Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
|
|
|
- Samuel Rafe Norris
- 9 years ago
- Views:
Transcription
1 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and electrons calculate the number of protons, neutrons, and electrons in an atom, given its atomic number interpret information on the periodic table construct model atoms define isotope SCHEDULE About 40 minutes For each team of four 6 beads, blue 6 beads, orange 6 beads, yellow colored pencils* (optional) 1 pipe cleaner, 40-cm 1 pipe cleaner, 50-cm 1 trough, clear, plastic For the class 1 roll fishing line illustrations of atoms from textbooks (optional)* 1 poster, Periodic Table of Elements 1 ruler, metric* 1 scissors* *provided by the teacher VOCABULARY atom electron element energy level isotope neutron nucleus periodic table proton subatomic particles MATERIALS For each student 1 Activity Sheet 4 1 periodic table chart PREPARATION Make a copy of Activity Sheet 4 for each student. Tear off one copy of the periodic table chart (from the pad of 40) for each student. Cut the fishing line into lengths of 60 cm (about 2 ft) each, two lengths per team. Each team of four will need two lengths of fishing line, two pipe cleaners (one of each length), and six each of the blue, orange, and yellow beads. Place the materials in a trough for easier handling. (In the chemistry laboratory, a trough is a pan that holds water into which other objects are placed. The troughs in the kit are used for that purpose as well as for holding activity materials, as here.) Each student will also need a periodic table chart for reference. delta science modules Matter and Change 37
2 4 Look for textbook illustrations of atoms to show the students. Display the poster of the Periodic Table of Elements in the classroom for reference in this and all subsequent activities. BACKGROUND INFORMATION Atoms are composed of protons, neutrons, and electrons, each of which is uniquely essential to the structure and function of the atom. The core of the atom is the nucleus, which consists of protons and neutrons. Most of the mass of an atom (about 99.9 percent) is in the nucleus, even though the nucleus occupies only a tiny part of the atom. If the nucleus of an atom were the size of a golf ball, the electrons would be about 2.5 km (1.5 mi) away. Each proton in the nucleus contains one positive charge. The neutron is roughly the same mass as the proton, but contains no charge. Electrons have a negative charge and are thousands of times less massive than either protons or neutrons. Electrons occupy cloud configurations at several distinct distances from the nucleus, called energy levels. Each consecutive distance away from the nucleus represents a higher energy level. Although there are many different energy levels, the students will model only the first and second levels in this activity. For your information, electron capacities for the first four levels are listed in Figure 4-1. Activity Sheet 4 Atomic Structure 1. Choose an atom from the list on the board. Make a model of this atom. Use one length of fishing line to string together the beads representing the protons and the beads representing the neutrons in the nucleus. Use the pipe cleaners to hold the beads representing the electrons in rings around the nucleus. Connect the model pieces with the other length of fishing line. 2. Fill in the blanks for the atom you built: Answers will vary. Name of Element Symbol Atomic Number Mass Number Number of Electrons Number of Protons 3. Classify the three main subatomic particles according to mass, charge, and location in the atom. Mass Charge Location Proton Neutron Electron What is an isotope? Nucleus Nucleus Outside nucleus, in energy levels An isotope is an atom of an element with the same atomic number and the same number of protons, but with a different number of neutrons. It has the same charge but a different mass number. 4. Using your model pieces, build an atom of lithium. Now modify your model to represent an isotope of lithium with a mass number of 8. Draw both models in the space below. lithium 6 lithium 8 Energy Level Electron Capacity Figure 4-1. Electron capacities for the first four energy levels. 38 activity 4 Atomic Structure
3 1 Guiding the Activity A boron atom contains five protons, five neutrons, and five electrons. Demonstrate for the class the modeling of a boron atom, as shown in Figure 4-2. Let the blue beads represent electrons, the orange beads represent neutrons, and the yellow beads, protons. Note: You may wish to assemble the boron atom model ahead of time. Additional Information 2 3 Write the words element and atom on the board. Tell the students that the model you have just assembled represents one atom of an element called boron. Ask, What is an element? What is an atom? Explain to the students that an atom is composed of three main subatomic particles. Under the word atom, write the term subatomic particles. Below that, write protons, neutrons, and electrons. Ask, What does it mean to say that protons, neutrons, and electrons are all subatomic particles? If necessary, explain that subatomic particles are the smaller parts that make up an atom. Write the word nucleus on the board. Explain that the nucleus, or center, of an atom is composed of protons and neutrons. A proton has one positive charge and a mass equal to one mass unit. A neutron has no charge, but has a mass that is also equal to 1 mass unit. Ask, What kind of charge does the nucleus as a whole have? Figure 4-2. A model boron atom. An element is a pure substance that cannot be broken down into any other substances. An atom is the smallest particle of an element that has all the properties of the element. It means that protons, neutrons, and electrons are the building blocks of atoms. A positive charge. delta science modules Matter and Change 39
4 4 Guiding the Activity Tell the students that the electrons are located around the nucleus. Electrons are so tiny that they have almost no mass, and each electron carries one negative charge. Hold up the model atom that you assembled. Ask, Which colors represent the protons, neutrons, and electrons? Again, hold up the model. Ask, How does the number of protons compare with the number of electrons? What can you conclude from this about the total charge of the atom? Write energy levels on the board. Explain that the electrons swirl around an atom at certain average distances from the nucleus. The areas where the electrons travel are called energy levels. In this model, energy levels are represented by rings. As the students observe the model, ask, Why do you think I put two electrons on the inner ring and three on the outer ring? Explain that only a certain number of electrons can be held in an energy level. The first energy level can hold two electrons. The second energy level can hold up to eight electrons. Ask, If we wanted to model the element carbon, which has six electrons, how many electrons (beads) would go on the inner ring? How many would go on the outer ring? If you have textbook illustrations of atoms, show them to the students now. Have the students note the various subatomic particles and how they are represented. Additional Information Although they will not be able to distinguish the protons from the neutrons, students should be able to figure out that the neutrons and protons are the orange and yellow beads and the electrons are the blue beads. Write the color code of the beads on the board for reference. The number of protons and the number of electrons are equal. Thus, the positive and negative charges balance and the atom is neutral. Accept whatever guesses the students may make. Students should respond that two electrons would go on the inner ring and four on the outer. Reinforce for the students that the electrons do not orbit around the nucleus like planets around the sun. Rather, they occupy a three-dimensional electron cloud a certain average distance from the nucleus. The pipe cleaners in the models are merely a way to hold the electrons (beads) in place; they do not describe the real positions or movement of the electrons. 40 activity 4 Atomic Structure
5 5 6 Guiding the Activity Write the term periodic table on the board and distribute a periodic table chart to each student. Give students time to study it. Ask, What information does the periodic table give us? Explain to the students that the atomic number of an atom tells the number of protons in the atom. And because, in a neutral atom, the number of protons and the number of electrons are equal, it also tells the number of electrons. The mass number (sometimes called the atomic mass) of the element tells the mass of one atom, which is approximately the sum of the number of protons and neutrons in the nucleus, since each proton and each neutron has a mass equal to one mass unit, and the electrons have virtually no mass. Give each team a trough containing two pipe cleaners (one of each length), six of each color bead (orange, yellow, and blue), and two lengths of fishing line. Distribute a copy of Activity Sheet 4 to each student. Write the words hydrogen, helium, lithium, beryllium, boron, and carbon, on the board. Have each team choose an element from this list to model. Instruct the students to use the periodic table to find the information they need, such as the number of electrons. Have them complete steps 1 3 of the activity sheet. Invite teams to share their models with the class. Have them tell what information they found on the periodic table and describe how they illustrated it in their models. Additional Information The periodic table is an orderly listing of all the elements, arranged from left to right and top to bottom in order of increasing atomic number. It shows the atomic number and the mass number of a single atom of each element. It also shows the element s name and symbol. Students may need to be reminded that the atomic number equals the number of protons (and the number of electrons). For these small atoms, the atomic number equals the number of neutrons as well. delta science modules Matter and Change 41
6 7 8 Guiding the Activity Next, tell the students that two atoms of the same element are not always identical. Remove a neutron from your demonstration model and ask, Is the charge of the atom affected by this change? Tell the students that, although the number of neutrons has changed, the model is still an atom of boron. Explain that atoms with the same number of protons but a different number of neutrons in their nuclei are called isotopes of an element. Write the term isotope on the board. Ask, Do two isotopes of the same atom have the same mass? Tell the students to complete the activity sheet. Discuss their answers with them. To wrap up, ask, What have you learned about the structure of the atom and the nature of subatomic particles from making a model of an atom? Ask, In what ways are the models you built in this activity different from real atoms? Additional Information No. The atom is still neutral because a neutron has no charge. No. Because the neutron has mass, the isotope that has more neutrons will have greater mass. Students should have learned the following: 1. Protons and neutrons are in the nucleus, and electrons are outside the nucleus. 2. Protons have a mass of 1 and a charge of Neutrons have a mass of 1 and no charge. 4. Electrons have virtually no mass and a charge of 1. Obviously the models are much bigger than real atoms. In the models, the electrons are the same size as the protons and neutrons. In real atoms, electrons are much smaller and much farther away from the nucleus (relatively speaking). Also, electrons do not travel in an orbit but may be a certain distance in any direction from the nucleus. 42 activity 4 Atomic Structure
7 R EINFORCEMENT Have the students pick from the periodic table an element with an atomic number between 1 and 14 and sketch an atom of that element using colored pens or pencils. Have them show the sketch to another student, who should compare the information in the drawing to that in the periodic table to figure out the identity of the element. Assessment Opportunity This Reinforcement also may be used as an ongoing assessment of students understanding of science concepts and skills. SCIENCE NOTEBOOKS Have students write their names on their periodic table charts and place them, along with their completed activity sheets, in their science notebooks. C LEANUP Leave the Periodic Table poster on display in the classroom. You may wish to decorate the classroom with the students atoms. When finished, put the beads and spool of fishing line back in the kit. The pipe cleaners should be straightened and returned to the kit as well. SCIENCE AT HOME Ask students to find three items in their homes that are composed of a pure element. For each item, they should use the periodic table to find out the symbol of the element the item is made of and the number of protons and neutrons in an atom of that element. delta science modules Matter and Change 43
8 Connections Science Extension Have cooperative-learning groups research theoretical models of the atom through history, from John Dalton s solid-sphere model (1807), to J. J. Thompson s plum pudding model (1903), Ernest Rutherford s nucleus-centered model (1911), Niels Bohr s model (1913), and finally to contemporary refinements. Suggest that students build models to illustrate and compare early theories of atomic structure. Ask volunteers to create a bulletin board time line showing the development of atomic models. Science and the Arts Let students use a variety of materials of their own choice to create models of atoms. Encourage students to be imaginative and creative, and suggest that they try to show electrons in different energy levels in a threedimensional cloud around the nucleus. Give students an opportunity to explain their models. Science and Careers Make sure students understand that radioactive elements are used to produce X-ray pictures of structures that cannot be observed directly. Invite a medical or industrial radiologist to visit the class and describe his or her work. Ask the visitor to bring X-ray photographs to show and explain to students. Science and Health Explain that radon is a colorless, odorless, radioactive gas that occurs naturally in soil and rock as a by-product of the decay of uranium. Suggest that students research the health hazards of radon exposure. (In high concentrations, radon can cause lung cancer.) Encourage students to find out about radon test kits that are available for home use. Students might enjoy researching the names and percentages of the elements in the human body. Suggest that they also find out the functions of various elements in maintaining good health. Ask volunteers to use this information to create a large poster for classroom display. Science and Language Arts If students have difficulty remembering the type of charge associated with each subatomic particle, suggest that they use the following mnemonic device: a Proton has a Positive charge; a NEUTRon is NEUTRal, or has no charge; and an electron has the remaining possibility, a negative charge. Ask students to find out how the elements got their names and symbols. Many elements were first named in Latin or Greek thus the symbols Pb for lead and Au for gold, for example. Other elements were named for their discoverers or place of discovery. Still others were named for specific properties such as color. Science and Social Studies Ask students to use a dictionary to determine the origin of the word atom (from the Greek atomos, meaning indivisible ). Encourage students to research the first application of this term and its later acceptance and use by the scientific community. (The Greek philosopher Democratus reasoned that if bits of matter were broken down into smaller and smaller particles, eventually a point would be reached at which a particle could not be divided any further. Democratus called this indivisible particle an atom. However, it was not until the 1800s that scientists were able to prove Democratus correct.) 44 activity 4 Atomic Structure
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
5.1 Evolution of the Atomic Model
 5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
Atoms and Elements [6th grade]
![Atoms and Elements [6th grade] Atoms and Elements [6th grade]](/thumbs/40/21183873.jpg) Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Atomic Theory Part 1
 Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
NOTES ON The Structure of the Atom
 NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Atomic structure. Resources and methods for learning about these subjects (list a few here, in preparation for your research):
 Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
The Models of the Atom
 The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
Chapter 4, Lesson 2: The Periodic Table
 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Development of the Atomic Theory
 Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
Unit 1 Practice Test. Matching
 Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
For convenience, we may consider an atom in two parts: the nucleus and the electrons.
 Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
An Atom Apart by Leslie Cargile
 Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Structure and Properties of Atoms
 PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these particles affect the properties of an atom (including identity,
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Introduction to Nuclear Physics
 Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
CHAPTER 4: ATOMS AND ELEMENTS
 CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
Bohr s Model of the Atom
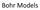 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Properties of Atoms and the Periodic Table
 Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Instructors Guide: Atoms and Their Isotopes
 Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Color Filters and Light
 activity 4 Color Filters and Light OBJECTIVES Students add to their understanding of subtractive color mixing by investigating the effect of filters on the color of light. The students shine white light
activity 4 Color Filters and Light OBJECTIVES Students add to their understanding of subtractive color mixing by investigating the effect of filters on the color of light. The students shine white light
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Atomic Calculations. 2.1 Composition of the Atom. number of protons + number of neutrons = mass number
 2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
Radioactivity & Particles
 Radioactivity & Particles Introduction... 2 Atomic structure... 2 How are these particles arranged?... 2 Atomic notation... 4 Isotopes... 4 What is radioactivity?... 5 Types of Radiation: alpha, beta and
Radioactivity & Particles Introduction... 2 Atomic structure... 2 How are these particles arranged?... 2 Atomic notation... 4 Isotopes... 4 What is radioactivity?... 5 Types of Radiation: alpha, beta and
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
Noble Gases. Outline Nobel Gas Elements Radon and Health Chemistry Homework
 Radon and Other Noble Gases The elements in the last column of the periodic table are all very stable, mono-atomic gases. Until 1962, they were called inert gases because they did not react with other
Radon and Other Noble Gases The elements in the last column of the periodic table are all very stable, mono-atomic gases. Until 1962, they were called inert gases because they did not react with other
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Elements and Atoms: The Building Blocks of Matter
 OpenStax-CNX module: m45998 1 Elements and Atoms: The Building Blocks of Matter OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By
OpenStax-CNX module: m45998 1 Elements and Atoms: The Building Blocks of Matter OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
NYC K-8 Science Scope and Sequence: PS Standard 4 - Properties of Matter: 3.1a, 3.3a-d MST Standard 1 Inquiry Skills MST Standard 4 Process Skills
 Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Student Exploration: Electron Configuration
 www.explorelearning.com Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund
www.explorelearning.com Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund
Chapter 2 Atoms and Molecules
 Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Levers for Lifting BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN ACTIVITY ASSESSMENT OPPORTUNITIES. Grade 3 Quarter 3 Activity 23
 activity Levers for Lifting BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade Quarter Activity SC.C... The student understands that the motion of an object can be described and measured. SC.H... The
activity Levers for Lifting BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade Quarter Activity SC.C... The student understands that the motion of an object can be described and measured. SC.H... The
Molecular Models & Lewis Dot Structures
 Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
KINDERGARTEN CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES
 KINDERGARTEN CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF KINDERGARTEN CHEMISTRY WEEK 1. PRE: Distinguishing the four types of matter. LAB: Classifying heavy and light rocks. POST:
KINDERGARTEN CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF KINDERGARTEN CHEMISTRY WEEK 1. PRE: Distinguishing the four types of matter. LAB: Classifying heavy and light rocks. POST:
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Transferring Solar Energy
 activity 14 Transferring Solar Energy BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 4 Quarter 2 Activity 14 SC.B.1.2.2 The student recognizes various forms of energy (e.g., heat, light, and electricity).
activity 14 Transferring Solar Energy BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 4 Quarter 2 Activity 14 SC.B.1.2.2 The student recognizes various forms of energy (e.g., heat, light, and electricity).
Name Date Class ELECTRONS IN ATOMS. Standard Curriculum Core content Extension topics
 13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
Radiation and the Universe Higher Exam revision questions and answers
 Radiation and the Universe Higher Exam revision questions and answers Madeley High School Q.The names of three different processes are given in List A. Where these processes happen is given in List B.
Radiation and the Universe Higher Exam revision questions and answers Madeley High School Q.The names of three different processes are given in List A. Where these processes happen is given in List B.
The Structure of the Atom
 The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
Question: Do all electrons in the same level have the same energy?
 Question: Do all electrons in the same level have the same energy? From the Shells Activity, one important conclusion we reached based on the first ionization energy experimental data is that electrons
Question: Do all electrons in the same level have the same energy? From the Shells Activity, one important conclusion we reached based on the first ionization energy experimental data is that electrons
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
Electrical Symbols PREPARATION OBJECTIVES SCHEDULE VOCABULARY MATERIALS. The students. For each student. For each team of two.
 activity 2 Electrical Symbols OBJECTIVES In this activity, students discover the usefulness of symbols used to identify parts of a circuit. The students draw and interpret circuit diagrams construct circuits
activity 2 Electrical Symbols OBJECTIVES In this activity, students discover the usefulness of symbols used to identify parts of a circuit. The students draw and interpret circuit diagrams construct circuits
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS
 ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
FIRST GRADE CHEMISTRY
 FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chemistry 1000 Lecture 2: Nuclear reactions and radiation. Marc R. Roussel
 Chemistry 1000 Lecture 2: Nuclear reactions and radiation Marc R. Roussel Nuclear reactions Ordinary chemical reactions do not involve the nuclei, so we can balance these reactions by making sure that
Chemistry 1000 Lecture 2: Nuclear reactions and radiation Marc R. Roussel Nuclear reactions Ordinary chemical reactions do not involve the nuclei, so we can balance these reactions by making sure that
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Objectives 404 CHAPTER 9 RADIATION
 Objectives Explain the difference between isotopes of the same element. Describe the force that holds nucleons together. Explain the relationship between mass and energy according to Einstein s theory
Objectives Explain the difference between isotopes of the same element. Describe the force that holds nucleons together. Explain the relationship between mass and energy according to Einstein s theory
Student Exploration: Electron Configuration
 Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Science 20. Unit A: Chemical Change. Assignment Booklet A1
 Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
Chemistry - Elements Electron Configurations The Periodic Table. Ron Robertson
 Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Lesson 6: Earth and the Moon
 Lesson 6: Earth and the Moon Reading Assignment Chapter 7.1: Overall Structure of Planet Earth Chapter 7.3: Earth s Interior More Precisely 7-2: Radioactive Dating Chapter 7.5: Earth s Magnetosphere Chapter
Lesson 6: Earth and the Moon Reading Assignment Chapter 7.1: Overall Structure of Planet Earth Chapter 7.3: Earth s Interior More Precisely 7-2: Radioactive Dating Chapter 7.5: Earth s Magnetosphere Chapter
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
OA4-13 Rounding on a Number Line Pages 80 81
 OA4-13 Rounding on a Number Line Pages 80 81 STANDARDS 3.NBT.A.1, 4.NBT.A.3 Goals Students will round to the closest ten, except when the number is exactly halfway between a multiple of ten. PRIOR KNOWLEDGE
OA4-13 Rounding on a Number Line Pages 80 81 STANDARDS 3.NBT.A.1, 4.NBT.A.3 Goals Students will round to the closest ten, except when the number is exactly halfway between a multiple of ten. PRIOR KNOWLEDGE
The University of Texas at Austin. Gravity and Orbits
 UTeach Outreach The University of Texas at Austin Gravity and Orbits Time of Lesson: 60-75 minutes Content Standards Addressed in Lesson: TEKS6.11B understand that gravity is the force that governs the
UTeach Outreach The University of Texas at Austin Gravity and Orbits Time of Lesson: 60-75 minutes Content Standards Addressed in Lesson: TEKS6.11B understand that gravity is the force that governs the
