Examination One. Biology 101. Dr. Jaeson T. Fournier
|
|
|
- Pierce Patterson
- 9 years ago
- Views:
Transcription
1 Examination One Biology 101 Dr. Jaeson T. Fournier
2 Examination Instructions: Answers are to be indicated on a scantron. Keep your work protected! This helps prevent dishonesty. The instructor will not answer questions pertaining to course content, the meaning of a test question, or comment on a test question. The instructor will only responded to a typographical error in a question. Read all questions, answers and instructions thoroughly. There are 75 questions. Academic dishonesty will not be tolerated as outlined in the course syllabus, which was provided on the first day of class. Take a deep breath, relax and then begin. 2 Dr. Jaeson T. Fournier
3 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. In living organisms, processes such as growth, repair, and nutrition are referred to as: a. development. b. metabolism. c. adaptation. d. genetics. e. homeostasis. 2. In all organisms, hereditary information is encoded within: a. DNA molecules. b. RNA molecules. c. protein molecules. d. lipid molecules. e. carbohydrate molecules. 3. Which of the following is the most basic level of chemical organization? a. cell b. molecule c. atom d. tissue e. organism 4. In an experiment, the control group: a. disproves the theory. b. proves the hypothesis. c. proves the prediction. d. does not contain the variable being tested. e. is similar to the test group in all aspects including the variable being tested. Figure 01-2 Use the figure to answer the following questions. 3 Dr. Jaeson T. Fournier
4 5. Refer to Figure Sequence A in the associated figure represents: a. a hypothesis. b. the experimental group. c. the control group. d. a sampling error. e. a deduction. Figure 02-2 Use the figure to answer the following questions. 6. Refer to Figure The type of bond illustrated in the accompanying figure is: a. an ionic bond. b. a polar bond c. a single covalent bond. d. a hydrogen bond. e. a double covalent bond. 7. Refer to Figure The accompanying figure represents: a. elemental helium. b. molecular hydrogen. c. molecular helium. d. a water molecule. e. molecular oxygen. 8. The representation H - O- H is known as: a. a structural formula. b. a simplest formula. c. a molecular formula. d. a Lewis structure. e. an orbital diagram. 9. The cohesiveness between water molecules is due largely to: a. hydrogen bonds. b. polar covalent bonds. c. nonpolar covalent bonds. d. ionic bonds. e. hydrophobic interactions. 10. What is the OH - concentration of a solution having a ph of 2? a. 1 x Dr. Jaeson T. Fournier
5 b. 1 x c. 1 x 10-7 d. 1 x 10-2 e. 1 x What aspect of long carbon chains makes them ideal for forming the backbones of long biomolecules? a. The carbon atom itself is strong and hard to split. b. Carbon can form a maximum of five covalent bonds with other atoms. c. Carbons can form a maximum of three covalent bonds with other atoms. d. Carbon-to-carbon covalent bonds are strong. e. Carbon-to-carbon hydrogen bonds are weak and transitory. 12. All of the following types of chemical bonds are responsible for maintaining the tertiary structure of this polypeptide except: a. ionic bonds. b. hydrogen bonds. c. hydrophobic interactions. d. disulfide bonds. e. peptide bonds. 5 Dr. Jaeson T. Fournier
6 13. If the differently shaded portions of this molecule represent different polypeptide chains, then this figure is representative of a. an amino acid. b. the quaternary structure of a protein. c. a steroid hormone. d. cellulose. e. a carotenoid. 14. ATP is important in living organisms because: a. like all other nucleic acids, it stores hereditary information. b. like RNA, it acts as a source code for the formation of proteins. c. it can transfer some of its energy to other chemicals. d. it is an important structural component of cell membranes. e. it is easily converted to starch for long-term storage. 15. Which portion of the following molecule is easily transferred and therefore responsible for the energy transfer property of this molecule? a. 1 b. 2 c. 3 d. 1 and 2 e. None of the above 6 Dr. Jaeson T. Fournier
7 16. It is advantageous for cells to be small because: a. a small cell size prevents a cell from weighing too much. b. a small cell size occupies less space in nature where space is limited. c. a small cell has a small volume relative to surface area, thereby increasing efficient transport. d. a small cell has a small surface area relative to volume, thereby facilitating ion balance. e. a small cell is better able to conserve energy than a larger cell. 17. Electron microscopes have a much higher resolution than either the human eye or any light microscope because: a. of their higher magnification. b. the lenses used are of much higher quality. c. of the very short (nanometer) wavelengths of electrons. d. the images are viewed on screens, rather than directly using an eyepiece or ocular lens. e. All of the above Figure 04-4 Use the figure to answer the following questions. 18. Refer to Figure The structures indicated by the arrows in the associated figure are: a. thylakoid lamellae. b. grana. c. cristae. d. matrices. e. plastids. 19. Refer to Figure The main process that occurs at the site of the structures marked by arrows in the figure is: a. protein synthesis. b. photosynthesis. c. conversion of food molecules to ATP. d. processing and packaging of proteins. e. transcription. 7 Dr. Jaeson T. Fournier
8 20. Refer to Figure The organelle featured in the accompanying figure: a. is present in a few prokaryotes. b. is the major site of protein synthesis in the cell. c. plays a vital role in packaging materials to be secreted. d. plays a central role in energy metabolism. e. is located in the nucleus. 21. A single cell in a smoker's lung has become cancerous. It doubles its DNA and divides much faster than a normal lung cell. The most likely change that would have caused this condition took place in the: a. nucleus. b. nucleolus. c. microtubule. d. mitochondria. e. lysosome. 22. The Human Genome Project: a. has examined the function of all human genes. b. identified which genes are responsible for which traits. c. will be used to produce animal tissues and organs for transplant into humans. d. has mapped the complete set of genes that make up the human genome. e. was completed in The cell theory states that all living organisms: a. grow and develop. b. respond to stimuli. c. are composed of basic units called cells. d. can move from one place to another in order to find food or to escape predators. e. can form a population of organisms that is able to adapt to the environment. 24. If a particular protein were being produced in excess of the cell's needs, then mechanisms intervene to stop production. a. metabolic b. homeostatic c. growth d. respiratory e. anabolic 8 Dr. Jaeson T. Fournier
9 Figure 01-1 Use the figure to answer the following questions. 25. Refer to Figure The sequence in the accompanying figure is a good example of: a. a response to a stimulus. b. reproduction. c. homeostasis. d. genetic variation. e. a population. 26. Which of the following is not a characteristic of all living organisms? a. adapt to their environment b. reproduce c. respond to stimuli d. multicellular e. movement 27. Genetic variation arises within asexual organisms by: a. the fusion of eggs and sperm. b. fertilization. c. the combination of genes from two different individuals of the same species. d. random mutation within the DNA of individuals. e. processes not fully understood by biologists at this time. 28. Among other things, prokaryotes differ from eukaryotes by: a. not having DNA. b. not having cells. c. having cell membranes. d. not having a nucleus. e. having membrane-bound organelles. 29. All of the members of the same species occupying the same area at the same time would be: a. an individual. b. a population. c. a community. d. an ecosystem. e. a biosphere. 30. The theory of evolution states that: 9 Dr. Jaeson T. Fournier
10 a. existing organisms can adapt to environmental changes. b. genetic information can pass from organism to organism by means of DNA. c. living organisms are composed of basic units called cells. d. living organisms contain substances produced by cells. e. organisms living today descended with modifications from previously existing forms. 31. What would be the ultimate effect on an ecosystem if decomposers were eliminated? a. The consumers would have to eat twice as much. b. The rate of photosynthesis would increase. c. All life would eventually cease as nutrients would no longer be available. d. Energy flow between producers and consumers would increase. e. Producers would outgrow consumers due to the excess of carbon dioxide. 32. Autotrophs (or producers): a. usually rely on sunlight as an energy source. b. produce food molecules, such as sugars. c. include algae, mosses, and vascular plants. d. synthesize complex molecules from CO 2, water, and energy. e. All of the above 33. A hypothesis is: a. a proposed explanation. b. a systematic thought process. c. an observation. d. a placebo. e. a conclusion. 34. In the experimental evaluation of a new drug, a placebo serves the purpose of: a. preventing errors in recording of the data. b. removing the bias of the physician in charge of the experiment. c. removing the potential psychological bias of the patient in the study. d. preventing sampling errors from compromising the results of the experiment. e. increasing the sample size. 35. Which of the following types of biologists would need an understanding of chemistry? a. molecular biologist b. ecologist c. evolutionary biologist d. botanist e. All of the above 36. An element is defined as a substance that: a. is composed of more than one kind of atom. b. is held together by covalent bonds. c. cannot be broken into simpler substances by chemical reactions. d. cannot burn. e. is soluble in both acid and water. 10 Dr. Jaeson T. Fournier
11 37. Which of the following elements is not responsible for a significant portion of the mass of living organisms? a. O b. S c. N d. H e. C 38. Chlorine has seven electrons in its valence shell. The number of electrons it must gain to complete its valence shell is: a. one. b. two. c. three. d. seven. e. eight. 39. The difference between an electrically neutral atom and an ion is that: a. an ion has an unequal number of protons and electrons, while an atom has an equal number. b. an ion has an equal number of protons and electrons, while an atom has an unequal number. c. an atom has an unequal number of neutrons and protons, while an ion has an equal number d. an atom has its electrons in orbitals, while an ion has its electrons in its nucleus. e. an atom must have an equal number of neutrons and electrons, while an ion does not. 40. Any chemical interaction between atoms: a. involves neutrons. b. may potentially involve any electron. c. involves protons. d. involves only valence electrons. e. involves only the nuclear subatomic particles. 41. In a water molecule, because oxygen is more electronegative than hydrogen, the shared electrons are more commonly found around the nucleus than the nucleus. a. oxygen; hydrogen b. hydrogen; oxygen c. hydrogen; other hydrogen d. oxygen; nitrogen e. nitrogen; oxygen 42. The covalent bond between a hydrogen atom and the oxygen atom in water is formed when: a. hydrogen gains an electron from oxygen. b. hydrogen and oxygen share an electron pair. c. hydrogen and oxygen both lose electrons from their outer shells. d. hydrogen and oxygen both gain electrons in their outer shells. e. hydrogen gains an electron from oxygen. 43. An atom becomes a cation if: a. it gains one or more electrons. b. it loses one or more electrons. c. it shares electrons. d. one or more of its electrons changes energy levels. e. it emits radiation. 11 Dr. Jaeson T. Fournier
12 44. In the formation of common table salt, sodium and chlorine interact because: a. sodium and chlorine share a pair of electrons. b. sodium and chlorine share two pairs of electrons. c. chlorine donates seven electrons to sodium. d. there is no electron exchange. e. sodium donates one electron to chlorine. 45. Which of the following is not a property of carbon? a. Carbon-to-carbon bonds are limited to single bonds. b. Carbon has four valence electrons. c. Carbon can form bonds to various other atoms. d. Two carbon atoms can share three electron pairs with each other. e. Carbon-to-carbon bonds are strong. 46. The number of electron pairs shared between carbon 2 and 3 in the figure below is: a. one. b. one and a half. c. two. d. three. e. None of the above 47. The chemical interactions of large hydrocarbons are largely determined by: a. their solubility in water. b. their functional groups. c. their polar nature. d. isomerization of these hydrocarbons into other forms. e. the hydrogens bonded to the carbon atoms. 48. Carbohydrate molecules: a. serve as structural components of human cell walls. b. form the regulatory compounds known as enzymes. c. are a source of energy. d. help protect vital organs from damage. e. contain the genetic information of a cell. 12 Dr. Jaeson T. Fournier
13 Figure 03-1 Use the figure to answer the following questions. 49. Refer to Figure The process illustrated in the figure is called: a. condensation. b. protein synthesis. c. hydrolysis. d. dehydration synthesis. e. denaturation. 50. A chemical reaction in which organic compounds are synthesized from their building blocks is called: a. hydrolysis. b. condensation. c. oxidation. d. reduction. e. dissociation. 51. The difference between a hexose and a pentose is that: a. a hexose is saturated, and a pentose is undersaturated. b. a hexose is hydrophilic, and a pentose is hydrophobic. c. a hexose always has six hydroxyl groups, and a pentose always has five. d. a hexose always has six carbons, but a pentose always has five carbons. e. a hexose can be polymerized, but a pentose cannot. 52. The most abundant molecules in this structure are: a. structural proteins. b. polysaccharides. c. triacylglycerols. d. phospholipids. e. polypeptides. 13 Dr. Jaeson T. Fournier
14 53. At which level of protein structure are peptide bonds most important? a. primary b. secondary c. tertiary d. quaternary e. globular 54. Evidence that all living cells have a common origin is provided by: a. the cell theory, which states that the cell is the basic unit of life. b. the fact that all new cells come from previously existing cells. c. the fact that cells are the building blocks of the most complex plants. d. basic similarities in cell structure and chemistry. e. the fact that cells are the smallest units that can carry out all life activities. 55. The function of the plasma membrane is to: a. serve as a highly selective barrier. b. completely isolate the cell from the external environment. c. equalize the chemical composition inside and outside the cell. d. allow cells to accumulate materials and energy. e. A and D 56. Differential centrifugation is a process that: a. separates different components of the cell that function differently. b. separates components of the cell that have a different chemical makeup. c. analyzes the chemical components of the cell. d. separates components of the cell that have different densities. e. allows researchers to view the contents of the cells. 57. A eukaryotic cell: a. is usually smaller than a prokaryotic cell. b. has its DNA concentrated in one area of the cell without a nuclear membrane. c. typically has a cell wall, in addition to a plasma membrane. d. is a bacteria-like organism. e. has a variety of membranous organelles. 58. Membranes facilitate all of the following except: a. facilitating the formation of energy-yielding gradients. b. acting as barriers to ions. c. acting as important "work benches" within cells. d. directing the synthesis of proteins. e. maintaining the identity of different cellular compartments. 59. If a toxin, such as a bacterial toxin, destroys ribosomes, what cellular activity will be affected first? a. protein synthesis b. DNA synthesis c. movement d. energy storage e. active transport 14 Dr. Jaeson T. Fournier
15 60. Proteins made on ribosomes may be further modified within the: a. lysosomes. b. nucleus. c. mitochondria. d. Golgi complex. e. peroxisomes. 61. The smooth endoplasmic reticulum: a. is absent in most plant cells. b. synthesizes proteins. c. provides structural support. d. synthesizes lipids. e. is required for ribosome synthesis. Figure 04-2 Use the figure to answer the following questions. 62. Refer to Figure The cellular structure indicated by the arrow is responsible for: a. lipid and fatty acid metabolism. b. protein synthesis. c. digestion of unused organelles. d. replication. e. None of the above 63. Which of the following pairs is correctly matched? a. chloroplast - storage of enzymes b. lysosome - powerhouse of the cell c. nucleolus - site of ribosomal subunit synthesis d. plastids - structural support of the cell e. Golgi complex - production of energy 15 Dr. Jaeson T. Fournier
16 64. A cellular structure found in plant but not animal cells is the: a. chloroplast. b. ribosome. c. endoplasmic reticulum. d. microtubule. e. microfilament. 65. Plants may respond to all of the following stimuli except: a. gravity. b. touch. c. sound. d. water. e. light. 66. The greatest number of plants and animals reproduce by: a. asexual reproduction. b. sexual reproduction. c. cell division within one individual. d. mutating into other individuals. e. natural selection. 67. Information in living organisms can be transmitted by: a. genes. b. hormones. c. electrical activity. d. neurotransmitters. e. All of the above 68. The organism in the figure below can be classified as: a. a animalae. b. a plantae c. a prokaryote. d. a funga e. a protista. 16 Dr. Jaeson T. Fournier
17 69. A functional structure that contains several different types of tissues is: a. an organ. b. a nucleus. c. an atom. d. a cell. e. an enzyme. 70. Evolution of a species operates at the level of: a. genes. b. individuals. c. populations. d. communities. e. ecosystems. 71. The ultimate source of genetic variation within a population is: a. mutations in DNA. b. adaptation of a species to environmental changes. c. homeostatic mechanisms that compensate for environmental changes. d. a sensory system that can detect an environmental change. e. a system of locomotion that allows an organism to escape environmental changes. 72. In the hypothetico-deductive approach to scientific thought processes, we begin with and make based on that information. a. premises; observations b. observations; premises c. observations; conclusions d. observations; inductions e. premises; conclusions 73. When a chemical reaction is at equilibrium: a. the forward reaction is going faster. b. the reverse reaction is going faster. c. the forward and reverse reactions are proceeding at equal rates. d. the forward reaction stops. e. the reverse reaction stops. 74. A covalent bond: a. can form only between identical atoms. b. involves a sharing of only one pair of electrons. c. is always polar. d. may be polar or nonpolar depending on the atoms involved. e. always forms between identical molecules. 75. Which of the following statements is true of proteins? a. Proteins lose some or all of their normal activity if their three-dimensional structure is disrupted. b. Proteins are composed of ribose, phosphate, and a nitrogen-containing base. c. The activity of proteins is independent of temperature and ph. d. Denaturation is usually reversible. e. All proteins are enzymes. 17 Dr. Jaeson T. Fournier
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Chapter 2: Cell Structure and Function pg. 70-107
 UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Given these characteristics of life, which of the following objects is considered a living organism? W. X. Y. Z.
 Cell Structure and Organization 1. All living things must possess certain characteristics. They are all composed of one or more cells. They can grow, reproduce, and pass their genes on to their offspring.
Cell Structure and Organization 1. All living things must possess certain characteristics. They are all composed of one or more cells. They can grow, reproduce, and pass their genes on to their offspring.
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Cells & Cell Organelles
 Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
1. When applying the process of science, which of these is tested? a. an observation b. a result c. a hypothesis d. a question e.
 BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
THE HISTORY OF CELL BIOLOGY
 SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
MCAS Biology. Review Packet
 MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
Organelles and Their Functions
 Organelles and Their Functions The study of cell organelles and their functions is a fascinating part of biology. The current article provides a brief description of the structure of organelles and their
Organelles and Their Functions The study of cell organelles and their functions is a fascinating part of biology. The current article provides a brief description of the structure of organelles and their
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Student name ID # 2. (4 pts) What is the terminal electron acceptor in respiration? In photosynthesis? O2, NADP+
 1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
Cytology. Living organisms are made up of cells. Either PROKARYOTIC or EUKARYOTIC cells.
 CYTOLOGY Cytology Living organisms are made up of cells. Either PROKARYOTIC or EUKARYOTIC cells. A. two major cell types B. distinguished by structural organization See table on handout for differences.
CYTOLOGY Cytology Living organisms are made up of cells. Either PROKARYOTIC or EUKARYOTIC cells. A. two major cell types B. distinguished by structural organization See table on handout for differences.
Chapter 4: A Tour of the Cell. 1. Cell Basics. Limits to Cell Size. 1. Cell Basics. 2. Prokaryotic Cells. 3. Eukaryotic Cells
 Chapter 4: A Tour of the Cell 1. Cell Basics 2. Prokaryotic Cells 3. Eukaryotic Cells 1. Cell Basics Limits to Cell Size There are 2 main reasons why cells are so small: If cells get too large: 1) there
Chapter 4: A Tour of the Cell 1. Cell Basics 2. Prokaryotic Cells 3. Eukaryotic Cells 1. Cell Basics Limits to Cell Size There are 2 main reasons why cells are so small: If cells get too large: 1) there
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Cell Structure & Function!
 Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Essentials of Human Anatomy & Physiology 11 th Edition, 2015 Marieb
 A Correlation of Essentials of Human Anatomy Marieb To the Next Generation Science Standards Life A Correlation of, HS-LS1 From Molecules to Organisms: Structures and Processes HS-LS1-1. Construct an explanation
A Correlation of Essentials of Human Anatomy Marieb To the Next Generation Science Standards Life A Correlation of, HS-LS1 From Molecules to Organisms: Structures and Processes HS-LS1-1. Construct an explanation
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
NO CALCULATORS OR CELL PHONES ALLOWED
 Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Unit I: Introduction To Scientific Processes
 Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Biology 101 Chapter 4 Cells as the Basic Unit of Life. The Cell Theory Major Contributors: Galileo = first observations made with a microscope
 Biology 101 Chapter 4 Cells as the Basic Unit of Life The Cell Theory Major Contributors: Galileo = first observations made with a microscope Robert Hooke = first to observe small compartments in dead
Biology 101 Chapter 4 Cells as the Basic Unit of Life The Cell Theory Major Contributors: Galileo = first observations made with a microscope Robert Hooke = first to observe small compartments in dead
Compartmentalization of the Cell. Objectives. Recommended Reading. Professor Alfred Cuschieri. Department of Anatomy University of Malta
 Compartmentalization of the Cell Professor Alfred Cuschieri Department of Anatomy University of Malta Objectives By the end of this session the student should be able to: 1. Identify the different organelles
Compartmentalization of the Cell Professor Alfred Cuschieri Department of Anatomy University of Malta Objectives By the end of this session the student should be able to: 1. Identify the different organelles
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Quick Hit Activity Using UIL Science Contests For Formative and Summative Assessments of Pre-AP and AP Biology Students
 Quick Hit Activity Using UIL Science Contests For Formative and Summative Assessments of Pre-AP and AP Biology Students Activity Title: Quick Hit Goal of Activity: To perform formative and summative assessments
Quick Hit Activity Using UIL Science Contests For Formative and Summative Assessments of Pre-AP and AP Biology Students Activity Title: Quick Hit Goal of Activity: To perform formative and summative assessments
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
CELLS: PLANT CELLS 20 FEBRUARY 2013
 CELLS: PLANT CELLS 20 FEBRUARY 2013 Lesson Description In this lesson we will discuss the following: The Cell Theory Terminology Parts of Plant Cells: Organelles Difference between plant and animal cells
CELLS: PLANT CELLS 20 FEBRUARY 2013 Lesson Description In this lesson we will discuss the following: The Cell Theory Terminology Parts of Plant Cells: Organelles Difference between plant and animal cells
Topic 3: Nutrition, Photosynthesis, and Respiration
 1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
2007 7.013 Problem Set 1 KEY
 2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
Cell Unit Practice Test #1
 ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
Cells. Structure, Function and Homeostasis
 Cells Structure, Function and Homeostasis Characteristics of Cells Basic unit of life anything alive is made of cells Plasma membrane (skin) that separates them from the environment. Skeletonsfor protection
Cells Structure, Function and Homeostasis Characteristics of Cells Basic unit of life anything alive is made of cells Plasma membrane (skin) that separates them from the environment. Skeletonsfor protection
Name Date Period. 2. When a molecule of double-stranded DNA undergoes replication, it results in
 DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Plasma Membrane hydrophilic polar heads
 The Parts of the Cell 3 main parts in ALL cells: plasma membrane, cytoplasm, genetic material this is about the parts of a generic eukaryotic cell Plasma Membrane -is a fluid mosaic model membrane is fluid
The Parts of the Cell 3 main parts in ALL cells: plasma membrane, cytoplasm, genetic material this is about the parts of a generic eukaryotic cell Plasma Membrane -is a fluid mosaic model membrane is fluid
The Living Cell from the Biology: The Science of Life Series. Pre-Test
 1 Pre-Test Directions: Answer each question TRUE OR FALSE. 1. The instructions for making proteins are stored in molecules of DNA. 2. Proteins are made in the nucleus. 3. All cells are surrounded by a
1 Pre-Test Directions: Answer each question TRUE OR FALSE. 1. The instructions for making proteins are stored in molecules of DNA. 2. Proteins are made in the nucleus. 3. All cells are surrounded by a
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
7.2 Cell Structure. Lesson Objectives. Lesson Summary. Cell Organization Eukaryotic cells contain a nucleus and many specialized structures.
 7.2 Cell Structure Lesson Objectives Describe the structure and function of the cell nucleus. Describe the role of vacuoles, lysosomes, and the cytoskeleton. Identify the role of ribosomes, endoplasmic
7.2 Cell Structure Lesson Objectives Describe the structure and function of the cell nucleus. Describe the role of vacuoles, lysosomes, and the cytoskeleton. Identify the role of ribosomes, endoplasmic
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Review of the Cell and Its Organelles
 Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
Multiple Choice Questions
 Chapter 5 THE FUNDAMENTAL UNIT OF LIFE Multiple Choice Questions 1. Which of the following can be made into crystal? (a) A Bacterium (b) An Amoeba (c) A Virus (d) A Sperm 2. A cell will swell up if (a)
Chapter 5 THE FUNDAMENTAL UNIT OF LIFE Multiple Choice Questions 1. Which of the following can be made into crystal? (a) A Bacterium (b) An Amoeba (c) A Virus (d) A Sperm 2. A cell will swell up if (a)
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT
 CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
CELL/ PHOTOSYNTHESIS/ CELLULAR RESPIRATION Test 2011 ANSWER 250 POINTS ANY WAY IN WHICH YOU WANT Completion: complete each statement. (1 point each) 1. All cells arise from. 2. The basic unit of structure
AP BIOLOGY 2006 SCORING GUIDELINES. Question 1
 AP BIOLOGY 2006 SCORING GUIDELINES Question 1 A major distinction between prokaryotes and eukaryotes is the presence of membrane-bound organelles in eukaryotes. (a) Describe the structure and function
AP BIOLOGY 2006 SCORING GUIDELINES Question 1 A major distinction between prokaryotes and eukaryotes is the presence of membrane-bound organelles in eukaryotes. (a) Describe the structure and function
H.W. 1 Bio 101 Prof. Fournier
 H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
The Cell Teaching Notes and Answer Keys
 The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
Cell and Membrane Practice. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Biological cell membranes
 Unit 14: Cell biology. 14 2 Biological cell membranes The cell surface membrane surrounds the cell and acts as a barrier between the cell s contents and the environment. The cell membrane has multiple
Unit 14: Cell biology. 14 2 Biological cell membranes The cell surface membrane surrounds the cell and acts as a barrier between the cell s contents and the environment. The cell membrane has multiple
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Introduction to the Cell: Plant and Animal Cells
 Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Lecture 4 Cell Membranes & Organelles
 Lecture 4 Cell Membranes & Organelles Structure of Animal Cells The Phospholipid Structure Phospholipid structure Encases all living cells Its basic structure is represented by the fluidmosaic model Phospholipid
Lecture 4 Cell Membranes & Organelles Structure of Animal Cells The Phospholipid Structure Phospholipid structure Encases all living cells Its basic structure is represented by the fluidmosaic model Phospholipid
3.1 AS Unit: Cells, Exchange and Transport
 3.1 AS Unit: Cells, Exchange and Transport Module 1: Cells 1.1.1 Cell Structure Candidates should be able to: (a) state the resolution and magnification that can be achieved by a light microscope, a transmission
3.1 AS Unit: Cells, Exchange and Transport Module 1: Cells 1.1.1 Cell Structure Candidates should be able to: (a) state the resolution and magnification that can be achieved by a light microscope, a transmission
An Overview of Cells and Cell Research
 An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
tissues are made of cells that work together, organs are )
 Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
cells - relatively simple cells - lack nuclear membrane and many organelles - bacteria and their relatives are all prokaryotic
 Cell Biology A cell is chemical system that is able to maintain its structure and reproduce. Cells are the fundamental unit of life. All living things are cells or composed of cells. 1 The interior contents
Cell Biology A cell is chemical system that is able to maintain its structure and reproduce. Cells are the fundamental unit of life. All living things are cells or composed of cells. 1 The interior contents
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT
 BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
Bacterial (Prokaryotic) Cell. Common features of all cells. Tour of the Cell. Eukaryotic Cell. Plasma Membrane defines inside from outside
 www.denniskunkel.com Tour of the Cell www.denniskunkel.com Today s Topics Properties of all cells Prokaryotes and Eukaryotes Functions of Major Cellular Organelles Information, Synthesis&Transport,, Vesicles
www.denniskunkel.com Tour of the Cell www.denniskunkel.com Today s Topics Properties of all cells Prokaryotes and Eukaryotes Functions of Major Cellular Organelles Information, Synthesis&Transport,, Vesicles
Date: Student Name: Teacher Name: Jared George. Score: 1) A cell with 1% solute concentration is placed in a beaker with a 5% solute concentration.
 Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
The Cell: Organelle Diagrams
 The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
Microscopes. Eukaryotes Eukaryotic cells are characterized by having: DNA in a nucleus that is bounded by a membranous nuclear envelope
 CH 6 The Cell Microscopy Scientists use microscopes to visualize cells too small to see with the naked eye. In a light microscope (LM), visible light is passed through a specimen and then through glass
CH 6 The Cell Microscopy Scientists use microscopes to visualize cells too small to see with the naked eye. In a light microscope (LM), visible light is passed through a specimen and then through glass
Eukaryotic Cell Structure: Organelles in Animal & Plant Cells Why are organelles important and how are plants and animals different?
 Why? Eukaryotic Cell Structure: Organelles in Animal & Plant Cells Why are organelles important and how are plants and animals different? The cell is the basic unit and building block of all living things.
Why? Eukaryotic Cell Structure: Organelles in Animal & Plant Cells Why are organelles important and how are plants and animals different? The cell is the basic unit and building block of all living things.
Comparing Plant And Animal Cells
 Comparing Plant And Animal Cells http://khanacademy.org/video?v=hmwvj9x4gny Plant Cells shape - most plant cells are squarish or rectangular in shape. amyloplast (starch storage organelle)- an organelle
Comparing Plant And Animal Cells http://khanacademy.org/video?v=hmwvj9x4gny Plant Cells shape - most plant cells are squarish or rectangular in shape. amyloplast (starch storage organelle)- an organelle
Biology Chapter 7 Practice Test
 Biology Chapter 7 Practice Test Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. The work of Schleiden and Schwann can be summarized by
Biology Chapter 7 Practice Test Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. The work of Schleiden and Schwann can be summarized by
Chapter 3. Cellular Structure and Function Worksheets. 39 www.ck12.org
 Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Eukaryotes. www.njctl.org PSI Biology Eukaryotes & Gene Expression
 Eukaryotes The Eukaryotic Cell Classwork 1. Identify two characteristics that are shared by all cells. 2. Suppose you are investigating a cell that contains a nucleus. Would you categorize this cell as
Eukaryotes The Eukaryotic Cell Classwork 1. Identify two characteristics that are shared by all cells. 2. Suppose you are investigating a cell that contains a nucleus. Would you categorize this cell as
Cellular Structure and Function
 Chapter Test A CHAPTER 7 Cellular Structure and Function Part A: Multiple Choice In the space at the left, write the letter of the term or phrase that best answers each question. 1. Which defines a cell?
Chapter Test A CHAPTER 7 Cellular Structure and Function Part A: Multiple Choice In the space at the left, write the letter of the term or phrase that best answers each question. 1. Which defines a cell?
Ms. Campbell Protein Synthesis Practice Questions Regents L.E.
 Name Student # Ms. Campbell Protein Synthesis Practice Questions Regents L.E. 1. A sequence of three nitrogenous bases in a messenger-rna molecule is known as a 1) codon 2) gene 3) polypeptide 4) nucleotide
Name Student # Ms. Campbell Protein Synthesis Practice Questions Regents L.E. 1. A sequence of three nitrogenous bases in a messenger-rna molecule is known as a 1) codon 2) gene 3) polypeptide 4) nucleotide
Plant and Animal Cells
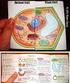 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
