CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
|
|
|
- Jeffery Holland
- 7 years ago
- Views:
Transcription
1 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1
2 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D) Page 2
3 3. Which of the following structures is that of D-mannose? A) B) C) D) 4. The structure of D-allose differs from that of D-glucose at which carbon atom? A) carbon #2 B) carbon #4 C) carbon #3 D) carbon #5 5. In which of the following pairs of monosaccharides do the members of the pair contain different numbers of carbon atoms? A) mannose and xylulose B) ribulose and arabinose C) mannose and galactose D) erythrose and threose Page 3
4 6. Which of the following statements conerning chiral centers in specific monosaccharides is incorrect? A) threose has two chiral centers B) erythrose has one chiral center C) tagatose has three chiral centers D) talose has four chiral centers 7. Which of the following terms correctly describes the relationship between D-psicose and L-psicose? A) enantiomers B) epimers C) diastereomers D) cis-trans isomers 8. Which of the following properties would be the same for D-lyxose and L-lyxose? A) interaction with plane polarized light B) solubility in chiral solvent C) freezing point D) reaction with (+)-2,3-butanediol Page 4
5 9. Which of the following structures represents a β-monosaccharide? A) B) C) D) Page 5
6 10. Which of the following monosaccharides has an open chain form that is a ketose? A) B) C) D) Page 6
7 11. Which of the following structures is a representation of a cyclic form of D-glucose? A) B) C) D) 12. Which of the following types of monosaccharides have cyclic forms that involve a 5- membered ring? A) ketopentoses and aldopentoses B) aldopentoses and ketohexoses C) ketopentoses and aldohexoses D) ketohexoses and aldohexoses 13. In which of the following pairs or carbohydrates are both members of the pair disaccharides? A) mannose and galactose B) maltose and glycogen C) sucrose and maltose D) cellobiose and cellulose Page 7
8 14. Which of the following substances will produce both galactose and glucose when it is hydrolyzed? A) sucrose B) maltose C) lactose D) amylose 15. In which of the following sugars is the bonding between monosaccharide units head-to head rather than head-to-tail? A) cellobiose B) maltose C) lactose D) sucrose 16. Which of the following is not a reducing sugar? A) psicose B) mannose C) cellulose D) cellobiose 17. The major structural difference between cellulose and starch is found in the A) identity of the monosaccharide units present. B) ring size of the monosaccharide units present. C) linkages between the monosaccharide units present. D) handedness of the monosaccharide units present. 18. Which of the following is a correct pairing of terms? A) heteropolysaccharide and starch B) homopolysaccharide and chitin C) branch-chain polysaccharide and cellulose D) unbranched-chain polysaccharide and glycogen 19. In which of the following polysaccharides are all glycosidic linkages of the β (1 4) type? A) starch B) cellulose C) glycogen D) amylopectin Page 8
9 20. Which of the following statements concerning carbohydrates is correct? A) They are the most abundant type of biochemical substance in the human body. B) They are all polyhydroxy aldehydes, polyhydroxyketones, or substances that yield such upon hydrolysis. C) They can exist in left-handed and right-handed forms, with the left-handed form being dominant in nature. D) They all have a sweet taste and therefore are also called sugars. 21. Which of the following monosaccharides are aldohexoses? A) glucose B) fructose C) ribose 22. Which of the following statements concerning the D- and L- forms of a monosaccharide is incorrect? A) They have structures that are nonsuperimposable mirror images of each other. B) They must contain the same number of chiral centers. C) They are enantiomers. 23. Which of the following monosaccharides has a structure in which two or more chiral centers are present? A) glyceraldehyde B) dihydroxyacetone C) glucose 24. The left- and right-handed forms of a monosaccharide with a single chiral center are A) enantiomers. B) diastereomers. C) epimers. Page 9
10 25. Which of the following properties would be the same for a pair of enantiomeric monosaccharides? A) interaction with plane-polarized light B) boiling point C) solubility in water 26. Which of the following monosaccharides has cyclic forms that involve a 6-membered ring? A) fructose B) galactose C) ribose 27. In which of the following disaccharides is a β (1 4) glycosidic linkage present? A) lactose B) sucrose C) maltose 28. Which of the following disaccharides produces two different monosaccharides upon hydrolysis? A) maltose B) sucrose C) cellobiose 29. Which of the following disaccharides does not have two cyclic forms (alpha and beta)? A) sucrose B) lactose C) maltose Page 10
11 30. Which of the following carbohydrates will give a positive Benedict's test? A) galactose B) sucrose C) starch 31. In which of the following pairs of carbohydrates are both members of the pair polysaccharides? A) cellulose and cellobiose B) starch and glycogen C) amylose and lactose 32. In which of the following carbohydrates are two different types of glycosidic linkages present? A) glycogen B) lactose C) amylose 33. Which of the following is a correct pairing of terms? A) storage polysaccharide and cellulose B) structural polysaccharide and starch C) acidic polysaccharide and glycogen 34. Which of the following statements concerning the positioning of groups in the Fischer projection for L-glucose is correct? A) right on both carbons 2 and 3 B) right on both carbons 3 and 4 C) left at carbon 3 and right at carbon 5 Page 11
12 35. Which of the following statements concerning the positioning of groups in the aworth projection for α D glucose is correct? A) up at carbons 1, 3, and 5 B) down at carbons 2, 3, and 4 C) up at carbons 1 and 3 and down at carbons 2 and 4 Use the following to answer questions 36-45: In each of the following multiple-choice questions, characterize EAC of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices a) All three statements are true. b) Two of the three statements are true. c) Only one of the statements is true. d) None of the statements is true. 36. Statements: (1) Glucose is both a hexose and a ketose. (2) There can never be more than two enantiomers for a molecule. (3) All common disaccharides have beta-one-four linkages. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. 37. Statements: (1) Complete hydrolysis of a oligosaccharide produces only monosaccharides. (2) Fructose, galactose, and ribose are all hexoses. (3) Both amylopectin and glycogen are branched glucose polymers. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. Page 12
13 38. Statements: (1) Diastereomers are stereoisomers whose molecules are mirror images of each other. (2) Both cyclic forms of glucose contain a six-membered carbon ring. (3) Complete hydrolysis of both maltose and cellobiose produce the same molecule. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. 39. Statements: (1) A Fischer projection is a three-dimensional notation for showing the spatial arrangements of groups of atoms about chiral centers in a molecule. (2) Cyclic forms of aldoses result from intermolecular hemiacetal formation. (3) All physical properties of enantiomers are the same. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. 40. Statements: (1) A positive Tollen's test classifies a monosaccharide as a reducing sugar. (2) The bond which links the two monosaccharides of a disaccharide together is a carbon-oxygen-carbon bond. (3) Monosaccharides cannot be broken done into simpler units by hydrolysis reactions. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. 41. Statements: (1) Enantiomers with more than three carbon atoms must have the same handedness. (2) The molecules glyceraldehyde and dihydroxyketone each contain one chiral center. (3) Glucose contain one more - group than does galactose. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. Page 13
14 42. Statements: (1) Cellulose and amylose are glucose polymers which differ in the type of glycosidic linkage present. (2) All monosaccharides are either aldoses and ketoses. (3) Enantiomers can never be epimers. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. 43. Statements: (1) The term sugar is a general designation for both monosaccharides and disaccharides. (2) The highest-numbered chiral center in a monosaccharide is used to determine D- or L-configuration. (3) Sucrose is a reducing sugar and lactose is a nonreducing sugar. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. 44. Statements: (1) Mirror images of an achiral molecule are identical. (2) All cyclic monosaccharides can exist in alpha- and beta-configurations. (3) Reaction of an alcohol with a cyclic monosaccharide produces a glycoside. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. 45. Statements: (1) The biochemical basis for different types of blood is a system of polysaccharide markers attached to red blood cell membranes. (2) Lactose intolerance is a genetic condition caused by excessive amounts of the enzyme lactase. (3) Aspartame, the most used artificial sweetener, is an undigestible trisaccharide. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true. Page 14
15 Use the following to answer questions 46-50: For each of the compound characterizations, select from the response list an appropriate structural formula. Responses may be used more than once or need not be used at all. a) C 2 C CO b) C 2 C C 2 c) C 2 C C C CO d) C 2 C C C C An achiral compound 47. A chiral compound with one chiral center 48. A chiral compound with two chiral centers stereoisomers exist for this compound different L-stereoisomers exist for this compound Page 15
16 Use the following to answer questions 51-55: For each of the stereochemical descriptions, select from the response list an appropriate set of structural formulas. Responses may be used more than once or need not be used at all. a) CO and CO O O C 2 C 2 b) CO C 2 and O CO C 2 c) CO and CO O C 2 C 2 d) CO and CO O C 2 C Enantiomers 52. Mirror images 53. Diastereomers 54. Optical isomers 55. Epimers Page 16
17 Use the following to answer questions 56-60: For each of the disaccharides or polysaccharides, select from the response list the correct hydrolysis products. Responses may be used more than once or need not be used at all. a) glucose only b) glucose and galactose c) glucose and fructose d) glucose and ribose 56. Sucrose 57. Lactose 58. Cellulose 59. Starch 60. Glycogen Use the following to answer questions 61-65: For each of the disaccharides or polysaccharides, select from the response list the correct characterization of the type of glycosidic linkages present in the compound. Responses may be used more than once or need not be used at all. a) α(1 4) only b) β(1 4) only c) α (1 4) and α (1 6) d) α, β(1 2) only 61. Sucrose 62. Lactose 63. Cellulose 64. Amylopectin Page 17
18 65. Chitin Use the following to answer questions 66-70: For each of the compound characterizations, select an appropriate structural formula from the response list. Responses may be used more than once or need not be used at all. a) C 2 O b) C 2 O OC 3 c) d) OC 2 O OC 2 C 2 O O 66. D-glucose 67. A beta monosaccharide 68. A glycoside 69. Open-chain form is an aldopentose Page 18
19 70. Open-chain form is a ketohexose Page 19
20 Answer Key 1. C 2. C 3. B 4. C 5. A 6. B 7. A 8. C 9. B 10. D 11. A 12. B 13. C 14. C 15. D 16. C 17. C 18. B 19. B 20. B 21. A 22. E 23. C 24. D 25. D 26. B 27. A 28. B 29. A 30. A 31. B 32. A 33. E 34. E 35. E 36. C 37. B 38. C 39. C 40. A 41. D 42. A 43. B 44. A Page 20
21 45. D 46. d 47. a 48. d 49. c 50. c 51. a 52. a 53. b 54. a 55. b 56. c 57. b 58. a 59. a 60. a 61. d 62. b 63. b 64. c 65. b 66. a 67. a 68. b 69. c 70. d Page 21
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
Solid Phase Peptide Synthesis
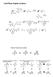 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
Qualitative Testing for Carbohydrates
 R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
CHEM 2353 Fundamentals of Organic Chemistry. Carbohydrates. Organic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R.
 hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
II. Chemistry of Sugars
 II. Chemistry of Sugars Sugars-or saccharides- are the most abundant bio-molecule on the planet. They are important in a number of biological roles. Most obviously you will know them as a major component
II. Chemistry of Sugars Sugars-or saccharides- are the most abundant bio-molecule on the planet. They are important in a number of biological roles. Most obviously you will know them as a major component
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Molecule Projections
 Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
CHEM 121. Chapter 19, Name: Date:
 CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
lt\ Nutrition a-glncose www.cambridge.org in this web service Cambridge University Press Glucose+ fructose Glucose+ galactose
 P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Stereochemistry Tutorial: Drawing Enantiomers and Diastereomers
 tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
The molecules of life. The molecules that make up living things are really big They are called macromolecules
 Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chapter 12 Organic Compounds with Oxygen and Sulfur
 Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Bulletin 887B. HPLC Carbohydrate Column Selection Guide
 9 North Harrison Road Bellefonte, PA -00 USA Telephone 00-- -9- Fax 00--0-9-0 email: supelco@sial.com sigma-aldrich.com/supelco HPLC Carbohydrate Column Selection Guide Because carbohydrates exhibit a
9 North Harrison Road Bellefonte, PA -00 USA Telephone 00-- -9- Fax 00--0-9-0 email: supelco@sial.com sigma-aldrich.com/supelco HPLC Carbohydrate Column Selection Guide Because carbohydrates exhibit a
Biofuel Feedstocks and Production
 Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Carbohydrate Analysis: Column Chemistries and Detection
 Carbohydrate Analysis: Column Chemistries and Detection Joe Romano Waters Corporation Carbohydrates in Feeds Methodology Forum AOAC 2007 Annual Meeting Anaheim, CA September 18, 2007 2007 Waters Corporation
Carbohydrate Analysis: Column Chemistries and Detection Joe Romano Waters Corporation Carbohydrates in Feeds Methodology Forum AOAC 2007 Annual Meeting Anaheim, CA September 18, 2007 2007 Waters Corporation
What happens to the food we eat? It gets broken down!
 Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Molecular Cell Biology
 Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Chapter 4. Carbohydrates: Bountiful Sources of Energy and Nutrients
 hapter 4 arbohydrates: Bountiful Sources of Energy and Nutrients hapter Objectives After reading this chapter, you will be able to: 1. Describe the difference between simple and complex carbohydrates,
hapter 4 arbohydrates: Bountiful Sources of Energy and Nutrients hapter Objectives After reading this chapter, you will be able to: 1. Describe the difference between simple and complex carbohydrates,
Page 1. 6. Which hydrocarbon is a member of the alkane series? (1) 1. Which is the structural formula of methane? (1) (2) (2) (3) (3) (4) (4)
 1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
Photosynthesis and Sucrose Production
 Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
McMush. Testing for the Presence of Biomolecules
 Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Chapter 15 Lecture Notes: Metabolism
 Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
Chapter 15 Lecture Notes: Metabolism Educational Goals 1. Define the terms metabolism, metabolic pathway, catabolism, and anabolism. 2. Understand how ATP is formed from ADP and inorganic phosphate (P
3Reactions of Sugars. Introduction. Mutarotation
 3eactions of Sugars Introduction 35 Mutarotation 35 xidation of Sugars 39 Glycoside Formation 40 Acid atalyzed Sugar eactions 42 Alkaline-atalyzed Sugar eactions 43 Summary 45 Vocabulary 47 eferences 47
3eactions of Sugars Introduction 35 Mutarotation 35 xidation of Sugars 39 Glycoside Formation 40 Acid atalyzed Sugar eactions 42 Alkaline-atalyzed Sugar eactions 43 Summary 45 Vocabulary 47 eferences 47
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
2. PHOTOSYNTHESIS. The general equation describing photosynthesis is light + 6 H 2 O + 6 CO 2 C 6 H 12 O 6 + 6 O 2
 2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
Sugar Identification using Polarimetry
 Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
chapter 5 Carbohydrates
 part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised)
 Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised) Course Title: Survey of Chemistry II, TR 1000 1115 Room NE-1260 Instructor: Dr. Jerry L. Poteat, Associate
Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised) Course Title: Survey of Chemistry II, TR 1000 1115 Room NE-1260 Instructor: Dr. Jerry L. Poteat, Associate
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391)
 NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
Photo Cell Resp Practice. A. ATP B. oxygen C. DNA D. water. The following equation represents the process of photosynthesis in green plants.
 Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
chromiiresistens Status Risk group
 Strain DSM 22788 Genus Leucobacter Species chromiiresistens Status Risk group L1 Type strain JG 31, CCOS 200, JCM 17813 Reference Author Sturm, G., Jacobs, J., Spröer, C., Schumann, P., Gescher, J. Title
Strain DSM 22788 Genus Leucobacter Species chromiiresistens Status Risk group L1 Type strain JG 31, CCOS 200, JCM 17813 Reference Author Sturm, G., Jacobs, J., Spröer, C., Schumann, P., Gescher, J. Title
Chapter 14 Glycolysis. Glucose. 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) TCA Cycle
 Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Digestive System Functions
 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
IR Applied to Isomer Analysis
 DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
Special organ structures and functions conduct these tasks through the successive parts of the overall system.
 Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
BERGEN COMMUNITY COLLEGE DIVISION OF MATHEMATICS, SCIENCE AND TECHNOLOGY DEPARTMENT OF PHYSICAL SCIENCES STUDENT COURSE OUTLINE
 BERGEN COMMUNITY COLLEGE DIVISION OF MATHEMATICS, SCIENCE AND TECHNOLOGY DEPARTMENT OF PHYSICAL SCIENCES STUDENT COURSE OUTLINE Course Title: Prerequisites: Course Description: Textbook: CHM 212 Organic
BERGEN COMMUNITY COLLEGE DIVISION OF MATHEMATICS, SCIENCE AND TECHNOLOGY DEPARTMENT OF PHYSICAL SCIENCES STUDENT COURSE OUTLINE Course Title: Prerequisites: Course Description: Textbook: CHM 212 Organic
PHOTOSYNTHESIS AND CELLULAR RESPIRATION
 reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
An Adventure in Stereochemistry: Alice in Mirror Image Land*
 An Adventure in Stereochemistry: Alice in Mirror Image Land* by Frank J. Dinan Department of Chemistry and Biochemistry Canisius College, Buffalo, NY Gordon T. Yee Department of Chemistry Virginia Polytechnic
An Adventure in Stereochemistry: Alice in Mirror Image Land* by Frank J. Dinan Department of Chemistry and Biochemistry Canisius College, Buffalo, NY Gordon T. Yee Department of Chemistry Virginia Polytechnic
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Get It Right. Answers. Chapter 1: The Science of Life. A biologist studies all living things.
 Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
CHEM 121. Chapter 17. Name: Date:
 CHEM 121. Chapter 17. Name: Date: 1. The elements present in a tertiary amine with two phenyl groups are A) carbon and nitrogen B) carbon, nitrogen and hydrogen C) carbon, nitrogen and oxygen D) carbon,
CHEM 121. Chapter 17. Name: Date: 1. The elements present in a tertiary amine with two phenyl groups are A) carbon and nitrogen B) carbon, nitrogen and hydrogen C) carbon, nitrogen and oxygen D) carbon,
1. The functional group present in carboxylic acids is called a A) carbonyl group. B) carboxyl group. C) carboxylate group. D) carbohydroxyl group.
 Name: Date: 1. The functional group present in carboxylic acids is called a A) carbonyl group. B) carboxyl group. C) carboxylate group. D) carbohydroxyl group. 2. Which of the following statements concerning
Name: Date: 1. The functional group present in carboxylic acids is called a A) carbonyl group. B) carboxyl group. C) carboxylate group. D) carbohydroxyl group. 2. Which of the following statements concerning
