Phys 234H Practice Final Exam (Note: this practice exam contains more questions than will the final, which will have 25 multiple-choice questions.
|
|
|
- Curtis Gray
- 9 years ago
- Views:
Transcription
1 Phys 234H Practice Final Exam (Note: this practice exam contains more questions than will the final, which will have 25 multiple-choice questions. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A kg block on a horizontal frictionless surface is attached to an ideal massless spring whose spring constant is The block is pulled from its equilibrium position at x = 0.00 m to a displacement x = m and is released from rest. The block then executes simple harmonic motion along the horizontal x-axis. When the displacement is what is the kinetic energy of the block? 1) A) 0.52 J B) 0.41 J C) 0.49 J D) 0.44 J E) 0.46 J 2) A long thin uniform rod of length 1.50 m is to be suspended from a frictionless pivot located at some point along the rod so that its pendulum motion takes 3.00 s. How far from the center of the rod should the pivot be located? 2) A) 8.40 cm B) 8.73 cm C) 23.4 cm D) 7.98 cm E) 7.52 cm 3) Four traveling waves are described by the following equations, where all quantities are measured in SI units and y represents the displacement. I: y = 0.12 cos(3x + 2t) II: y = 0.15 sin(6x - 3t) III: y = 0.23 cos(3x + 6t) IV: y = sin(1.5x - t) Which of these waves have the same speed? 3) A) I and II B) III and IV C) I and III D) I and IV E) II and III 4) A heavy stone of mass m is hung from the ceiling by a thin 8.25-g wire that is 65.0 cm long. When you gently pluck the upper end of the wire, a pulse travels down the wire and returns 7.84 ms later, having reflected off the lower end. The stone is heavy enough to prevent the lower end of the wire from moving. What is the mass m of the stone? 4) A) 8.90 kg B) 349 kg C) 227 kg D) 35.6 kg E) 23.1 kg 5) When light goes from one material into another material having a HIGHER index of refraction 5) A) its speed increases, its wavelength decreases, and its frequency stays the same. B) its speed decreases but its wavelength and frequency both increase. C) its speed decreases but its frequency and wavelength stay the same. D) its speed and wavelength decrease, but its frequency stays the same. E) its speed, wavelength, and frequency all decrease. 6) The speed of light in a material is What is the critical angle of a light ray at the interface between the material and a vacuum? 6) A) 24 B) 30 C) 27 D) 21 7) A convex lens has a focal length f. An object is placed at a distance between f and 2f on a line perpendicular to the center of the lens. The image formed is located at what distance from the lens? 7) A) between the lens and f B) between f and 2f C) farther than 2f D) 2f
2 E) f 8) A goldfish bowl is spherical, 8.0 cm in radius. A goldfish is swimming 3.0 cm from the wall of the bowl. Where does the fish appear to be to an observer outside? The index of refraction of water is Neglect the effect of the glass wall of the bowl. 8) A) 3.9 cm inside the bowl B) 1.7 cm inside the bowl C) 3.0 cm inside the bowl D) 3.3 cm inside the bowl E) 2.5 cm inside the bowl 9) In a double-slit experiment, if the slit separation is increased, which of the following happens to the interference pattern shown on the screen? 9) A) The maxima stay at the same position. B) The minima and maxima stay at the same position. C) The maxima get further apart. D) The minima get closer together. E) The minima stay at the same position. 10) At most, how many bright fringes can be formed on each side of the central bright fringe (not counting the central bright fringe) when light of 625 nm falls on a double slit whose spacing is 10) A) 1 B) 2 C) 3 D) 4 E) 5 11) A thin layer of oil (n = 1.25) is on top of a puddle of water (n = 1.33). If normally incident 500-nm light is strongly reflected, what is the minimum nonzero thickness of the oil layer? 11) A) 200 nm B) 100 nm C) 150 nm D) 400 nm E) 250 nm 12) A slit of width 2.0 μm is used in a single slit experiment with light of wavelength 650 nm. If the intensity at the central maximum is I0, what is the intensity 10 from the center? 12) A) 0.43I0 B) 0.50I0 C) 0.53I0 D) 0.35I0 E) 0.030I0 13) When monochromatic light illuminates a grating with 7000 lines per centimeter, its second order maximum is at What is the wavelength of the light? 13) A) 452 nm B) 363 nm C) 752 nm D) 336 nm E) 633 nm 14) A rocket is moving at 1/4 the speed of light relative to Earth. At the center of this rocket, a light suddenly flashes. To an observer at rest on Earth 14) A) the light will reach the front of the rocket before it reaches the back of the rocket. B) the light will reach the front of the rocket after it reaches the back of the rocket. C) the light will reach the front of the rocket at the same instant that it reaches the back of the rocket. 15) A set of twins, Andrea and Courtney, are initially 10 years old. While Courtney remains on Earth, Andrea rides on a spaceship which travels away from Earth at a speed of 0.60c for five years (as measured by Courtney), then immediately turns around and comes back at 0.60c. When Andrea returns, Courtney is 20 years old. How old is Andrea upon her return? 15)
3 A) 12 y B) 10 y C) 20 y D) 18 y E) 15 y 16) In a certain particle accelerator, a proton has a kinetic energy that is equal to its rest energy. What is the speed of the proton relative to the accelerator? 16) A) 0.87c B) 0.50c C) 0.71c D) 0.25c E) 0.75c 17) A proton in a certain particle accelerator has a kinetic energy that is equal to its rest energy. What is the momentum of the proton as measured by a physicist working with the accelerator? (c = m/s, m proton = kg) 17) A) kg m/s B) kg m/s C) kg m/s D) kg m/s E) kg m/s 18) Monochromatic light strikes a metal surface and electrons are ejected from the metal. If the intensity of the light is increased, what will happen to the ejection rate and maximum energy of the electrons? 18) A) greater ejection rate; same maximum energy B) same ejection rate; same maximum energy C) same ejection rate; greater maximum energy D) greater ejection rate; greater maximum energy 19) Light of wavelength 400 nm falls on a metal surface having a work function 1.70 ev. What is the maximum kinetic energy of the photoelectrons emitted from the metal? (c = m/s, h = J s = ev s, 1 ev = J) 19) A) 1.41 ev B) 3.11 ev C) 4.52 ev D) 2.82 ev E) 1.70 ev 20) An electron inside a hydrogen atom is confined to within a space of nm. What is the minimum uncertainty in the electron's velocity? ( h = J s, mel = kg) 20) A) m/s B) m/s C) m/s D) m/s E) m/s 21) At absolute temperature T, a black body radiates its peak intensity at wavelength λ. At absolute temperature 2T, what would be the wavelength of the peak intensity? 21) A) λ/2 B) λ/16 C) λ D) 2λ E) 16λ 22) Light shines through atomic hydrogen gas. It is seen that the gas absorbs light readily at a wavelength of nm. What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level. (h = J s, c = m/s, 1 ev = J, the Rydberg constant is R = m-1) 22) A) 14 B) 21 C) 16 D) 11
4 23) An electric current through a tungsten filament maintains its temperature at 2800 K. Assume the tungsten filament behaves as an ideal radiator at that temperature. If the radiating area of the filament is m2, at what rate does it radiate energy? (σ = W/m2 K4, Wien displacement law constant is m K) 23) A) 10 W B) 7.0 W C) 11.5 W D) 5.5 W E) 8.5 W 24) A set of five possible wave functions is given below, where L is a positive real number. ψ1(x) = Ae-x, for all x ψ2(x) = A cos x, for all x ψ3(x) = ψ4(x) = ψ5(x) = Which of the five possible wave functions are normalizable? (There may be more than one correct choice.) 24) A) ψ2(x) B) ψ3(x) C) ψ4(x) D) ψ1(x) E) ψ5(x) 25) Find the value of A to normalize the wave function ψ(x) =. 25) A) 1/L B) 1/ C) 1/L2 D) 1. E) 26) An electron is trapped in an infinite square well (a box) of width Find the wavelength of photons emitted when the electron drops from the n = 5 state to the n = 1 state in this system. (c = m/s, h = J s, mel = kg) 26) A) 5.45 μm B) 6.49 μm C) 5.91 μm D) 7.07 μm 27) If two electrons in the same atom have the same four quantum numbers, then they must have the same energy. 27) A) true B) false C) They cannot both have the same four quantum numbers. 28) An electron in a hydrogen atom has orbital quantum number l = 7. How many possible values of the magnetic quantum number ml could it have? 28) A) 6 B) 15 C) 33 D) 7 E) 98 29) An atom has completely filled inner shells and a single valence electron in an excited p state. The filled inner shells have an orbital momentum equal to zero. What is the magnitude of the orbital angular momentum L of the atom? 29) A) 1.7ħ B) 1.4ħ C) 2.0ħ D) 1.0ħ E) 1.2ħ SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
5 30) The energy of an electron in the p-level of an atom is changed in the presence of a magnetic field of magnitude 4.6 T. What is the difference between the largest and smallest possible energies? (Bohr magneton = μb = J/T) 30) 31) For a solid in which the occupation of the energy states is given by the Fermi-Dirac distribution, the probability that a certain state is occupied at a temperature T0 is If the temperature is doubled to 2T0, what is the probability that the same state is occupied? Assume that the Fermi energy does not change with temperature. 31) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 32) A rotating diatomic molecule has rotational quantum number l. The energy DIFFERENCE between adjacent energy levels 32) A) is independent of l. B) is the same for all changes in l. C) decreases as l increases. D) increases as l increases. 33) A diatomic molecule has ev of rotational energy in the l = 2 quantum state. What is its rotational energy in the l = 1 quantum state? 33) A) 8.7 µev B) 3.4 µev C) 5.3 µev D) 4.1 µev E) 7.8 µev 34) Which of the following statements about the atomic nucleus is correct? (There may be more than one correct choice.) 34) A) All nuclei have nearly the same density. B) Large nuclei are denser than light nuclei. C) As the number of nucleons increases the binding energy per nucleon always increases. D) A nucleus containing 20 nucleons will have approximately twice the radius as a nucleus containing 10 nucleons. E) The nucleus is held together more by the electrical force than by the gravitational force. 35) A radioactive nuclide of atomic number Z emits an electron, then the daughter nuclide emits a gamma ray. What is the atomic number of the resulting nuclide after both processes? 35) A) Z + 1 B) Z + 2 C) Z - 3 D) Z - 2 E) Z ) What would be the expected radius of the nucleus of Sr? (R 0 = 1.2 fm) 36) A) 1.2 fm B) 4.0 fm C) 0.11 pm D) 0.54 pm E) 5.4 fm 37) What is the binding energy per nucleon for Al? The neutral Al atom has a mass of u; a neutral hydrogen atom has a mass of u; a neutron has a mass of u; and a proton has a mass of u. (1 u = MeV/c2) 37) A) 2.8 MeV B) 8.3 MeV C) 3.4 MeV D) 5.4 MeV E) 6.7 MeV 38) A certain substance has a half-life of 5.0 hours. How many nuclei of the substance are required to give an initial activity of 6.0 μci? 1 Ci = 3.7 Bq. 38)
6 A) 5.8 B) 8.5 C) 2.4 D) 6.3 E) ) The stability of Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: He: u K: u Ca: Sc Ti: u u u The Sc nuclide is 39) A) subject to alpha decay only. B) subject to β- decay only. C) not subject to alpha, β+, or β- decay. D) subject to β+ decay only. E) subject to β+ or β- decay, but not to alpha decay.
7 1) D 2) B 3) D 4) D 5) D 6) B 7) C 8) E 9) D 10) C 11) A 12) D 13) E 14) B 15) D 16) A 17) A 18) A 19) A 20) C 21) A 22) A 23) B 24) B, C 25) B 26) B 27) C 28) B 29) B 30) J 31) ) D 33) A 34) A 35) A 36) E 37) B 38) A 39) B
How To Understand Light And Color
 PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
Physics 111 Homework Solutions Week #9 - Tuesday
 Physics 111 Homework Solutions Week #9 - Tuesday Friday, February 25, 2011 Chapter 22 Questions - None Multiple-Choice 223 A 224 C 225 B 226 B 227 B 229 D Problems 227 In this double slit experiment we
Physics 111 Homework Solutions Week #9 - Tuesday Friday, February 25, 2011 Chapter 22 Questions - None Multiple-Choice 223 A 224 C 225 B 226 B 227 B 229 D Problems 227 In this double slit experiment we
Basic Nuclear Concepts
 Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
Nuclear Physics. Nuclear Physics comprises the study of:
 Nuclear Physics Nuclear Physics comprises the study of: The general properties of nuclei The particles contained in the nucleus The interaction between these particles Radioactivity and nuclear reactions
Nuclear Physics Nuclear Physics comprises the study of: The general properties of nuclei The particles contained in the nucleus The interaction between these particles Radioactivity and nuclear reactions
Main properties of atoms and nucleus
 Main properties of atoms and nucleus. Atom Structure.... Structure of Nuclei... 3. Definition of Isotopes... 4. Energy Characteristics of Nuclei... 5. Laws of Radioactive Nuclei Transformation... 3. Atom
Main properties of atoms and nucleus. Atom Structure.... Structure of Nuclei... 3. Definition of Isotopes... 4. Energy Characteristics of Nuclei... 5. Laws of Radioactive Nuclei Transformation... 3. Atom
Review for Test 3. Polarized light. Action of a Polarizer. Polarized light. Light Intensity after a Polarizer. Review for Test 3.
 Review for Test 3 Polarized light No equation provided! Polarized light In linearly polarized light, the electric field vectors all lie in one single direction. Action of a Polarizer Transmission axis
Review for Test 3 Polarized light No equation provided! Polarized light In linearly polarized light, the electric field vectors all lie in one single direction. Action of a Polarizer Transmission axis
A-level PHYSICS (7408/1)
 SPECIMEN MATERIAL A-level PHYSICS (7408/1) Paper 1 Specimen 2014 Morning Time allowed: 2 hours Materials For this paper you must have: a pencil a ruler a calculator a data and formulae booklet. Instructions
SPECIMEN MATERIAL A-level PHYSICS (7408/1) Paper 1 Specimen 2014 Morning Time allowed: 2 hours Materials For this paper you must have: a pencil a ruler a calculator a data and formulae booklet. Instructions
PHYSICS PAPER 1 (THEORY)
 PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light
 AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light Name: Period: Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Reflection,
AP Physics B Ch. 23 and Ch. 24 Geometric Optics and Wave Nature of Light Name: Period: Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Reflection,
Practice Test SHM with Answers
 Practice Test SHM with Answers MPC 1) If we double the frequency of a system undergoing simple harmonic motion, which of the following statements about that system are true? (There could be more than one
Practice Test SHM with Answers MPC 1) If we double the frequency of a system undergoing simple harmonic motion, which of the following statements about that system are true? (There could be more than one
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES. PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points
 TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
226 Chapter 15: OSCILLATIONS
 Chapter 15: OSCILLATIONS 1. In simple harmonic motion, the restoring force must be proportional to the: A. amplitude B. frequency C. velocity D. displacement E. displacement squared 2. An oscillatory motion
Chapter 15: OSCILLATIONS 1. In simple harmonic motion, the restoring force must be proportional to the: A. amplitude B. frequency C. velocity D. displacement E. displacement squared 2. An oscillatory motion
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Practice final for Basic Physics spring 2005 answers on the last page Name: Date:
 Practice final for Basic Physics spring 2005 answers on the last page Name: Date: 1. A 12 ohm resistor and a 24 ohm resistor are connected in series in a circuit with a 6.0 volt battery. Assuming negligible
Practice final for Basic Physics spring 2005 answers on the last page Name: Date: 1. A 12 ohm resistor and a 24 ohm resistor are connected in series in a circuit with a 6.0 volt battery. Assuming negligible
UNIVERSITY OF SASKATCHEWAN Department of Physics and Engineering Physics
 UNIVERSITY OF SASKATCHEWAN Department of Physics and Engineering Physics Physics 111.6 MIDTERM TEST #4 March 15, 2007 Time: 90 minutes NAME: (Last) Please Print (Given) STUDENT NO.: LECTURE SECTION (please
UNIVERSITY OF SASKATCHEWAN Department of Physics and Engineering Physics Physics 111.6 MIDTERM TEST #4 March 15, 2007 Time: 90 minutes NAME: (Last) Please Print (Given) STUDENT NO.: LECTURE SECTION (please
The rate of change of velocity with respect to time. The average rate of change of distance/displacement with respect to time.
 H2 PHYSICS DEFINITIONS LIST Scalar Vector Term Displacement, s Speed Velocity, v Acceleration, a Average speed/velocity Instantaneous Velocity Newton s First Law Newton s Second Law Newton s Third Law
H2 PHYSICS DEFINITIONS LIST Scalar Vector Term Displacement, s Speed Velocity, v Acceleration, a Average speed/velocity Instantaneous Velocity Newton s First Law Newton s Second Law Newton s Third Law
Chem 1A Exam 2 Review Problems
 Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
............... [2] At the time of purchase of a Strontium-90 source, the activity is 3.7 10 6 Bq.
![............... [2] At the time of purchase of a Strontium-90 source, the activity is 3.7 10 6 Bq. ............... [2] At the time of purchase of a Strontium-90 source, the activity is 3.7 10 6 Bq.](/thumbs/40/21837699.jpg) 1 Strontium-90 decays with the emission of a β-particle to form Yttrium-90. The reaction is represented by the equation 90 38 The decay constant is 0.025 year 1. 90 39 0 1 Sr Y + e + 0.55 MeV. (a) Suggest,
1 Strontium-90 decays with the emission of a β-particle to form Yttrium-90. The reaction is represented by the equation 90 38 The decay constant is 0.025 year 1. 90 39 0 1 Sr Y + e + 0.55 MeV. (a) Suggest,
Homework #10 (749508)
 Homework #10 (749508) Current Score: 0 out of 100 Description Homework on quantum physics and radioactivity Instructions Answer all the questions as best you can. 1. Hewitt10 32.E.001. [481697] 0/5 points
Homework #10 (749508) Current Score: 0 out of 100 Description Homework on quantum physics and radioactivity Instructions Answer all the questions as best you can. 1. Hewitt10 32.E.001. [481697] 0/5 points
1) The time for one cycle of a periodic process is called the A) wavelength. B) period. C) frequency. D) amplitude.
 practice wave test.. Name Use the text to make use of any equations you might need (e.g., to determine the velocity of waves in a given material) MULTIPLE CHOICE. Choose the one alternative that best completes
practice wave test.. Name Use the text to make use of any equations you might need (e.g., to determine the velocity of waves in a given material) MULTIPLE CHOICE. Choose the one alternative that best completes
PHYS 101-4M, Fall 2005 Exam #3. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 PHYS 101-4M, Fall 2005 Exam #3 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A bicycle wheel rotates uniformly through 2.0 revolutions in
PHYS 101-4M, Fall 2005 Exam #3 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A bicycle wheel rotates uniformly through 2.0 revolutions in
13- What is the maximum number of electrons that can occupy the subshell 3d? a) 1 b) 3 c) 5 d) 2
 Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
PHYS 222 Spring 2012 Final Exam. Closed books, notes, etc. No electronic device except a calculator.
 PHYS 222 Spring 2012 Final Exam Closed books, notes, etc. No electronic device except a calculator. NAME: (all questions with equal weight) 1. If the distance between two point charges is tripled, the
PHYS 222 Spring 2012 Final Exam Closed books, notes, etc. No electronic device except a calculator. NAME: (all questions with equal weight) 1. If the distance between two point charges is tripled, the
Photons. ConcepTest 27.1. 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy. Which has more energy, a photon of:
 ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
C B A T 3 T 2 T 1. 1. What is the magnitude of the force T 1? A) 37.5 N B) 75.0 N C) 113 N D) 157 N E) 192 N
 Three boxes are connected by massless strings and are resting on a frictionless table. Each box has a mass of 15 kg, and the tension T 1 in the right string is accelerating the boxes to the right at a
Three boxes are connected by massless strings and are resting on a frictionless table. Each box has a mass of 15 kg, and the tension T 1 in the right string is accelerating the boxes to the right at a
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Physical Quantities, Symbols and Units
 Table 1 below indicates the physical quantities required for numerical calculations that are included in the Access 3 Physics units and the Intermediate 1 Physics units and course together with the SI
Table 1 below indicates the physical quantities required for numerical calculations that are included in the Access 3 Physics units and the Intermediate 1 Physics units and course together with the SI
Physics 30 Worksheet # 14: Michelson Experiment
 Physics 30 Worksheet # 14: Michelson Experiment 1. The speed of light found by a Michelson experiment was found to be 2.90 x 10 8 m/s. If the two hills were 20.0 km apart, what was the frequency of the
Physics 30 Worksheet # 14: Michelson Experiment 1. The speed of light found by a Michelson experiment was found to be 2.90 x 10 8 m/s. If the two hills were 20.0 km apart, what was the frequency of the
6) How wide must a narrow slit be if the first diffraction minimum occurs at ±12 with laser light of 633 nm?
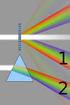 Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
AS COMPETITION PAPER 2008
 AS COMPETITION PAPER 28 Name School Town & County Total Mark/5 Time Allowed: One hour Attempt as many questions as you can. Write your answers on this question paper. Marks allocated for each question
AS COMPETITION PAPER 28 Name School Town & County Total Mark/5 Time Allowed: One hour Attempt as many questions as you can. Write your answers on this question paper. Marks allocated for each question
Chapters 21-29. Magnetic Force. for a moving charge. F=BQvsinΘ. F=BIlsinΘ. for a current
 Chapters 21-29 Chapter 21:45,63 Chapter 22:25,49 Chapter 23:35,38,53,55,58,59 Chapter 24:17,18,20,42,43,44,50,52,53.59,63 Chapter 26:27,33,34,39,54 Chapter 27:17,18,34,43,50,51,53,56 Chapter 28: 10,11,28,47,52
Chapters 21-29 Chapter 21:45,63 Chapter 22:25,49 Chapter 23:35,38,53,55,58,59 Chapter 24:17,18,20,42,43,44,50,52,53.59,63 Chapter 26:27,33,34,39,54 Chapter 27:17,18,34,43,50,51,53,56 Chapter 28: 10,11,28,47,52
PHYSICAL QUANTITIES AND UNITS
 1 PHYSICAL QUANTITIES AND UNITS Introduction Physics is the study of matter, its motion and the interaction between matter. Physics involves analysis of physical quantities, the interaction between them
1 PHYSICAL QUANTITIES AND UNITS Introduction Physics is the study of matter, its motion and the interaction between matter. Physics involves analysis of physical quantities, the interaction between them
Arrangement of Electrons in Atoms
 CHAPTER 4 PRE-TEST Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the following orbital
CHAPTER 4 PRE-TEST Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the following orbital
Physics 9e/Cutnell. correlated to the. College Board AP Physics 1 Course Objectives
 Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
PHYS 211 FINAL FALL 2004 Form A
 1. Two boys with masses of 40 kg and 60 kg are holding onto either end of a 10 m long massless pole which is initially at rest and floating in still water. They pull themselves along the pole toward each
1. Two boys with masses of 40 kg and 60 kg are holding onto either end of a 10 m long massless pole which is initially at rest and floating in still water. They pull themselves along the pole toward each
Boardworks AS Physics
 Boardworks AS Physics Vectors 24 slides 11 Flash activities Prefixes, scalars and vectors Guide to the SI unit prefixes of orders of magnitude Matching powers of ten to their SI unit prefixes Guide to
Boardworks AS Physics Vectors 24 slides 11 Flash activities Prefixes, scalars and vectors Guide to the SI unit prefixes of orders of magnitude Matching powers of ten to their SI unit prefixes Guide to
Light as a Wave. The Nature of Light. EM Radiation Spectrum. EM Radiation Spectrum. Electromagnetic Radiation
 The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
5. The Nature of Light. Does Light Travel Infinitely Fast? EMR Travels At Finite Speed. EMR: Electric & Magnetic Waves
 5. The Nature of Light Light travels in vacuum at 3.0. 10 8 m/s Light is one form of electromagnetic radiation Continuous radiation: Based on temperature Wien s Law & the Stefan-Boltzmann Law Light has
5. The Nature of Light Light travels in vacuum at 3.0. 10 8 m/s Light is one form of electromagnetic radiation Continuous radiation: Based on temperature Wien s Law & the Stefan-Boltzmann Law Light has
Code number given on the right hand side of the question paper should be written on the title page of the answerbook by the candidate.
 Series ONS SET-1 Roll No. Candiates must write code on the title page of the answer book Please check that this question paper contains 16 printed pages. Code number given on the right hand side of the
Series ONS SET-1 Roll No. Candiates must write code on the title page of the answer book Please check that this question paper contains 16 printed pages. Code number given on the right hand side of the
The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS. Friday, June 20, 2014 1:15 to 4:15 p.m.
 P.S./PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Friday, June 20, 2014 1:15 to 4:15 p.m., only The possession or use of any communications device
P.S./PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Friday, June 20, 2014 1:15 to 4:15 p.m., only The possession or use of any communications device
Wave Function, ψ. Chapter 28 Atomic Physics. The Heisenberg Uncertainty Principle. Line Spectrum
 Wave Function, ψ Chapter 28 Atomic Physics The Hydrogen Atom The Bohr Model Electron Waves in the Atom The value of Ψ 2 for a particular object at a certain place and time is proportional to the probability
Wave Function, ψ Chapter 28 Atomic Physics The Hydrogen Atom The Bohr Model Electron Waves in the Atom The value of Ψ 2 for a particular object at a certain place and time is proportional to the probability
MASS DEFECT AND BINDING ENERGY
 MASS DEFECT AND BINDING ENERGY The separate laws of Conservation of Mass and Conservation of Energy are not applied strictly on the nuclear level. It is possible to convert between mass and energy. Instead
MASS DEFECT AND BINDING ENERGY The separate laws of Conservation of Mass and Conservation of Energy are not applied strictly on the nuclear level. It is possible to convert between mass and energy. Instead
Atomic Structure Ron Robertson
 Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS. Wednesday, June 17, 2015 1:15 to 4:15 p.m.
 P.S./PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Wednesday, June 17, 2015 1:15 to 4:15 p.m., only The possession or use of any communications
P.S./PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Wednesday, June 17, 2015 1:15 to 4:15 p.m., only The possession or use of any communications
Physics 2A, Sec B00: Mechanics -- Winter 2011 Instructor: B. Grinstein Final Exam
 Physics 2A, Sec B00: Mechanics -- Winter 2011 Instructor: B. Grinstein Final Exam INSTRUCTIONS: Use a pencil #2 to fill your scantron. Write your code number and bubble it in under "EXAM NUMBER;" an entry
Physics 2A, Sec B00: Mechanics -- Winter 2011 Instructor: B. Grinstein Final Exam INSTRUCTIONS: Use a pencil #2 to fill your scantron. Write your code number and bubble it in under "EXAM NUMBER;" an entry
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Sample Questions for the AP Physics 1 Exam
 Sample Questions for the AP Physics 1 Exam Sample Questions for the AP Physics 1 Exam Multiple-choice Questions Note: To simplify calculations, you may use g 5 10 m/s 2 in all problems. Directions: Each
Sample Questions for the AP Physics 1 Exam Sample Questions for the AP Physics 1 Exam Multiple-choice Questions Note: To simplify calculations, you may use g 5 10 m/s 2 in all problems. Directions: Each
Masses in Atomic Units
 Nuclear Composition - the forces binding protons and neutrons in the nucleus are much stronger (binding energy of MeV) than the forces binding electrons to the atom (binding energy of ev) - the constituents
Nuclear Composition - the forces binding protons and neutrons in the nucleus are much stronger (binding energy of MeV) than the forces binding electrons to the atom (binding energy of ev) - the constituents
Sample Exercise 6.1 Concepts of Wavelength and Frequency
 Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
PHYA5/1. General Certificate of Education Advanced Level Examination June 2012. Unit 5 Nuclear and Thermal Physics Section A
 Centre Number Surname Candidate Number For Examinerʼs Use Other Names Candidate Signature Examinerʼs Initials General Certificate of Education Advanced Level Examination June 2012 Question 1 2 Mark Physics
Centre Number Surname Candidate Number For Examinerʼs Use Other Names Candidate Signature Examinerʼs Initials General Certificate of Education Advanced Level Examination June 2012 Question 1 2 Mark Physics
104 Practice Exam 2-3/21/02
 104 Practice Exam 2-3/21/02 1. Two electrons are located in a region of space where the magnetic field is zero. Electron A is at rest; and electron B is moving westward with a constant velocity. A non-zero
104 Practice Exam 2-3/21/02 1. Two electrons are located in a region of space where the magnetic field is zero. Electron A is at rest; and electron B is moving westward with a constant velocity. A non-zero
Astronomy 110 Homework #04 Assigned: 02/06/2007 Due: 02/13/2007. Name:
 Astronomy 110 Homework #04 Assigned: 02/06/2007 Due: 02/13/2007 Name: Directions: Listed below are twenty (20) multiple-choice questions based on the material covered by the lectures this past week. Choose
Astronomy 110 Homework #04 Assigned: 02/06/2007 Due: 02/13/2007 Name: Directions: Listed below are twenty (20) multiple-choice questions based on the material covered by the lectures this past week. Choose
2, 8, 20, 28, 50, 82, 126.
 Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of magic nuclei for which either the number of neutrons
Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of magic nuclei for which either the number of neutrons
Objectives 404 CHAPTER 9 RADIATION
 Objectives Explain the difference between isotopes of the same element. Describe the force that holds nucleons together. Explain the relationship between mass and energy according to Einstein s theory
Objectives Explain the difference between isotopes of the same element. Describe the force that holds nucleons together. Explain the relationship between mass and energy according to Einstein s theory
State Newton's second law of motion for a particle, defining carefully each term used.
 5 Question 1. [Marks 20] An unmarked police car P is, travelling at the legal speed limit, v P, on a straight section of highway. At time t = 0, the police car is overtaken by a car C, which is speeding
5 Question 1. [Marks 20] An unmarked police car P is, travelling at the legal speed limit, v P, on a straight section of highway. At time t = 0, the police car is overtaken by a car C, which is speeding
Experiment #12: The Bohr Atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes, Holder, and Variac Flashlight
 Experiment #12: The Bohr Atom Purpose: To observe the visible spectrum of hydrogen and helium and verify the Bohr model of the hydrogen atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes,
Experiment #12: The Bohr Atom Purpose: To observe the visible spectrum of hydrogen and helium and verify the Bohr model of the hydrogen atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes,
2 ATOMIC SYSTEMATICS AND NUCLEAR STRUCTURE
 2 ATOMIC SYSTEMATICS AND NUCLEAR STRUCTURE In this chapter the principles and systematics of atomic and nuclear physics are summarised briefly, in order to introduce the existence and characteristics of
2 ATOMIC SYSTEMATICS AND NUCLEAR STRUCTURE In this chapter the principles and systematics of atomic and nuclear physics are summarised briefly, in order to introduce the existence and characteristics of
Candidate Number. General Certificate of Education Advanced Level Examination June 2014
 entre Number andidate Number Surname Other Names andidate Signature General ertificate of Education dvanced Level Examination June 214 Physics PHY4/1 Unit 4 Fields and Further Mechanics Section Wednesday
entre Number andidate Number Surname Other Names andidate Signature General ertificate of Education dvanced Level Examination June 214 Physics PHY4/1 Unit 4 Fields and Further Mechanics Section Wednesday
Chemistry 102 Summary June 24 th. Properties of Light
 Chemistry 102 Summary June 24 th Properties of Light - Energy travels through space in the form of electromagnetic radiation (EMR). - Examples of types of EMR: radio waves, x-rays, microwaves, visible
Chemistry 102 Summary June 24 th Properties of Light - Energy travels through space in the form of electromagnetic radiation (EMR). - Examples of types of EMR: radio waves, x-rays, microwaves, visible
Answer the following questions by marking the BEST answer choice on the answer sheet
 Answer the following questions by marking the BEST answer choice on the answer sheet 1. What is the average speed of a car that travels a total distance of 320 meters in 2.6 minutes? a. 2.1 m/s b. 120
Answer the following questions by marking the BEST answer choice on the answer sheet 1. What is the average speed of a car that travels a total distance of 320 meters in 2.6 minutes? a. 2.1 m/s b. 120
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level
 Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *0123456789* PHYSICS 9702/02 Paper 2 AS Level Structured Questions For Examination from 2016 SPECIMEN
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *0123456789* PHYSICS 9702/02 Paper 2 AS Level Structured Questions For Examination from 2016 SPECIMEN
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS
 DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
Nuclear Physics and Radioactivity
 Nuclear Physics and Radioactivity 1. The number of electrons in an atom of atomic number Z and mass number A is 1) A 2) Z 3) A+Z 4) A-Z 2. The repulsive force between the positively charged protons does
Nuclear Physics and Radioactivity 1. The number of electrons in an atom of atomic number Z and mass number A is 1) A 2) Z 3) A+Z 4) A-Z 2. The repulsive force between the positively charged protons does
The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS
 PS/PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Wednesday, June 24, 2009 9:15 a.m. to 12:15 p.m., only The answer sheet for Part A and Part B
PS/PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Wednesday, June 24, 2009 9:15 a.m. to 12:15 p.m., only The answer sheet for Part A and Part B
FIZIKA ANGOL NYELVEN
 ÉRETTSÉGI VIZSGA 2011. május 17. FIZIKA ANGOL NYELVEN KÖZÉPSZINTŰ ÍRÁSBELI VIZSGA 2011. május 17. 8:00 Az írásbeli vizsga időtartama: 120 perc Pótlapok száma Tisztázati Piszkozati NEMZETI ERŐFORRÁS MINISZTÉRIUM
ÉRETTSÉGI VIZSGA 2011. május 17. FIZIKA ANGOL NYELVEN KÖZÉPSZINTŰ ÍRÁSBELI VIZSGA 2011. május 17. 8:00 Az írásbeli vizsga időtartama: 120 perc Pótlapok száma Tisztázati Piszkozati NEMZETI ERŐFORRÁS MINISZTÉRIUM
Curriculum for Excellence. Higher Physics. Success Guide
 Curriculum for Excellence Higher Physics Success Guide Electricity Our Dynamic Universe Particles and Waves Electricity Key Area Monitoring and Measuring A.C. Monitoring alternating current signals with
Curriculum for Excellence Higher Physics Success Guide Electricity Our Dynamic Universe Particles and Waves Electricity Key Area Monitoring and Measuring A.C. Monitoring alternating current signals with
Tennessee State University
 Tennessee State University Dept. of Physics & Mathematics PHYS 2010 CF SU 2009 Name 30% Time is 2 hours. Cheating will give you an F-grade. Other instructions will be given in the Hall. MULTIPLE CHOICE.
Tennessee State University Dept. of Physics & Mathematics PHYS 2010 CF SU 2009 Name 30% Time is 2 hours. Cheating will give you an F-grade. Other instructions will be given in the Hall. MULTIPLE CHOICE.
SURFACE TENSION. Definition
 SURFACE TENSION Definition In the fall a fisherman s boat is often surrounded by fallen leaves that are lying on the water. The boat floats, because it is partially immersed in the water and the resulting
SURFACE TENSION Definition In the fall a fisherman s boat is often surrounded by fallen leaves that are lying on the water. The boat floats, because it is partially immersed in the water and the resulting
Atomic and Nuclear Physics Laboratory (Physics 4780)
 Gamma Ray Spectroscopy Week of September 27, 2010 Atomic and Nuclear Physics Laboratory (Physics 4780) The University of Toledo Instructor: Randy Ellingson Gamma Ray Production: Co 60 60 60 27Co28Ni *
Gamma Ray Spectroscopy Week of September 27, 2010 Atomic and Nuclear Physics Laboratory (Physics 4780) The University of Toledo Instructor: Randy Ellingson Gamma Ray Production: Co 60 60 60 27Co28Ni *
The Phenomenon of Photoelectric Emission:
 The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total
 Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
Chapter NP-5. Nuclear Physics. Nuclear Reactions TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 NUCLEAR REACTIONS 2.0 NEUTRON INTERACTIONS
 Chapter NP-5 Nuclear Physics Nuclear Reactions TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 2.0 NEUTRON INTERACTIONS 2.1 ELASTIC SCATTERING 2.2 INELASTIC SCATTERING 2.3 RADIATIVE CAPTURE 2.4 PARTICLE
Chapter NP-5 Nuclear Physics Nuclear Reactions TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 2.0 NEUTRON INTERACTIONS 2.1 ELASTIC SCATTERING 2.2 INELASTIC SCATTERING 2.3 RADIATIVE CAPTURE 2.4 PARTICLE
Convex Mirrors. Ray Diagram for Convex Mirror
 Convex Mirrors Center of curvature and focal point both located behind mirror The image for a convex mirror is always virtual and upright compared to the object A convex mirror will reflect a set of parallel
Convex Mirrors Center of curvature and focal point both located behind mirror The image for a convex mirror is always virtual and upright compared to the object A convex mirror will reflect a set of parallel
Name Date Class ELECTRONS IN ATOMS. Standard Curriculum Core content Extension topics
 13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
Basics of Nuclear Physics and Fission
 Basics of Nuclear Physics and Fission A basic background in nuclear physics for those who want to start at the beginning. Some of the terms used in this factsheet can be found in IEER s on-line glossary.
Basics of Nuclear Physics and Fission A basic background in nuclear physics for those who want to start at the beginning. Some of the terms used in this factsheet can be found in IEER s on-line glossary.
AP Physics - Chapter 8 Practice Test
 AP Physics - Chapter 8 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A single conservative force F x = (6.0x 12) N (x is in m) acts on
AP Physics - Chapter 8 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A single conservative force F x = (6.0x 12) N (x is in m) acts on
WAVES AND ELECTROMAGNETIC RADIATION
 WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
F N A) 330 N 0.31 B) 310 N 0.33 C) 250 N 0.27 D) 290 N 0.30 E) 370 N 0.26
 Physics 23 Exam 2 Spring 2010 Dr. Alward Page 1 1. A 250-N force is directed horizontally as shown to push a 29-kg box up an inclined plane at a constant speed. Determine the magnitude of the normal force,
Physics 23 Exam 2 Spring 2010 Dr. Alward Page 1 1. A 250-N force is directed horizontally as shown to push a 29-kg box up an inclined plane at a constant speed. Determine the magnitude of the normal force,
Physics 41 HW Set 1 Chapter 15
 Physics 4 HW Set Chapter 5 Serway 8 th OC:, 4, 7 CQ: 4, 8 P: 4, 5, 8, 8, 0, 9,, 4, 9, 4, 5, 5 Discussion Problems:, 57, 59, 67, 74 OC CQ P: 4, 5, 8, 8, 0, 9,, 4, 9, 4, 5, 5 Discussion Problems:, 57, 59,
Physics 4 HW Set Chapter 5 Serway 8 th OC:, 4, 7 CQ: 4, 8 P: 4, 5, 8, 8, 0, 9,, 4, 9, 4, 5, 5 Discussion Problems:, 57, 59, 67, 74 OC CQ P: 4, 5, 8, 8, 0, 9,, 4, 9, 4, 5, 5 Discussion Problems:, 57, 59,
Candidate Number. General Certificate of Education Advanced Level Examination June 2012
 entre Number andidate Number Surname Other Names andidate Signature General ertificate of Education dvanced Level Examination June 212 Physics PHY4/1 Unit 4 Fields and Further Mechanics Section Monday
entre Number andidate Number Surname Other Names andidate Signature General ertificate of Education dvanced Level Examination June 212 Physics PHY4/1 Unit 4 Fields and Further Mechanics Section Monday
AP1 Oscillations. 1. Which of the following statements about a spring-block oscillator in simple harmonic motion about its equilibrium point is false?
 1. Which of the following statements about a spring-block oscillator in simple harmonic motion about its equilibrium point is false? (A) The displacement is directly related to the acceleration. (B) The
1. Which of the following statements about a spring-block oscillator in simple harmonic motion about its equilibrium point is false? (A) The displacement is directly related to the acceleration. (B) The
Waves and Light Extra Study Questions
 Waves and Light Extra Study Questions Short Answer 1. Determine the frequency for each of the following. (a) A bouncing spring completes 10 vibrations in 7.6 s. (b) An atom vibrates 2.5 10 10 times in
Waves and Light Extra Study Questions Short Answer 1. Determine the frequency for each of the following. (a) A bouncing spring completes 10 vibrations in 7.6 s. (b) An atom vibrates 2.5 10 10 times in
Rutgers Analytical Physics 750:228, Spring 2016 ( RUPHY228S16 )
 1 of 13 2/17/2016 5:28 PM Signed in as Weida Wu, Instructor Help Sign Out Rutgers Analytical Physics 750:228, Spring 2016 ( RUPHY228S16 ) My Courses Course Settings University Physics with Modern Physics,
1 of 13 2/17/2016 5:28 PM Signed in as Weida Wu, Instructor Help Sign Out Rutgers Analytical Physics 750:228, Spring 2016 ( RUPHY228S16 ) My Courses Course Settings University Physics with Modern Physics,
Energy. Mechanical Energy
 Principles of Imaging Science I (RAD119) Electromagnetic Radiation Energy Definition of energy Ability to do work Physicist s definition of work Work = force x distance Force acting upon object over distance
Principles of Imaging Science I (RAD119) Electromagnetic Radiation Energy Definition of energy Ability to do work Physicist s definition of work Work = force x distance Force acting upon object over distance
Magnetism. d. gives the direction of the force on a charge moving in a magnetic field. b. results in negative charges moving. clockwise.
 Magnetism 1. An electron which moves with a speed of 3.0 10 4 m/s parallel to a uniform magnetic field of 0.40 T experiences a force of what magnitude? (e = 1.6 10 19 C) a. 4.8 10 14 N c. 2.2 10 24 N b.
Magnetism 1. An electron which moves with a speed of 3.0 10 4 m/s parallel to a uniform magnetic field of 0.40 T experiences a force of what magnitude? (e = 1.6 10 19 C) a. 4.8 10 14 N c. 2.2 10 24 N b.
Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect
 Objectives: PS-7.1 Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect Illustrate ways that the energy of waves is transferred by interaction with
Objectives: PS-7.1 Physical Science Study Guide Unit 7 Wave properties and behaviors, electromagnetic spectrum, Doppler Effect Illustrate ways that the energy of waves is transferred by interaction with
Practice Exam Three Solutions
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics Physics 8.01T Fall Term 2004 Practice Exam Three Solutions Problem 1a) (5 points) Collisions and Center of Mass Reference Frame In the lab frame,
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics Physics 8.01T Fall Term 2004 Practice Exam Three Solutions Problem 1a) (5 points) Collisions and Center of Mass Reference Frame In the lab frame,
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
ATOMIC SPECTRA. Apparatus: Optical spectrometer, spectral tubes, power supply, incandescent lamp, bottles of dyed water, elevating jack or block.
 1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
9. The kinetic energy of the moving object is (1) 5 J (3) 15 J (2) 10 J (4) 50 J
 1. If the kinetic energy of an object is 16 joules when its speed is 4.0 meters per second, then the mass of the objects is (1) 0.5 kg (3) 8.0 kg (2) 2.0 kg (4) 19.6 kg Base your answers to questions 9
1. If the kinetic energy of an object is 16 joules when its speed is 4.0 meters per second, then the mass of the objects is (1) 0.5 kg (3) 8.0 kg (2) 2.0 kg (4) 19.6 kg Base your answers to questions 9
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS
 PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
Chapter 6. Work and Energy
 Chapter 6 Work and Energy The concept of forces acting on a mass (one object) is intimately related to the concept of ENERGY production or storage. A mass accelerated to a non-zero speed carries energy
Chapter 6 Work and Energy The concept of forces acting on a mass (one object) is intimately related to the concept of ENERGY production or storage. A mass accelerated to a non-zero speed carries energy
Physics 30 Worksheet #10 : Magnetism From Electricity
 Physics 30 Worksheet #10 : Magnetism From Electricity 1. Draw the magnetic field surrounding the wire showing electron current below. x 2. Draw the magnetic field surrounding the wire showing electron
Physics 30 Worksheet #10 : Magnetism From Electricity 1. Draw the magnetic field surrounding the wire showing electron current below. x 2. Draw the magnetic field surrounding the wire showing electron
Calculating particle properties of a wave
 Calculating particle properties of a wave A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency f of the wave via Planck: E = h f (1) A particle can
Calculating particle properties of a wave A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency f of the wave via Planck: E = h f (1) A particle can
Candidate Number. General Certificate of Education Advanced Level Examination June 2010
 entre Number andidate Number Surname Other Names andidate Signature General ertificate of Education dvanced Level Examination June 1 Physics PHY4/1 Unit 4 Fields and Further Mechanics Section Friday 18
entre Number andidate Number Surname Other Names andidate Signature General ertificate of Education dvanced Level Examination June 1 Physics PHY4/1 Unit 4 Fields and Further Mechanics Section Friday 18
The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS. Tuesday, June 22, 2010 9:15 a.m. to 12:15 p.m.
 PS/PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Tuesday, June 22, 2010 9:15 a.m. to 12:15 p.m., only The answers to all questions in this examination
PS/PHYSICS The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING PHYSICS Tuesday, June 22, 2010 9:15 a.m. to 12:15 p.m., only The answers to all questions in this examination
The content is based on the National Science Teachers Association (NSTA) standards and is aligned with state standards.
 Literacy Advantage Physical Science Physical Science Literacy Advantage offers a tightly focused curriculum designed to address fundamental concepts such as the nature and structure of matter, the characteristics
Literacy Advantage Physical Science Physical Science Literacy Advantage offers a tightly focused curriculum designed to address fundamental concepts such as the nature and structure of matter, the characteristics
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
