A Brief History of. Atomic Theory
|
|
|
- Arabella Elliott
- 9 years ago
- Views:
Transcription
1 A Brief History of Atomic Theory The man of science does not want to discover in order to know - he wants to know in order to discover. - Alfred Whitehead Douglas Gilliland Honors Physical Sarasota High
2 Atomic Models JJ Thomson B C Rutherford Dalton Bohr
3 DEMOCRITUS BC The Greek philosopher Democritus proposed that all matter was made of small, unbreakable particles he called atoms which means unbreakable. He believed that atoms were too small to be seen. Philosophers are not scientists. They do not test their ideas. Instead they use reasoning to back up their beliefs. To them, human reasoning was superior to experimentation. Democritus used an example of a beach to support his theory. From afar, the beach appears to be a solid mass. But up close one finds that a beach is made of small grains of sand too small to be seen from a distance.
4 DEMOCRITUS BC Democritus proposed that the shape of an atom determined the properties of that substance. Fire atoms had sharp points. This is the reason it hurt you if you put your hand in a fire. Wine atoms were spheres. This is the reason that wine rolled around in a glass. Clay atoms were jagged. This is the reason why clay clumps together when it is molded. Fire Wine Clay
5 ARISTOTOLE The famous philosopher Aristotle, who also lived at that time, argued that all matter was made of only four elements. For the next two thousand years, Aristotle overshadowed Democritus. Finally, in the early 1800s, the atomist s theory was revived by John Dalton.
6 JOHN DALTON Nationality: English In 1809, Dalton by proposing the following: a) All matter was made of atoms. b) Atoms were solid spheres. c) Atoms of different elements differed in mass. d) Atoms were indivisible and indestructible. e) Atoms combine to form compounds. +
7 J.J. THOMSON Nationality: English The son of an English bookseller, Joseph John Thomson dreamed of being an engineer. At 14 he entered college but his father s early death made it impossible for him to pay the extra engineering fees so he changed his major to physics. Just as he graduated another Englishman made a discovery that would alter JJ s life forever. In 1875 Sir William Crookes constructed a glass tube with two metal plates inside it. He removed almost all the air from the tube and sealed it. When he connected the plates to the + and - terminals of a high voltage battery he observed a mysterious glowing ray. From experiments he was able to prove the ray was matter, Sir William Crookes
8 THE CROOKE S TUBE This is the instrument that Mr. Gilliland demonstrated to the class in the lab during this presentation. When connect to a high voltage current it produced a blue ray. Crooke found that this ray consisted of matter.
9 J.J. THOMSON Nationality: English Before you can understand Thomson s experiment, you need to understand 3 properties about electrical charges: a) There are two types of electrical charge: positive and negative. + - b) Opposite charges attract. + - c) Like charges repel
10 J.J. THOMSON Nationality: English Thomson took Crooke s tube and added two plates inside the tube and connected them with a wire. When the plates were not charged, the ray shot straight. Glass Ray of Matter Vacuum Tube Metal Plates (no charge) Battery
11 J.J. THOMSON Nationality: English But when he charged the plates a strange thing happened! + -
12 J.J. THOMSON Nationality: English When he reversed the charges on the plates he observed the ray reversed its position. - +
13 J.J. THOMSON Nationality: English From his observations Thomson concluded that, because the ray was always attracted to the + plate and repelled by the -, it was composed of negatively charged matter. One hundred years earlier, Dalton had proposed that atoms were neutral, solid spheres. Thomson s experiment disproved Dalton s theory. Thomson and other scientists knew that all atoms are neutral. His new model proposed that matter was made of atoms with negatively charged particles embedded in a positive cloud. He called these negative particles electrons. The negative particles and positive cloud neutralized each other so the atom had an overall neutral charge.
14 J.J. THOMSON Nationality: English Thomson s model was called the Plum Pudding Model was named after a popular dessert in England at that time. It was the first model to propose that smaller charged particles make up the atom. JJ Thomson with his cathode ray tube Plum Pudding Model, 1897 Thomson s model lasted less than two decades but it was first to propose the existence of subatomic particles. In 1911 another scientist who worked in Thomson s lab improved on his atomic model.
15 ERNEST RUTHERFORD Nationality: New Zealander Ernest Rutherford was born in 1871, the second of 11 children born to a New Zealand farmer. After graduating from college he traveled to England to work at the Cavendish Laboratory doing research with Hans Geiger on radioactivity. One type of radioactivity is when an atom throws out a positively charged particle from the nucleus. This particle was called an alpha particle (α). Rutherford and Geiger in the Cavendish Lab Rutherford used this alpha particle to investigate the structure of the atom.
16 ERNEST RUTHERFORD U Nationality: New Zealander + Uranium is a radioactive element that gives off Rutherford encased uranium in lead (which absorbs alpha particles). This produced a beam of alpha particles traveling in a straight line. He fired these positive particles at a thin piece of gold (dense metal). A screen around the gold to detect positive particles (alpha particles). Rutherford used these positive particles to investigate the makeup of the atom. where the alpha particles were traveling.
17 ERNEST RUTHERFORD Nationality: New Zealander Goil Foil Movie Rutherford shot alpha particles at a thin sheet of gold to observe what happened when the positive α particles passes through the gold atoms. If Thompson s model was correct the alpha particles should pass through the diffused positive cloud with ease.
18 ERNEST RUTHERFORD Nationality: New Zealander From his observations Rutherford concluded that the atom had a dense, positive central nucleus composed of + charged protons. He stated that the electrons orbited the nucleus - like planets orbiting the Sun. In 1909 Rutherford proposed his Planetary Model of the Atom. - His model created positively charged protons located in the nucleus and placed electrons in orbit around the nucleus - like planets around the sun.
19 A YouTube Movie on Rutherford s Experiment For Entertainment Purposes Only!
20 NIELS BOHR Nationality: Danish In 1913 Niels Bohr proposed that electrons are arranged in up to seven specific energy levels. Since it is the model we will use in this class you will do a computer program to teach you how to draw the electrons in their proper levels. The arrangement of these electrons in certain energy levels determines the chemical and physical properties of the elements.
21 JAMES CHADWICK Nationality: English In 1932 James Chadwick discovered a 3rd subatomic particle in the atom. He called this particle a neutron because it was neutral. He proposed that neutrons resided in the nucleus of the atom along with protons.
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
5.1 Evolution of the Atomic Model
 5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
Development of the Atomic Theory
 Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
The Models of the Atom
 The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
NOTES ON The Structure of the Atom
 NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS
 ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
Atomic Calculations. 2.1 Composition of the Atom. number of protons + number of neutrons = mass number
 2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
For convenience, we may consider an atom in two parts: the nucleus and the electrons.
 Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
Atomic Theory Part 1
 Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
CHAPTER 4: ATOMS AND ELEMENTS
 CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
Atoms and Elements [6th grade]
![Atoms and Elements [6th grade] Atoms and Elements [6th grade]](/thumbs/40/21183873.jpg) Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
Answers to Review Questions for Atomic Theory Quiz #1
 Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
The Structure of the Atom
 The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Atomic Theory: History of the Atom
 Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Lecture 3 September 14, 2009 Atomic Models: Rutherford & Bohr
 Welcome to 3.091 Lecture 3 September 14, 2009 Atomic Models: Rutherford & Bohr 1 Periodic Table Quiz 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37
Welcome to 3.091 Lecture 3 September 14, 2009 Atomic Models: Rutherford & Bohr 1 Periodic Table Quiz 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37
Ernest Rutherford Atomic Model 1911. Plum Pudding Model J.J. Thomson 1897
 1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Radioactivity & Particles
 Radioactivity & Particles Introduction... 2 Atomic structure... 2 How are these particles arranged?... 2 Atomic notation... 4 Isotopes... 4 What is radioactivity?... 5 Types of Radiation: alpha, beta and
Radioactivity & Particles Introduction... 2 Atomic structure... 2 How are these particles arranged?... 2 Atomic notation... 4 Isotopes... 4 What is radioactivity?... 5 Types of Radiation: alpha, beta and
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
Cathode Rays Figure 1: Figure 2:
 Cathode Rays The first ideas about electrons came from experiments with cathode-ray tubes. A forerunner of neon signs, fluorescent lights, and TV picture tubes, a typical cathode-ray tube is a partially
Cathode Rays The first ideas about electrons came from experiments with cathode-ray tubes. A forerunner of neon signs, fluorescent lights, and TV picture tubes, a typical cathode-ray tube is a partially
Review for Atomic Theory Quiz #1
 Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
Unit 1 Practice Test. Matching
 Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
EARLY ATOMIC THEORY AND STRUCTURE
 CHAPTER 5 EARLY ATOMIC THEORY AND STRUCTURE SOLUTIONS TO REVIEW QUESTIONS 1. Elements are composed of indivisable particles called atoms. Atoms of the same element have the same properties; atoms of different
CHAPTER 5 EARLY ATOMIC THEORY AND STRUCTURE SOLUTIONS TO REVIEW QUESTIONS 1. Elements are composed of indivisable particles called atoms. Atoms of the same element have the same properties; atoms of different
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
NYC K-8 Science Scope and Sequence: PS Standard 4 - Properties of Matter: 3.1a, 3.3a-d MST Standard 1 Inquiry Skills MST Standard 4 Process Skills
 Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Atomic structure. Resources and methods for learning about these subjects (list a few here, in preparation for your research):
 Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total
 Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
Light as a Wave. The Nature of Light. EM Radiation Spectrum. EM Radiation Spectrum. Electromagnetic Radiation
 The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
Introduction to Nuclear Physics
 Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Charges, voltage and current
 Charges, voltage and current Lecture 2 1 Atoms and electrons Atoms are built up from Positively charged nucleus Negatively charged electrons orbiting in shells (or more accurately clouds or orbitals) -
Charges, voltage and current Lecture 2 1 Atoms and electrons Atoms are built up from Positively charged nucleus Negatively charged electrons orbiting in shells (or more accurately clouds or orbitals) -
Basic Nuclear Concepts
 Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
ELEMENTS OF PHYSICS MOTION, FORCE, AND GRAVITY
 1 Pre-Test Directions: This will help you discover what you know about the subject of motion before you begin this lesson. Answer the following true or false. 1. Aristotle believed that all objects fell
1 Pre-Test Directions: This will help you discover what you know about the subject of motion before you begin this lesson. Answer the following true or false. 1. Aristotle believed that all objects fell
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Name Date Class ELECTRONS IN ATOMS. Standard Curriculum Core content Extension topics
 13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
Molecular Models & Lewis Dot Structures
 Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure Ron Robertson
 Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
Objectives 404 CHAPTER 9 RADIATION
 Objectives Explain the difference between isotopes of the same element. Describe the force that holds nucleons together. Explain the relationship between mass and energy according to Einstein s theory
Objectives Explain the difference between isotopes of the same element. Describe the force that holds nucleons together. Explain the relationship between mass and energy according to Einstein s theory
Credits Copyright, Utah State Office of Education, 2015.
 Credits Copyright, Utah State Office of Education, 2015. Unless otherwise noted, the contents of this book are licensed under the Creative Commons Attribution NonCommercial ShareAlike license. Detailed
Credits Copyright, Utah State Office of Education, 2015. Unless otherwise noted, the contents of this book are licensed under the Creative Commons Attribution NonCommercial ShareAlike license. Detailed
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
An Atom Apart by Leslie Cargile
 Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Chemistry. The student will be able to identify and apply basic safety procedures and identify basic equipment.
 Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Flame Tests & Electron Configuration
 Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Instructors Guide: Atoms and Their Isotopes
 Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Force on Moving Charges in a Magnetic Field
 [ Assignment View ] [ Eðlisfræði 2, vor 2007 27. Magnetic Field and Magnetic Forces Assignment is due at 2:00am on Wednesday, February 28, 2007 Credit for problems submitted late will decrease to 0% after
[ Assignment View ] [ Eðlisfræði 2, vor 2007 27. Magnetic Field and Magnetic Forces Assignment is due at 2:00am on Wednesday, February 28, 2007 Credit for problems submitted late will decrease to 0% after
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* PHYSICS 0625/04 Paper 4 Theory (Extended) For Examination from 2016 SPECIMEN PAPER 1
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* PHYSICS 0625/04 Paper 4 Theory (Extended) For Examination from 2016 SPECIMEN PAPER 1
Structure and Properties of Atoms
 PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
PS-6.2 Explain the factors that determine potential and kinetic energy and the transformation of one to the other.
 PS-6.1 Explain how the law of conservation of energy applies to the transformation of various forms of energy (including mechanical energy, electrical energy, chemical energy, light energy, sound energy,
PS-6.1 Explain how the law of conservation of energy applies to the transformation of various forms of energy (including mechanical energy, electrical energy, chemical energy, light energy, sound energy,
Tro's "Introductory Chemistry", Chapter 4
 1 Introductory Chemistry, 3 rd Edition Nivaldo Tro Atoms and Elements Opening figure showing a shore scene with molecules of O 2, N 2, triethyl amine (CH 3 CH 2 ) 3 N, and rocks made of silicates containing
1 Introductory Chemistry, 3 rd Edition Nivaldo Tro Atoms and Elements Opening figure showing a shore scene with molecules of O 2, N 2, triethyl amine (CH 3 CH 2 ) 3 N, and rocks made of silicates containing
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS
 Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
Lab 1. Charges and Electrostatics
 Physics 2020, Fall 2005 Lab 1 page 1 of 8 Lab 1. Charges and Electrostatics INTRODUCTION: Most modern applications of electricity involve moving electric charges or current electricity. Historically, however,
Physics 2020, Fall 2005 Lab 1 page 1 of 8 Lab 1. Charges and Electrostatics INTRODUCTION: Most modern applications of electricity involve moving electric charges or current electricity. Historically, however,
Bohr s Model of the Atom
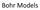 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
GCSE Additional Science Physics Contents Guide
 GCSE Additional Science Contents Guide Copyright Boardworks Ltd 2007 Boardworks Ltd The Gallery 54 Marston Street Oxford OX4 1LF 08703 50 55 60 [email protected] www.boardworks.co.uk 04-07 contains
GCSE Additional Science Contents Guide Copyright Boardworks Ltd 2007 Boardworks Ltd The Gallery 54 Marston Street Oxford OX4 1LF 08703 50 55 60 [email protected] www.boardworks.co.uk 04-07 contains
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Chapter 2: The Atom. Structure of the atom
 18 Chapter 2: The Atom An atom is the smallest particle of an element, having the same chemical properties as the bulk element. The first accurate theory explaining the nature of matter was Dalton s Atomic
18 Chapter 2: The Atom An atom is the smallest particle of an element, having the same chemical properties as the bulk element. The first accurate theory explaining the nature of matter was Dalton s Atomic
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
THIRD GRADE PHYSICS 3 WEEKS LESSON PLANS AND ACTIVITIES
 THIRD GRADE PHYSICS 3 WEEKS LESSON PLANS AND ACTIVITIES APPLIED SCIENCE OVERVIEW OF THIRD GRADE SCIENCE AND MATH WEEK 1. PRE: Comparing objects mathematically. LAB: Predicting and measuring objects. POST:
THIRD GRADE PHYSICS 3 WEEKS LESSON PLANS AND ACTIVITIES APPLIED SCIENCE OVERVIEW OF THIRD GRADE SCIENCE AND MATH WEEK 1. PRE: Comparing objects mathematically. LAB: Predicting and measuring objects. POST:
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Chapter 20 Electrostatics and Coulomb s Law 20.1 Introduction electrostatics. 20.2 Separation of Electric Charge by Rubbing
 I wish to give an account of some investigations which have led to the conclusion that the carriers of negative electricity are bodies, which I have called corpuscles, having a mass very much smaller than
I wish to give an account of some investigations which have led to the conclusion that the carriers of negative electricity are bodies, which I have called corpuscles, having a mass very much smaller than
Time allowed: 1 hour 45 minutes
 GCSE PHYSICS Foundation Tier Paper 1F F Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the Physics Equation Sheet (enclosed). Instructions Answer
GCSE PHYSICS Foundation Tier Paper 1F F Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the Physics Equation Sheet (enclosed). Instructions Answer
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004. Name (print) SSN
 CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
Monday 21 May 2012 Morning
 THIS IS A NEW SPECIFICATION H Monday 21 May 2012 Morning GCSE TWENTY FIRST CENTURY SCIENCE PHYSICS A A182/02 Modules P4 P5 P6 (Higher Tier) *A135280612* Candidates answer on the Question Paper. A calculator
THIS IS A NEW SPECIFICATION H Monday 21 May 2012 Morning GCSE TWENTY FIRST CENTURY SCIENCE PHYSICS A A182/02 Modules P4 P5 P6 (Higher Tier) *A135280612* Candidates answer on the Question Paper. A calculator
Models of the Atom and periodic Trends Exam Study Guide
 Name 1. What is the term for the weighted average mass of all the naturally occurring isotopes of an element? ans: atomic mass 2. Which is exactly equal to 1/12 the mass of a carbon -12 atom? ans: atomic
Name 1. What is the term for the weighted average mass of all the naturally occurring isotopes of an element? ans: atomic mass 2. Which is exactly equal to 1/12 the mass of a carbon -12 atom? ans: atomic
3 Atomic Structure 15
 3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
ANSWER KEY. Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take!
 ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
WAVES AND ELECTROMAGNETIC RADIATION
 WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
Basics of Nuclear Physics and Fission
 Basics of Nuclear Physics and Fission A basic background in nuclear physics for those who want to start at the beginning. Some of the terms used in this factsheet can be found in IEER s on-line glossary.
Basics of Nuclear Physics and Fission A basic background in nuclear physics for those who want to start at the beginning. Some of the terms used in this factsheet can be found in IEER s on-line glossary.
