CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES. Section B: Carbohydrates - Fuel and Building Material
|
|
|
- Rhoda Chapman
- 7 years ago
- Views:
Transcription
1 CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES Section B: Carbohydrates - Fuel and Building Material 1. Sugars, the smallest carbohydrates, serve as fuel and carbon sources 2. Polysaccharides, the polymers of sugars, have storage and structural roles
2 Introduction Carbohydrates include both sugars and polymers. The simplest carbohydrates are monosaccharides or simple sugars. Disaccharides, double sugars, consist of two monosaccharides joined by a condensation reaction. Polysaccharides are polymers of monosaccharides.
3 1. Sugars, the smallest carbohydrates serve as a source of fuel and carbon sources Monosaccharides generally have molecular formulas that are some multiple of CH 2 O. For example, glucose has the formula C 6 H 12 O 6. Most names for sugars end in -ose. Monosaccharides have a carbonyl group and multiple hydroxyl groups. If the carbonly group is at the end, the sugar is an aldose, if not, the sugars is a ketose. Glucose, an aldose, and fructose, a ketose, are structural isomers.
4 Monosaccharides are also classified by the number of carbons in the backbone. Glucose and other six carbon sugars are hexoses. Five carbon backbones are pentoses and three carbon sugars are trioses. Monosaccharides may also exist as enantiomers. For example, glucose and galactose, both sixcarbon aldoses, differ in the spatial arrangement around asymmetrical carbons.
5 Fig. 5.3
6 Monosaccharides, particularly glucose, are a major fuel for cellular work. They also function as the raw material for the synthesis of other monomers, including those of amino acids and fatty acids. Fig. 5.4
7 Two monosaccharides can join with a glycosidic linkage to form a dissaccharide via dehydration. Maltose, malt sugar, is formed by joining two glucose molecules. Sucrose, table sugar, is formed by joining glucose and fructose and is the major transport form of sugars in plants. Fig. 5.5a
8 While often drawn as a linear skeleton, in aqueous solutions monosaccharides form rings. Fig. 5.5
9 2. Polysaccharides, the polymers of sugars, have storage and structural roles Polysaccharides are polymers of hundreds to thousands of monosaccharides joined by glycosidic linkages. One function of polysaccharides is as an energy storage macromolecule that is hydrolyzed as needed. Other polysaccharides serve as building materials for the cell or whole organism.
10 Starch is a storage polysaccharide composed entirely of glucose monomers. Most monomers are joined by 1-4 linkages between the glucose molecules. One unbranched form of starch, amylose, forms a helix. Branched forms, like amylopectin, are more complex. Fig. 5.6a
11 Plants store starch within plastids, including chloroplasts. Plants can store surplus glucose in starch and withdraw it when needed for energy or carbon. Animals that feed on plants, especially parts rich in starch, can also access this starch to support their own metabolism.
12 Animals also store glucose in a polysaccharide called glycogen. Glycogen is highly branched, like amylopectin. Humans and other vertebrates store glycogen in the liver and muscles but only have about a one day supply. Fig. 5.6b Insert Fig. 5.6b - glycogen
13 While polysaccharides can be built from a variety of monosaccharides, glucose is the primary monomer used in polysaccharides. One key difference among polysaccharides develops from 2 possible ring structure of glucose. These two ring forms differ in whether the hydroxyl group attached to the number 1 carbon is fixed above (beta glucose) or below (alpha glucose) the ring plane. Fig. 5.7a
14 Starch is a polysaccharide of alpha glucose monomers. Fig. 5.7
15 Structural polysaccharides form strong building materials. Cellulose is a major component of the tough wall of plant cells. Cellulose is also a polymer of glucose monomers, but using beta rings. Fig. 5.7c
16 While polymers built with alpha glucose form helical structures, polymers built with beta glucose form straight structures. This allows H atoms on one strand to form hydrogen bonds with OH groups on other strands. Groups of polymers form strong strands, microfibrils, that are basic building material for plants (and humans).
17 Fig. 5.8
18 The enzymes that digest starch cannot hydrolyze the beta linkages in cellulose. Cellulose in our food passes through the digestive tract and is eliminated in feces as insoluble fiber. As it travels through the digestive tract, it abrades the intestinal walls and stimulates the secretion of mucus. Some microbes can digest cellulose to its glucose monomers through the use of cellulase enzymes. Many eukaryotic herbivores, like cows and termites, have symbiotic relationships with cellulolytic microbes, allowing them access to this rich source of energy.
19 Another important structural polysaccharide is chitin, used in the exoskeletons of arthropods (including insects, spiders, and crustaceans). Chitin is similar to cellulose, except that it contains a nitrogen-containing appendage on each glucose. Pure chitin is leathery, but the addition of calcium carbonate hardens the chitin. Chitin also forms the structural support for the cell walls of many fungi. Fig. 5.9
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
lt\ Nutrition a-glncose www.cambridge.org in this web service Cambridge University Press Glucose+ fructose Glucose+ galactose
 P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
Qualitative Testing for Carbohydrates
 R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
The molecules of life. The molecules that make up living things are really big They are called macromolecules
 Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Digestive System Functions
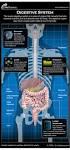 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
THE HISTORY OF CELL BIOLOGY
 SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
Solid Phase Peptide Synthesis
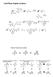 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Molecular Cell Biology
 Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Photosynthesis and Sucrose Production
 Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photo Cell Resp Practice. A. ATP B. oxygen C. DNA D. water. The following equation represents the process of photosynthesis in green plants.
 Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Biological Molecules
 Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Biofuel Feedstocks and Production
 Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
1. When applying the process of science, which of these is tested? a. an observation b. a result c. a hypothesis d. a question e.
 BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
Structure of proteins
 Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Chapter 48. Nutrients in Food. Carbohydrates, Proteins, and Lipids. Carbohydrates, Proteins, and Lipids, continued
 Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Chapter 14 Glycolysis. Glucose. 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) TCA Cycle
 Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Weds 5/20/15. Membranes - finish last lecture outline. Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system
 Membranes - finish last lecture outline Weds 5/20/15 Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system structures and functions // accessory organs mechanism
Membranes - finish last lecture outline Weds 5/20/15 Digestive System Nutrition Types of digestion & digestive systems Vertebrate digestive system structures and functions // accessory organs mechanism
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Special organ structures and functions conduct these tasks through the successive parts of the overall system.
 Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Topic 4: Digestion and Nutrition
 Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 4: Digestion and Nutrition THE CONTENTS OF FOOD Food contains nutrients: Nutrients include: 1. 2. 3. 4. 5. Nutrients must be small enough to enter our cells. If they are too large they must be digested
Topic 3: Nutrition, Photosynthesis, and Respiration
 1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Unit B Understanding Animal Body Systems. Lesson 1 Understanding Animal Digestion
 Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
Unit B Understanding Animal Body Systems Lesson 1 Understanding Animal Digestion 1 Terms Absorption Amino acids Anus Avian Bile Cecum Chyme Crop Cud Digestion Digestive system Enzymes Eructated Feces Gizzard
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Student name ID # 2. (4 pts) What is the terminal electron acceptor in respiration? In photosynthesis? O2, NADP+
 1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
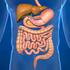 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Chapter 2: Cell Structure and Function pg. 70-107
 UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
Chapter 25: Metabolism and Nutrition
 Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
PROTEINS THE PEPTIDE BOND. The peptide bond, shown above enclosed in the blue curves, generates the basic structural unit for proteins.
 Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Chapter 15 Lecture Notes: Metabolism
 Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
Fatty Acids carboxylic acids
 Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Get It Right. Answers. Chapter 1: The Science of Life. A biologist studies all living things.
 Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
chapter 5 Carbohydrates
 part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
H.W. 1 Bio 101 Prof. Fournier
 H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 4. Chemical Energy
 hapter 4 hemical Energy Perhaps the most convenient form in which to store energy is chemical energy. The foods we eat, combined with the oxygen we breathe, store energy that our bodies extract and convert
hapter 4 hemical Energy Perhaps the most convenient form in which to store energy is chemical energy. The foods we eat, combined with the oxygen we breathe, store energy that our bodies extract and convert
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
