Atoms and Elements [6th grade]
|
|
|
- Randell Watts
- 9 years ago
- Views:
Transcription
1 Trinity University Digital Trinity Understanding by Design: Complete Collection Understanding by Design Summer Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected] Follow this and additional works at: Repository Citation Wray, Jennifer J., "Atoms and Elements [6th grade]" (2015). Understanding by Design: Complete Collection. Paper This Instructional Material is brought to you for free and open access by the Understanding by Design at Digital Trinity. It has been accepted for inclusion in Understanding by Design: Complete Collection by an authorized administrator of Digital Trinity. For more information, please contact [email protected].
2 Unit: Atoms and Elements Grade: 6 th Grade Stage 1: Desired Results Understandings Students will understand that Scientific truth changes based on new learning. All physical things are made of tiny units put together. Models are used to help us understand concepts. Scientists can use indirect evidence to learn about things that can t be seen or touched. Standards: Colorado Academic Standards, 6 th Grade Physical Science: 1. All matter is made of atoms, which are far too small to see directly through a light microscope. Elements have unique atoms and thus, unique properties. Atoms themselves are made of even smaller particles. Colorado Academic Standards, 6 th Grade Research and Reasoning: 1. Individual and group research projects require obtaining information on a topic from a variety of sources and organizing it for presentation. Core Knowledge Science: 1. Scientists have developed models of atoms; while these models have changed over time as scientists make new discoveries, the models help us imagine what we cannot see. 2. Elements have atoms of only one kind. 1. The Periodic Table: organizes elements with common properties. 2. Atomic symbol and atomic number 3. Some well-known elements and their symbols. Essential Questions 1. What are all things made of? 2. How can you study something you can t see? 3. How can something true change? 4. Why do we have models? Knowledge Students will know Atoms are the building blocks of matter. Atoms are made of smaller particles called protons, electrons, and neutrons with charges. Scientific ideas concerning atoms have changed over time. Elements are made of one type of atom with unique properties. Common elements. The Periodic Table of Elements organizes elements based on their properties, such as their atomic symbol and atomic number. How to conduct research on a particular element. How to present acquired information. Skills Students will be able to Break scientific ideas into their building blocks (ex: apply knowledge of atoms to cells in biology) Research a topic and present information in writing and a model. Describe how understandings can change with the addition of new learning. Use indirect observation to study something.
3 Stage 2: Assessment Evidence Performance Task: Adopt an Element After learning about atoms and gaining an understanding of how the Periodic Table of Elements is organized, students will chose an element that they would like to Adopt. Students will conduct research on their element and create a 3-dimensional model of their choice. In addition to the model, there will be a written component that provides details and interesting facts about their element. The assignment and rubric are in the appendix. Other evidence: Quizzes Notes Models Stage 3: Learning Activities (Steps taken to get students to answer Stage 1 questions and complete performance task) Lesson 1 What are things made of? Objective: Scientists have been investigating what things are made of for a very long time and they conclude that all things are made of atoms. (Students write down objective in their science notebook.) Pre-Assessment: On a notecard, have students draw a picture of an atom. Students can discuss what ideas they may or may not have about the word. If students do not have any background knowledge on atoms, instead ask, What are things made of? After students write, draw, and discuss collect the cards. Students rip out a page from their science notebooks. Instruct students to rip their paper in half and continue to do so until they make the absolute tiniest piece possible. What do you think eventually happens? o Eventually, we would get down to something so small that we couldn t even see if with a microscope. We call these objects atoms. Atoms are the basic units of matter. (Students write definition in notebooks) Matter is anything that has mass and volume. Students will then read an article about the scientific history of discovering the atom. Any article will work but here is a link to one: y_of_atoms.pdf Students will take notes using some kind of strategy that emphasizes sequencing (Schools that use Thinking Maps can use a Flow Map). Stress to students that science is always changing and research and discoveries are constantly being made, how exciting! Homework: Students finish reading and taking notes about the article. Lesson 2 Mystery Box Objective: Because atoms cannot be seen, scientist study them using indirect evidence. How do scientist know about atoms? How can you learn about something you can t see? We can use indirect evidence. Indirect evidence is evidence gained by observation. Working in groups, students receive some type of box with about 4 items in it. Students must use the questions to guide them through their exploration. (Questions are in the appendix) When they have answered questions and hypothesized about what is in their box they may open the box and see what it is. Then, groups can trade boxes or put in items of their own choice for others to figure out. Summary Discussion: Scientists use many tools to help them explore atoms. Indirect observation means using observation to gain new understanding. New tools and understandings are always being introduced so our understanding of the atom changes with new information.
4 Students finish their observation guide for homework. Lesson 3 Structure of an Atom (2 days) Objective: Students can explain what an atom looks like. So, if we can t see or touch atoms, what do they look like? o Students take notes on this website (or any other website describing the 3 main subatomic particles: protons, electrons, neutrons), depending on access to computers they may do it alone or as a class. o In their science notebooks they should have these three definitions Proton: positively charged particle found in the nucleus. Electron: Negatively charged particle outside of the nucleus. Neutron: Neutral particles found in the nucleus. Once students have the language and understanding of the three particles then discuss again how understandings have changed and give a history of atomic theory. o Go back to the article from Lesson 1 and have students draw pictures in their notes of each stage of development. This can be done through whole group lecture, group work, or this video is also very informative, although a little bit above a typical 6 th grader but with great visuals: Ancient Greeks (Democritus and Leucippus): 300s BC believed that everything was made of Fire, Earth, Air, Water, and Aether. They believed that there was something that could not be broken down into smaller pieces - atomos. John Dalton: 1805 Students will draw a plain circle for their model. Without knowing about protons or electrons, John Dalton proposed that atoms cannot be divided into smaller particles and elements are made of atoms. (A similar idea to the Greeks, just resurfaced thousands of years later.) J.J. Thomson s Plum Pudding Model: 1897 JJ Thomson discovered that there were positive and negative charges in an atom using a tube and electric charges. He proposed that negatively charged particles were floating around in a pudding of positively charged substance. Students label, date and draw the plum pudding model in their journals. The Rutherford Model: 1894 Rutherford discovered there was a positively charged nucleus! He conducted an experiment using gold foil and made observations to do this. Students draw the model with a nucleus and electrons orbiting around. Bohr Model: 1911 Bohr suggested there were energetic levels of electrons orbiting the nucleus. Students draw a model in their notebook with a nucleus and electrons orbiting in concentric circles. Erwin Schrodinger: 1926 Schrodinger concluded that it is impossible to know where an electron will be. Therefore, instead of modeling electrons using predictable patterns, he depicts them in a cloud or cotton candy and used mathematical equations to find the probability of where the electrons were most likely to be. Students draw a picture in their notes of the atom with an electron cloud around a nucleus. Below is an example of possible models to draw in their notebooks.
5 o Students get into groups of 6. Each student gets a particle. Tell them they are now a Helium atom. There should be 2 Protons, 2 Electrons, and 2 Neutrons. You could use name tags to reinforce the point. Then, go through each model and have students act how an atom would behave in that model. Students should also verbally practice saying the name of each model and talking about each particle. (Sentence structures projected on the board are good for this: This is the model. The electrons. The protons and neutrons.) This activity can be done as a summary or after talking about each model. Ticket out the door: Students draw and label an atom using the cloud model. If you want more worksheets or activities, I found I really liked this website: o Lesson 4 Build Your Own Atom Objective: Students will model the structure of an atom. Start class with this video: o I always introduce this video as Mind Blowing because it describes how small atoms are. This is a difficult abstract concept for 6 th graders and the video shows many examples. Understanding how small atoms are makes the discoveries even more amazing and sheds light on the importance of models in science. It even addresses the big idea that models are not perfect but help us understand concepts. Then, challenge students to build their own 3 D model using classroom materials. Teachers can provide things like cotton balls, toothpicks, string, marshmallows or anything else around the room. They use the instruction sheet to guide them found in the appendix. Homework: Study for Quiz Lesson 5 It s Elemental! Objective: Elements are made of the same type of atom and are organized on the Periodic Table of Elements. Students take quiz on the first 4 lessons. (Quiz is in the appendix) Students will read an article (any on what elements are will do, here is an example from Chemkids: o Key Points that should be in their notes: Atoms can be organized by how many protons, electrons and neutrons they have. An element is a substance that is made of only one type of atom. All of the known materials in the universe are made from elements. There are currently 118 known elements. o This is a fun video that very quickly goes through the elements:
6 o Project a Periodic Table of Elements. Tell students that the Periodic Table of Elements organizes all of the elements based on common properties. 2 main groups are metals and non-metals. o Then, tell what each symbol and number mean for the elements. Students should draw a model in their notebook and label and define the atomic number, atomic mass, element name, and symbol. Students complete a Periodic Table Scavenger Hunt. Below are some examples from online: o -%20Periodic%20Table%20Scavenger%20Hunt.pdf o file:///c:/users/ncate/downloads/periodic-table-scavenger-hunt-reviewing%20(4).pdf Lesson 6 Getting to Know Common Elements Objective: Students get familiar with Periodic Table and memorize common elements and their symbols. Start class with a review. Half the class will have an element on a notecard and half of the class will have numbers. They must use the Periodic Table to match up with their partner. Review how the periodic table is organized and how to read it. Then, discuss metals and non-metals. This is a good online article to ground the discussion/note taking: Core Knowledge has a list of common elements that people should know. With the remainder of class, students use notecards to make flashcards of these elements. Element and atomic number on one side, atomic symbol and possibly a picture on the other. o Hydrogen H, Helium He, Carbon C, Nitrogen N, Oxygen O, Sodium Na, Aluminum Al, Silicon Si, Chlorine Cl, Iron Fe, Copper Cu, Silver Ag, Gold Au Students finish their flashcards and study for homework. **There is also a good PBS Nova video on elements called Hunting the Elements. It is currently on Youtube at Lesson 7 Start Adopt an Atom project Objective: Students will begin researching their chosen element. You will need computers for this lesson! Pass out Performance Task and discuss what is expected. Then, students will begin conducting research on their specific element. I have found the following websites helpful: o o Students use class time to research, plan, and write their paper to accompany their model. References: The assessment is adapted from ScienceSpot.net Other references embedded in text. Appendix: Below you will find accompanying documents. Name Date Adopt an Element
7 Performance Task You ve thought about it a lot, and now you are ready to adopt... an atom that is! You will choose an atom to adopt from the Periodic Table of Elements. We have learned that scientists use models to help them understand concepts more clearly, so here is your chance! You will create a 3 dimensional model of an atom based on the properties of your specific element. You also will get to explore your atom more by investigating its properties and unique aspects on the internet. You will have class time to research and write but most of the model building will be done at home. Use your imagination and creativity in selecting materials for this project. It can be presented as a free standing model, suspended from something like a coat hanger, mounted on a poster board, or something else that you have in mind. Your model needs to have the correct number of protons, neutrons, and electrons with their charges labeled. It needs to have positively charged nucleus and space in between the electrons and nucleus. You may use the Rutherford, Bohr, or Cloud model to represent your element, please identify which one you chose on your model. For the written piece, you need to use the Element Fact Sheet to guide your research. It should include all the information from the fact sheet, any additional information, clear transitions, and proper grammar. Use the rubric to make sure you have included all the requirements. Now, go adopt an element! Name Element name Element Fact Sheet Date
8 Symbol Atomic Number Atomic Weight Number of Protons Boiling Point Classification Normal Phase Discovered by Metal Nonmetal Metalloid Solid Liquid Gas What are three interesting facts you learned about your element? Why did you choose to adopt this element? Now, use this information to write a neat written piece describing your element. To earn full credit you must include all the information on this fact sheet! Be sure to include your sources at the bottom of your piece. Name Rubric for Adopt an Element Date Expectation: Model labels and correctly shows the protons, neutrons, and electrons of an atom. Charges are labeled and there is clearly a nucleus with spaced in the middle of the electrons. All components are included and correctly labeled. 16 points Model has most of the elements but is lacking 1 or 2 parts points Model is lacking 3 4 components points Model is lacking 5 or more components. 0-7 points 16 points
9 Model is neat and creative. 8 points Written piece includes information from the Element Fact Sheet 16 points Written piece has structure. There should be an opening, transitions, and closing. Proper grammar is used. 8 points Effort 6 points Total Score /54 Student goes above and beyond to use materials in an extremely creative fashion. The model is neat and clear. 8 points All facts are included. 16 points Written piece reads nicely and has a good flow. Errors do not impede the meaning. 8 points Students effort is clearly observed in class and reflected in their project. Time is managed and used wisely. 6 points Model used materials creatively and combines them neatly and clearly. The piece is missing 1 3 facts points Written piece is choppy at times or 1 component is missing. Errors confuse reader at times. 6 7 points Student uses most of time wisely. Student displays effort in class and project reflects this. 4-5 points Model looks messy and materials were not used very creatively. The piece is missing 4-6 facts points Written piece is unclear at times or is missing 2-3 components. Errors confuse reader often. 4 5 points Student uses some of class time wisely. Work choices are reflected in project. 2-3 points Model is copied directly from another source or not turned in. The piece is missing 7 or more facts. 0-5 points Piece is unclear and does not include an opening, transitions, or a closing. Errors significantly hold the reader back. 0-3 points Student uses little of their class time wisely. Their project suffers because of this. 0-1 point Name Date Mystery Box Use with Lesson 2 Atoms are crazy small! Not even with a traditional microscope can we see them. However, scientist have a good idea of what they look like, how is this possible? As scientists, we can use indirect evidence to study and support our ideas about things. Indirect evidence means we make predictions and theories based on our observations. You, as a scientist, will be using observations to make a hypothesis of something you cannot see. Materials Box with objects inside from your teacher
10 Triple beam balance Procedure 1. Obtain a box from your teacher. 2. Without being able to look inside your box, use strategies to figure out what is inside. For example, you may weigh, shake, tip, slide, or make comparisons to other objects. 3. After each action, record your observations. 4. Based on your observation, sketch the object(s) in your box. Include as many characteristics as you can. Observations Make a table to display your observations. Record your action and what you observed. Analysis 1. How is this activity similar to what scientists have done to learn about the atom? 2. How could you test your model of the box contents to see if you are correct? 3. What tools could help you study the contents of your box more? 4. Open your box. How does your hypothesis compare to the actual contents? Conclusions 1. What can we learn from this about the current model of an atom?
11 2. What did you like about this activity? What would you do differently next time? 3. What did you learn today? Lesson Adapted from: Name Challenge: Build an Atom Date Use with Lesson 4 Now that you know all about atoms and their subatomic particles. You will build one! Think about the structure and components of an atom. Now, look at the materials in the classroom and think about items could be used to model the parts of an atom. First, list the materials you will use below: Now, draw out and label your design (Label protons, neutrons, electrons and their charges):
12 Next, build it! Finally, make conclusions. Think about the questions below and write down your thoughts. What was difficult? What worked well? How does this apply to what scientists do? Did your model perfectly show an atom? What are the things about your model that aren t really the way an atom is? Name Atom Quiz Use after Lesson 4 Date 1. What is the smallest unit that matter is made up of? 2. How do scientists study atoms? 3. Who developed the Plum Pudding" model of an atom? 4. What was significant about the Rutherford model?
13 5. What did Schrodinger propose about electrons? How did that change his model? 6. Why are there so many different models of an atom? 7. Draw an atom using the cloud model. Label the protons, electrons, and neutrons with their charges.
Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Atomic Theory Part 1
 Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
PERIODIC TABLE. reflect
 reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
Development of the Atomic Theory
 Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
Development of the Atomic Theory Atom The smallest particle into which an element can be divided and still be the same substance. Element A pure substance that cannot be separated into simpler substances
An Atom Apart by Leslie Cargile
 Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Have you ever walked through a cloud of gnats on a hot summer, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
Atomic structure. Resources and methods for learning about these subjects (list a few here, in preparation for your research):
 Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
The Models of the Atom
 The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
The Models of the Atom All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas, consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals,
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
NOTES ON The Structure of the Atom
 NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
CHAPTER 4: ATOMS AND ELEMENTS
 CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
CHAPTER 4: ATOMS AND ELEMENTS Problems: 1-70 then after Chapter 9, complete 71-94, 103-104, 107-108, 113-114 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element All matter
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
5.1 Evolution of the Atomic Model
 5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
5.1 Evolution of the Atomic Model Studying the atom has been a fascination of scientists for hundreds of years. Even Greek philosophers, over 2500 years ago, discussed the idea of there being a smallest
Chapter 4, Lesson 2: The Periodic Table
 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Unit 2 Atomic Structure
 Unit 2 Atomic Structure Big Idea: Atomic structure explains patterns in the behavior of elements and allows us to predict the chemical and physical behavior of a given element. The organization of elements
Unit 2 Atomic Structure Big Idea: Atomic structure explains patterns in the behavior of elements and allows us to predict the chemical and physical behavior of a given element. The organization of elements
FIRST GRADE CHEMISTRY
 FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Atomic Calculations. 2.1 Composition of the Atom. number of protons + number of neutrons = mass number
 2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
Properties of Atoms and the Periodic Table
 Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
Properties of Atoms and the Periodic Table Section 3 The Periodic Table Skim Section 3 and write three questions based on your brief preview. 1. Accept all reasonable answers. How are the elements organized
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Atomic Theory: History of the Atom
 Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
Atomic Theory: History of the Atom Atomic Theory: experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). 1. Law of Conservation of Mass -During
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004. Name (print) SSN
 CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Session 1 What Is Matter? Properties and Classification of Matter
 Session 1 What Is Matter? Properties and Classification of Matter What is matter? This question at first seems simple matter is all around us. Yet how do we define it? What does a block of cheese have
Session 1 What Is Matter? Properties and Classification of Matter What is matter? This question at first seems simple matter is all around us. Yet how do we define it? What does a block of cheese have
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
The Structure of Water Introductory Lesson
 Dana V. Middlemiss Fall 2002 The Structure of Water Introductory Lesson Abstract: This is an introduction to the chemical nature of water and its interactions. In particular, this lesson will explore evaporation,
Dana V. Middlemiss Fall 2002 The Structure of Water Introductory Lesson Abstract: This is an introduction to the chemical nature of water and its interactions. In particular, this lesson will explore evaporation,
The Structure of the Atom
 The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
The Structure of the Atom Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc. Section 4. Early Ideas About Matter pages 02 05 Section 4. Assessment page 05. Contrast the methods
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Unit 1 Practice Test. Matching
 Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Unit 1 Practice Test Matching Match each item with the correct statement below. a. proton d. electron b. nucleus e. neutron c. atom 1. the smallest particle of an element that retains the properties of
Chapter 2 Atoms and Molecules
 Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
About the course GENERAL CHEMISTRY. Recommended literature: Chemistry: science of the matter. Responsible for the course: Dr.
 About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Chemical Composition Review Mole Calculations Percent Composition. Copyright Cengage Learning. All rights reserved. 8 1
 Chemical Composition Review Mole Calculations Percent Composition Copyright Cengage Learning. All rights reserved. 8 1 QUESTION Suppose you work in a hardware store and a customer wants to purchase 500
Chemical Composition Review Mole Calculations Percent Composition Copyright Cengage Learning. All rights reserved. 8 1 QUESTION Suppose you work in a hardware store and a customer wants to purchase 500
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
Bohr s Model of the Atom
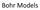 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
NYC K-8 Science Scope and Sequence: PS Standard 4 - Properties of Matter: 3.1a, 3.3a-d MST Standard 1 Inquiry Skills MST Standard 4 Process Skills
 Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Modeling Rutherford s Experiment A Teacher s Guide Cornell Laboratory for Accelerator-based Sciences and Education Author: Lora K. Hine Lesson: http://www.lepp.cornell.edu/education/teacherresources.html
Session 2 The Particle Nature of Matter: Solids, Liquids, and Gases
 Session 2 The Particle Nature of Matter: Solids, Liquids, and Gases What explanation might account for the differences between the states of matter, as well as explain its different properties? Session
Session 2 The Particle Nature of Matter: Solids, Liquids, and Gases What explanation might account for the differences between the states of matter, as well as explain its different properties? Session
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Molecular Models & Lewis Dot Structures
 Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Chapter Test B. Chapter: Measurements and Calculations
 Assessment Chapter Test B Chapter: Measurements and Calculations PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.
Assessment Chapter Test B Chapter: Measurements and Calculations PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.
THE PERIODIC TABLE O F T H E E L E M E N T S. The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27)
 THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
Trends of the Periodic Table Basics
 Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
 Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
For convenience, we may consider an atom in two parts: the nucleus and the electrons.
 Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Atomic structure A. Introduction: In 1808, an English scientist called John Dalton proposed an atomic theory based on experimental findings. (1) Elements are made of extremely small particles called atoms.
Name Date Class CHAPTER 1 REVIEW. Answer the following questions in the space provided.
 CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
Accommodated Lesson Plan on Solving Systems of Equations by Elimination for Diego
 Accommodated Lesson Plan on Solving Systems of Equations by Elimination for Diego Courtney O Donovan Class: Algebra 1 Day #: 6-7 Grade: 8th Number of Students: 25 Date: May 12-13, 2011 Goal: Students will
Accommodated Lesson Plan on Solving Systems of Equations by Elimination for Diego Courtney O Donovan Class: Algebra 1 Day #: 6-7 Grade: 8th Number of Students: 25 Date: May 12-13, 2011 Goal: Students will
What s in a Mole? Molar Mass
 LESSON 10 What s in a Mole? Molar Mass OVERVIEW Key Ideas Lesson Type Lab: Groups of 4 Chemists compare moles of substances rather than masses because moles are a way of counting atoms. When considering
LESSON 10 What s in a Mole? Molar Mass OVERVIEW Key Ideas Lesson Type Lab: Groups of 4 Chemists compare moles of substances rather than masses because moles are a way of counting atoms. When considering
Name: Teacher: Pd. Date:
 Name: Teacher: Pd. Date: STAAR Tutorial : Energy and Matter: Elements, Compounds, and Chemical Equations: 6.5C Differentiate between elements and compounds on the most basic level. 8.5F Recognize whether
Name: Teacher: Pd. Date: STAAR Tutorial : Energy and Matter: Elements, Compounds, and Chemical Equations: 6.5C Differentiate between elements and compounds on the most basic level. 8.5F Recognize whether
KINDERGARTEN CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES
 KINDERGARTEN CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF KINDERGARTEN CHEMISTRY WEEK 1. PRE: Distinguishing the four types of matter. LAB: Classifying heavy and light rocks. POST:
KINDERGARTEN CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF KINDERGARTEN CHEMISTRY WEEK 1. PRE: Distinguishing the four types of matter. LAB: Classifying heavy and light rocks. POST:
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS
 ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
ATOMS: ATOMIC STRUCTURE QUESTIONS AND ANSWERS QUESTION ONE: MODELS OF THE ATOM (2011;1) At different times scientists have proposed various descriptions or models of the atom to match experimental evidence
Answers to Review Questions for Atomic Theory Quiz #1
 Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b
7-5.5. Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including:
 7-5.5 Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including: NaCl [salt], H 2 O [water], C 6 H 12 O 6 [simple sugar], O 2 [oxygen
7-5.5 Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including: NaCl [salt], H 2 O [water], C 6 H 12 O 6 [simple sugar], O 2 [oxygen
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1. A chemical equation. (C-4.4)
 Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
Review for Atomic Theory Quiz #1
 Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
Review for Atomic Theory Quiz #1 Practice Multiple Choice Questions: 1. Which of the following is/are quantitative physical property(s) of matter? a) mass c) density b) volume d) all of the above 2. Which
Chapter 2 Atoms, Molecules, and Ions
 Chapter 2 Atoms, Molecules, and Ions 1. Methane and ethane are both made up of carbon and hydrogen. In methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a ration of 3:1 by mass. In ethane,
Chapter 2 Atoms, Molecules, and Ions 1. Methane and ethane are both made up of carbon and hydrogen. In methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a ration of 3:1 by mass. In ethane,
Ions & Their Charges Worksheet
 Ions & Their Charges Worksheet Name Date Teacher Diagram of charges based on groups on the periodic table including transition metals and noble gases: IA IIA Transition IIIA IVA VA VIA VIIA VIIIA metals
Ions & Their Charges Worksheet Name Date Teacher Diagram of charges based on groups on the periodic table including transition metals and noble gases: IA IIA Transition IIIA IVA VA VIA VIIA VIIIA metals
