CHEM 2353 Fundamentals of Organic Chemistry. Carbohydrates. Organic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R.
|
|
|
- Robert McCormick
- 7 years ago
- Views:
Transcription
1 hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University 1 arbohydrates and Biochemistry arbohydrates are compounds of tremendous biological importance: they provide energy through oxidation they supply carbon for the synthesis of cell components they serve as a form of stored chemical energy they form part of the structures of some cells and tissues arbohydrates, along with lipids, proteins, nucleic acids, and other compounds are known as biomolecules because they are closely associated with living organisms. Biochemistry is the study of the chemistry of biomolecules and living organisms. 2
2 hapter 7 Notes lassification of arbohydrates 3 arbohydrates arbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis 2 ribose The term carbohydrate comes from the fact that when you heat sugars, you 4 get carbon and water.
3 hapter 7 Notes lasses of arbohydrates Monosaccharides contain a single polyhydroxy aldehyde or ketone unit (saccharo is Greek for sugar ) (e.g., glucose, fructose). Disaccharides consist of two monosaccharide units linked together by a covalent bond (e.g., sucrose). ligosaccharides contain from 3 to 10 monosaccharide units (e.g., raffinose). 5 lasses of arbohydrates lasses of arbohydrates Polysaccharides contain very long chains of hundreds or thousands of monosaccharide units, which may be either in straight or branched chains (e.g., cellulose, glycogen, starch). 6
4 hapter 7 Notes The Stereochemistry of arbohydrates 7 Stereoisomers Glyceraldehyde, the simplest carbohydrate, exists in two isomeric forms that are mirror images of each other: 2 L-glyceraldehyde 2 D-glyceraldehyde These forms are stereoisomers of each other. Glyceraldehyde is a chiral molecule it cannot be superimposed on its mirror image. The two mirrorimage forms of glyceraldehyde are enantiomers of each other. 8
5 hapter 7 Notes hiral arbons hiral molecules have the same relationship to each other that your left and right hands have when reflected in a mirror. Achiral objects can be superimposed on the mirror images for example, drinking glasses, spheres, and cubes. Any carbon atom which is connected to four different groups will be chiral, and will have two nonsuperimposable mirror images; it is a chiral carbon or a center of chirality. if any of the two groups on the carbon are the same, the carbon atom cannot be chiral. Many organic compounds, including carbohydrates, contain more than one chiral carbon. 9 Examples: hiral arbon Atoms Identify the chiral carbon atoms (if any) in each of the following molecules: l l Br l l l l F l
6 hapter 7 Notes Examples: hiral arbons in arbohydrates Identify the chiral carbons (if any) in the following carbohydrates: 2 erythrose glucose n Rule When a molecule has more than one chiral carbon, each carbon can possibly be arranged in either the right-hand or left-hand form, thus if there are n chiral carbons, there are 2 n possible stereoisomers. Maximum number of possible stereoisomers = 2 n 3 3 holesterol has 2 8 = 256 possible stereoisomers. (But Nature makes only one!) 12
7 hapter 7 Notes Fischer Projections Fischer projections are a convenient way to represent mirror images in two dimensions. Place the carbonyl group at or near the top and the last achiral 2 at the bottom L-glyceraldehyde 2 D-glyceraldehyde 13 Naming Stereoisomers When there is more than one chiral center in a carbohydrate, look at the chiral carbon farthest from the carbonyl group: if the hydroxy group points to right when the carbonyl is up it is the D-isomer, and when the hydroxy group points to the left, it is the L-isomer. 2 L-erythrose 2 D-erythrose 14
8 hapter 7 Notes Examples: Fischer Projections Draw Fischer projections of D and L lactic acid: 3 2 Given the structure for D-glucose, draw the structure of L- glucose: Draw Fischer projections of D and L alanine: 3 N D-glucose 15 Examples: Fischer Projections Identify the following compounds as D or L isomers, and draw their mirror images. 2 lyxose 2 2 fructose 16
9 hapter 7 Notes What s s So Great About hiral Molecules? Molecules which are enantiomers of each other have exactly the same physical properties (melting point, boiling point, index of refraction, etc.) but not their interaction with polarized light. Polarized light vibrates only in one plane; it results from passing light through a polarizing filter. 17 ptical Activity A levorotatory ( ) substance rotates polarized light to the left. [E.g., l-glucose; (-)-glucose] A dextrorotatory (+) substance rotates polarized light to the right. [E.g., d-glucose; (+)-glucose] Molecules which rotate the plane of of polarized light are optically active. Most biologically important molecules are chiral, and hence are optically active. ften, living systems contain only one of all of the possible stereochemical forms of a compound. In some cases, one form of a molecule is beneficial, and the enantiomer is a poison (e.g., thalidomide). 18
10 hapter 7 Notes Monosaccharides 19 lassification of Monosaccharides The monosaccharides are the simplest of the carbohydrates, since they contain only one polyhydroxy aldehyde or ketone unit. Monosaccharides are classified according to the number of carbon atoms they contain: No. of lass of carbons Monosaccharide 3 triose 4 tetrose 5 pentose 6 hexose The presence of an aldehyde is indicated by the prefix aldo- and a ketone by the prefix keto-. 20
11 hapter 7 Notes lassification of Monosaccharides Thus, glucose is an aldohexose (aldehyde + 6 s) and ribulose is a ketopentose (ketone + 5 s) 2 2 glucose an aldohexose 2 ribulose a ketopentose 21 Examples: lassifying Monosaccharides lassify the following monosaccharides:
12 hapter 7 Notes The Family of D-aldosesD (L-forms not shown) 2 D-glyceraldehyde Aldotriose 2 1 = 2 2 D-erythrose 2 D-threose Aldotetroses 2 2 = 4 Aldopentoses 2 3 = 8 2 D-ribose 2 D-arabinose 2 D-xylose 2 D-lyxose 23 The Family of D-aldosesD (L-forms not shown) Aldohexoses 2 4 = 16 2 D-allose 2 D-altrose 2 D-glucose 2 D-mannose 2 D-gulose 2 D-idose 2 D-galactose 2 D-talose 24
13 hapter 7 Notes The Family of D-ketosesD (L-forms not shown) 2 2 Dihydroxyacetone Ketotriose 2 0 = D-erythrulose Ketotetroses 2 1 = Ketopentoses 2 2 = 4 2 D-ribulose 2 D-xylulose 25 The Family of D-ketosesD (L-forms not shown) Ketohexoses 2 3 = 8 2 D-Psicose 2 D-Fructose 2 D-Sorbose 2 D-Tagatose 26
14 hapter 7 Notes Physical Properties of Monosaccharides Most monosaccharides have a sweet taste (fructose is sweetest; 73% sweeter than sucrose). They are solids at room temperature. They are extremely soluble in water: Despite their high molecular weights, the presence of large numbers of groups make the monosaccharides much more water soluble than most molecules of similar MW. Glucose can dissolve in minute amounts of water to make a syrup (1 g / 1 ml 2). 27 Physical Properties of Monosaccharides Table 7.2 The relative sweetness of sugars (sucrose = 1.00) Sugar Lactose Galactose Maltose Xylose Glucose Sucrose Invert sugar Fructose Relative Sweetness Type Disaccharide Monosaccharide Disaccharide Monosaccharide Monosaccharide Disaccharide Mixture of glucose and fructose Monosaccharide 28
15 hapter 7 Notes hemical Properties of Monosaccharides Monosaccharides do not usually exist in solution in their open-chain forms: an alcohol group can add into the carbonyl group in the same molecule to form a pyranose ring containing a stable cyclic hemiacetal or hemiketal. 2 2 D-glucose 2 a pyranose ring β-d-glucose β-up cyclic hemiacetals α-d-glucose α-down 29 aworth structures 2 α-d-glucose 36% 1 Glucose Anomers 1 In the pyranose form of glucose, carbon-1 is chiral, and thus two stereoisomers are possible: one in which the group points down (α-hydroxy group) and one in which the group points up (βhydroxy group). These forms are anomers of each other, and carbon-1 is called the anomeric carbon. 2 D-glucose 0.02% 2 β-d-glucose 64% 1 30
16 hapter 7 Notes Fructose Anomers Fructose closes on itself to form a furanose ring: a furanose ring D-fructose 2 β-hydroxy α-hydroxy 2 α-d-fructose β-d-fructose 31 Drawing Furanose and Pyranose Rings Monosaccharides are often represented using the aworth structures shown below for furanose and pyranose rings. The remaining groups on the ring point up or down depending on the identity of the sugar. 2 always above ring for D-saccharides 2 Furanose ring Pyranose ring 32
17 hapter 7 Notes Examples: Anomers With Friends Like These, Who Needs Anomers? Identify the structures below as being the α- or β- forms, and draw the structure of their anomers: 2 2 ribose galactose 33 xidation of Monosaccharides Aldehydes and ketones that have an group on the carbon next to the carbonyl group react with a basic solution of u 2+ (Benedict s reagent) to form a red-orange precipitate of copper(i) oxide (u 2 ). Sugars that undergo this reaction are called reducing sugars. (All of the monosaccharides are reducing sugars.) Reducing sugar + u 2+ oxidation product deep blue solution + u 2 red-orange ppt 34
18 hapter 7 Notes P Formation of Phosphate Esters 2 P 2 glucose 6-phosphate fructose 6-phosphate Phosphate esters can form at the 6-carbon of aldohexoses and aldoketoses. Phosphate esters of monosaccharides are found in the sugar-phosphate backbone of DNA and RNA, in ATP, and as intermediates in the metabolism of carbohydrates in the body Glycoside Formation The hemiacetal and hemiketal forms of monosaccharides can react with alcohols to form acetal and ketal structures called glycosides. The new carbon-oxygen bond is called the glycosidic linkage. 2 α-d-glucose methyl α-d-glycopyranoside methyl β-d-glycopyranoside 36
19 hapter 7 Notes Glycoside Formation nce the glycoside is formed, the ring can no longer open up to the open-chain form. Glycosides, therefore, are not reducing sugars. Glycoside + u 2+ NR 37 Examples: Glycoside Formation Identify the glycosidic linkage in each of the following molecules:
20 hapter 7 Notes Important Monosaccharides 2 2 β-d-ribose 2 Forms the sugar backbone of ribonucleic acid (RNA) β-d-deoxyribose Forms the sugar backbone of deoxyribonucleic acid (DNA) β-d-galactose Incorporated with glucose into lactose (milk sugar) 39 Important Monosaccharides 2 β-d-glucose Also known as dextrose and blood sugar; present in honey and fruits. Glucose is metabolized in the body for energy. ther sugars absorbed into the body must be converted to glucose by the liver. 2 2 β-d-fructose Also known as levulose and fruit sugar. Fructose is the sweetest of the monosaccharides. It is present in honey (1:1 ratio with glucose), fruits, and corn syrup. It is often used to sweeten foods, since less fructose is needed to achieve the same degree of sweetness. 40
21 hapter 7 Notes Disaccharides 41 Disaccharides Two monosaccharides can be linked together through a glycosidic linkage to form a disaccharide α-d-glucose α-d-glucose 2 2 cyclic hemiacetal α(1 4) glycosidic linkage Maltose
22 hapter 7 Notes 2 α-d-glucose α-d-glucose Maltose Also known as malt sugar. It is found in germinating grain (such as barley), and is formed during the hydrolysis of starch to glucose during digestion. Because it has a hemiacetal group, it is a reducing sugar. Important Disaccharides 2 β(1 4) glycosidic linkage 2 β-d-galactose 2 α-d-glucose Lactose Also known as milk sugar. Lactose constitutes 5% of cow's milk and 7% of human milk. It is digested by the enzyme lactase Important Disaccharides α-d-glucose 2 α 1 β 2 glycosidic linkage β-d-fructose Sucrose Also known as table sugar. Both anomeric carbons of glucose and fructose are tied together in the glycosidic linkage; thus neither ring can open, and sucrose is not a reducing sugar. Sucrose is abundant in sugar cane and sugar beets; maple syrup contains about 65% sucrose, with glucose and fructose present as well; caramel is the solid residue formed from heating sucrose. A flavoring agent called invert sugar is produced by the hydrolysis of sucrose under acidic conditions, which breaks it apart into glucose and fructose; invert sugar is sweeter than sucrose because of the fructose. Some of the sugar found in honey is formed in this fashion; invert sugar is also produced in jams and jellies prepared from acid-containing fruits. 44
23 hapter 7 Notes ligosaccharides 45 ligosaccharides ligosaccharides contain from 3 to 10 monosaccharide units. 2 β-d-galactose β-d-fructose α-d-glucose Raffinose An oligosaccharide found in peas and beans; largely undigested until reaching the intestinal flora in the large intestine, releasing hydrogen, carbon dioxide, and methane) 46
24 hapter 7 Notes Polysaccharides 47 Polysaccharides Polysaccharides contain hundreds or thousands of carbohydrate units. Polysaccharides are not reducing sugars, since the anomeric carbons are connected through glycosidic linkages. We will consider three kinds of polysaccharides, all of which are polymers of glucose: starch, glycogen, and cellulose. 48
25 hapter 7 Notes Starch Starch is a polymer consisting of D-glucose units. Starches (and other glucose polymers) are usually insoluble in water because of the high molecular weight, but they can form thick colloidal suspensions with water. There are two forms of starch: amylose and amylopectin. 49 Starch Amylose Amylose consists of long, unbranched chains of glucose (from 1000 to 2000 molecules) connected by α(1 4) glycosidic linkages. 10%-20% of the starch in plants is in this form. Amylose forms helices (coils) which can trap molecules of iodine, forming a characteristic deep blue-purple color. (Iodine is often used as a test for the presence of starch.) α(1 4) glycosidic linkage 50
26 hapter 7 Notes Starch Amylopectin Amylopectin consists of long chains of glucose (up to 10 5 molecules) connected by α(1 4) glycosidic linkages, with α(1 6) branches every 24 to 30 glucose units along the chain. 80%-90% of the starch in plants is in this form α(1 6) branch point 2 α(1 4) glycosidic linkage 51 Glycogen Glycogen, also known as animal starch, is structurally similar to amylopectin, containing both α(1 4) glycosidic linkages and α(1 6) branch points. Glycogen is even more highly branched, however, with branches occuring every 8 to 12 glucose units. Glycogen is abundant in the liver and muscles; on hydrolysis it forms glucose, which maintains normal blood sugar level and provides energy. Amylose Amylopectin/Glycogen 52
27 hapter 7 Notes ellulose ellulose is a polymer consisting of long, unbranched chains of D-glucose connected by β(1 4) glycosidic linkages; it may contain from 300 to 3000 glucose units in one molecule β(1 4) glycosidic linkage 53 ellulose Because of the β-linkages, cellulose has a different overall shape from amylose, forming extended straight chains which hydrogen bond to each other, resulting in a very rigid structure. ellulose is an important structural polysaccharide, and is the single most abundant organic compound on earth. It is the material in plant cell walls that provides strength and rigidity; wood is 50% cellulose. Most animals lack the enzymes needed to digest cellulose, although it does provide needed roughage (dietary fiber) to stimulate contraction of the intestines and thus help pass food along through the digestive system. 54
28 hapter 7 Notes ellulose Some animals, such as cows, sheep, and horses, can process cellulose through the use of colonies of bacteria in the digestive system which are capable of breaking cellulose down to glucose; ruminants use a series of stomachs to allow cellulose a longer time to digest. Some other animals such as rabbits reprocess digested food to allow more time for the breakdown of cellulose to occur. ellulose is also important industrially, from its presence in wood, paper, cotton, cellophane, rayon, linen, nitrocellulose (guncotton), photographic films (cellulose acetate), etc. 55 Nitrocellulose, elluloid, and Rayon Guncotton (German, schiessbaumwolle) is cotton which has been treated with a mixture of nitric and sulfuric acids. It was discovered by hristian Friedrich Schönbein in 1845, when he used his wife s cotton apron to wipe up a mixture of nitric and sulfuric acids in his kitchen, which vanished in a flash of flame when it dried out over a fire. Schönbein attempted to market it as a smokeless powder, but it combusted so readily it was dangerous to handle. Eventually its use was replaced by cordite (James Dewar and Frederick Abel, 1891), a mixture of nitrocellulose, nitroglycerine, and petroleum jelly, which could to extruded into cords. elluloid (John yatt, 1869) was the first synthetic plastic, made by combining partially nitrated cellulose with alcohol and ether and adding camphor to make it softer and more malleable. It was used in manufacturing synthetic billiard balls (as a replacement for ivory), photographic film, etc.; it was eventually replaced by less flammable plastics. Rayon (Louis Marie hardonnet, 1884) consists of partially nitrated cellulose mixed with solvents and extruded through small holes, allowing the solvent to evaporate; rayon was a sensation when introduced since it was a good substitute for silk, but it was still highly flammable. 56
29 hapter 7 Notes ow Sweet It Is! Sugar Substitutes 57 Saccharin N Saccharin The first of the artificial sweeteners, saccharin is noncaloric and about 500 times sweeter than sugar. It was discovered in 1879 by onstantine Fahlberg, a chemistry student at Johns opkins University working for Ira Remsen; he noticed that the bread he was eating was unusually sweet, and went back to his lab bench and tasted all of the compounds he had been working with that day to find the compound responsible. It was marketed commercially as a non-nutritive sweetener very quickly, especially for use by diabetics. It was banned in some areas for some time because it was a suspected carcinogen. S 58
30 hapter 7 Notes Aspartame (NutraSweet) 3 N Aspartame (NutraSweet) N 2 Aspartame (NutraSweet) is about 160 times sweeter than sugar; it is composed of the amino acids aspartic acid and phenylalanine, neither of which has a sweet taste. It was discovered at Searle by James Schlatter in 1965, who was preparing intermediates for the synthesis of a tetrapedtide for an anti-ulcer project. Schlatter had spilled some of the dipeptide intermediate on his hands, and noticed later that there was a strong, sweet taste; he went back to his bench and tasted the dipeptide and found that it indeed was extremely sweet. Aspartame is sensitive to heat, so it cannot be used in cooked foods, and it decomposes slowly in liquids, reducing their shelf life. 59 N S 2 - N K + yclamate Discovered by Michael Sveda in 1937, who noticed that a cigar that he was smoking in the lab tasted especially sweet. yclamates were banned by the FDA in S 3 Acesulfame-K (Sunette) Approved by the FDA in 1988; 200 times sweeter than sugar; noncaloric; heat-stable and can be used during cooking 60
31 hapter 7 Notes 2 2 Sorbitol A sugar alcohol; incompletely absorbed during digestion, and contribute fewer calories than carbohydrates; found naturally in fruits; used commercially in sugar-free candies, cookies, chewing gum, etc. l 2 "galactose" l 2 2 l "fructose" Sucralose A non-caloric artificial sweetener approved by the FDA in The glucose in sucrose is replaced by a galactose, and three of the groups are replaced by l atoms. The molecule still tastes sweet, but is not metabolized in the body. 61 The Maillard Reaction The Maillard Reaction occurs between foods containing carbohydrates and proteins under heating; it results in a complex mixture of products and the development of a brown color. It is observed in the grilling or browning of meats, the formation of crust on baked breads, the boiling of maple syrup, the brewing of beer, the roasting of coffee and cocoa beans, the roasting of nuts, and other sources. In addition to the darkened colors, many complex flavors are developed. There are hundreds of compounds produced during these reactions, not many of which are well-characterized. Furfural 62
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Solid Phase Peptide Synthesis
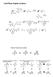 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Qualitative Testing for Carbohydrates
 R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
What impacts blood glucose levels?
 What you eat and how much you eat has an impact on your blood glucose levels. Your blood glucose level reflects how well your diabetes is controlled. There are many aspects to eating for target BG (Blood
What you eat and how much you eat has an impact on your blood glucose levels. Your blood glucose level reflects how well your diabetes is controlled. There are many aspects to eating for target BG (Blood
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Name Date Period. Keystone Review Enzymes
 Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Chapter 4. Carbohydrates: Bountiful Sources of Energy and Nutrients
 hapter 4 arbohydrates: Bountiful Sources of Energy and Nutrients hapter Objectives After reading this chapter, you will be able to: 1. Describe the difference between simple and complex carbohydrates,
hapter 4 arbohydrates: Bountiful Sources of Energy and Nutrients hapter Objectives After reading this chapter, you will be able to: 1. Describe the difference between simple and complex carbohydrates,
Photo Cell Resp Practice. A. ATP B. oxygen C. DNA D. water. The following equation represents the process of photosynthesis in green plants.
 Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
Name: ate: 1. Which molecule supplies the energy for cellular functions?. TP. oxygen. N. water 2. Photosynthesis The following equation represents the process of photosynthesis in green plants. What happens
ASPARTAME The Study of a Peptide Bond
 ASPARTAME The Study of a Peptide Bond INTRODUCTION Aspartame, commonly known as NutraSweet or Equal, is the most popular artificial, low-calorie sweetener available to consumers today. Chemically, aspartame
ASPARTAME The Study of a Peptide Bond INTRODUCTION Aspartame, commonly known as NutraSweet or Equal, is the most popular artificial, low-calorie sweetener available to consumers today. Chemically, aspartame
Biology 13A Lab #13: Nutrition and Digestion
 Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes.... 102 Introduction...... 103 Food Chemistry & Nutrition.... 104 Activity 1: Testing for the Presence
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
ORGANIC COMPOUNDS IN THREE DIMENSIONS
 (adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
(adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
Digestive System Functions
 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
How To Eat Healthily
 Pierce College Putman/NUTR& 101 Unit 04 Practice Exam: Carbohydrates 1. Which is not a monosaccharide? a. lactose b. glucose c. fructose d. galactose 2. Used for immediate energy in the body: a. carbohydrates
Pierce College Putman/NUTR& 101 Unit 04 Practice Exam: Carbohydrates 1. Which is not a monosaccharide? a. lactose b. glucose c. fructose d. galactose 2. Used for immediate energy in the body: a. carbohydrates
II. Chemistry of Sugars
 II. Chemistry of Sugars Sugars-or saccharides- are the most abundant bio-molecule on the planet. They are important in a number of biological roles. Most obviously you will know them as a major component
II. Chemistry of Sugars Sugars-or saccharides- are the most abundant bio-molecule on the planet. They are important in a number of biological roles. Most obviously you will know them as a major component
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
PHOTOSYNTHESIS AND CELLULAR RESPIRATION
 reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
Biology for Science Majors
 Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Photosynthesis and Sucrose Production
 Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES
 PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
Which of the following can be determined based on this model? The atmosphere is the only reservoir on Earth that can store carbon in any form. A.
 Earth s Cycles 1. Models are often used to explain scientific knowledge or experimental results. A model of the carbon cycle is shown below. Which of the following can be determined based on this model?
Earth s Cycles 1. Models are often used to explain scientific knowledge or experimental results. A model of the carbon cycle is shown below. Which of the following can be determined based on this model?
10-ml Graduated cylinder 40 ml 3% Hydrogen peroxide solution (found in stores) Straight-edged razor blade Scissors and Forceps (tweezers)
 Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
The functional properties of sugar
 The functional properties of sugar These days, sugar comes in many varieties and can therefore be used in many different food products. Sugar has a range of unique properties that, either individually
The functional properties of sugar These days, sugar comes in many varieties and can therefore be used in many different food products. Sugar has a range of unique properties that, either individually
Enzymes. Chapter 3. 3.1 Enzymes and catalysts. Vital mistake. What is an enzyme?
 Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Chapter 3 Enzymes Vital mistake We may not be able to see them, but enzymes are absolutely crucial to the lives of ourselves and all other living organisms. The Quarter Horse (Figure 3.1) is a breed of
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Determination of Specific Nutrients in Various Foods. Abstract. Humans need to consume food compounds such as carbohydrates, proteins, fats,
 Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
USING THE FOOD LABEL TO FIND ITEMS THAT MEET THE EAT SMART IN PARKS GUIDELINES
 USING THE FOOD LABEL TO FIND ITEMS THAT MEET THE EAT SMART IN PARKS GUIDELINES FOOD LABELS Food Nacho chips label Although one serving of chips (1 ounce) meets the calorie guideline, 3 ounces of chips
USING THE FOOD LABEL TO FIND ITEMS THAT MEET THE EAT SMART IN PARKS GUIDELINES FOOD LABELS Food Nacho chips label Although one serving of chips (1 ounce) meets the calorie guideline, 3 ounces of chips
1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. 2. (U4C1L4:G13) Another name for fiber in a diet is.
 Cadet Name: Date: 1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. A) True B) False 2. (U4C1L4:G13) Another name for fiber in a diet is. A) vegetables B) laxative C) fruit D)
Cadet Name: Date: 1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. A) True B) False 2. (U4C1L4:G13) Another name for fiber in a diet is. A) vegetables B) laxative C) fruit D)
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
McMush. Testing for the Presence of Biomolecules
 Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Alkanes. Chapter 1.1
 Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
HOW YEAST WORKS 2011, 1997 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright is included.
 HOW YEAST WORKS 2011, 1997 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright is included. Materials Needed active dry yeast, 6 packages or a jar
HOW YEAST WORKS 2011, 1997 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright is included. Materials Needed active dry yeast, 6 packages or a jar
Molecule Projections
 Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
The Huntington Library, Art Collections, and Botanical Gardens. How Sweet It Is: Enzyme Action in Seed Germination
 The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
What happens to the food we eat? It gets broken down!
 Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body? What happens to the food we eat? It gets broken down!
Molecular Cell Biology
 Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
chapter 5 Carbohydrates
 part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
part two The Energy-Yielding Nutrients and Alcohol chapter 5 Carbohydrates Chapter utline Carbohydrates An Introduction Structures and Functions of Simple Carbohydrates Monosaccharides: Glucose, Fructose,
Relevance of Sweeteners to Health and Diet. Fiastuti Witjaksono Department of Nutrition Faculty of Medicine Universitas Indonesia
 Relevance of Sweeteners to Health and Diet Fiastuti Witjaksono Department of Nutrition Faculty of Medicine Universitas Indonesia Sweetener A sugar substitute Food additive that duplicates the effect of
Relevance of Sweeteners to Health and Diet Fiastuti Witjaksono Department of Nutrition Faculty of Medicine Universitas Indonesia Sweetener A sugar substitute Food additive that duplicates the effect of
Chapter 12 Organic Compounds with Oxygen and Sulfur
 Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Topic 3: Nutrition, Photosynthesis, and Respiration
 1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
1. Base your answer to the following question on the chemical reaction represented below and on your knowledge of biology. If this reaction takes place in an organism that requires sunlight to produce
