calculating mass of atoms (electrons are not used to calculate the mass of an atom)
6 (4) Sign/symbol of electron: e - 3) Different Atoms different elements, they all have different properties a) How is every element different? i) Atomic Number (1) Atoms of every different element have their own number of protons (2) #of protons = atomic number (3) # of protons is the sure way to identify an element. (4) Exercise (look to p. 154) (a) Atomic # of each, draw the box from the periodic table: (b) Carbon - C (c) Oxygen - O (d) Gold Au (e) Mercury - Hg (f) Your own example? (i) Identify the element 1. #of protons = a. 47 b. 28 c. 2 b) Isotopes i) Isotopes are atoms of the same element. ii) Isotopes have the same number of protons, but they do not have the same number of neutrons. iii) Example: Hydrogen-1 has no neutrons. Hydrogen-2 has 1 neutron. They are atoms of the same element (same atomic #), but they are not exactly the same. iv) Mass Number (1) The mass of a single atom. (2) Mass number = # of protons + # of neutrons mass = p + n n = mass " p (3) Mass numbers are used to name isotopes. (a) Way they are named: Name of element mass # (b) Example: Fig.5 p. 92 (4) Exercise-mass numbers of different atoms; Figure out number of protons and neutrons in each isotope: (a) Carbon-14: (b) Lithium-5: (c) Helium-4: c) Atomic Mass i) Weighted average of the masses of all the naturally occurring isotopes of that element ii) What does this statement mean? (1) Average mass of all the atoms of a single element found in the world. iii) Math focus (1) Calculate the atomic mass of boron, which occurs naturally as 20% boron-10 and 80% boron-11. OBJECTIVE: Explain why elements in a group often have similar properties. 1) What causes elements in a group to have similar properties? a) The reason is the number of electrons in the atoms outer shell/level (valence electrons). b) Why would these valence electrons cause similar properties?
7 i) The number of electrons in the outer shell/level of an atom determines how it behaves and how it can bond to other atoms. 2) Number of electrons allowed in each shell for the main group of elements: a) 1 st shell 2 electrons b) 2 nd shell 8 electrons c) 3 rd shell 8 electron 3) Calculating the valence electrons of an element/atom a) 1 st find out the number of electrons in the atom i) If an atom is neutral (no charge), how many electrons are in the atom? The number of electrons must equal the number of protons if the atom is neutral (no charge.) b) 2 nd figure out how many electrons go into each shell c) 3 rd count the amount of electrons in the outer shell d) Examples of neutral atoms: i) Helium ii) Lithium iii) Carbon iv) Magnesium v) Neon vi) Oxygen *Look at PT/answers Assessment of Activities in Notes: 1. Number of protons given: What is the charge and the mass as a result of the protons assuming the mass of a proton is 1 amu? a. Example: 8 protons charge is +8; mass is 8 amu 2. Number of neutrons given: What is the charge and the mass as a result of the neutrons assuming the mass of a neutron is 1 amu? a. Example: 5 neutrons charge is 0; mass is 5 amu 3. Number of electrons given: What is the charge and the mass as a result of the electrons assuming the mass of an electron to be 0 amu? a. Example: 6 electrons charge is -6; mass is 0 amu 4. Element given: Find the number of protons a. Example: Helium number of protons is two 5. Element given: Find the atomic number a. Example: Lithium atomic number is 3 6. Atomic number given: Find the element. a. Example: Atomic number is 5 Element is Boron 7. Number of protons given: Find the element.
8 a. Example: number of protons is 6 Carbon 8. Isotope name given: Determine the mass of the atom. a. Example: Carbon -12; mass = 12 amu 9. Isotope name given: Determine the number of protons, neutrons, and electrons if the atom is neutral. a. Example: Carbon-14; # of protons = 6, # of neutrons = 8, # of electrons = Mass percentages given: Determine the atomic mass of the element. a. Example: If Boron-10 accounts for 20% of Boron found in nature, and Boron-11 accounts for 80%, what is the atomic mass of Boron? i. (.8)(11amu) + (.2)(10amu) = 10.8 amu 11. Element given: Determine the number of valence electrons assuming the atom is neutral. a. Example: Sodium - # of valence electrons = 1 Differentiated Instruction for Notes: Make sure to assist students with learning difficulties and provide the typed out steps above for solving each type of problem in the notes. Don t provide answers unless difficulties persist and give more problems to the student. Activity B Element Project Each student will choose an element and complete a project regarding their element. Artifacts: Each student will draw, design, or make a model of an atom of their element. Each student will also type up a report on their element that meet a list of requirements. -Atomic Model Aspect of Element Project -Diagram of atom with correct number of protons, neutrons, electrons, and valence electrons -Calculations or shown work for number of protons, neutrons, electrons, and valence electrons -Research on element such as where it is found in nature and its uses -List of some elements that bond to students sample -Requirements for Typed Aspect Element Project -Atomic Number
9 - Student must look at the periodic table to find the atomic number. It s the number found above the chemical symbol. -Atomic Weight - Student must research the percent weight of each isotope of their element. Limit the number of isotopes to five, and make sure to instruct the students that the percentages must sum up to 100%. -Number of protons -Number of protons = atomic number -Number of neutrons -Students must choose an isotope because different isotopes of the same element have a different number of neutrons. # of neutrons = mass number - # of protons. -Number of electrons -The number of electrons equals the number of protons if the element is neutral. -Number of valence electrons -The number of valence electrons can be determined by using the following instructions: 1 st find out the number of electrons in the atom 2 nd figure out how many electrons go into each shell 3 rd count the amount of electrons in the outer shell -Calculation of number of atoms in a 5 gram sample Example : Carbon MassOfCarbonAtom(g) =12amu* 1x10"24 g 1amu =1.2x10"23 g MassOfSample #ofatoms = MassOfCarbonAtom = 5g 1.2x10 = "23 5x1023 CarbonAtoms -Uses of elements This research would include where the atom is found and its industrial uses. -List of elements that can bond -Compound Formulas This is just an exercise to introduce students as to how to write compounds.
10 Differentiated Instruction for Activity B -Students with learning difficulties can simply do a research project on an element and the common compounds that it is found in. Activity C Website Activity Each student will be required to design a webpage that contains the information provided in the Element Project (Activity B) including atomic number, atomic mass, melting point, boiling point, where the element is found, the element s uses in industry, and how the element was discovered. This part of the unit is cross-curricular in that students need to work in a computer lab. Web design software is needed, and the assistance of a computer/technology instructor may be necessary in assisting the students in designing their pages. Activity D Inquiry Activity Have students conduct their own investigation using a scientific method where they test for the physical and/or chemical properties of a Main Group element or a compound that is seen in their everyday lives with that element present in the compound. Inquiry requirement in conclusion: Why do elements possess certain properties? Why do other elements have similar properties? The goal is to see whether students can incorporate what they know about electrons and, in particular, valence electrons to explain why groups of elements have similar properties. Time must be allotted for students to discuss some of their research, their findings, and the periodic table. The teacher should try to limit the guidance given to the students, however they should make it available if the class as a whole struggles with the conclusion. - Some elements will not be practical if they cannot be found in safe sample, so compounds can be used as long as it is explained why certain elements cannot be tested and why compounds have different properties. - One of the main objectives is that students use a system or a method to figure out some simple properties of substances such as density, state of matter, or melting point. The method should include research, procedures, variables, observations, and a conclusion that explains why elements and compounds have certain properties. Assessment of Activity D
11 REUBRIC Title/Problem Statement -Properties being diagnosed are introduced and labeled. Research -Properties of elements vs. properties of compounds are explained. Each property being tested is defined and discussed in a way that will lead to students making a logical procedure to find out that property. Procedure -The procedure is logical and practical in finding out the properties. Variables -Measurements and conditions that are not the result of the procedure are labeled as independent variables. The measurements that are a result of the procedure are labeled as dependent. Observations -The observations are listed and any work required to calculate the property is shown. Conclusion -The conclusion explains WHY these elements or compounds possess certain properties. The student discusses how structure determines function. 5 points 25 points 25 points 10 points 10 points 25 points Bibliography 1. Brady, J. Holum. J. Fundamentals of Chemistry John Wiley and Sons. p Smoot, R. Price, J. Smith, R. Chemistry: A Modern Course p , 120, 349.
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
5.1 Evolution of the Atomic Model
 5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
NOTES ON The Structure of the Atom
 NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
Atomic Calculations. 2.1 Composition of the Atom. number of protons + number of neutrons = mass number
 2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
Development of the Atomic Theory
 Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
The Models of the Atom
 The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Atomic Theory Part 1
 Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
For convenience, we may consider an atom in two parts: the nucleus and the electrons.
 Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
Unit 1 Practice Test. Matching
 Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
The Structure of the Atom
 The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
Atomic Theory: History of the Atom
 Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS
 Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Instructors Guide: Atoms and Their Isotopes
 Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
About the course GENERAL CHEMISTRY. Recommended literature: Chemistry: science of the matter. Responsible for the course: Dr.
 About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Chemistry. The student will be able to identify and apply basic safety procedures and identify basic equipment.
 Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Introduction to Chemistry Exam 2 Practice Problems 1 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1.Atoms consist principally of what three
Introduction to Chemistry Exam 2 Practice Problems 1 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1.Atoms consist principally of what three
Atomic structure. Resources and methods for learning about these subjects (list a few here, in preparation for your research):
 Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Answers to Review Questions for Atomic Theory Quiz #1
 Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
CH3 Stoichiometry. The violent chemical reaction of bromine and phosphorus. P.76
 CH3 Stoichiometry The violent chemical reaction of bromine and phosphorus. P.76 Contents 3.1 Counting by Weighing 3.2 Atomic Masses 3.3 The Mole 3.4 Molar Mass 3.5 Percent Composition of Compounds 3.6
CH3 Stoichiometry The violent chemical reaction of bromine and phosphorus. P.76 Contents 3.1 Counting by Weighing 3.2 Atomic Masses 3.3 The Mole 3.4 Molar Mass 3.5 Percent Composition of Compounds 3.6
Nuclear Structure. particle relative charge relative mass proton +1 1 atomic mass unit neutron 0 1 atomic mass unit electron -1 negligible mass
 Protons, neutrons and electrons Nuclear Structure particle relative charge relative mass proton 1 1 atomic mass unit neutron 0 1 atomic mass unit electron -1 negligible mass Protons and neutrons make up
Protons, neutrons and electrons Nuclear Structure particle relative charge relative mass proton 1 1 atomic mass unit neutron 0 1 atomic mass unit electron -1 negligible mass Protons and neutrons make up
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Bohr s Model of the Atom
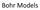 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
Matter. Atomic weight, Molecular weight and Mole
 Matter Atomic weight, Molecular weight and Mole Atomic Mass Unit Chemists of the nineteenth century realized that, in order to measure the mass of an atomic particle, it was useless to use the standard
Matter Atomic weight, Molecular weight and Mole Atomic Mass Unit Chemists of the nineteenth century realized that, in order to measure the mass of an atomic particle, it was useless to use the standard
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms and Elements [6th grade]
![Atoms and Elements [6th grade] Atoms and Elements [6th grade]](/thumbs/40/21183873.jpg) Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, jwray@alum.trinity.edu
Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, jwray@alum.trinity.edu
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
CHAPTER 4: ATOMS AND ELEMENTS
 CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
The Mole Concept and Atoms
 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 4 24 September 2013 Calculations and the Chemical Equation The Mole Concept and Atoms Atoms are exceedingly
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 4 24 September 2013 Calculations and the Chemical Equation The Mole Concept and Atoms Atoms are exceedingly
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004. Name (print) SSN
 CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS
 ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
Chemistry - Elements Electron Configurations The Periodic Table. Ron Robertson
 Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Introduction to Chemistry
 1 Copyright ç 1996 Richard Hochstim. All rights reserved. Terms of use. Introduction to Chemistry In Chemistry the word weight is commonly used in place of the more proper term mass. 1.1 Atoms, Ions, and
1 Copyright ç 1996 Richard Hochstim. All rights reserved. Terms of use. Introduction to Chemistry In Chemistry the word weight is commonly used in place of the more proper term mass. 1.1 Atoms, Ions, and
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D
 Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
The Mole Concept. The Mole. Masses of molecules
 The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
Atoms, Elements, and the Periodic Table (Chapter 2)
 Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Chemical Calculations: The Mole Concept and Chemical Formulas. AW Atomic weight (mass of the atom of an element) was determined by relative weights.
 1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
Chemical Calculations: Formula Masses, Moles, and Chemical Equations
 Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
Greatest Discoveries With Bill Nye: Chemistry Teacher s Guide
 Teacher s Guide Grade Level: 6 8 Curriculum Focus: Physical Science Lesson Duration: Two class periods Program Oxygen and Atoms Explore atomic and molecular structure and see how oxygen was first isolated.
Teacher s Guide Grade Level: 6 8 Curriculum Focus: Physical Science Lesson Duration: Two class periods Program Oxygen and Atoms Explore atomic and molecular structure and see how oxygen was first isolated.
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
Unit 2 Atomic Structure
 Unit 2 Atomic Structure Big Idea: Atomic structure explains patterns in the behavior of elements and allows us to predict the chemical and physical behavior of a given element. The organization of elements
Unit 2 Atomic Structure Big Idea: Atomic structure explains patterns in the behavior of elements and allows us to predict the chemical and physical behavior of a given element. The organization of elements
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
What s in a Mole? Molar Mass
 LESSON 10 What s in a Mole? Molar Mass OVERVIEW Key Ideas Lesson Type Lab: Groups of 4 Chemists compare moles of substances rather than masses because moles are a way of counting atoms. When considering
LESSON 10 What s in a Mole? Molar Mass OVERVIEW Key Ideas Lesson Type Lab: Groups of 4 Chemists compare moles of substances rather than masses because moles are a way of counting atoms. When considering
Chapter 2 Atoms and Molecules
 Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
 Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Review for Atomic Theory Quiz #1
 Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
Chemical Changes. Measuring a Chemical Reaction. Name(s)
 Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
An Atom Apart by Leslie Cargile
 Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Ernest Rutherford Atomic Model 1911. Plum Pudding Model J.J. Thomson 1897
 1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Regents Review Atom & PT Part 2 Worksheet Mr. Beauchamp
 Regents Review Atom & PT Part 2 Worksheet Mr. Beauchamp Base your answers to questions 1 through 3 on the information below. The accepted values for the atomic mass and percent natural abundance of each
Regents Review Atom & PT Part 2 Worksheet Mr. Beauchamp Base your answers to questions 1 through 3 on the information below. The accepted values for the atomic mass and percent natural abundance of each
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1. A chemical equation. (C-4.4)
 Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
