ELECTRONIC DEVICES MENJANA MINDA KREATIF DAN INOVATIF
|
|
|
- Molly Carr
- 7 years ago
- Views:
Transcription
1 INTRODUCTION TO ELECTRONIC DEVICES MENJANA MINDA KREATIF DAN INOVATIF
2 Introduction What is Electronics? Electronic Devices? Electronic Systems? introduction
3 Electronics: The branch of physics that deals with the emission and effects of electrons ; and the use of electronic devices. Science of the motion of charges in a gas, vacuum or semiconductor.
4 Electronic devices: An electronic building block packaged in a discrete form with two or more connecting leads or metallic pads. Components are connected together to create an electronic circuit with a particular function. E.g.: an amplifier, radio receiver, or oscillator. Active components are sometimes called devices.
5 Electronic systems: Composed of subsystems or electronic circuits, which may include amplifiers signal sources, power supplies etc E.g.: Laptop, DVD players, ipod, PDA, mobile phones
6 What we are learning? Electronic Devices: Diodes Transistor (BJT) Transistor (FET)
7 CHAPTER 1 Semiconductor Material
8 Semiconductor Material 1.1 Atomic Structure Materials Classification 1.3 Energy Band 1.4 Covalent Bonds Conduction in Semiconductors 1.6 p type and n type semiconductors
9 11Atomic 1.1 structure All matters on earth made of atoms (made up of elements or combination of elements). All atoms consist of electrons, protons, and neutrons. An atom is the smallest particle of an element that retains the characteristics of that element.
10 Bohr s Atomic Structuret According to Bohr, atoms have a planetary structure that consists of a central nucleus, surrounded d by orbiting electrons. Nucleus contains protons and neutrons. Electrons (-) orbits the nucleus Nucleus : Protons (+) Neutrons (neutral)
11 Cont.. Each type of atom has a certain number of electrons and protons that distinguishes it from atoms of other elements. H: 1 proton, 1 He: 2 proton, 2 electron neutron, 2 electron
12 Helium Atom Each electron has its own orbit which corresponds to different energy levels. Similar energy levels (orbits) are grouped into energy bands called shells.
13 Electron Shells and Orbits Each electron travels on its own orbit. The different orbit corresponds to different energy level. In an atom, orbits are grouped into energy bands known as shells. Each shell has a fixed max no. of electrons at allowed energy levelsl
14 The Energy Band Concept The number of electrons in shell 1-4 can be calculated as: N e = 2n 2 Electron orbits the nucleus at certain distances The outermost t shell is called the valence shell. Electrons on this shell are called valence electrons
15 Valence Electrons The outermost shell is called the valence shell and electrons at this layer are called valence electrons. Valence electrons contribute to chemical reactions and bonding within the structure of a material and determine its electrical properties. Maximum number of valence electron is 8. An atom is stable if it has 8 valence electrons; eg: Neon (10), Argon (18), Krypton (36).
16 Cont.. The number of valence electrons determines the ability of material to conduct current. The less complete a shell is filled to capacity (max 8) the more conductive the material is. If an atom of a material carries 1 to 3 valence electrons, the material is a conductor because the atom has more tendency to lose its valence electrons which become free electrons. (Copper, Aluminium)
17 Cont If an atom of a material carries 5 to 8 valence electron, the material is an insulator because the atom has more tendency to gain free electrons to complete its shell. (Argon, Neon) If an atom of a material carries 4 valence electrons, the material is a semiconductor because it is not easy for the atom to lose or gain any electrons. (Silicon, Germanium)
18 Cont A Silicon atom has 4 electrons in its valence ring. This makes it a semiconductor. A Copper atom has only 1electron in its valence ring. This makes it a good conductor.
19 Semiconductor Material 1.1 Atomic Structure Materials Classification Insulators, Conductors, and Semiconductor 1.3 Energy Band Covalent Bonds 1.5 Conduction in Semiconductors 1.6 p type and n type semiconductors
20 Materials Classification Semiconductors, conductors and insulators How are they different? What is the difference between silicon and germanium semiconductor? Why is Si more widely used compared to Ge?
21 Cont.. Conduction band and valence electrons determines the electrical properties of a material.
22 Conductor A conductor is a material that easily conducts electrical current. The best conductors are (with one valence electron) eg: copper, silver, gold and aluminium. In a conductor, the valence band and the conductor band overlaps ( 0.01 ev). Only a little energy or voltage is needed for the electron to jump into conduction band.
23 Insulator An insulator is a material that does not conduct electrical current under normal circumstances. Energy gap in an insulator is very wide ( 6eV). Valence electron requires a large electric field to gain enough energy to jump into conduction band.
24 Semiconductor A semiconductor is between a conductor and insulator. A semiconductor in its pure (intrinsic) state is neither a good conductor nor a good insulator. Most common semiconductor are silicon, germanium and carbon. A semiconductor has a conductivity level between the extreme of an insulator and a conductor.
25 Cont.. Compare silicon and germanium. Which one is better and why?
26 Semiconductor Material 1.1 Atomic Structure 1.2 Materials Classification 1.3 Energy Band - Energy Level - Valence Band, Conduction Band, Energy Gap 1.4 Covalent Bonds 1.5 Conduction in Semiconductors 1. 6 p type and n type semiconductors
27 13Energy 1.3 Band ENERGY in an electron is of two types - kinetic (energy of motion) and potential (energy of position). Each material has its own set of permissible energy levels for the electrons in its atomic structure. Energy level in an atom is measured in ELECTRON VOLT (ev): 1 ev = x J Electrons that orbits within an energy level will have similar amount of energy.
28 Energy Level More energy Less energy Energy increases as the distance from the nucleus increases.
29 Energy Gap When an electron acquires sufficient additional energy, it can leave the valence shell and become a free electron and exists in the conduction band. The energy difference between the valence band and conduction band is called the energy gap. Energy gap: the amount of energy that a valence electron must have to jump into the conduction band. Once in conduction band, the electron is free to move throughout the material and is not tied to any atom.
30 Cont.. Energy diagrams for the three types of materials:
31 Semiconductor Material 1.1 Atomic Structure Materials Classification 1.3 Energy Band 1.4 Covalent Bonds 1.5 Conduction in Semiconductors 1.6 p type and n type semiconductors
32 Covalent Bonds In a pure silicon or germanium crystal, the four valence electrons of one atom form a bonding arrangement with four adjoining atoms. This bonding of atoms, strengthened by the sharing of electrons, is called covalent bonding is a method by which atoms complete their valence shell by sharing valence electrons with other atoms
33 Cont.. The atoms are electrically stable because their valence shells are complete.
34 Cont.. Certain atoms will combine in this way to form a crystal structure. Silicon and Germanium atoms combine in this way in their intrinsic i i or pure state. t Covalent Bonds in a silicon crystal
35 Review 1. In the atomic structure of a semiconductor, within which energy band do free electrons exist? Within which energy band do valence electrons exist? 2. Why is current established more easily in a semiconductor than in an insulator?
36 Previously.. Diodes and transistors are all made of semiconductor material. Bohr model: electrons circle the nucleus. (planetary-type) Atomic structure of a material determines its ability to conduct or insulate. The orbit paths (energy band) of the electrons surrounding the nucleus are called shells. Each shell has a defined number of electrons it will hold. The outer shell is called the valence shell. The valence shell determines the ability of material to conduct current. Energy gap: difference in energy levels between the conduction and valence bands. (forbidden band) Covalent bonding is a bonding of two or more atoms by the interaction of their valence electrons to form a crystal structure.
37 Semiconductor Material 1.1 Atomic Structure Materials Classification 1.3 Energy Band 1.4 Covalent Bonds Conduction in Semiconductors 1.6 p type and n type semiconductors
38 15C 1.5 Conduction in Semiconductors How is current produced in a semiconductor?
39 Conduction in Semiconductors Unexcited (no external energy) silicon atom. (0 K)
40 Electron-Hole Pair Excited silicon atom. (Room temperature) t
41 Conduction Electrons and Holes When an intrinsic silicon crystal gains sufficient heat (thermal energy), some valence electrons could break their covalent bonds to jump the gap into conduction band, becoming free electrons. Free electrons are also called conduction electrons, (negative charge). This vacancy in the valence band is called a hole (positive charge). For every electron raised to the conduction band, there is 1 hole in the valence band creating electron-hole pair. When a conduction electron loses energy and falls back into a When a conduction electron loses energy and falls back into a hole, this is called recombination.
42 Cont.. There is an equal number of holes in the valence band created when these electrons jump into the conduction band.
43 Cont.. Free electrons tend to search for positive holes, while holes tends to attract electrons to reform covalent bonds. (Opposites attract) With the existence of holes and electrons, current can be produced when a voltage is applied across the terminals.
44 Current In Semiconductor There are 2 types of current flow: Electron current Hole current
45 Electron Current When voltage is applied across a piece of intrinsic silicon, free electrons are easily attracted towards the positive end. This movement caused the electron current.
46 Hole Current Holes created when valence electrons escaped. Valence electrons remaining at valence band are still attached to their atoms. They could only move into a nearby hole with little change in its energy level, thus leaving another hole behind. The hole appears to move from one place to the next in the crystal structure. t This movement of hole is called hole current.
47 Cont... When a valence electron moves left to right to fill a hole while leaving another hole behind, the hole has effectively moved from right to left. Gray arrows indicate effective movement of a hole. FIGURE 1 13 Hole current in intrinsic silicon.
48 Cont... Electron current - + Hole current The direction of hole current is normally used as the The direction of hole current is normally used as the conventional current direction.
49 Electron and Hole Current Electron Current Movement of free electrons in the conduction band. Electron posses the conduction band. Hole Current Movement of valence band electron in the valence band to fill up the hole left when an electron moves into the conduction band. Electron posses the valence band energy.
50 Review 1. At room temperature, an intrinsic semiconductor has some holes in it due to. 2. The merging g of a free electron and a hole is called. 3. electrons are responsible for current in a material. 4. Hole current occurs at the level.
51 Semiconductor Material 1.1 Atomic Structure Materials Classification 1.3 Energy Band 1.4 Covalent Bonds 1.5 Conduction in Semiconductors 1.6 p type and n type semiconductors -Doping -majority and minority carriers
52 Doping A process of adding impurities to intrinsic (pure) Silicon and Germanium to improve the conductivity of the semiconductor material. 2 types of semiconductor material that are subjected to doping process which are: N-type P-type
53 Cont.. 2 types of semiconductor material that are subjected to doping process which are: N-type P-type tpe 2 types of elements used doping are: Trivalent element with 3 valence electrons Pentavalent element with 5 valence electrons
54 N-type Semiconductor Pentavalent impurity atoms are added Arsenic (As), phosphorus (P),bismuth (Bi), Pentavalent also known as a donor atoms since they donate electrons. When a pentavalent atom is added to an intrinsic semiconductor, it ll readily donate it s 5 th electron, as a result becomes n-type extrinsic semiconductor.
55 N-type Semiconductor Each pentavalent atom forms covalent bond with 4 adjacent Si atom Since only 4 electrons are needed to form a covalent bond, leaving an extra electron becomes a free electron when each pentavalent atom is added d In n-type material a electrons eecto sae are majority carrier, and holes the minority carrier
56 P-type Semiconductor Trivalent ( with 3 valence electrons) impurity atoms are added Aluminium (Al), boron (B), indium (In),gallium (Ga) Trivalent also known as a acceptor atom since they accpet electrons. When a trivalent atom is added to an intrinsic semiconductor, it ll readily accept free electron, as a result becomes p-type extrinsic semiconductor.
57 P-type Semiconductor Each trivalent atom forms covalent bond with 4 adjacent Si atom. Since 4 electrons are needed to form a covalent bond, causes an existence of hole in the covalent bonding. It also causes a lack of valence electrons in the B atoms In p-type material holes are majority carrier, and electron the minority carrier
58 Review.. 1. What s the difference between a pentavalent atom and a trivalent t atom? 2. By what process are the majority carriers produced? 3. By what process are the minority carriers produced? 4. What s the difference between intrinsic and extrinsic semiconductors?
Semiconductors, diodes, transistors
 Semiconductors, diodes, transistors (Horst Wahl, QuarkNet presentation, June 2001) Electrical conductivity! Energy bands in solids! Band structure and conductivity Semiconductors! Intrinsic semiconductors!
Semiconductors, diodes, transistors (Horst Wahl, QuarkNet presentation, June 2001) Electrical conductivity! Energy bands in solids! Band structure and conductivity Semiconductors! Intrinsic semiconductors!
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Solid-State Physics: The Theory of Semiconductors (Ch. 10.6-10.8) SteveSekula, 30 March 2010 (created 29 March 2010)
 Modern Physics (PHY 3305) Lecture Notes Modern Physics (PHY 3305) Lecture Notes Solid-State Physics: The Theory of Semiconductors (Ch. 10.6-10.8) SteveSekula, 30 March 2010 (created 29 March 2010) Review
Modern Physics (PHY 3305) Lecture Notes Modern Physics (PHY 3305) Lecture Notes Solid-State Physics: The Theory of Semiconductors (Ch. 10.6-10.8) SteveSekula, 30 March 2010 (created 29 March 2010) Review
The Physics of Energy sources Renewable sources of energy. Solar Energy
 The Physics of Energy sources Renewable sources of energy Solar Energy B. Maffei Bruno.maffei@manchester.ac.uk Renewable sources 1 Solar power! There are basically two ways of using directly the radiative
The Physics of Energy sources Renewable sources of energy Solar Energy B. Maffei Bruno.maffei@manchester.ac.uk Renewable sources 1 Solar power! There are basically two ways of using directly the radiative
Crystalline solids. A solid crystal consists of different atoms arranged in a periodic structure.
 Crystalline solids A solid crystal consists of different atoms arranged in a periodic structure. Crystals can be formed via various bonding mechanisms: Ionic bonding Covalent bonding Metallic bonding Van
Crystalline solids A solid crystal consists of different atoms arranged in a periodic structure. Crystals can be formed via various bonding mechanisms: Ionic bonding Covalent bonding Metallic bonding Van
Understanding the p-n Junction by Dr. Alistair Sproul Senior Lecturer in Photovoltaics The Key Centre for Photovoltaic Engineering, UNSW
 Understanding the p-n Junction by Dr. Alistair Sproul Senior Lecturer in Photovoltaics The Key Centre for Photovoltaic Engineering, UNSW The p-n junction is the fundamental building block of the electronic
Understanding the p-n Junction by Dr. Alistair Sproul Senior Lecturer in Photovoltaics The Key Centre for Photovoltaic Engineering, UNSW The p-n junction is the fundamental building block of the electronic
3. Diodes and Diode Circuits. 3. Diodes and Diode Circuits TLT-8016 Basic Analog Circuits 2005/2006 1
 3. Diodes and Diode Circuits 3. Diodes and Diode Circuits TLT-8016 Basic Analog Circuits 2005/2006 1 3.1 Diode Characteristics Small-Signal Diodes Diode: a semiconductor device, which conduct the current
3. Diodes and Diode Circuits 3. Diodes and Diode Circuits TLT-8016 Basic Analog Circuits 2005/2006 1 3.1 Diode Characteristics Small-Signal Diodes Diode: a semiconductor device, which conduct the current
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Lecture 2 - Semiconductor Physics (I) September 13, 2005
 6.012 - Microelectronic Devices and Circuits - Fall 2005 Lecture 2-1 Lecture 2 - Semiconductor Physics (I) September 13, 2005 Contents: 1. Silicon bond model: electrons and holes 2. Generation and recombination
6.012 - Microelectronic Devices and Circuits - Fall 2005 Lecture 2-1 Lecture 2 - Semiconductor Physics (I) September 13, 2005 Contents: 1. Silicon bond model: electrons and holes 2. Generation and recombination
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Theory of Transistors and Other Semiconductor Devices
 Theory of Transistors and Other Semiconductor Devices 1. SEMICONDUCTORS 1.1. Metals and insulators 1.1.1. Conduction in metals Metals are filled with electrons. Many of these, typically one or two per
Theory of Transistors and Other Semiconductor Devices 1. SEMICONDUCTORS 1.1. Metals and insulators 1.1.1. Conduction in metals Metals are filled with electrons. Many of these, typically one or two per
Solid State Detectors = Semi-Conductor based Detectors
 Solid State Detectors = Semi-Conductor based Detectors Materials and their properties Energy bands and electronic structure Charge transport and conductivity Boundaries: the p-n junction Charge collection
Solid State Detectors = Semi-Conductor based Detectors Materials and their properties Energy bands and electronic structure Charge transport and conductivity Boundaries: the p-n junction Charge collection
Yrd. Doç. Dr. Aytaç Gören
 H2 - AC to DC Yrd. Doç. Dr. Aytaç Gören ELK 2018 - Contents W01 Basic Concepts in Electronics W02 AC to DC Conversion W03 Analysis of DC Circuits W04 Transistors and Applications (H-Bridge) W05 Op Amps
H2 - AC to DC Yrd. Doç. Dr. Aytaç Gören ELK 2018 - Contents W01 Basic Concepts in Electronics W02 AC to DC Conversion W03 Analysis of DC Circuits W04 Transistors and Applications (H-Bridge) W05 Op Amps
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
electron configuration
 electron configuration Electron Configuration Knowing the arrangement of electrons in atoms will better help you understand chemical reactivity and predict an atom s reaction behavior. We know when n=1
electron configuration Electron Configuration Knowing the arrangement of electrons in atoms will better help you understand chemical reactivity and predict an atom s reaction behavior. We know when n=1
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
ELECTRICAL CONDUCTION
 Chapter 12: Electrical Properties Learning Objectives... How are electrical conductance and resistance characterized? What are the physical phenomena that distinguish conductors, semiconductors, and insulators?
Chapter 12: Electrical Properties Learning Objectives... How are electrical conductance and resistance characterized? What are the physical phenomena that distinguish conductors, semiconductors, and insulators?
COURSE: PHYSICS DEGREE: COMPUTER ENGINEERING year: 1st SEMESTER: 1st
 COURSE: PHYSICS DEGREE: COMPUTER ENGINEERING year: 1st SEMESTER: 1st WEEKLY PROGRAMMING WEE K SESSI ON DESCRIPTION GROUPS GROUPS Special room for LECTU PRAC session RES TICAL (computer classroom, audiovisual
COURSE: PHYSICS DEGREE: COMPUTER ENGINEERING year: 1st SEMESTER: 1st WEEKLY PROGRAMMING WEE K SESSI ON DESCRIPTION GROUPS GROUPS Special room for LECTU PRAC session RES TICAL (computer classroom, audiovisual
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
2. John Dalton did his research work in which of the following countries? a. France b. Greece c. Russia d. England
 CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
CHAPTER 3 1. Which combination of individual and contribution is not correct? a. Antoine Lavoisier - clarified confusion over cause of burning b. John Dalton - proposed atomic theory c. Marie Curie - discovered
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
BASIC ELECTRONICS TRANSISTOR THEORY. December 2011
 AM 5-204 BASIC ELECTRONICS TRANSISTOR THEORY December 2011 DISTRIBUTION RESTRICTION: Approved for Public Release. Distribution is unlimited. DEPARTMENT OF THE ARMY MILITARY AUXILIARY RADIO SYSTEM FORT
AM 5-204 BASIC ELECTRONICS TRANSISTOR THEORY December 2011 DISTRIBUTION RESTRICTION: Approved for Public Release. Distribution is unlimited. DEPARTMENT OF THE ARMY MILITARY AUXILIARY RADIO SYSTEM FORT
ELECTRICAL FUNDAMENTALS
 General Electricity is a form of energy called electrical energy. It is sometimes called an "unseen" force because the energy itself cannot be seen, heard, touched, or smelled. However, the effects of
General Electricity is a form of energy called electrical energy. It is sometimes called an "unseen" force because the energy itself cannot be seen, heard, touched, or smelled. However, the effects of
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Student Exploration: Electron Configuration
 Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
Chapter 7. Electron Structure of the Atom. Chapter 7 Topics
 Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
PERIODIC TABLE. reflect
 reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
Noble Gases. Outline Nobel Gas Elements Radon and Health Chemistry Homework
 Radon and Other Noble Gases The elements in the last column of the periodic table are all very stable, mono-atomic gases. Until 1962, they were called inert gases because they did not react with other
Radon and Other Noble Gases The elements in the last column of the periodic table are all very stable, mono-atomic gases. Until 1962, they were called inert gases because they did not react with other
Conduction in Semiconductors
 Chapter 1 Conduction in Semiconductors 1.1 Introduction All solid-state devices, e.g. diodes and transistors, are fabricated from materials known as semiconductors. In order to understand the operation
Chapter 1 Conduction in Semiconductors 1.1 Introduction All solid-state devices, e.g. diodes and transistors, are fabricated from materials known as semiconductors. In order to understand the operation
Be (g) Be + (g) + e - O (g) O + (g) + e -
 2.13 Ionisation Energies Definition :First ionisation energy The first ionisation energy is the energy required when one mole of gaseous atoms forms one mole of gaseous ions with a single positive charge
2.13 Ionisation Energies Definition :First ionisation energy The first ionisation energy is the energy required when one mole of gaseous atoms forms one mole of gaseous ions with a single positive charge
Diodes and Transistors
 Diodes What do we use diodes for? Diodes and Transistors protect circuits by limiting the voltage (clipping and clamping) turn AC into DC (voltage rectifier) voltage multipliers (e.g. double input voltage)
Diodes What do we use diodes for? Diodes and Transistors protect circuits by limiting the voltage (clipping and clamping) turn AC into DC (voltage rectifier) voltage multipliers (e.g. double input voltage)
Electronics Technology Fundamentals
 Lindem 11. jan 09 Electronics Technology Fundamentals Chapter 1 Principles of Electricity 1 1.1 The Starting Point Atomic Structure Atom smallest particle of matter that retains the physical characteristics
Lindem 11. jan 09 Electronics Technology Fundamentals Chapter 1 Principles of Electricity 1 1.1 The Starting Point Atomic Structure Atom smallest particle of matter that retains the physical characteristics
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D
 Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PS-6.2 Explain the factors that determine potential and kinetic energy and the transformation of one to the other.
 PS-6.1 Explain how the law of conservation of energy applies to the transformation of various forms of energy (including mechanical energy, electrical energy, chemical energy, light energy, sound energy,
PS-6.1 Explain how the law of conservation of energy applies to the transformation of various forms of energy (including mechanical energy, electrical energy, chemical energy, light energy, sound energy,
18.2 Comparing Atoms. Atomic number. Chapter 18
 As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
As you know, some substances are made up of only one kind of atom and these substances are called elements. You already know something about a number of elements you ve heard of hydrogen, helium, silver,
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
WAVES AND ELECTROMAGNETIC RADIATION
 WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
5.4 Trends in the Periodic Table
 5.4 Trends in the Periodic Table Think about all the things that change over time or in a predictable way. For example, the size of the computer has continually decreased over time. You may become more
5.4 Trends in the Periodic Table Think about all the things that change over time or in a predictable way. For example, the size of the computer has continually decreased over time. You may become more
Unit 3.2: The Periodic Table and Periodic Trends Notes
 Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Unit 3.2: The Periodic Table and Periodic Trends Notes The Organization of the Periodic Table Dmitri Mendeleev was the first to organize the elements by their periodic properties. In 1871 he arranged the
Semiconductor I. Semiconductors. germanium. silicon
 Basic Electronics Semiconductor I Materials that permit flow of electrons are called conductors (e.g., gold, silver, copper, etc.). Materials that block flow of electrons are called insulators (e.g., rubber,
Basic Electronics Semiconductor I Materials that permit flow of electrons are called conductors (e.g., gold, silver, copper, etc.). Materials that block flow of electrons are called insulators (e.g., rubber,
Electron Configurations, Isoelectronic Elements, & Ionization Reactions. Chemistry 11
 Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
Electron Configurations, Isoelectronic Elements, & Ionization Reactions Chemistry 11 Note: Of the 3 subatomic particles, the electron plays the greatest role in determining the physical and chemical properties
Bohr s Model of the Atom
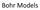 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Multi-electron atoms
 Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Section 11.3 Atomic Orbitals Objectives
 Objectives 1. To learn about the shapes of the s, p and d orbitals 2. To review the energy levels and orbitals of the wave mechanical model of the atom 3. To learn about electron spin A. Electron Location
Objectives 1. To learn about the shapes of the s, p and d orbitals 2. To review the energy levels and orbitals of the wave mechanical model of the atom 3. To learn about electron spin A. Electron Location
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Introduction to Nuclear Physics
 Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Introduction to Nuclear Physics 1. Atomic Structure and the Periodic Table According to the Bohr-Rutherford model of the atom, also called the solar system model, the atom consists of a central nucleus
Photovoltaics photo volt Photovoltaic Cells Crystalline Silicon Cells Photovoltaic Systems
 1 Photovoltaics Photovoltaic (PV) materials and devices convert sunlight into electrical energy, and PV cells are commonly known as solar cells. Photovoltaics can literally be translated as light-electricity.
1 Photovoltaics Photovoltaic (PV) materials and devices convert sunlight into electrical energy, and PV cells are commonly known as solar cells. Photovoltaics can literally be translated as light-electricity.
 Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Unit 12 Practice Test
 Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
History of the Atom & Atomic Theory
 Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Chapter 5 History of the Atom & Atomic Theory You re invited to a Thinking Inside the Box Conference Each group should nominate a: o Leader o Writer o Presenter You have 5 minutes to come up with observations
Characteristic curves of a solar cell
 Related Topics Semi-conductor, p-n junction, energy-band diagram, Fermi characteristic energy level, diffusion potential, internal resistance, efficiency, photo-conductive effect, acceptors, donors, valence
Related Topics Semi-conductor, p-n junction, energy-band diagram, Fermi characteristic energy level, diffusion potential, internal resistance, efficiency, photo-conductive effect, acceptors, donors, valence
FUNDAMENTAL PROPERTIES OF SOLAR CELLS
 FUNDAMENTAL PROPERTIES OF SOLAR CELLS January 31, 2012 The University of Toledo, Department of Physics and Astronomy SSARE, PVIC Principles and Varieties of Solar Energy (PHYS 4400) and Fundamentals of
FUNDAMENTAL PROPERTIES OF SOLAR CELLS January 31, 2012 The University of Toledo, Department of Physics and Astronomy SSARE, PVIC Principles and Varieties of Solar Energy (PHYS 4400) and Fundamentals of
ANSWER KEY. Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take!
 ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
Trends of the Periodic Table Basics
 Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Basics Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Atomic structure. Resources and methods for learning about these subjects (list a few here, in preparation for your research):
 Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Atomic structure This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/1.0/,
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Voltage, Current, Resistance, Capacitance and Inductance
 Voltage, Current, Resistance, Capacitance and Inductance Really basic electrical engineering. 1 Electricity and conductors Electricity is the movement of electrons. Electrons move easily through a conductor
Voltage, Current, Resistance, Capacitance and Inductance Really basic electrical engineering. 1 Electricity and conductors Electricity is the movement of electrons. Electrons move easily through a conductor
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
Ionic and Metallic Bonding
 Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
How To Write A Periodic Table
 Spring 2008 hemistry 2000 Midterm #1A / 50 marks INSTRUTINS 1) Please read over the test carefully before beginning. You should have 5 pages of questions and a periodic table. 2) If you need extra space,
Spring 2008 hemistry 2000 Midterm #1A / 50 marks INSTRUTINS 1) Please read over the test carefully before beginning. You should have 5 pages of questions and a periodic table. 2) If you need extra space,
Sample Exercise 12.1 Calculating Packing Efficiency
 Sample Exercise 12.1 Calculating Packing Efficiency It is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is the fraction of space in a crystal
Sample Exercise 12.1 Calculating Packing Efficiency It is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is the fraction of space in a crystal
Chapter 2 Atoms, Ions, and the Periodic Table
 Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Chapter 2 Atoms, Ions, and the Periodic Table 2.1 (a) neutron; (b) law of conservation of mass; (c) proton; (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i)
Copyrighted by Gabriel Tang B.Ed., B.Sc.
 Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
FYS3410 - Vår 2015 (Kondenserte fasers fysikk) http://www.uio.no/studier/emner/matnat/fys/fys3410/v15/index.html
 FYS3410 - Vår 015 (Kondenserte fasers fysikk) http://www.uio.no/studier/emner/matnat/fys/fys3410/v15/index.html Pensum: Introduction to Solid State Physics by Charles Kittel (Chapters 1-9 and 17, 18, 0,
FYS3410 - Vår 015 (Kondenserte fasers fysikk) http://www.uio.no/studier/emner/matnat/fys/fys3410/v15/index.html Pensum: Introduction to Solid State Physics by Charles Kittel (Chapters 1-9 and 17, 18, 0,
3 Atomic Structure 15
 3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
TRENDS IN THE PERIODIC TABLE
 Noble gases Period alogens Alkaline earth metals Alkali metals TRENDS IN TE PERIDI TABLE Usual charge +1 + +3-3 - -1 Number of Valence e - s 1 3 4 5 6 7 Electron dot diagram X X X X X X X X X 8 Group 1
Noble gases Period alogens Alkaline earth metals Alkali metals TRENDS IN TE PERIDI TABLE Usual charge +1 + +3-3 - -1 Number of Valence e - s 1 3 4 5 6 7 Electron dot diagram X X X X X X X X X 8 Group 1
Chemistry: The Periodic Table and Periodicity
 Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
Chemistry: The Periodic Table and Periodicity Name: per: Date:. 1. By what property did Mendeleev arrange the elements? 2. By what property did Moseley suggest that the periodic table be arranged? 3. What
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
Course description: Introduces the student to basic electricity with an emphasis on Ohms Law.
 The following is presented for information purposes only and comes with no warranty. See http://www.bristolwatch.com/ Course Title: Basic Electricity and Ohms Law Course description: Introduces the student
The following is presented for information purposes only and comes with no warranty. See http://www.bristolwatch.com/ Course Title: Basic Electricity and Ohms Law Course description: Introduces the student
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Chemistry - Elements Electron Configurations The Periodic Table. Ron Robertson
 Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Chemistry - Elements Electron Configurations The Periodic Table Ron Robertson History of Chemistry Before 16 th Century Alchemy Attempts (scientific or otherwise) to change cheap metals into gold no real
Composition of the Atmosphere. Outline Atmospheric Composition Nitrogen and Oxygen Lightning Homework
 Molecules of the Atmosphere The present atmosphere consists mainly of molecular nitrogen (N2) and molecular oxygen (O2) but it has dramatically changed in composition from the beginning of the solar system.
Molecules of the Atmosphere The present atmosphere consists mainly of molecular nitrogen (N2) and molecular oxygen (O2) but it has dramatically changed in composition from the beginning of the solar system.
Gamma and X-Ray Detection
 Gamma and X-Ray Detection DETECTOR OVERVIEW The kinds of detectors commonly used can be categorized as: a. Gas-filled Detectors b. Scintillation Detectors c. Semiconductor Detectors The choice of a particular
Gamma and X-Ray Detection DETECTOR OVERVIEW The kinds of detectors commonly used can be categorized as: a. Gas-filled Detectors b. Scintillation Detectors c. Semiconductor Detectors The choice of a particular
Unit 2 Periodic Behavior and Ionic Bonding
 Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
