Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: Time: Minutes Presenters: 2-4
|
|
|
- Matilda Boone
- 9 years ago
- Views:
Transcription
1 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: Time: Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter Explain the relationship between atoms, elements, molecules and compounds Build a model of an atom and a molecule Interpret element information from the Periodic Table Discuss the historical development of the study of matter, including contributions of notable scientists. Standards This lesson aligns with the following National Science Content Standards: Physical Science, 5-8, 9-12 History & Nature of Science, 5-8 Materials Introduction Atoms PowerPoint slides (overheads or flashdrive) Periodic Table poster Molecule building kit Periodic Table placemats Atoms activity Large marshmallows 2 different colors Small colored marshmallows Round toothpicks Atom Models Appendix A Atoms activity sheet Appendix B Miscellaneous Answer keys Appendix H Periodic Table handout- Appendix I Molecules activity Small gumdrops (yellow only) Large gumdrops (green, black, red) Round toothpicks Colored pencils (yellow, green, black, red) Molecules activity sheet App. C Molecules pictures Appendix D Elements Extension Periodic Table Cards Appendix E Periodic Table activity sheet - Appendix F Glue sticks 11 x 14 construction paper Colored pencils Orbitals activity sheet Appendix G Small round colored stickers or colored markers Revision Date: 8/9/ Micron Technology Foundation, Inc. All Rights Reserved
2 Preparation Ask the teacher to divide the class into at least two groups. If there are enough volunteers, groups can be split to do identical activities, allowing for a greater hands-on experience. Build the molecules shown in Appendix D using the Molecule building kit. Make copies of the Periodic Table handout - Appendix I for each student and have available at the stations. If Periodic Table placemats are available, have one at each station. Put the Periodic Table poster where it can be referred to during the introduction. Atoms activity: (Set up two stations of this activity if there are enough volunteers) Display Atom Models Appendix A Provide Atoms activity sheets Appendix B, one per student. Only use the first page of the appendix for the standard activity; copy both pages back-to-back if the Ions & Isotopes extension is going to be used. Put the large marshmallows in bowls or bins, one color to a bowl. Put all of the small marshmallows in one additional bowl. Have toothpicks available within easy reach of all students in the working group. Molecules activity: (Set up two stations of this activity if there are enough volunteers) Provide Molecules activity sheet Appendix C, one per student. Sort the gumdrops by color. Have toothpicks available within easy reach of all students in the group. Use the Molecules Pictures Appendix D as a reference. All students will need access to yellow, green, black and red colored pencils. The Elements activity works best as an additional classroom activity to reinforce and review the structure of the Periodic Table. Elements activity: Divide class into groups of 5 or 6 students. Prepare one set per group of Periodic Table Cards Appendix E by copying them (onto cardstock, if possible) and cutting them apart ahead of time. Provide Periodic Table activity sheets Appendix F, one per student. Have the following colored pencils available: green, pink, blue, purple, orange, red, tan and yellow. Each group will also need a glue stick and an 11 x 14 piece of construction paper. The Elements lesson extension may be included as a third station or used by itself. Elements Upper Level extension: Copy Orbitals activity sheets Appendix D (onto cardstock, if possible). Provide small round stickers or a colored marker, one color per student. 2
3 Introduction Have the volunteers introduce themselves and give a brief description of their backgrounds. Use the Atoms PowerPoint slides (also found at ) for the introduction. Slide 1: Introduction Q: What is everything made of? Every building, every person, every object? A: Everything is made up of matter. Matter is anything that takes up space and has mass. Anything that is material is made of matter in fact both words come from the same Latin root meaning stuff. Q: What is matter made of? A: Matter is made up of molecules, and molecules are made up of atoms. Slide 2: Democritus About 2400 years ago, a Greek philosopher named Democritus ( B.C.) thought a lot about what things were made of. One day while slicing an apple, he wondered how small he could slice it. He figured that everything that could be touched could be divided again and again until there was a piece left that was so small it couldn t be cut. It turns out that he had the right idea, and that smallest piece we now know as the atom. The word atom comes from an ancient Greek word that means uncuttable. Democritus could not see an atom (as we can today), but he had figured out something very important. His atom is what we talk about today as an element. Q: Give an example of an element. A: Answers will vary; Examples include hydrogen, oxygen, gold, etc. Refer to a Periodic Table for additional elements. In the mid 17th century, scientists began to prove the existence of specific elements, or pure substances that couldn t be cut into other pieces. This led scientists to discover the elements and atoms that make up all matter. The types of scientists who study atoms are chemists and physicists. At the beginning of the 20th century, scientists found that Democritus atom actually could be cut into smaller pieces, called sub-atomic particles. Slide 3: Atom Models Q: What are the parts of an atom? A: Nucleus, electron, proton, neutron 3
4 The nucleus is at the center of the atom. It is made up of protons and neutrons. Moving around outside of the nucleus are the electrons. In 1915 Nucleus a scientist named Niels Bohr proposed a model of - the atom that illustrates the atomic structure, called the planetary model or the Bohr model. Refer to the picture of the atom on the slide and explain how the electrons look like planets Protons + + Electrons orbiting the nucleus sun. Proton comes from the Greek word for first. Q: What type of charge does a proton have? A: Protons have a positive charge. Neutrons - Note: Typically, positively charged particles would repel each other, but they are held together in the nucleus with a force called the strong atomic force. This is the strongest force in the universe. The other part of the nucleus is the neutron. Neutrons are about the same size as protons. The word neutron comes from the Latin word for neutral. Q: What charge does a neutron have? A: The neutron has no charge it is neutral. The third particle of an atom is the electron. Electrons are much smaller than the protons or the neutrons (almost 2000 times smaller). It is easy to illustrate them orbiting around the nucleus using the Bohr model, although they actually move in a cloud. Q: What type of charge do electrons have? A: Electrons are negative. All atoms in the universe are made up of the same basic particles: the proton, the neutron and the electron. The different combinations of those particles combine to make different elements, which combine to make different molecules. 4
5 Slide 4: Periodic Table All of the known elements are organized into a table called the Periodic Table of Elements. Each box on the Periodic Table represents an element, organized according to its atomic number and atomic mass. Each element is represented by a letter, or letters, which is its atomic symbol. Generally the symbol is the first one or two letters of the element s name, although several elements symbols come from their name in Latin. Some elements have names that relate to famous scientists or where it was discovered. Point out some of the names of the elements, specifically: Sodium (Na, #11) name comes from the Latin Natrium Copper (Cu, #29) name comes from the Latin Cuprum Einsteinium (Es, #99) named after Albert Einstein Berkelium (Bk, #97) and Californium (Cf, #98) named after the Berkeley, California lab where they were discovered. Elements have a specific atomic configuration and properties. Each element has an atomic number, equal to the number of protons in that atom. In fact, the number of protons in an atom determines what element it is. Q: How is the Periodic Table arranged with respect to the number of protons an atom has? A: The Periodic Table is arranged in increasing Atomic Number, which corresponds to an increasing number of protons in each element. Q: How many elements are there? A: There are 117 known elements. 90 of them are naturally occurring elements, and scientists have been able to create 27 more in the laboratory. If you are using the PowerPoint presentation included on the flash drive in the kit, you can click on the symbol on the slide to access a fun song about the Elements. You must be connected to the internet to access this song. Note: In 1869, Dmitri Mendeleyev was credited with putting together the Periodic Table of Elements. He listed all of the known elements and grouped them together based on their properties. Mendeleyev was able to organize the table in its present form even though many of the elements hadn t been discovered yet. Although Mendeleev is credited with developing the Periodic Table, many scientists contributed to its development. The organization was done in such a way that as new elements have been discovered, they fit right where they are supposed to on the Periodic Table. It is no coincidence that once the periodic table was arranged by atomic number, the elements that were close to one other ended up having very similar properties. 5
6 The elements in the Periodic Table are arranged according to their atomic structure. We can determine the number of protons, neutrons and electrons in an atom by looking at the information given in this table. If you are using the flash drive PowerPoint, an additional click will pull up a large element box. Point out the location of specific parts of the element box, including the atomic number, atomic symbol, chemical name, atomic mass. Slide 5: Sodium Let s look at a familiar element, sodium. Q: What is the atomic number for sodium? A: Sodium has an atomic number of 11. Q: How many protons does it have? A: Sodium has eleven protons; eleven is the Atomic Number, which is equal to the number of protons. Atomic Number Atomic Symbol Name Atomic Mass 11 Na Sodium (23) The number of protons plus the number of neutrons equals the Atomic Mass of an element, because each one is approximately equal to one Atomic Mass Unit (AMU). The mass of the electrons is negligible because they are so small. Q: If the number of protons as well as the Atomic Mass of an element is known, how can the number of neutrons be determined? A: The number of neutrons can be determined by subtracting the number of protons from the Atomic Mass (rounded to the nearest whole number). Q: What is the atomic mass of sodium? A: The atomic mass of sodium is (whole number = 23) Q: How many neutrons does the sodium atom have? A: The sodium atom has twelve neutrons. Subtract the Atomic Number (11) from the Atomic Mass (23) to get 12. Q: In order for the charge of the atom to be balanced, how many electrons does an atom have? A: An atom must have the same number of electrons (negative charge) as protons (positive charge) in order for it to be balanced. The atom will have no overall charge. Q: How many electrons does the sodium atom have? A: The sodium atom has eleven electrons to balance the 11 protons. 6
7 Slide 6: Atoms, Molecules, Matter Atoms are the building blocks of molecules, and molecules are the building blocks of matter. Molecules are extremely small. In one spoonful of sugar there are approximately 300 billion, billion molecules of sugar! Molecules can be made up of atoms of the same element, or molecules can be made up of a combination of atoms of different elements. Q: Name a molecule that is made up of atoms of the same element. A: Hydrogen (H 2), oxygen (O 2) and nitrogen (N 2). Slide 7: Single element molecules A molecule is formed when two or more atoms join together chemically. Combinations of two or more elements are called compounds. All compounds are molecules but not all molecules are compounds. Molecules can also join together to form larger molecules. Q: Name a molecule that is also a compound, or is made up of atoms of different elements. A: The most familiar answers will be water (H 2O), carbon dioxide (CO 2) or salt (NaCl). Slide 8: Compounds Look at the model of the water molecule. It is noted as H 2O. Since it is made up of more than one element, it is also a compound. Q: What does the 2 represent in the formula for water? A: There are two hydrogen atoms for every one oxygen atom in a molecule of water. The molecular formula for table sugar is C 12H 22O 11. Write this compound formula on the board. Q: What are the different elements in this compound? A: Carbon, hydrogen and oxygen. Q: How many atoms of each element are in the compound? A: There are 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. This is how combinations of elements are noted in compounds; the element symbols followed by the number of atoms of that element in that compound. The number of atoms in the combination is determined by how the atom is structured but that is a future topic! Slide 9: Stations Today we will be exploring the basic building blocks of atoms, and how atoms and elements form together to become molecules and compounds. Have the class divide into four groups; two groups will do an Atoms station and two groups will do a Molecules station. If there are not enough volunteers, the groups can be combined for two stations. 7
8 Atoms Station Refer to the Atom Models picture Appendix A and the Periodic Table Appendix I for this activity. Review with the students the basics of the atomic structure, including the names of the parts of an atom and their charges, the basis of what makes an element (number of protons), and how to determine the number of sub-atomic elements in an element. Atoms Activity Pass out the Atoms activity sheets Appendix B, one to each student. Have students fill out their activity sheets to determine the numbers of sub-atomic particles in each atom on the activity sheet. Next have the students use the marshmallows to build an atom model. Suggest students start with a Helium atom. If time allows, have students build the Lithium or Beryllium atom. It will be required to build these additional atoms in order to do the Extension activity. Step 1: Determine the number of sub-atomic particles in each atom and complete the activity sheet. Step 2: Construct a helium atom model. Start with the nucleus. The large colored marshmallows are the protons, and the large white marshmallows are the neutrons. Connect the nucleus marshmallows together using toothpicks. Step 3: Use small colored marshmallows for the electrons. Attach the proper number of electrons to the nucleus using the toothpicks. Step 4: Construct additional atom models if there is time. 8
9 Atoms Extension Ions & Isotopes If it is desired to do the Atoms Extension activity, prepare the activity sheet by copying both pages of the Atoms activity sheet Appendix B back-to-back. Use slide 10 Ions & Isotopes for this extension. A neutral atom is an atom with an equal number of electrons and protons, which is equal to the atomic number. The atoms you constructed are neutral atoms and have no net charge. Atoms can be altered by changing the number of neutrons or electrons. Ions Ions are atoms with extra electrons or missing electrons. The atom s electron configuration determines if it is an ion. Two examples of elements that form ions are sodium (Na) and chlorine (Cl), which form an ionic bond to make Sodium Chloride, or table salt. Q: Sodium loses an electron to bond with chlorine. Does it become a positive or a negative ion? A: It becomes positive because it lost a negative charge, and is noted Na+. Q: What happens to chlorine in order to bond to the sodium ion? A: Chlorine gains an electron, becoming a negative ion noted as Cl-. Students may have already constructed a lithium atom from the main activity. Have them use this atom to construct an ion. Step 1: Construct a lithium atom with the marshmallows Step 2: Turn it into an ion by removing one of the electrons. Step 3: Record the information on the Atoms: Ions/Isotopes activity sheet. 9
10 Isotopes Some elements have isotopes of that element. An isotope of an element has the same number of protons (and electrons) as that element, but a different number of neutrons. If you change the number of protons an atom has, you change what element it is. If you change the number of neutrons an atom has, you make an isotope of that element. Carbon is an example of this. Carbon has an isotope called carbon-14 (C-14), which is used to carbon-date organic objects. Q: Based on the atomic number and atomic mass of carbon, how many neutrons does it have? A: Carbon has 6 neutrons. Carbon-14 has 8 neutrons, or 2 more than regular or elemental carbon. Q: Why is it called carbon-14? A: The 14 is the total number of protons and neutrons, or All isotopes are noted in this way. Have students use either the lithium or the beryllium atom they made from the previous activity to make an isotope. Step 1: Take either the lithium or the beryllium atom you made and add or subtract a neutron to make an isotope. Step 2: Record the information on the Atoms: Ions/Isotopes activity sheet. Example: Beryllium, Be, Atomic number 9, has 4 protons and 5 neutrons. To make Be-10, you need to add one neutron to the model of the Beryllium atom. 10
11 Molecules Station Refer to the Molecules picture Appendix D for this activity. Review with students the structure of molecules, noting the difference between same-element molecules, and multielement molecules, or compounds. Molecules Activity Give each student a Molecules activity sheet. Guide the students through the activity according to the steps. When finished, compare the gumdrop models to pictures of molecules (Appendix D), or to models from a molecule kit made ahead of time. Step 1: Color in the Molecule Color Key with colored pencils as indicated. Step 2: Determine the number and type of elements in each molecule and write it down on the activity sheet. Step 3: Draw and color the molecule models using the colored pencils. Step 4: Make at least one of the molecule models using appropriately colored gumdrops and toothpicks. Conclusion Everything in the world is made up of atoms. Q: What determines how atoms and molecules are structured? A: The arrangement of the sub-atomic particles in the atom; the electrons, protons and neutrons. The arrangement of sub-atomic particles within each atom determines not only what type of element it is, but how it combines to form molecules and how it reacts in the physical world. The makeup of the entire world is dependent on the configuration of individual atoms. Understanding the chemistry and physics of the atom helps us understand our world. 11
12 Elements Activity This activity is best suited as an extension to the Atoms & Molecules activities. Divide the students into groups of 4 or 5, and distribute the Periodic Table cards Appendix E evenly to each group. Give each student a Periodic Table activity sheet Appendix F. Write the color key and the card pattern (given below) on the board. Students will need a Periodic Table - Appendix I to do this activity. Sample Card This activity is adapted from Periodic Table Basics taken from The Science Spot by T. Trimpe Atomic # Step 1: The card has an element box with a chemical symbol on it. Complete the information in the Element box by filling in the atomic number, element name & atomic mass. Step 2: Determine the number of protons, neutrons and electrons for each element. Write it in the appropriate place on the card. H Element Name Atomic mass E = Step 3: Color the element box on the card according to the key: White Green Pink Blue Purple Orange Red Tan Yellow H Li O Be F B C N He Na S Mg Cl Al Si P Ne Ar Step 4: Arrange the cards in whatever pattern makes the most sense. Discuss with the students their reasons for choosing the pattern they did. Then have them rearrange their cards in the following pattern and glue them on the construction paper. Have them notice how this is similar (or different from) the pattern on the Periodic Table. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar Step 5: Answer the questions on the activity sheet using the information on your Periodic Table that you made. Discuss the answers to the questions with the students, asking each member of the group to explain a different question. 12
13 Elements Upper Level Extension The following information involves a more in-depth explanation of Elements and the Periodic Table. Individual lesson objectives will determine if the following material is appropriate. Slides are appropriate for this extension. The rows of the Periodic Table are called periods, and correspond to how the electrons are grouped for the elements in that row. Point out the periods on the Periodic Table. Slide 12 Orbital Shells & Periods The electrons are arranged in orbital shells around the nucleus, with specific patterns of electrons in each shell. This arrangement helps determine how different atoms and elements bond together to become molecules and compounds. Elements in any one period are only similar because they have the same number of orbitals. They do not typically have other similar characteristics. The number of the period is the same as the number of orbitals for those elements. Period 1 elements fill electrons in the 1 st orbital shell. 1 st orbital shell Electron Nucleus He Period 2 elements fill electrons in the 2 nd orbital shell. The 1 st shell is full. 2 nd orbital shell Electron Empty N 3 rd orbital shell Period 3 elements fill electrons in the 3 rd orbital shell. The 1 st & 2 nd shells are full. Si Refer to the Periodic Table, pointing out lithium, carbon and oxygen. Q: What Period are these elements in, and how many orbital shells would each atom have? A: Each element in this group is in Period 2, and so each atom has two orbital shells. Gold has the chemical symbol of Au. Have the students find Au on the Periodic Table. Q: How many orbital shells does it have and why? A: It has six orbital shells because it is in Period 6. 13
14 Slide 13 - Groups The columns of the Periodic Table are called groups. Point out the groups on the Periodic Table. Elements in the same group typically have similar chemical properties, which has to do with the similar configuration of the electrons for those elements. Each element in a group has the same number of electrons in its outermost orbital, which makes them have similar bonding characteristics. Some Periodic Tables have elements in different colors. Q: Is there a pattern to the color arrangements of the elements on the Periodic Table? A: Yes, in most cases the elements that are the same color are adjacent to each other. The most notable patterns are the two columns on the far right of the Periodic Table. Q: What do you think this represents? A: Elements in the same group have the most similar properties. The elements in the last two groups have very similar properties to each other, but properties that are very different than the rest of the elements. Slide 14 Valence Electrons The electrons in the outermost orbital are called valence electrons. The group number tells you how many valence electrons an element has. Each shell can only hold a certain number of electrons. The first shell only holds two electrons, and the next shells hold eight. Refer to the Periodic Table. Look at carbon and silicon on the Periodic Table. Q: What group number are they in? A: They are in group IV, or 4. Q: How many valence electrons would each element have, since they are in group IV? A: There are four valence electrons in the outermost orbital shell of those elements, and all other elements in that Group. Element 6 C Carbon 14 Si Silicon Group IV Valence Electrons Atoms are in their most stable state when they have a full outer shell. In order to maintain a full outer shell, atoms will gain or lose electrons. Since most of the outer shells hold eight electrons, this is called the octet rule, because the atom wants to have a full octet (eight) of electrons. This rule, or the potential to gain or lose an electron to maintain a full shell, is what governs how elements combine with one another. 14
15 Orbitals Activity: Materials for this activity include Orbitals activity sheets Appendix G and small round stickers divided by colors (or different colored markers), one color per group. You will need to refer to the Periodic Table for this activity. Give each student an activity sheet and a sheet of sticker dots (all one color) or a colored marker. Assign each student one of the elements listed below. Elements: Li, B, N, F, Mg, Si, S, Ar If there are more than 8 students in the group, also assign He, Na, P, Cl. Step 1: Write down the name of the element you were assigned. Step 2: Determine the Atomic number, Atomic mass (rounded), and the number of protons, neutrons and electrons for the element. Record it on your activity sheet. Step 3: Fill in the period number of the element. This is how many orbitals it has. Step 4: Fill in the group number of the element. This is how many electrons are in the outermost orbital. Step 5: Starting at the innermost orbital, put stickers on (or color in) the spaces corresponding to the total number of electrons. You must fill up one orbital before moving to the next one. 15
16 Appendix A Atoms and Molecules Atom Models Nucleus - Protons + + Electrons Neutrons - Bohr model of the atom 3-D model of the atom 16
17 Appendix B Atoms & Molecules ATOMS Name: _ Refer to a Periodic Table and the Key below to fill out this table for each element. Start with helium as your first atom to make. 1. Fill out the table below with the correct values. 2. Assemble the nucleus using the proper number of large colored and white marshmallows, sticking them together with toothpicks. 3. Select the proper number of small colored marshmallows (all one color) as your electrons. Attach them one at a time to the nucleus with toothpicks, away from the nucleus. ATOM ATOMIC SYMBOL ATOMIC NUMBER NUMBER OF PROTONS (see key) ATOMIC MASS (ROUNDED) NUMBER OF NEUTRONS (see key) NUMBER OF ELECTRONS (see key) Hydrogen H Helium Lithium Beryllium Atomic Number Atomic Symbol Name Atomic Mass 1 H Hydrogen KEY Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Subtract Atomic Number from Atomic Mass (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) 17
18 Appendix B Atoms & Molecules Name: _ ATOMS: Ions & Isotopes Refer to a Periodic Table and the Key below to fill out this table for each element. 1. Assemble the nucleus using the proper number of large colored and white marshmallows. Stick them together with toothpicks. 2. Select the proper number of small colored marshmallows (all one color) as your electrons. Attach them one at a time to the nucleus with toothpicks. 3. Turn the lithium atom into an ion, and note the information. 4. Turn either the lithium atom or the beryllium atom into an isotope. Record what you did. ATOM Lithium ATOMIC SYMBOL ATOMIC NUMBER NUMBER OF PROTONS (see key) ATOMIC MASS NUMBER OF NEUTRONS (see key) NUMBER OF ELECTRONS (see key) Beryllium Lithium Ion Isotope: Atomic Number Atomic Symbol Name Atomic Mass 3 Li Lithium 7 KEY Number of Protons = Atomic Number Number of Neutrons = Subtract Atomic Number from Atomic Mass Number of Electrons = Number of Protons Ions: Add or subtract an electron from the element Isotope: Add or subtract a neutron from the element 18
19 Appendix C Atoms & Molecules Name: _ Molecules 1. Color in the Molecule Color Key molecules with colored pencils as indicated. 2. Determine the number of elements in each molecule, and write it down. 3. Draw and color the molecule with the correct number of elements. 4. Make each molecule model using appropriately colored gumdrops and toothpicks. Molecule Elements Draw It! Molecule Color Key Water H 2 O H = O = C = Hydrogen (yellow) Oxygen (red) Carbon Dioxide CO 2 Ammonia NH 3 H = O = C = H = O = C = Nitrogen (green) Carbon (black) Methane CH 4 H = O = C = 19
20 Appendix D Atoms & Molecules Molecule Pictures H 2 (Hydrogen) O 2 (Oxygen) N 2 (Nitrogen) NaCl (Salt) H 2 O (Water) CO 2 (Carbon Dioxide) NH 3 (Ammonia) CH 4 (Methane) 20
21 Appendix E Atoms & Molecules Periodic Table Cards Pg. 1 of 2 Sample Card 1 H Hydrogen H _ He _ Li _ Be _ 1.00 P = 1 N = 0 E= 1 B C N O F _ 21
22 Appendix E Atoms & Molecules Periodic Table Cards Pg. 2 of 2 Ne Na Mg Al Si _ P S Cl Ar H _ 22
23 Appendix G Atoms & Molecules Name Periodic Table Activity sheet Use the periodic table you made to answer each question 1. How are the atomic numbers and the atomic masses of the elements related to how the elements are arranged on the Periodic Table? 2. How does the number of electrons relate to the arrangement? What is the difference in the number of electrons in a 3 rd period element and the 2 nd period element above it? 3. Do some elements next to each other have the same number of neutrons? How is that possible? 4. How are the colors arranged, and what conclusions can be drawn from this arrangement? Referring to the table below, write the name and number of the group above each color group on the periodic table you made. Green Blue Orange Red Tan Pink Purple Yellow Group I Group II Group III Group IV Group V Group VI Group VII Group VIII Alkali Metals Alkaline Earth Metals Boron Family Carbon Family Nitrogen Family Oxygen Family Halides Noble Gases 5. Compare the location of the Metals groups in relation to the Noble Gases group. What is the significance of their locations on the Periodic Table? 6. Which groups have names that help you to remember where certain elements are located? 23
24 Appendix G Atoms & Molecules Name: _ Orbitals P N Element = Atomic Number = Number of Protons = Atomic Number = Atomic Mass (rounded to nearest whole number) = Number of Neutrons = Atomic Mass Atomic Number = Number of Electrons = Number of Protons = Period = = Number of Orbitals Group = = Number of electrons in outermost orbital (These are called valence electrons) 24
25 Appendix H Atoms & Molecules ATOMS Answer Key Refer to a Periodic Table and the Key below to fill out this table for each element. Start with helium as your first atom to make. 1. Fill out the table below with the correct values. 2. Assemble the nucleus using the proper number of large colored and white marshmallows. Stick them together with toothpicks. 3. Select the proper number of small colored marshmallows (all one color) as your electrons. Attach them one at a time to the nucleus with toothpicks. ATOM ATOMIC SYMBOL ATOMIC NUMBER NUMBER OF PROTONS ATOMIC MASS (ROUNDED) NUMBER OF NEUTRONS (MASS ATOMIC NUMBER) NUMBER OF ELECTRONS Hydrogen H Helium He Lithium Li Beryllium Be Atomic Number Atomic Symbol Name Atomic Mass 1 H Hydrogen (1) KEY Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) 25
26 ATOMS: Ions & Isotopes Answer Key Refer to a Periodic Table and the Key below to fill out this table for each element. 1. Assemble the nucleus using the proper number of large colored and white marshmallows. Stick them together with toothpicks. 2. Select the proper number of small colored marshmallows (all one color) as your electrons. Attach them one at a time to the nucleus with toothpicks. 3. Turn the lithium atom into an ion, and note the information. 4. Turn either the lithium atom or the beryllium atom into an isotope. Record what you did. ATOM ATOMIC ATOMIC # ATOMIC # # SYMBOL NUMBER PROTONS MASS NEUTRONS ELECTRONS Lithium Li Beryllium Be Lithium Ion Li Isotope: Be Be Atomic Number Atomic Symbol Name Atomic Mass 3 Li Lithium 7 26 KEY Number of Protons = Atomic Number Number of Neutrons = Atomic Mass Atomic Number Number of Electrons = Number of Protons Ions: Add or subtract an electron from the element Isotope: Add or subtract a neutron from the element
27 Sample Card Periodic Table Cards ANSWER KEY Pg. 1 of 2 Atomic # H Element Name H Hydrogen He Helium Li Lithium Be Beryllium Atomic mass P = 1 N = 0 E =1 P = 2 N = 2 E = 2 P = 3 N = 4 E = 3 P = 4 N = 5 E = B C N O F Boron Carbon Nitrogen Oxygen Fluorine P = 5 N = 6 P = 6 N = 6 P = 7 N = 7 P = 8 N = 8 P = 9 N = 10 E = 5 E = 6 E = 7 E = 8 E = 9 27
28 Periodic Table Cards ANSWER KEY Pg. 2 of Ne Na Mg Al Si Neon Sodium Magnesium Aluminum Silicon P = 10 N = 10 P = 11 N = 12 P = 12 N = 12 P = 13 N = 14 P = 14 N = 14 E = 10 E = 11 E = 12 E = 13 E = P S Cl Ar H Phosphorus Sulfur Chlorine Argon Hydrogen P = 15 N = 16 P = 16 N = 16 P = 17 N = 18 P = 18 N = 22 P = 1 N = 0 E = 15 E = 16 E = 17 E = 18 E = 1 28
29 Name Periodic Table Activity sheet Answer key Use your periodic table to answer each question 1. How are the atomic numbers and the atomic masses of the elements related to how the elements are arranged on the Periodic Table? The elements are arranged in increasing atomic number, which also corresponds to increasing atomic mass. 2. How does the number of electrons relate to the arrangement? What is the difference in the number of electrons in a 3 rd period element and the 2 nd period element above it? The elements are arranged in increasing number of electrons. Each element in the 3 rd row has eight more electrons than the element above it in the 2 nd row. 3. Do some elements next to each other have the same number of neutrons? How is that possible? Yes, some of the elements next to each other have the same number of neutrons, but they are different elements because they have a different number of protons. 4. How are the colors arranged, and what conclusions can be drawn from this arrangement? The colors line up in columns. The elements in each column have similar properties. Elements are organized into families (groups) according to their physical and chemical properties. Notice that the elements that are the same color fall into the same group. 5. Compare the location of the Metals groups in relation to the Noble Gases group. What is the significance of their locations on the Periodic Table? The Metals groups are on the far left of the Table, and the Noble Gases are on the far right. There is a very big difference in the structure of the elements from one side of the Table to the other. 6. Which groups have names that help you to remember where certain elements are located? Groups III, IV, V and VI are all named after the element at the top of the group. Knowing the names of these groups helps to locate where those elements are on the Periodic Table. 29
30 Orbital Shell Cards Answer Key (pg. 1 of 2) Element = Li (Lithium) Atomic Number = 3 Atomic Mass = 6.9 Number of Protons = 3 Number of Neutrons = 7 Number of Electrons = 3 Period = 2 Group = I Element = B (Boron) Atomic Number = 5 Atomic Mass = 10.8 Number of Protons = 5 Number of Neutrons = 6 Number of Electrons = 5 Period = 2 Group = III Element = N (Nitrogen) Atomic Number = 7 Atomic Mass = 14.4 Number of Protons = 7 Number of Neutrons = 7 Number of Electrons = 7 Period = 2 Group = V Element = F (Fluorine) Atomic Number = 9 Atomic Mass = Number of Protons = 9 Number of Neutrons = 10 Number of Electrons = 9 Period = 2 Group = VII Element = Mg (Magnesium) Atomic Number = 12 Atomic Mass = 24.3 Number of Protons = 12 Number of Neutrons = 12 Number of Electrons = 12 Period = 3 Group = II Element = Si (Silicon) Atomic Number = 14 Atomic Mass = 28.1 Number of Protons = 14 Number of Neutrons = 14 Number of Electrons = 14 Period = 3 Group = IV Element = S (Sulfur) Atomic Number = 16 Atomic Mass = 32.1 Number of Protons = 16 Number of Neutrons = 16 Number of Electrons = 16 Period = 3 Group = VI Element = Ar (Argon) Atomic Number = 18 Atomic Mass = 39.9 Number of Protons = 18 Number of Neutrons = 22 Number of Electrons = 18 Period = 3 Group = VIII 30
31 Element = He (Helium) Atomic Number = 2 Atomic Mass = 4.0 Number of Protons = 2 Number of Neutrons = 2 Number of Electrons = 2 Period = 1 Group = VIII Orbital Shell Cards Answer Key (pg. 2 of 2) Element = Na (Sodium) Atomic Number = 11 Atomic Mass = Number of Protons = 11 Number of Neutrons = 12 Number of Electrons = 11 Period = 3 Group = I Element = P (Phosphorus) Atomic Number = 15 Atomic Mass = Number of Protons = 15 Number of Neutrons = 16 Number of Electrons = 15 Period = 3 Group = V Element = Cl (Chlorine) Atomic Number = 17 Atomic Mass = Number of Protons = 17 Number of Neutrons = 18 Number of Electrons = 17 Period = 3 Group = VII Element = Atomic Number = Atomic Mass = Number of Protons = Number of Neutrons = Number of Electrons = Period = Group = Element = Atomic Number = Atomic Mass = Number of Protons = Number of Neutrons = Number of Electrons = Period = Group = Element = Atomic Number = Atomic Mass = Number of Protons = Number of Neutrons = Number of Electrons = Period = Group = Element = Atomic Number = Atomic Mass = Number of Protons = Number of Neutrons = Number of Electrons = Period = Group = 31
32 Molecules - Answer Key Molecule Elements Draw It! Molecule Color Key Water H 2 O H = 2 O = 1 N = 0 C = 0 Hydrogen (yellow) Oxygen (red) Carbon Dioxide CO 2 H = 0 O = 2 N = 0 C = 1 Nitrogen (green) Carbon (black) Ammonia NH 3 H = 3 O = 0 N = 1 C = 0 Methane CH 4 H = 4 O = 0 N = 0 C = 1 32
33 Appendix I Atoms & Molecules 33
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
PERIODIC TABLE. reflect
 reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Molecular Models & Lewis Dot Structures
 Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
Student Exploration: Electron Configuration
 Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
Ions & Their Charges Worksheet
 Ions & Their Charges Worksheet Name Date Teacher Diagram of charges based on groups on the periodic table including transition metals and noble gases: IA IIA Transition IIIA IVA VA VIA VIIA VIIIA metals
Ions & Their Charges Worksheet Name Date Teacher Diagram of charges based on groups on the periodic table including transition metals and noble gases: IA IIA Transition IIIA IVA VA VIA VIIA VIIIA metals
Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations.
 The Periodic Table Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations. Vertical Rows are called Families or Groups.
The Periodic Table Horizontal Rows are called Periods. Elements in the same period have the same number of energy levels for ground state electron configurations. Vertical Rows are called Families or Groups.
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
Chapter 7 Periodic Properties of the Elements
 Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Trends of the Periodic Table Basics
 Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Bonding Practice Problems
 NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 5 Periodic Table. Dmitri Mendeleev: Russian Chemist credited with the discovery of the periodic table.
 Chapter 5 Periodic Table Dmitri Mendeleev: Russian Chemist credited with the discovery of the periodic table. How did he organize the elements? According to similarities in their chemical and physical
Chapter 5 Periodic Table Dmitri Mendeleev: Russian Chemist credited with the discovery of the periodic table. How did he organize the elements? According to similarities in their chemical and physical
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Ionic and Metallic Bonding
 Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
neutrons are present?
 AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
Bohr s Model of the Atom
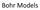 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Directions: T. Trimpe 2005 http://sciencespot.net/
 Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
Chapter 4, Lesson 2: The Periodic Table
 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Chemistry - Elements Electron Configurations The Periodic Table. Ron Robertson
 Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
19.2 Chemical Formulas
 In the previous section, you learned how and why atoms form chemical bonds with one another. You also know that atoms combine in certain ratios with other atoms. These ratios determine the chemical formula
In the previous section, you learned how and why atoms form chemical bonds with one another. You also know that atoms combine in certain ratios with other atoms. These ratios determine the chemical formula
Chapter 3, Elements, Atoms, Ions, and the Periodic Table
 1. Which two scientists in 1869 arranged the elements in order of increasing atomic masses to form a precursor of the modern periodic table of elements? Ans. Mendeleev and Meyer 2. Who stated that the
1. Which two scientists in 1869 arranged the elements in order of increasing atomic masses to form a precursor of the modern periodic table of elements? Ans. Mendeleev and Meyer 2. Who stated that the
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D
 Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
THE PERIODIC TABLE O F T H E E L E M E N T S. The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27)
 THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
Lewis Dot Structures of Atoms and Ions
 Why? The chemical properties of an element are based on the number of electrons in the outer shell of its atoms. We use Lewis dot structures to map these valence electrons in order to identify stable electron
Why? The chemical properties of an element are based on the number of electrons in the outer shell of its atoms. We use Lewis dot structures to map these valence electrons in order to identify stable electron
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Section 1: Arranging the Elements Pages 106-112
 Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Electron Configurations, Isoelectronic Elements, & Ionization Reactions. Chemistry 11
 Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Chapter Test. Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a.
 Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Name: Worksheet: Electron Configurations. I Heart Chemistry!
 1. Which electron configuration represents an atom in an excited state? 1s 2 2s 2 2p 6 3p 1 1s 2 2s 2 2p 6 3s 2 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name:
1. Which electron configuration represents an atom in an excited state? 1s 2 2s 2 2p 6 3p 1 1s 2 2s 2 2p 6 3s 2 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name:
Chapter 2 Atoms, Molecules, and Ions
 Chapter 2 Atoms, Molecules, and Ions 1. Methane and ethane are both made up of carbon and hydrogen. In methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a ration of 3:1 by mass. In ethane,
Chapter 2 Atoms, Molecules, and Ions 1. Methane and ethane are both made up of carbon and hydrogen. In methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a ration of 3:1 by mass. In ethane,
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS
 Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
TOPIC 7. CHEMICAL CALCULATIONS I - atomic and formula weights.
 TOPIC 7. CHEMICAL CALCULATIONS I - atomic and formula weights. Atomic structure revisited. In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually
TOPIC 7. CHEMICAL CALCULATIONS I - atomic and formula weights. Atomic structure revisited. In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
CHEMISTRY BONDING REVIEW
 Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Periodic Table. 1. In the modern Periodic Table, the elements are arranged in order of increasing. A. atomic number B. mass number
 Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Atomic Theory Part 1
 Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
TRENDS IN THE PERIODIC TABLE
 Noble gases Period alogens Alkaline earth metals Alkali metals TRENDS IN TE PERIDI TABLE Usual charge +1 + +3-3 - -1 Number of Valence e - s 1 3 4 5 6 7 Electron dot diagram X X X X X X X X X 8 Group 1
Noble gases Period alogens Alkaline earth metals Alkali metals TRENDS IN TE PERIDI TABLE Usual charge +1 + +3-3 - -1 Number of Valence e - s 1 3 4 5 6 7 Electron dot diagram X X X X X X X X X 8 Group 1
NOTES ON The Structure of the Atom
 NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table
 Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lesson Topics Covered SCH 3UI Unit 2 Outline Up to Quiz #1 Atomic Theory and the Periodic Table 1 Note: History of Atomic Theory progression of understanding of composition of matter; ancient Greeks and
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance
 Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
Instructors Guide: Atoms and Their Isotopes
 Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Chemistry: The Periodic Table and Periodicity
 Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
Ionic and Covalent Bonds
 Ionic and Covalent Bonds Ionic Bonds Transfer of Electrons When metals bond with nonmetals, electrons are from the metal to the nonmetal The becomes a cation and the becomes an anion. The between the cation
Ionic and Covalent Bonds Ionic Bonds Transfer of Electrons When metals bond with nonmetals, electrons are from the metal to the nonmetal The becomes a cation and the becomes an anion. The between the cation
Copyrighted by Gabriel Tang B.Ed., B.Sc.
 Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
Atoms and Elements [6th grade]
![Atoms and Elements [6th grade] Atoms and Elements [6th grade]](/thumbs/40/21183873.jpg) Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, [email protected]
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Find a pair of elements in the periodic table with atomic numbers less than 20 that are an exception to the original periodic law.
 Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Introduction to Chemistry Exam 2 Practice Problems 1 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1.Atoms consist principally of what three
Introduction to Chemistry Exam 2 Practice Problems 1 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1.Atoms consist principally of what three
Look at a periodic table to answer the following questions:
 Look at a periodic table to answer the following questions: 1. What is the name of group 1? 2. What is the name of group 2? 3. What is the name of group 17? 4. What is the name of group 18? 5. What is
Look at a periodic table to answer the following questions: 1. What is the name of group 1? 2. What is the name of group 2? 3. What is the name of group 17? 4. What is the name of group 18? 5. What is
Modelling Compounds. 242 MHR Unit 2 Atoms, Elements, and Compounds
 6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
