Yarışma Sınavı. 5 Which one is related with red blood cells?
|
|
|
- Evelyn Marsh
- 10 years ago
- Views:
Transcription
1 1 Which one is a genetic disease in which a person's blood does not clot properly? sickle cell anaemia anaemia thalesshemia haemophilia colourblindness 5 Which one is related with red blood cells? destroy disease causing organisms carry oxygen to the tissues can move like amoebas liquid part of the blood help to clot blood 2 Which structures are strong tissues that hold bones together at joints? villi ligaments bone marrow cartilage fatty tissue 6 Which element is found as a diatomic gas in nature? rgon Oxygen Neon Helium Iron Compounds of which element are used to disinfect drinking water and swimming pools? 8 Which group of elements in the periodic table never form compounds in chemical reactions? Francium Hydrogen Chlorine Magnesium Sodium VII VI I II VIII 1
2 9 Which of the following glands release both hormone and digestive juices? pancreas testes thyroid gland pituitary gland adrenal gland 12 Where is ball and socket joint found? skull fingers knees shoulders elbows 10 Which part of your eyes controls the amount of light entering the eye? pupil cornea iris lens retina 11 Which structure in the umbilical cord carries carbondioxide and other wastes away from the fet us? placenta vein villi gland artery 13 What are the products of respiration? nitrogen and oxygen carbondioxide and water oxygen and carbondioxide water and oxygen glucose and oxygen 14 Which element in the periodic table is known as the 'most reactive metal'? Magnesium Francium Iron Lithium Flourine 15 Which one is not found in a DN molecule? a deoxyribose sugar a phosphate group a ribose sugar a cytosine nucleotide an adenine nucleotide 2
3 16 Which one is a kind of solid solution? sugar solution steel alcohol solution air coffee 20 What will the weight of a man be on the moon if he has a mass of 60 kg? 100 N 60 N 10 kg 600 N 60 kg Which one is related with arteries? they are the places where exchange of gases take place they carry blood to the heart they are used to find the pulse rate they are the thinnest blood vessels they are only one-cell thick 19 What is the function of the bone marrow? to produce blood cells to give body its shape to help digestion of food to store calcium and phosphorus to supply food and oxygen 22 water pump uses 0.5 kw of power to raise 100kg of water by 10m. How long does it take to pump water? s 2 s 20 s s 50 s 3
4 23 Which one is related with metals? They have low densities and low melting points than non-metals They gain electrons in chemical reactions They have 1, 2 or 3 valence electrons They form anions They are brittle 26 Two healthy parents had three childen. The third was found to have a certain disease although the other two were normal. What can you say about the genotypes of the parents? Bb and BB BB and BB Bb and Bb Bb and bb bb and bb 24 Which statement is true for chamical changes? State changes are examples of chemical changes Chemical changes cannot be reversed easily In a chemical change only the size and shape of the substance changes No new substances are made Heat is not produced in chemical changes 27 What is blood clotting? Solidification of blood Giving out oxygen from the blood Increasing the amount of blood Loss of blood through a broken blood vessel Contraction of the blood vessels 25 Which of the following factors cannot reduce friction? lubricating surfaces rolling the objects pushing on a smooth surface separating the surfaces in contact with air pulling on a rough surface 28 I. tmospheric pressure is highest at sea level. II. mount of oxygen increases as you climb up a mountain. III. In space, pressure inside our body is greater than the outside pressure. Which sentences are correct? I and III I and II I, II and III II and III only I 4
5 29 The area of the large piston in a hydraulic lift is 100 times the area of the small one. How much force is needed to lift a weight of 6000N? 60N 6N 600N 6000N N 32 Why do you sense the taste of foods less when you get cold? because you cannot eat too much because your body temperature increases because your nose is clogged because your mouth is clogged because your body loses energy 30 Which one is responsible for the exchange of materials in and out of the cell? mitochondria cell membrane nucleus nuclear membrane ribosome 33 Which of the organelles below are found both in plant and animal cells? golgi body and endoplasmic reticulum mitochondria and chloroplasts centrioles and vacuoles cell membrance and cell wall cell wall and ribosomes If a car travels 100 km with a constant speed of 20 m/s, how long does the journey take? 5000 s 5 s 120 s 0.2 s s 5
6 35 Which of the following sentences is not true? Humans, insects and wind help pollination Pollination is the joining of an egg and a sperm Self pollination requres only one parent fter pollination pollen grains produce a pollen tube Fruit is produced from the ovary 39 Which one is not true for compounds? They can only be separated by chemical methods They are composed of elements only They are always homogenous Each substance which forms it keeps its own properties None of them 36 Which one is not true for RN molecule? It is made by DN It contains the base uracil It forms a shape called a double helix It is found both in the nucleus and cytoplasm It has lower molecular weight than DN 40 Which one is true for gravitatinal force? It is different in different places It is directly proportional to the distance between bodies It is inversely proportional to the mass of the bodies It is a scalar quantity It can be measured by using a barometer 37 Which one is false for cations? They are formed by losing electrons They join with anions to form ionic compounds They lose electrons to become a neutral atom They are positively charged atoms They gain electrons to become a neutral atom 41 Which one is not correct for Noble gases? They do not undergo chemical reactions They are used in lighting They are diatomic gases They have full outer shells They never form compounds 38 Which is incorrect for identical twins? They are always same sex They look exactly alike They are develeoped from two eggs and two sperms They are formed from one zygote None of them 42 Which one is not related with acids? They have slippery feeling They have a sour taste They turn blue litmus paper to red They produce salt by neutralization reaction They produce hydrogen gas when react with metals 6
7 43 I. Liquid in the cochlea vibrates II. Brain interprets nerve impulses III. Three tiny bones vibrate IV. Pinna collects sound waves V. Ear drum vibrates Which is the appropriate order for hearing? II, IV, V, III, I IV, V, II, III, I IV, V, III, I, II V, III, IV, II, I III, II, V, I, IV 47 Solubility of NaCl at 80ºC is 32g / 100ml water. Which one is the unsaturated solution of NaCl at 80ºC? 16 g NaCl in 50 ml water 40 g NaCl in 100 ml water 64 g NaCl in 200 ml water 108 g NaCl in 400 ml water 90 g NaCl in 300 ml water 44 Which one is not a property of covalent compounds? 45 They do not form ions in solutions They are hard solids They are formed between non-metal atoms They have low melting points They are formed by sharing of valence electrons 48 Which one is not correct for chromosomes? They consist of proteins and DN molecules They are found in pairs in the nucleus They have different shapes and lengths Each chromosome has one pair of genes on it They are found in the nucleus 49 Which one is not true for solutions? Particles in them cannot be separated by filtering They are heterogenous mixtures Particles in them do not settle down after some time They are transparent Particles are evenly distributed in the solution 46 7
8 50 TEST BİTTİ CEVPLRINIZI KONTROL EDİNİZ 8
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Name Date Period. 2. When a molecule of double-stranded DNA undergoes replication, it results in
 DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes.
 LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
CHAPTER 9 BODY ORGANIZATION
 CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Introduction to the Cell: Plant and Animal Cells
 Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Cells & Cell Organelles
 Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Date: Student Name: Teacher Name: Jared George. Score: 1) A cell with 1% solute concentration is placed in a beaker with a 5% solute concentration.
 Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
Biology Chapter 7 Practice Test
 Biology Chapter 7 Practice Test Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. The work of Schleiden and Schwann can be summarized by
Biology Chapter 7 Practice Test Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. The work of Schleiden and Schwann can be summarized by
Comparing Plant And Animal Cells
 Comparing Plant And Animal Cells http://khanacademy.org/video?v=hmwvj9x4gny Plant Cells shape - most plant cells are squarish or rectangular in shape. amyloplast (starch storage organelle)- an organelle
Comparing Plant And Animal Cells http://khanacademy.org/video?v=hmwvj9x4gny Plant Cells shape - most plant cells are squarish or rectangular in shape. amyloplast (starch storage organelle)- an organelle
Cell Structure & Function!
 Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
7.2 Cells: A Look Inside
 CHAPTER 7 CELL STRUCTURE AND FUNCTION 7.2 Cells: A Look Inside Imagine a factory that makes thousands of cookies a day. Ingredients come into the factory, get mixed and baked, then the cookies are packaged.
CHAPTER 7 CELL STRUCTURE AND FUNCTION 7.2 Cells: A Look Inside Imagine a factory that makes thousands of cookies a day. Ingredients come into the factory, get mixed and baked, then the cookies are packaged.
BIO 137: CHAPTER 1 OBJECTIVES
 BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Cell and Membrane Practice. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
COMPARISON OF PLANT AND ANIMAL CELLS SIMILARITIES IN PLANT & ANIMAL CELLS
 COMPARISON OF PLANT AND ANIMAL CELLS Cells vary widely in structure and function, even within the same organism. The human body, for example, has more than 200 different types of cells, each with a specialized
COMPARISON OF PLANT AND ANIMAL CELLS Cells vary widely in structure and function, even within the same organism. The human body, for example, has more than 200 different types of cells, each with a specialized
The chemical reactions inside cells are controlled by enzymes. Cells may be specialised to carry out a particular function.
 12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
Chapter 3. Cellular Structure and Function Worksheets. 39 www.ck12.org
 Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Science 20. Unit A: Chemical Change. Assignment Booklet A1
 Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
REVIEW for BIOLOGY UNIT TEST
 REVIEW for BIOLOGY UNIT TEST NOTE: The Unit Test will cover everything we have learned in the Biology Unit, starting from cell structures, cell division, various organ systems, disorders, organ donation,
REVIEW for BIOLOGY UNIT TEST NOTE: The Unit Test will cover everything we have learned in the Biology Unit, starting from cell structures, cell division, various organ systems, disorders, organ donation,
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
tissues are made of cells that work together, organs are )
 Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
UNIT 1 - Living Organisms and the Environment Situations. Cells
 Lesson Summaries HUMAN AND SOCIAL BIOLOGY UNIT 1 - Living Organisms and the Environment Situations Lesson 2 Cells OBJECTIVES At the end of this lesson you will be able to: a) Describe the structure of
Lesson Summaries HUMAN AND SOCIAL BIOLOGY UNIT 1 - Living Organisms and the Environment Situations Lesson 2 Cells OBJECTIVES At the end of this lesson you will be able to: a) Describe the structure of
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 6. Solution, Acids and Bases
 Chapter 6 Solution, Acids and Bases Mixtures Two or more substances Heterogeneous- different from place to place Types of heterogeneous mixtures Suspensions- Large particles that eventually settle out
Chapter 6 Solution, Acids and Bases Mixtures Two or more substances Heterogeneous- different from place to place Types of heterogeneous mixtures Suspensions- Large particles that eventually settle out
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Plant and Animal Cells
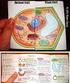 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
B2 1 Cells, Tissues and Organs
 B2 Cells, Tissues and Organs 5 minutes 5 marks Page of 7 Q. The diagram shows a bacterium. On the drawing, name the structures labelled A, B, C and D. (Total 4 marks) Q2. (a) The diagrams show cells containing
B2 Cells, Tissues and Organs 5 minutes 5 marks Page of 7 Q. The diagram shows a bacterium. On the drawing, name the structures labelled A, B, C and D. (Total 4 marks) Q2. (a) The diagrams show cells containing
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
By Casey Schmidt and Wendy Ford
 By Casey Schmidt and Wendy Ford Body systems Digestive System Circulatory System Respiratory System Excretory System Immune System Reproductive System Nervous System Muscular System Skeletal System Endocrine
By Casey Schmidt and Wendy Ford Body systems Digestive System Circulatory System Respiratory System Excretory System Immune System Reproductive System Nervous System Muscular System Skeletal System Endocrine
IIn our high tech world, one of the hottest areas of development
 Topic 1.1 Why are cells important? Key Concepts Studying cells helps us understand how organisms function. Cellular organelles work together to carry out life functions. Cellular processes enable organisms
Topic 1.1 Why are cells important? Key Concepts Studying cells helps us understand how organisms function. Cellular organelles work together to carry out life functions. Cellular processes enable organisms
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Type of Chemical Bonds
 Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
Review of the Cell and Its Organelles
 Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
ANSWER KEY. Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take!
 ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
Cellular Respiration
 Cellular Respiration Cellular Respiration Text, Diagrams, Assessments, and Link to Standards Focus Questions 1) What is cellular respiration? 2) How is cellular respiration connected to breathing? 3) If
Cellular Respiration Cellular Respiration Text, Diagrams, Assessments, and Link to Standards Focus Questions 1) What is cellular respiration? 2) How is cellular respiration connected to breathing? 3) If
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Part B 2. Allow a total of 15 credits for this part. The student must answer all questions in this part.
 Part B 2 Allow a total of 15 credits for this part. The student must answer all questions in this part. 51 [1] Allow 1 credit for 3 Mg(s) N 2 (g) Mg 3 N 2 (s). Allow credit even if the coefficient 1 is
Part B 2 Allow a total of 15 credits for this part. The student must answer all questions in this part. 51 [1] Allow 1 credit for 3 Mg(s) N 2 (g) Mg 3 N 2 (s). Allow credit even if the coefficient 1 is
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
The Cell Teaching Notes and Answer Keys
 The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Respiration occurs in the mitochondria in cells.
 B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Animal & Plant Cell Slides
 Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Membrane Structure and Function
 Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
7 Answers to end-of-chapter questions
 7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
7 Answers to end-of-chapter questions Multiple choice questions 1 B 2 B 3 A 4 B 5 A 6 D 7 C 8 C 9 B 10 B Structured questions 11 a i Maintenance of a constant internal environment within set limits i Concentration
MCAS Biology. Review Packet
 MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
(1) e.g. H hydrogen that has lost 1 electron c. anion - negatively charged atoms that gain electrons 16-2. (1) e.g. HCO 3 bicarbonate anion
 GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* CHEMISTRY 0620/03 Paper 3 Theory (Core) For Examination from 2016 SPECIMEN PAPER 1 hour
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* CHEMISTRY 0620/03 Paper 3 Theory (Core) For Examination from 2016 SPECIMEN PAPER 1 hour
Video Links: Differences Between Plant and Animal Cells http://www.youtube.com/watch?v=mwz4ptp_qeu
 Comparing Animal and Plant Cells by Annie Plant and animal cells are known as Eukaryotic cells which contain a nucleus and other genetic material enclosed within membranes. (Science Daily, n.d.) The primary
Comparing Animal and Plant Cells by Annie Plant and animal cells are known as Eukaryotic cells which contain a nucleus and other genetic material enclosed within membranes. (Science Daily, n.d.) The primary
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Chapter 5 Organelles. Lesson Objectives List the organelles of the cell and their functions. Distinguish between plant and animal cells.
 Chapter 5 Organelles Lesson Objectives List the organelles of the cell and their functions. Distinguish between plant and animal cells. Check Your Understanding What is a cell? How do we visualize cells?
Chapter 5 Organelles Lesson Objectives List the organelles of the cell and their functions. Distinguish between plant and animal cells. Check Your Understanding What is a cell? How do we visualize cells?
B2 Revision. Subject Module Date Biology B2 13 TH May (am)
 B2 Revision Subject Module Date Biology B2 13 TH May (am) Useful websites www.aqa.org.uk This website contains the specifications that we follow and also has a large number of past papers and mark schemes
B2 Revision Subject Module Date Biology B2 13 TH May (am) Useful websites www.aqa.org.uk This website contains the specifications that we follow and also has a large number of past papers and mark schemes
Cell Unit Practice Test #1
 ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Fifth Grade Cells: Structures and Processes Assessment
 Fifth Grade Cells: Structures and Processes Assessment 1a. All living things are made up of. a. cells b. tissues c. organisms d. systems 1b. All living things are made up of. 1c. Explain what cells are
Fifth Grade Cells: Structures and Processes Assessment 1a. All living things are made up of. a. cells b. tissues c. organisms d. systems 1b. All living things are made up of. 1c. Explain what cells are
CHAPTER 2 : CELL AS THE BASIC UNIT OF LIFE
 CHAPTER 2 : CELL AS THE BASIC UNIT OF LIFE Parts of microscope : An instrument that magnifies minute objects so they can be seen easily. It is one of the most important tools of science. Physicians and
CHAPTER 2 : CELL AS THE BASIC UNIT OF LIFE Parts of microscope : An instrument that magnifies minute objects so they can be seen easily. It is one of the most important tools of science. Physicians and
Cells and Systems Unit 2 Test
 Cells and Systems Unit 2 Test Student Name Class 1. Characteristics of living organisms include all of the following, EXCEPT... A. they need energy and produce wastes B. they reproduce and grow C. they
Cells and Systems Unit 2 Test Student Name Class 1. Characteristics of living organisms include all of the following, EXCEPT... A. they need energy and produce wastes B. they reproduce and grow C. they
Introduction to Animal Systems
 Human Body Systems Introduction to Animal Systems Recurring Themes in Biology 1. Correlation between structure and function( seen at many levels) 2. Life is organized at many levels from Smallest ----
Human Body Systems Introduction to Animal Systems Recurring Themes in Biology 1. Correlation between structure and function( seen at many levels) 2. Life is organized at many levels from Smallest ----
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
ATOMS. Multiple Choice Questions
 Chapter 3 ATOMS AND MOLECULES Multiple Choice Questions 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv)
Chapter 3 ATOMS AND MOLECULES Multiple Choice Questions 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv)
Science Standard Articulated by Grade Level Strand 5: Physical Science
 Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Chapter Test A. Elements, Compounds, and Mixtures MULTIPLE CHOICE. chemically combined? MIXs2 a. element b. compound c. mixture d.
 Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
The Living Cell from the Biology: The Science of Life Series. Pre-Test
 1 Pre-Test Directions: Answer each question TRUE OR FALSE. 1. The instructions for making proteins are stored in molecules of DNA. 2. Proteins are made in the nucleus. 3. All cells are surrounded by a
1 Pre-Test Directions: Answer each question TRUE OR FALSE. 1. The instructions for making proteins are stored in molecules of DNA. 2. Proteins are made in the nucleus. 3. All cells are surrounded by a
Chapter 4: A Tour of the Cell. 1. Cell Basics. Limits to Cell Size. 1. Cell Basics. 2. Prokaryotic Cells. 3. Eukaryotic Cells
 Chapter 4: A Tour of the Cell 1. Cell Basics 2. Prokaryotic Cells 3. Eukaryotic Cells 1. Cell Basics Limits to Cell Size There are 2 main reasons why cells are so small: If cells get too large: 1) there
Chapter 4: A Tour of the Cell 1. Cell Basics 2. Prokaryotic Cells 3. Eukaryotic Cells 1. Cell Basics Limits to Cell Size There are 2 main reasons why cells are so small: If cells get too large: 1) there
RAD 223. Radiography physiology. Lecture Notes. First lecture: Cell and Tissue
 RAD 223 Radiography physiology Lecture Notes First lecture: Cell and Tissue Physiology: the word physiology derived from a Greek word for study of nature. It is the study of how the body and its part work
RAD 223 Radiography physiology Lecture Notes First lecture: Cell and Tissue Physiology: the word physiology derived from a Greek word for study of nature. It is the study of how the body and its part work
Sugar or Salt? Ionic and Covalent Bonds
 Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
7 TH GRADE FINAL EXAM PRACTICE TEST. Part I: Cells. 1. The cell grows to its mature size during. a. mitosis b. prophase c. telophase d.
 7 TH GRADE FINAL EXAM PRACTICE TEST Part I: Cells 1. The cell grows to its mature size during a. mitosis b. prophase c. telophase d. interphase 2. The final stage of the cell cycle is called a. interphase
7 TH GRADE FINAL EXAM PRACTICE TEST Part I: Cells 1. The cell grows to its mature size during a. mitosis b. prophase c. telophase d. interphase 2. The final stage of the cell cycle is called a. interphase
Composition of nucleus. Priority Vocabulary: Electron, Proton, Neutron, Nucleus, Isotopes, Atomic Number, Atomic Mass, Element, Electron Shell,
 Lake County, Lakeview, 9 th grade, Physical Science, Brent Starr Standard: H1P1 Explain how atomic structure is related to the properties of elements and their position in the Periodic Table. Explain how
Lake County, Lakeview, 9 th grade, Physical Science, Brent Starr Standard: H1P1 Explain how atomic structure is related to the properties of elements and their position in the Periodic Table. Explain how
EXAMPLE EXERCISE 4.1 Change of Physical State
 EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
C E L L O. Recommended Age: 6 years-8 years Time: 45 minutes prep, additional 3 hours for Jello to set
 C E L L O Recommended Age: 6 years-8 years Time: 45 minutes prep, additional 3 hours for Jello to set The smallest and simplest unit of life is called a cell. Cells are divided into two types of cells
C E L L O Recommended Age: 6 years-8 years Time: 45 minutes prep, additional 3 hours for Jello to set The smallest and simplest unit of life is called a cell. Cells are divided into two types of cells
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Cells, tissues and organs
 Chapter 8: Cells, tissues and organs Cells: building blocks of life Living things are made of cells. Many of the chemical reactions that keep organisms alive (metabolic functions) take place in cells.
Chapter 8: Cells, tissues and organs Cells: building blocks of life Living things are made of cells. Many of the chemical reactions that keep organisms alive (metabolic functions) take place in cells.
Biology 101 Chapter 4 Cells as the Basic Unit of Life. The Cell Theory Major Contributors: Galileo = first observations made with a microscope
 Biology 101 Chapter 4 Cells as the Basic Unit of Life The Cell Theory Major Contributors: Galileo = first observations made with a microscope Robert Hooke = first to observe small compartments in dead
Biology 101 Chapter 4 Cells as the Basic Unit of Life The Cell Theory Major Contributors: Galileo = first observations made with a microscope Robert Hooke = first to observe small compartments in dead
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
Multiple Choice Questions
 Chapter 5 THE FUNDAMENTAL UNIT OF LIFE Multiple Choice Questions 1. Which of the following can be made into crystal? (a) A Bacterium (b) An Amoeba (c) A Virus (d) A Sperm 2. A cell will swell up if (a)
Chapter 5 THE FUNDAMENTAL UNIT OF LIFE Multiple Choice Questions 1. Which of the following can be made into crystal? (a) A Bacterium (b) An Amoeba (c) A Virus (d) A Sperm 2. A cell will swell up if (a)
Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test
 Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test NAME Section 7.1 The Mole: A Measurement of Matter A. What is a mole? 1. Chemistry is a quantitative science. What does this term mean?
Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test NAME Section 7.1 The Mole: A Measurement of Matter A. What is a mole? 1. Chemistry is a quantitative science. What does this term mean?
Chapter 5, Lesson 3 Why Does Water Dissolve Salt?
 Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
ANSWER KEY. Acids, Bases, and Solutions. Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries,
 Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter 6 Assessment. Name: Class: Date: ID: A. Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
PLANT AND ANIMAL CELL ORGANELLES
 reflect The heart is an example of an organ. Think for a minute about your body. It s organized into parts that perform specific functions. For example, your heart functions to help transport materials
reflect The heart is an example of an organ. Think for a minute about your body. It s organized into parts that perform specific functions. For example, your heart functions to help transport materials
DNA, RNA, Protein synthesis, and Mutations. Chapters 12-13.3
 DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
