Orbitals / Bonding / Shape Mixing Atomic Orbitals Hybridization of s and p orbitals. Orbitals / Bonding / Shape Atomic s and p orbitals
|
|
|
- Loreen Potter
- 7 years ago
- Views:
Transcription
1 The study of carbon-containing compounds. Organic compounds contain backbones comprised of chains and/or rings of carbon and hydrogen atoms. Commonly used formulas are empirical, molecular, structural (bond-line, condensed and 3-D), which are most commonly used over empirical, molecular formulas. Bonding Arrangements Orbitals / Bonding / Shape Atomic s and p orbitals Orbitals / Bonding / Shape Mixing Atomic Orbitals Hybridization of s and p orbitals
2 The atomic orbitals used in bond formation determine the bond angles sp 2 hybridization Tetrahedral bond angle: Electron pairs spread themselves into space as far from each other as possible A Triple Bond sp-hybridization A triple bond consists of one σ bond and two π bonds with a bond order of 3. Triple bonds are shorter and stronger than double bonds There is a bond angle of the sp carbon: 180 Each C atom is tetrahedral with sp 3 hybridized orbitals. Each C atom is linear with sp hybridized orbitals. Each C atom is trigonal planar with sp 2 hybridized orbitals. There is no rotation about the C=C bond in alkenes. Each C--C bond is the same length; shorter than a C-C bond: longer than a C=C bond. The concept of resonance is used to explain this phenomena.
3 An acyclic (noncyclic) hydrocarbon alkane has 12 carbon atoms. How many hydrogen atoms would one molecule of the compound possess? It is easy to rotate about the C-C bond in alkanes. A. 12 B. 24 C. 26 D. It would depend on more than just the number of carbon atoms; it cannot be predicted without more information. Molecular Representations Molecular formula:?? Empirical Formula:?? Bond-Line Structure??: Empirical Formula, Molecular Formula, Structure: (Lewis, Kekule, Condensed, Line), Visual Model: wireframe, stick, ball & stick, space filling, electrostatic, energy surface
4 Examples of Alkyl Substituents 2,3-dimethylbutane has how many carbon atoms in its longest continuous carbon chain? A. 2 B. 3 C. 4 D. 6 How many carbon atoms are present per molecule in the compound 3-methyl-4-ethyloctane? How many of those are present on the side chains (branches) only? A. 11 total; 3 on branches B. 15 total; 7 on branches C. 12 total; 3 on branches D. 15 total; 2 on branches Draw a bond-line structure for: 4-ethyl-3,5-dimethylnonane
5 Different Kinds of Alkyl Carbon Atoms Different Kinds of sp 3 Carbon and Associated Hydrogen Atoms 1. Notice that methyl itself is not considered. 2. Notice the number of H atoms: 1 o = 2H; 2 o = 1H; 3 o = 0 3. This distinction is not limited to halides, but applies to all sp 3 hybridized carbon atoms with a substituent, eg. OH (alcohols), etc. 4. For non-substituted C atoms an H atom replaces the substituent. eg. R-CH 2 -H = R-CH 3 = primary, etc. Draw a bond-line structure for: cis-4-methyl-2-hexene
6 How many hydrogen atoms would be part of one molecule of cyclopentene? A. 4 B. 5 C. 8 D. 10 Draw a bond-line structure for: 4-methyl-1-pentyne Aromatic structures are formally related to benzene. Resonance forms provide for delocalized π electrons leading to equal bond lengths. The net result is represented as a circle in the ring.
7 Hydrocarbons / Oil Refining Bond-line structures (omitting H atoms). ketone aldehyde carboxylic acid ester (carboxylic acid ester) Identify the functional groups in the following molecule. A) Alcohol, amide, carboxylic acid B) Aldehyde, amine, ester C) Alcohol, amine, carboxylic acid D) Aldehyde, amide, ketone
8
9 An important biological alcohol is cholesterol. Cells cannot survive without it!
10 [O] Oxidation: adding O, N or X; or removing hydrogens [H] Reduction: adding hydrogens or removing O, N or X [O] Oxidation: adding O, N or X; or removing hydrogens [H] Reduction: adding hydrogens or removing O, N or X Indicate if the reaction in the direction of the arrow is respectively oxidation [O] or reduction [H]. Alcohol Oxidation: (Removing 2 hydrogen atoms) 1 o alcohols produce aldehydes, which can oxidize further 2 o alcohols produce ketones 3 o alcohols do not react Name an oxidation product of 2-butanol. A. Butanoic acid B. 2-butanal C. Butanone D. Butanal Which of the following possible starting materials would be best used to prepare benzoic acid in one step using an oxidation reaction? A. Benzaldehyde B. 2-phenylethylalcohol C. Benzene D. Phenol
11 Esters are often associated with the aromas and tastes of fruits. The ester methyl butyrate is associated with apples. What compounds would be observed if this ester were to break down into its original components? A. Methanal and butanone B. Methanol and butanone C. Methanol and butanoic acid D. Methanoic acid and butanol Saponification, hydrolysis of an ester in the presence of a base, is the reverse of esterification. Fats saponify to give fatty acids plus glycerols. (eg.triglycerides)
12 The compound diethyl amine that can be used as a curing agent in some epoxy materials would have how many hydrogen atoms per molecule? A. 7 B. 10 C. 11 D. 12 Enantiomers (Chiral: Non-superimposable Mirror Images) Diastereomers (Chiral: Non-superimposable Non-Mirror Images; multiple chiral centers) Molecules which have the same molecular formula, but differ in the arrangement of their atoms, are called isomers. Constitutional (or structural) isomers differ in their bonding sequence. Stereoisomers differ only in the arrangement of the atoms in space.
13 cis or (Z-) same side trans or (E-) across cis-trans isomers are geometric isomers. There must be two different groups on the sp 2 carbons. No cis-trans isomers in top two isomers, only the bottom two. (+) dextrorotatory (-) levorotatory The mirror image of an enantiomer will rotate the plane of polarized light by the same amount in the opposite direction. Eg (+) d-carvone +62 o (caraway) and (-) l-carvone -62 o (spearmint). What about a 50:50 (racemic) mixture?
14 The monosaccharide mannose has how many chiral carbon centers? A. None B. Two C. Four D. Six Each sp 3 carbon atom with four different substituents are chiral. Tartaric acid has 2 chiral carbon atoms. Cholesterol can have how many possible stereoisomers?
15 Louis Pasteurʼs lab notebook page (1848) A. B. C. D. A. & B. = enantiomers A. & C. and B. & C. = diastereomers A. = naturally occuring form found in wine D. = racemic mixture (50% A. & 50% B.) Macromolecules which are made from small molecules, monomers, or co-monomers which structurally repeat themselves. Monomer Polymer Ethylene Polyethylene Vinyl chloride Polyvinyl chloride PVC Tetrafluoroethylene Teflon Proteins Amino Acids A macromolecule which is a poly-amide. Using a condensation mechanism predict the number of hydrogen atoms found in one unit of the theoretical copolymer dimer formed between hexamethylenediamine and oxalic acid
16 The synthetic polymer polyethylene is made from the monomer ethene or also referred to as ethylene. The polymer has no carbon branching. Polypropylene is made from the monomer propene. As propene monomers are added together, a chain with methyl branches can form. In such a chain how many carbon atoms would be between each branch. Note: these carbon atom(s) themselves would have no branches. A. 1 B. 2 C. 3 D. none Waste / Recycling? ~250 billion pounds produced annually, worldwide.
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Page 1. 6. Which hydrocarbon is a member of the alkane series? (1) 1. Which is the structural formula of methane? (1) (2) (2) (3) (3) (4) (4)
 1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
ORGANIC COMPOUNDS IN THREE DIMENSIONS
 (adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
(adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 12 Organic Compounds with Oxygen and Sulfur
 Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Survival Organic Chemistry Part I: Molecular Models
 Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
1. The functional group present in carboxylic acids is called a A) carbonyl group. B) carboxyl group. C) carboxylate group. D) carbohydroxyl group.
 Name: Date: 1. The functional group present in carboxylic acids is called a A) carbonyl group. B) carboxyl group. C) carboxylate group. D) carbohydroxyl group. 2. Which of the following statements concerning
Name: Date: 1. The functional group present in carboxylic acids is called a A) carbonyl group. B) carboxyl group. C) carboxylate group. D) carbohydroxyl group. 2. Which of the following statements concerning
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Unit 2 Review: Answers: Review for Organic Chemistry Unit Test
 Unit 2 Review: Answers: Review for Organic Chemistry Unit Test 2. Write the IUPAC names for the following organic molecules: a) acetone: propanone d) acetylene: ethyne b) acetic acid: ethanoic acid e)
Unit 2 Review: Answers: Review for Organic Chemistry Unit Test 2. Write the IUPAC names for the following organic molecules: a) acetone: propanone d) acetylene: ethyne b) acetic acid: ethanoic acid e)
Molecular Models Experiment #1
 Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Alkanes. Chapter 1.1
 Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Chapter 4 Lecture Notes
 Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391)
 NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
Alcohols An alcohol contains a hydroxyl group ( OH) attached to a carbon chain. A phenol contains a hydroxyl group ( OH) attached to a benzene ring.
 Chapter : rganic Compounds with xygen Alcohols, Ethers Alcohols An alcohol contains a hydroxyl group ( H) attached to a carbon chain. A phenol contains a hydroxyl group ( H) attached to a benzene ring.
Chapter : rganic Compounds with xygen Alcohols, Ethers Alcohols An alcohol contains a hydroxyl group ( H) attached to a carbon chain. A phenol contains a hydroxyl group ( H) attached to a benzene ring.
Alcohols. Copyright 2009 by Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. CH 3 CH 2 CH 2 OH 1-propanol OH
 Chapter 12 rganic Compounds with xygen and Sulfur 12.1 Alcohols, Thiols, and Ethers Alcohols An alcohol contains a hydroxyl group ( ) attached to a carbon chain. A phenol contains a hydroxyl group ( )
Chapter 12 rganic Compounds with xygen and Sulfur 12.1 Alcohols, Thiols, and Ethers Alcohols An alcohol contains a hydroxyl group ( ) attached to a carbon chain. A phenol contains a hydroxyl group ( )
Chemistry 1110 Organic Chemistry IUPAC Nomenclature
 hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
Ch17_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch17_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which molecule is a carboxylic acid? A) 1) B) C) D) E) CH3 CH2 CH2 NH2 2) Which molecule
Ch17_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which molecule is a carboxylic acid? A) 1) B) C) D) E) CH3 CH2 CH2 NH2 2) Which molecule
IUPAC System of Nomenclature
 IUPAC System of Nomenclature The IUPAC (International Union of Pure and Applied Chemistry) is composed of chemists representing the national chemical societies of several countries. ne committee of the
IUPAC System of Nomenclature The IUPAC (International Union of Pure and Applied Chemistry) is composed of chemists representing the national chemical societies of several countries. ne committee of the
CHEM 203 Exam 1. KEY Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 1 KEY Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. _D C 1. Which of the following elements is a large percentage of both
CHEM 203 Exam 1 KEY Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. _D C 1. Which of the following elements is a large percentage of both
NOMENCLATURE OF ORGANIC COMPOUNDS 2010, 2003, 1980, by David A. Katz. All rights reserved.
 NMENCLATURE F RGANIC CMPUNDS 2010, 2003, 1980, by David A. Katz. All rights reserved. rganic chemistry is the chemistry of carbon compounds. Carbon has the ability to bond with itself to form long chains
NMENCLATURE F RGANIC CMPUNDS 2010, 2003, 1980, by David A. Katz. All rights reserved. rganic chemistry is the chemistry of carbon compounds. Carbon has the ability to bond with itself to form long chains
CH 102 Practice Exam 2 PCC-Sylvania
 CH 102 Practice Exam 2 PCC-Sylvania True/False Indicate if the statement is true or false. 1.Tertiary alcohols are not easily oxidized. 2.Secondary alcohols can be oxidized to aldehydes. 3.Primary alcohols
CH 102 Practice Exam 2 PCC-Sylvania True/False Indicate if the statement is true or false. 1.Tertiary alcohols are not easily oxidized. 2.Secondary alcohols can be oxidized to aldehydes. 3.Primary alcohols
Alcohols. Characterized by OH group Name: add ol. to name of hydrocarbon. Methanol. Butanol. Sterno. Alcohols burn in air. A mixture of ethanol +
 1 2 3 Functional Groups Alcohols Structures of Alcohols haracterized by group Name: add ol to name of hydrocarbon 3 5 : how many structural isomers? See D-RM Screens 11.5 & 11.6 Methanol Butanol 1-propanol
1 2 3 Functional Groups Alcohols Structures of Alcohols haracterized by group Name: add ol to name of hydrocarbon 3 5 : how many structural isomers? See D-RM Screens 11.5 & 11.6 Methanol Butanol 1-propanol
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models
 EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
Chapter 13 Carboxylic Acids, Esters, Amines, and Amides. Carboxylic Acids. Names and Sources of Some Carboxylic Acids. IUPAC Names
 Chapter 13 Carboxylic Acids, Esters, Amines, and Amides 13.1 Carboxylic Acids Carboxylic Acids A carboxylic acid contains a carboxyl group, which is a carbonyl group (C=) attached to a hydroxyl group (
Chapter 13 Carboxylic Acids, Esters, Amines, and Amides 13.1 Carboxylic Acids Carboxylic Acids A carboxylic acid contains a carboxyl group, which is a carbonyl group (C=) attached to a hydroxyl group (
Determining the Structure of an Organic Compound
 Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants In the 19 th and early 20 th
Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants In the 19 th and early 20 th
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
for excitation to occur, there must be an exact match between the frequency of the applied radiation and the frequency of the vibration
 ! = 1 2"c k (m + M) m M wavenumbers! =!/c = 1/" wavelength frequency! units: cm 1 for excitation to occur, there must be an exact match between the frequency of the applied radiation and the frequency
! = 1 2"c k (m + M) m M wavenumbers! =!/c = 1/" wavelength frequency! units: cm 1 for excitation to occur, there must be an exact match between the frequency of the applied radiation and the frequency
HOMEWORK PROBLEMS: IR SPECTROSCOPY AND 13C NMR. The peak at 1720 indicates a C=O bond (carbonyl). One possibility is acetone:
 HMEWRK PRBLEMS: IR SPECTRSCPY AND 13C NMR 1. You find a bottle on the shelf only labeled C 3 H 6. You take an IR spectrum of the compound and find major peaks at 2950, 1720, and 1400 cm -1. Draw a molecule
HMEWRK PRBLEMS: IR SPECTRSCPY AND 13C NMR 1. You find a bottle on the shelf only labeled C 3 H 6. You take an IR spectrum of the compound and find major peaks at 2950, 1720, and 1400 cm -1. Draw a molecule
Please read and sign the Honor Code statement below:
 CHEM 3311 Exam #1 Name Dr. Minger June 8, 2015 Please read and sign the Honor Code statement below: I pledge that on my honor, as a University of Colorado at Boulder student, I have neither given nor received
CHEM 3311 Exam #1 Name Dr. Minger June 8, 2015 Please read and sign the Honor Code statement below: I pledge that on my honor, as a University of Colorado at Boulder student, I have neither given nor received
Chapter 18: Organic Chemistry
 h 18 Page 1 hapter 18: rganic hemistry rganic chemistry is a branch of chemistry that focuses on compounds that contain carbon (Exceptions:, 2, 3 2-, and N - ) Even though organic compounds only contain
h 18 Page 1 hapter 18: rganic hemistry rganic chemistry is a branch of chemistry that focuses on compounds that contain carbon (Exceptions:, 2, 3 2-, and N - ) Even though organic compounds only contain
Polymers: Introduction
 Chapter Outline: Polymer Structures Hydrocarbon and Polymer Molecules Chemistry of Polymer Molecules Molecular Weight and Shape Molecular Structure and Configurations Copolymers Polymer Crystals Optional
Chapter Outline: Polymer Structures Hydrocarbon and Polymer Molecules Chemistry of Polymer Molecules Molecular Weight and Shape Molecular Structure and Configurations Copolymers Polymer Crystals Optional
Question Bank Organic Chemistry-I
 Question Bank Organic Chemistry-I 1. (a) What do you understand by the following terms : (i) Organic chemistry (ii) Organic compounds (iii) Catenation? [3] (b) Why are there very large number of organic
Question Bank Organic Chemistry-I 1. (a) What do you understand by the following terms : (i) Organic chemistry (ii) Organic compounds (iii) Catenation? [3] (b) Why are there very large number of organic
the double or triple bond. If the multiple bond is CH 3 C CCHCCH 3
 Alkenes, Alkynes, and Aromatic ompounds Alkenes and Alkynes Unsaturated contain carbon-carbon double and triple bond to which more hydrogen atoms can be added. Alkenes: carbon-carbon double bonds Alkynes:
Alkenes, Alkynes, and Aromatic ompounds Alkenes and Alkynes Unsaturated contain carbon-carbon double and triple bond to which more hydrogen atoms can be added. Alkenes: carbon-carbon double bonds Alkynes:
Question (3): What are the different types of covalent bonds found in carbons compounds? Briefly explain with examples.
 CLASS: X NCERT (CBSE) Chemistry: For Class 10 Page : 1 Question (1): What is organic chemistry? Organic chemistry is the study of carbon compounds of living matter i.e., plants and animals (CO 2, carbonates,
CLASS: X NCERT (CBSE) Chemistry: For Class 10 Page : 1 Question (1): What is organic chemistry? Organic chemistry is the study of carbon compounds of living matter i.e., plants and animals (CO 2, carbonates,
Introduction to Biodiesel Chemistry Terms and Background Information
 Introduction to Biodiesel Chemistry Terms and Background Information Basic rganic Chemistry rganic chemistry is the branch of chemistry that deals with organic compounds. rganic compounds are compounds
Introduction to Biodiesel Chemistry Terms and Background Information Basic rganic Chemistry rganic chemistry is the branch of chemistry that deals with organic compounds. rganic compounds are compounds
Copyright 2010 Pearson Education, Inc. Chapter Fourteen 1
 An alcohol has an OH bonded to an alkyl group; a phenol has an OH bonded directly to an aromatic ring; and an ether has an O bonded to two organic groups. Chapter Fourteen 1 Ethyl alcohol, dimethyl ether,
An alcohol has an OH bonded to an alkyl group; a phenol has an OH bonded directly to an aromatic ring; and an ether has an O bonded to two organic groups. Chapter Fourteen 1 Ethyl alcohol, dimethyl ether,
PRACTICE PROBLEMS, CHAPTERS 1-3
 PRATIE PRBLEMS, APTERS 1-3 (overed from h. 3: Alkane and Alkyl alide nomenclature only) 1. The atomic number of boron is 5. The correct electronic configuration of boron is: A. 1s 2 2s 3 B. 1s 2 2p 3.
PRATIE PRBLEMS, APTERS 1-3 (overed from h. 3: Alkane and Alkyl alide nomenclature only) 1. The atomic number of boron is 5. The correct electronic configuration of boron is: A. 1s 2 2s 3 B. 1s 2 2p 3.
IR Applied to Isomer Analysis
 DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
An Introduction to Organic Chemistry
 An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch 13_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) In organic chemistry, the term unsaturated means a molecule A) which contains one or more
Ch 13_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) In organic chemistry, the term unsaturated means a molecule A) which contains one or more
4/18/2011. 9.8 Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic ring Some substituents activate the ring, making it more reactive than benzene
9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic ring Some substituents activate the ring, making it more reactive than benzene
Q.1 Draw out some suitable structures which fit the molecular formula C 6 H 6
 Aromatic compounds GE 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out some suitable structures which fit the molecular formula
Aromatic compounds GE 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out some suitable structures which fit the molecular formula
ALCOHOLS: Properties & Preparation
 ALLS: Properties & Preparation General formula: R-, where R is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol.
ALLS: Properties & Preparation General formula: R-, where R is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol.
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch14_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Compounds with the -OH group attached to a saturated alkane-like carbon are known as A)
Ch14_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Compounds with the -OH group attached to a saturated alkane-like carbon are known as A)
Q.1 Draw structures for, and name, all carboxylic acids with formula :-
 arboxylic acids F4 1 ARBXYLI AIDS Structure contain the carboxyl functional group includes a carbonyl (=) group and a hydroxyl (-) group the bonds are in a planar arrangement are isomeric with esters :-
arboxylic acids F4 1 ARBXYLI AIDS Structure contain the carboxyl functional group includes a carbonyl (=) group and a hydroxyl (-) group the bonds are in a planar arrangement are isomeric with esters :-
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MLEULAR REPRESENTATINS AND INFRARED SPETRSPY A STUDENT SULD BE ABLE T: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give examples
MLEULAR REPRESENTATINS AND INFRARED SPETRSPY A STUDENT SULD BE ABLE T: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give examples
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
1.15 Bonding in Methane and Orbital Hybridization
 1.15 Bonding in Methane and Orbital Hybridization Structure of Methane tetrahedral bond angles = 109.5 bond distances = 110 pm but structure seems inconsistent with electron configuration of carbon Electron
1.15 Bonding in Methane and Orbital Hybridization Structure of Methane tetrahedral bond angles = 109.5 bond distances = 110 pm but structure seems inconsistent with electron configuration of carbon Electron
H 3 C CH 2 CH 2 CH 2 CH 2 CH 3. Copyright 2012 Nelson Education Ltd. Chapter 1: Organic Compounds 1.1-1
 Section 1.1: Alkanes Mini Investigation: Arranging Carbon Atoms, page 10 A. Three different molecules of C 5 H 12 are possible. B. Five arrangements are possible for C 6 H 14, as predicted: H 3 C CH 2
Section 1.1: Alkanes Mini Investigation: Arranging Carbon Atoms, page 10 A. Three different molecules of C 5 H 12 are possible. B. Five arrangements are possible for C 6 H 14, as predicted: H 3 C CH 2
Chapter 9 - Covalent Bonding: Orbitals
 Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Experiment 11. Infrared Spectroscopy
 Chem 22 Spring 2010 Experiment 11 Infrared Spectroscopy Pre-lab preparation. (1) In Ch 5 and 12 of the text you will find examples of the most common functional groups in organic molecules. In your notebook,
Chem 22 Spring 2010 Experiment 11 Infrared Spectroscopy Pre-lab preparation. (1) In Ch 5 and 12 of the text you will find examples of the most common functional groups in organic molecules. In your notebook,
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. What is the hybridization of the indicated atom in the following molecule?
 Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
Unit X: Polymers Test 1.1
 ame: Unit X: Polymers Test 1.1 Multiple hoice Questions 1 through 9 pertain to the reactions on the last two pages of this test. Where multiple answers exist only one need be reported. 1. Which process
ame: Unit X: Polymers Test 1.1 Multiple hoice Questions 1 through 9 pertain to the reactions on the last two pages of this test. Where multiple answers exist only one need be reported. 1. Which process
Molecule Projections
 Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
UNIT (9) CARBOXYLIC ACIDS, ESTERS, AMINES, AND AMIDES
 UNIT (9) CARBXYLIC ACIDS, ESTERS, AMINES, AND AMIDES 9.1 Carboxylic Acids The functional group in carboxylic acids is called the carboxyl group. A carboxyl group is a carbonyl group (C = ) with a hydroxyl
UNIT (9) CARBXYLIC ACIDS, ESTERS, AMINES, AND AMIDES 9.1 Carboxylic Acids The functional group in carboxylic acids is called the carboxyl group. A carboxyl group is a carbonyl group (C = ) with a hydroxyl
AROMATIC COMPOUNDS A STUDENT SHOULD BE ABLE TO:
 A STUDENT SHULD BE ABLE T: ARMATIC CMPUNDS 1. Name benzene derivatives given the structures, and draw the structures given the names. This includes: Monosubstituted benzenes named as derivatives of benzene:
A STUDENT SHULD BE ABLE T: ARMATIC CMPUNDS 1. Name benzene derivatives given the structures, and draw the structures given the names. This includes: Monosubstituted benzenes named as derivatives of benzene:
Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain.
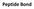 Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
17.2 REACTIONS INVOLVING ALLYLIC AND BENZYLIC RADICALS
 17. REACTINS INVLVING ALLYLIC AND BENZYLIC RADICALS 793 As Eq. 17. shows, the products derived from the reaction of water at the ring carbons are not formed. The reason is that these products are not aromatic
17. REACTINS INVLVING ALLYLIC AND BENZYLIC RADICALS 793 As Eq. 17. shows, the products derived from the reaction of water at the ring carbons are not formed. The reason is that these products are not aromatic
Infrared Spectroscopy
 Infrared Spectroscopy 1 Chap 12 Reactions will often give a mixture of products: OH H 2 SO 4 + Major Minor How would the chemist determine which product was formed? Both are cyclopentenes; they are isomers.
Infrared Spectroscopy 1 Chap 12 Reactions will often give a mixture of products: OH H 2 SO 4 + Major Minor How would the chemist determine which product was formed? Both are cyclopentenes; they are isomers.
Organic Chemistry Tenth Edition
 Organic Chemistry Tenth Edition T. W. Graham Solomons Craig B. Fryhle Welcome to CHM 22 Organic Chemisty II Chapters 2 (IR), 9, 3-20. Chapter 2 and Chapter 9 Spectroscopy (interaction of molecule with
Organic Chemistry Tenth Edition T. W. Graham Solomons Craig B. Fryhle Welcome to CHM 22 Organic Chemisty II Chapters 2 (IR), 9, 3-20. Chapter 2 and Chapter 9 Spectroscopy (interaction of molecule with
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Reactions of Fats and Fatty Acids
 Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
STANDARD ANSWERS AND DEFINITIONS
 Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
Chapter 22 Carbonyl Alpha-Substitution Reactions
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 22 Carbonyl Alpha-Substitution Reactions The α Position The carbon next to the carbonyl group is designated as being in the α position Electrophilic
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 22 Carbonyl Alpha-Substitution Reactions The α Position The carbon next to the carbonyl group is designated as being in the α position Electrophilic
Organic Functional Groups Chapter 7. Alcohols, Ethers and More
 Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
INFRARED SPECTROSCOPY (IR)
 INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
passing through (Y-axis). The peaks are those shown at frequencies when less than
 Infrared Spectroscopy used to analyze the presence of functional groups (bond types) in organic molecules The process for this analysis is two-fold: 1. Accurate analysis of infrared spectra to determine
Infrared Spectroscopy used to analyze the presence of functional groups (bond types) in organic molecules The process for this analysis is two-fold: 1. Accurate analysis of infrared spectra to determine
Symmetric Stretch: allows molecule to move through space
 BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised)
 Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised) Course Title: Survey of Chemistry II, TR 1000 1115 Room NE-1260 Instructor: Dr. Jerry L. Poteat, Associate
Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised) Course Title: Survey of Chemistry II, TR 1000 1115 Room NE-1260 Instructor: Dr. Jerry L. Poteat, Associate
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
These instructions are for a classroom activity which supports OCR A Level Chemistry A.
 Lesson Element Keyword activities Instructions for teachers These instructions are for a classroom activity which supports OCR A Level Chemistry A. Just a minute! To run this activity you will need a set
Lesson Element Keyword activities Instructions for teachers These instructions are for a classroom activity which supports OCR A Level Chemistry A. Just a minute! To run this activity you will need a set
Organic Spectroscopy. UV - Ultraviolet-Visible Spectroscopy. !! 200-800 nm. Methods for structure determination of organic compounds:
 Organic Spectroscopy Methods for structure determination of organic compounds: X-ray rystallography rystall structures Mass spectroscopy Molecular formula -----------------------------------------------------------------------------
Organic Spectroscopy Methods for structure determination of organic compounds: X-ray rystallography rystall structures Mass spectroscopy Molecular formula -----------------------------------------------------------------------------
DETERMINACIÓN DE ESTRUCTURAS ORGÁNICAS (ORGANIC SPECTROSCOPY) IR SPECTROSCOPY
 DETERMINACIÓN DE ESTRUCTURAS ORGÁNICAS (ORGANIC SPECTROSCOPY) IR SPECTROSCOPY Hermenegildo García Gómez Departamento de Química Instituto de Tecnología Química Universidad Politécnica de Valencia 46022
DETERMINACIÓN DE ESTRUCTURAS ORGÁNICAS (ORGANIC SPECTROSCOPY) IR SPECTROSCOPY Hermenegildo García Gómez Departamento de Química Instituto de Tecnología Química Universidad Politécnica de Valencia 46022
CHEM 121. Chapter 19, Name: Date:
 CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
Synthesis of Isopentyl Acetate
 Experiment 8 Synthesis of Isopentyl Acetate Objectives To prepare isopentyl acetate from isopentyl alcohol and acetic acid by the Fischer esterification reaction. Introduction Esters are derivatives of
Experiment 8 Synthesis of Isopentyl Acetate Objectives To prepare isopentyl acetate from isopentyl alcohol and acetic acid by the Fischer esterification reaction. Introduction Esters are derivatives of
Mass Spec - Fragmentation
 Mass Spec - Fragmentation An extremely useful result of EI ionization in particular is a phenomenon known as fragmentation. The radical cation that is produced when an electron is knocked out of a neutral
Mass Spec - Fragmentation An extremely useful result of EI ionization in particular is a phenomenon known as fragmentation. The radical cation that is produced when an electron is knocked out of a neutral
Laboratory 22: Properties of Alcohols
 Introduction Alcohols represent and important class of organic molecules. In this experiment you will study the physical and chemical properties of alcohols. Solubility in water, and organic solvents,
Introduction Alcohols represent and important class of organic molecules. In this experiment you will study the physical and chemical properties of alcohols. Solubility in water, and organic solvents,
CHEM 51LB EXP 1 SPECTROSCOPIC METHODS: INFRARED AND NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 CHEM 51LB EXP 1 SPECTRSCPIC METHDS: INFRARED AND NUCLEAR MAGNETIC RESNANCE SPECTRSCPY REACTINS: None TECHNIQUES: IR Spectroscopy, NMR Spectroscopy Infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy
CHEM 51LB EXP 1 SPECTRSCPIC METHDS: INFRARED AND NUCLEAR MAGNETIC RESNANCE SPECTRSCPY REACTINS: None TECHNIQUES: IR Spectroscopy, NMR Spectroscopy Infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Lipids. There are 2 types of lipids; those that contain the structural component of a fatty acid; and
 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted from cells using
Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted from cells using
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
PROTEINS THE PEPTIDE BOND. The peptide bond, shown above enclosed in the blue curves, generates the basic structural unit for proteins.
 Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Chapter 15 Radical Reactions. Radicals are reactive species with a single unpaired electron, formed by
 Chapter 15 Radical Reactions Radicals are reactive species with a single unpaired electron, formed by homolysis of a covalent bond; a radical contains an atom that does not have an octet of electrons,
Chapter 15 Radical Reactions Radicals are reactive species with a single unpaired electron, formed by homolysis of a covalent bond; a radical contains an atom that does not have an octet of electrons,
Chapter 6. Alkenes: Structure and Stability
 hapter 6. Alkenes: Structure and Stability Steric Acid (saturated fatty acid) Linoleic Acid (unsaturated fatty acid) Degrees of unsaturation saturated hydrocarbon n 2n2 cycloalkane (1 ring) n 2n alkene
hapter 6. Alkenes: Structure and Stability Steric Acid (saturated fatty acid) Linoleic Acid (unsaturated fatty acid) Degrees of unsaturation saturated hydrocarbon n 2n2 cycloalkane (1 ring) n 2n alkene
CHEM 101 Exam 4. Page 1
 CEM 101 Exam 4 Form 1 (White) November 30, 2001 Page 1 Section This exam consists of 8 pages. When the exam begins make sure you have one of each. Print your name at the top of each page now. Show your
CEM 101 Exam 4 Form 1 (White) November 30, 2001 Page 1 Section This exam consists of 8 pages. When the exam begins make sure you have one of each. Print your name at the top of each page now. Show your
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
How to Interpret an IR Spectrum
 How to Interpret an IR Spectrum Don t be overwhelmed when you first view IR spectra or this document. We have simplified the interpretation by having you only focus on 4/5 regions of the spectrum. Do not
How to Interpret an IR Spectrum Don t be overwhelmed when you first view IR spectra or this document. We have simplified the interpretation by having you only focus on 4/5 regions of the spectrum. Do not
