ABG Outline and Background for Carbonic Acid/Bicarbonate Buffer System
|
|
|
- Sandra Walker
- 7 years ago
- Views:
Transcription
1 1 ABG Outline and Background for Carbonic Acid/Bicarbonate Buffer System Importance of ph Normal blood ph is between 7.35 and Blood ph below 7.35 is known as acidosis. Blood ph above 7.45 is known as alkalosis. Values less than 6.8 or greater than 7.8 often result in death. The body s ph influences the function of enzymes and thus the speed of cellular reactions, cell permeability, and the integrity of cell structure. Effect of ph Changes on the Body The major effect of acidosis is depression of the central nervous system. When the ph of the blood falls below 7.35, the central nervous system malfunctions, and the individual becomes disoriented and possibly comatose as the condition worsens. A major effect of alkalosis is hyperexcitability of the nervous system. When the ph of the blood rises above 7.45, the nervous system can generate impulses without normal stimuli. Peripheral nerves are affected first, resulting in spontaneous nervous stimulation of muscles. Spasms and tetanic contractions and possibly extreme nervousness or convulsions result. Severe alkalosis can cause death as a result of spasms of the respiratory muscles. Respiratory vs. Metabolic Acidosis/Alkalosis Acidosis and alkalosis are categorized by the cause of the condition. Respiratory acidosis or respiratory alkalosis results from abnormalities of the respiratory system. Metabolic acidosis or metabolic alkalosis results from all causes other than abnormal respiratory functions. Although chemical buffers help resist changes in the ph of body fluids, the respiratory system and the Urinary system (kidneys) are the primary systems that regulate the ph of the body fluids. Malfunctions of either the respiratory system or the kidneys can result in acidosis or alkalosis.
2 2 Regulation of Acid Base Balance Three regulatory systems maintain the body s ph: 1) Chemical buffers, 1 st line of defense (react in seconds). 2) Respiratory system (lungs), 2 nd line of defense (react in minutes). 3) Renal system (kidneys), 3 rd line of defense (react in hours to days). Is the most powerful and lasts the longest. Kidneys don t kid around! Chemical Buffers Chemical Buffers resist changes in ph when an acid or base is added. They are found in intracellular fluid, interstitial fluid, and blood If an acidic solution is added, the buffer will combine with the extra H + ions to help maintain the ph. If a basic solution is added, the buffer will release H + ions to combine with the base to help maintain the ph. The body has three main chemical buffer systems: 1. The carbonic acid:bicarbonate buffer system. 2. The protein buffer system (include intracellular proteins and hemoglobin protein) 3. The phosphate buffer system (HPO4 2- ) Carbonic Acid:Bicarbonate Buffer System Maintains a relatively constant plasma ph of about 7.4 and counteracts any force that would alter this. In this system, carbon dioxide (CO2) combines with water (H2O) to form carbonic acid (H2CO3), most of which in turn rapidly dissociates to form hydrogen ions (H + ) and bicarbonate (HCO3 ) as shown in the above reaction. Remember that carbon dioxide is produced as a waste product during aerobic cellular respiration. Basically, CO2 is not an acid but is acts like an acid because it can combine with water to form carbonic acid, H+ acts as an acid, and HCO3 acts as a base. Most bicarbonate is produced by RBCs during the chloride shift. Although H+ and HCO3 are created in a 1:1 ratio the intracellular buffering of H+ by hemoglobin is a major reason the two ions do not appear in the plasma in the same concentration. The HCO3 in the plasma is then available to buffer H+ from metabolic sources. Any disturbance of the system will be compensated by a shift in the chemical equilibrium by the law of mass action. Example: If hydrogen ions are added, some of those hydrogen ions will associate with bicarbonate, forming carbonic acid and drive the equilibrium to the left, resulting in a smaller net increase of acidity than if it was just plain water. The carbonic acid will dissociate into carbon dioxide and water and the respiratory system would increase breathing rate to eliminate the excess CO2.
3 Example: If carbon dioxide is added, the equilibrium will shift to the right. More carbon dioxide will combine with water to form more carbonic acid. The carbonic acid would dissociate and more hydrogen ions and bicarbonate ions will be produced. The production of H+ from CO2 and H2O is the single biggest source of acid input under normal conditions. Note: Acid-base physiology focuses primarily on acids because there are many more acid sources compared to base sources. Many metabolic intermediates and foods are organic acids that ionize and contribute H+ to body fluids. Examples of organic acids include acidic fruits, amino acids, fatty acids, citric acid cycle intermediates, and lactic acid produced by anaerobic respiration. In cases such diabetes mellitus there is the production of ketoacids. For these reasons, the body uses far more resources removing excess acids (H+). 3 Respiratory System Acid-Base Regulation The Respiratory System regulates the amount of carbon dioxide (CO2) in the blood, which combines with H2O to form H2CO3. Chemoreceptor's in the brain and carotid/aortic bodies sense ph changes and send information to medulla oblongata which varies the rate and depth of breathing to regulate CO2 levels. The partial pressure of arterial CO2 (PCO2) reflects the level of unbound CO2 in the blood. Since PCO2 is regulated by respiration, abnormalities that alter the PCO2 levels are referred to as respiratory acidosis (high PCO2) and respiratory alkalosis (low PCO2). Respiratory Acidosis Respiratory acidosis (high PCO2) is caused by hypoventilation and develops when the lungs don t adequately eliminate CO2. Respiratory acidosis causes a ph below 7.35 and a PCO2 above 45 mm Hg. HCO3 is normal. hypoventilation can result from: Diseases that affect the lungs (COPD, emphysema, asthma, pulmonary edema, pneumonia, cystic fibrosis), Diseases of the nerves and muscles of the chest that impair the mechanics of breathing (Myasthenia Gravis, Amyotrophic Lateral Sclerosis). Physical trauma such as a broken rib Drugs that slow a patient s respirations such as sedatives.
4 Respiratory Acidosis and the Bicarbonate Buffer System Hypoventilation increases CO2, which by the law of mass action will cause the equilibrium to shift to the right. This increases carbonic acid (H2CO3) which dissociates and increases H+ ions resulting in a decrease in ph 4 Lungs Kidneys Kidneys compensate by secreting more hydrogen ions in the urine and increase reabsorption and production of bicarbonate, but can take up to 24 hrs to be fully effective. Treatment: Improve ventilation. May need to administer drugs such as bronchodilators to improve breathing and, in severe cases, use mechanical ventilation. Respiratory Alkalosis Respiratory alkalosis (low PCO2) is caused by hyperventilation and develops when the lungs eliminate too much CO2. Respiratory alkalosis causes a ph above 7.45 and a PCO2 below 35 mm Hg. HCO3 is normal. Hyperventilation can result from: Fear and anxiety (most common cause). Hypoxemia (inadequate oxygen levels in blood) caused by pulmonary disease, congestive heart failure, or high altitudes. Hypermetabolic states caused by fever, anemia, or thyrotoxicosis. Respiratory Alkalosis and the Bicarbonate Buffer System Hyperventilation decreases CO2 levels, which by the law of mass action, will cause the equilibrium to shift to the left. This reduces H+ concentrations causing a rise in ph (more alkaline). Lungs Kidneys Kidneys compensate by conserving (reabsorb) hydrogen ions and release more bicarbonate in the urine. This may take up to 24 hours to be fully effective. Thus, the kidneys are not effective if respiratory alkalosis develops quickly. However, they are very effective if respiratory alkalosis develops slowly. For example, the kidneys are not effective in compensating for respiratory alkalosis that occurs in response to hyperventilation triggered by emotions, which usually begins quickly and subsides within minutes or hours. However if
5 alkalosis results from staying at a high altitude over a 2 or 3 day period, the kidneys play a significant role in helping to compensate. Treatment: Goal is to slow the breathing rate. If anxiety is the cause, encourage the patient to slow his or her breathing. If pain is causing rapid, shallow breathing, provide pain relief. Breathing into a paper bag allows a patient to rebreathe CO2, raising the level of CO2 in the blood. 5
6 Overview of Carbonic Acid:Bicarbonate (H2CO3:HCO3-) Buffer System and Link to Respiratory and Urinary Systems Carbonic Acid:Bicarbonate Buffer System is considered the most important chemical buffer because it is coupled to the respiratory system and urinary system Hypoventilation increases CO2 which by the law of mass action, will increase carbonic acid (H2CO3) and increase H+ ions causing respiratory acidosis (lowers ph) Hyperventilation decreases dissolved CO2, which by the law of mass action, reduces H+ concentrations causing respiratory alkalosis (raises ph) Response to respiratory acidosis: Increased HCO3- reabsorption and synthesis (puts base into the blood), and increased H+ secretion into the urine raises blood ph (becomes more alkaline). Response to respiratory alkalosis: Decreased HCO3- reabsorption and synthesis, decreased H+ secretion into the urine lowers blood ph (becomes more acidic) 6
7 Metabolic Acidosis Metabolic acidosis is caused by an increase in acid production or decreased bicarbonate (HCO3-) levels that are not caused by respiratory problems. Metabolic acidosis causes a HCO3- below 22 meq/l and a ph below PaCO2 is normal. Metabolic acidosis can result from: Renal disease or failure resulting in an inability to excrete acids or excess loss of bicarbonate (base) or inability of kidneys to make bicarbonate. Excess acid production: Diabetic ketoacidosis Starvation resulting in increased plasma fatty acids and ketoacidosis Lactic acidosis caused by anaerobic metabolism due to poor oxygenated blood perfusion. Diarrhea resulting in excess HCO3- loss Compensation for metabolic acidosis include: Respiratory system: The reduced ph stimulates the respiratory center, which causes hyperventilation (Kussmaul breathing deep labored rapid breathing). As carbon dioxide is eliminated at a greater rate there is a shift to the left in the carbonic acid/bicarbonate equilibrium and excess hydrogen ions are eliminated. This counteracts the metabolic acidosis. Kidneys increase rate of H+ secretion and bicarbonate ion reabsorption. Kidneys can also produce bicarbonate to offset the acidosis. However this may take up to 24 hours to be fully functional. 7 Lungs Kidneys
8 Metabolic Alkalosis Metabolic alkalosis is caused by a decrease or loss of metabolic acids or an increase in bicarbonate concentrations that is not caused by respiratory problems.. Metabolic alkalosis causes a HCO3 above 26 meq/l and a ph above PaCO2 is normal. Metabolic alkalosis can result from: Vomiting resulting in loss of stomach acid. H+ loss through the kidneys Excessive ingestion of antacids (increased bicarbonate) Constipation, in which excessive bicarbonate is reabsorbed Compensation for metabolic alkalosis include: Respiratory System: The increased ph inhibits the respiratory center, which causes hypoventilation and increases carbon dioxide accumulates in the blood. Carbon dioxide reacts with water to produce carbonic acid shifting the carbonic acid/bicarbonate equilibrium to the right and the excess hydrogen ions help counter act the metabolic alkalosis Kidneys decrease the rate of H + secretion into the urine and decrease bicarbonate ion reabsorption and production. However this may take up to 24 hours to be maximally effective. Metabolic Acid-Base Imbalances/Compensation 8
9 9 Arterial Blood Gas (ABG) ABG is a diagnostic test is used to assess the effectiveness of your patient s ventilation and acid-base balance. A low partial pressure of oxygen (PO2) suggests that a person is not getting enough oxygen. Normal PO2 values: mmhg, Normal oxygen saturation values (breathing room air) (SaO2): % Note: PO2 is not the same as SaO2 that you take with the pulse oximeter, also known as the glow finger. Details of PaO2, SaO2, and CaO2 will be presented later Three results are essential for evaluating acid-base balance: 1) ph: Indicates whether the person is in acidosis or alkalosis but it does not indicate the cause. 2) PCO2: Abnormal values of PCO2 may indicate whether the acidosis/alkalosis is caused by the respiratory system or if the patient is compensating for a metabolic disturbance. 3) HCO3-: Abnormal values of the HCO3- indicate the acidosis/alkalosis is metabolic or if the patient is compensating for a respiratory disturbance. Normal values of ph, PCO2, and HCO3-. You will need to memorize these values. ph: 7.35 to 7.45 Average : 7.4 PCO2 : 35 to 45 mm Hg Average: 40 mm Hg HCO3-: 22 to 26 meq/l Average: 24 meq/l Note: PO2 and PCO2 can be written as PaO2 and PaCO2. The a stands for arterial. Since the first word is arterial in ABG often the small a is left off. Fast Facts on Acid-Base Balance The more hydrogen ion (H+) in the blood, the lower the ph. The less H+ in the blood, the higher the ph. When partial pressure of arterial carbon dioxide (PCO2) rises, ph falls. When PCO2 falls, ph rises. In respiratory acid-base disorders, ph and PCO2 move in opposite directions. Bicarbonate (HCO3 ) remains normal until compensation occurs. In metabolic acid-base disorders, ph and HCO3 move in the same direction. PCO2 remains normal until compensation occurs.
10 10 Arterial Blood Gas Naming ABGs are identified by three names. Example: Uncompensated respiratory acidosis First we identify the last name. It is an acidosis, an alkalosis, or is it normal? If it is normal we are finished all is good. Second we identify the middle name. Is it respiratory or metabolic? Third, we identify the first name. Is it uncompensated, partially or fully compensated? Terms Used In ABG Results Terms used to label abnormal ABG results: Respiratory Acidosis (uncompensated, partially compensated, compensated) Respiratory Alkalosis (uncompensated, partially compensated, compensated) Metabolic Acidosis (uncompensated, partially compensated, compensated) Metabolic Alkalosis (uncompensated, partially compensated, compensated) Compensating for imbalances Compensation is when the renal and respiratory systems make adjustments to regain acidbase balance. The lungs compensate (respond) to a metabolic disorder by increasing or decreasing ventilation and thus the concentration of carbon dioxide. The renal system compensates for a respiratory disorder by regulating the amount of H+ and HCO3- that is reabsorbed or secreted producing more acidic or more alkaline urine. Partial compensation is when there is a respiratory or metabolic response, but the ph remains abnormal. If the ph returns to normal, the response is called complete compensation. Correction occurs when the values for both components of the buffer pair (carbonic acid and bicarbonate) return to normal levels.
11 Determination of Abnormal ABG Values In analyzing ABG results you compare the ph, PCO2, and HCO3- values to the normal values. Will allow you to determine if your patient is normal or in respiratory or metabolic acidosis or alkalosis.. For ph anything less than 7.35 is acidic and anything greater than 7.45 is a basic. For PCO2 (NOTE: it is the opposite) anything less than 35 mm Hg is basic and anything greater than 45 mm Hg is acidic. For HCO3- anything less than 22 meq/l is acidic and anything greater than 26 meq/l is basic. Write out the following table to help you understand. Normal Values ph: 7.35 to 7.45 PCO2: 35 to 45 mm Hg HCO3-: 22 to 26 meq/l 11 TIC-TAC-TOE Method for ABG Analysis Tic-Tac-Toe method to determine acid-base balances: The column that the ph is in tells whether the patient has acidosis, alkalosis, or is normal. The position of the PCO2, and HCO3- reveals the origin of any acid-base balance. If the ph and the HCO3- fall in the same column other than normal, the problem is metabolic. If the ph and the PCO2 fall in the same column other than normal the problem is respiratory. To determine compensation we look at the value that didn t come into alignment with the ph If the value that is not in alignment with the ph is in the normal column, there is no compensation. If the value that is not in alignment with the ph is in the far opposite column, there is partial compensation. If an ABG shows full compensation, the ph will be normal. To determine if it is an acidosis or alkalosis you look at which it is closer to. To set up a tic tac toe grid, label each column as acid, ph, and base. It should look like this:
12 Practice Problem 1 ABG results are the following: ph 7.24 PCO2 75 mm Hg HCO3 28 meq/l Analysis of Practice Problem 1 ABG results are the following: ph 7.24, PCO2 75, HCO3 28 Draw your tic tac toe lay out. Analyze your ph. Ask yourself is it normal, basic, or acidic? Since the ph is less than 7.35 making it an acid, place it under the acid column. Analyze your PCO2. Ask yourself is it normal, basic, or acidic? Since the PCO2 is greater than 45 making it an acid, place it under the acid column along with ph. Remember PCO2 is the opposite and the normal is Analyze your HCO3. Ask yourself is it normal, basic, or acidic? Since HCO3 is greater than 26 making it basic, place it under the base column because the value is considered basic. Your tic tac toe lay out should look like this: Since your ph is acidic you know that you have acidosis going on but is it respiratory or metabolic acidosis. Since PCO2 represents respiratory and it is under the acid column with your ph you have respiratory acidosis. But is it fully compensated, partially compensated, or uncompensated respiratory acidosis? Look at your HCO3. Since your HCO3 is under basic, the kidneys are trying to balance the body s acidosis by becoming more basic so it is partially compensating. The condition is Partially Compensated Respiratory Acidosis. Note: If HCO3 was under the normal column the kidneys would not be activated to compensate and therefore, it would be considered uncompensated respiratory acidosis. Practice Problem 2 ABG results are: ph 7.50 PCO2 36 mm Hg HCO3 32 meq/l Analysis of Practice Problem 2 Here is what your tic tac toe grid should look like: Analyze your ph. Ask yourself is it normal, basic, or acidic? Since the ph is greater than 7.45 making it a basic, place it under the base column. Analyze your PCO2. Ask yourself is it normal, basic, or acidic? Since the PCO2 is between it is normal, place it under the normal column. Analyze your HCO3. Ask yourself is it normal, basic, or acidic? Since HCO3 is greater than 26 making it is basic, place it under the base column. 12
13 Since your ph is basic you know you have alkalosis, but is it respiratory or metabolic? Since the HCO3 (which represents metabolic) is under you basic column with ph, it is a metabolic issue. So your patient is in: Metabolic Alkalosis. Now is it fully compensated, partially compensated, or uncompensated metabolic alkalosis? Look at the PCO2! Since it is under the normal column that means the lungs have NOT tried to help out the body s system by making increasing the PCO2 levels and making the blood more acidic. So the body is not compensating. Answer: Uncompensated Metabolic Alkalosis Practice Problem 3 Ms. Doe, a 75 year old diabetic, has a long history of non-compliance with her insulin. She was recently admitted to the hospital with the following ABG results: ph 7.26 PCO2 42 mm Hg HCO3 17 meq/l Analysis of Practice Problem 3 Analysis: Since the ph and the HCO3- both fall under the ACID column (three in a row), Ms. Doe has Metabolic Acidosis. The pco2 is normal designating that no respiratory compensation has occurred. Thus Ms. Doe has Uncompensated Metabolic Acidosis. Practice Problem 4 Ms. Doe presented with the following ABGs: ph 7.26 PCO2 32 mm Hg HCO3-17 meq/l Analysis of Practice Problem 4 Analysis: Because the PCO2 was alkaline instead of normal, it is placed under the alkaline column. This reflects that the respiratory system (PCO2) has begun to compensate for the metabolic acidosis (HCO3-) with a resulting respiratory alkalosis. The patient will likely have a high respiratory rate (Kussmaul breathing ) as she blows off lots of CO2 to try to raise the ph. Therefore, with these values, Ms. Doe s diagnosis is now Partially Compensated Metabolic Acidosis. 13
14 Practice Problem 5 Mr. Smith presented with the following ABGs: ph 7.49 PCO2 60 mm Hg HCO3-30 meq/l Analysis of Practice Problem 5 Analysis: Because the ph and HCO3- are alkaline instead of normal, they are placed under the alkaline column. The PCO2 is in the acidic column which indicates that the respiratory system has begun to compensate for the metabolic alkalosis (HCO3 ) with a resulting respiratory acidosis. The patient will likely have a low respiratory rate as they hold onto CO2 to try to lower the ph. Therefore, with these values, Mr. Smith s diagnosis is Partially Compensated Metabolic Alkalosis. Practice Problem 6 Full Compensation Example Mr. Dalton presented with the following ABGs: ph 7.38 PCO2 60 mm Hg HCO3-27 meq/l Analysis of Practice Problem 6 PCO2 ph HCO3- Analysis: The ph is normal but on the acid side of 7.4 (half-way between 7.35 and 7.45). This problem is considered to the respiratory because between the two, the ph is more on the acidic side, the same side as the PCO2. HCO3 is alkaline. This ABG is fully compensated respiratory acidosis because the ph is normal and the renal system (metabolic system) is able to compensate by increasing the bicarbonate levels to keep the ph in the normal range. Practice Problem 7 Full Compensation Mr. Dalton presented with the following ABGs: ph 7.36 Analysis of Practice Problem 7 Analysis: PCO2 30 mm Hg HCO3-15 meq/l HCO3- ph PCO2 The ph is normal but on the acid side of 7.4 (half-way between 7.35 and 7.45). This problem is considered to the metabolic because between the two, the ph is more on the acidic side, the same side as the HCO3. PCO2 is alkaline. This ABG is fully compensated metabolic acidosis because the ph is normal and the respiratory system is able to compensate by decreasing the carbon dioxide level to keep the ph in the normal range. 14
15 15 Additional Practice ABGs 1. ph: 7.32, PCO2: 21, HCO3-: ph: 7.27, PCO2: 49, HCO3-: ph: 7.29, PCO2: 50, HCO3-: ph: 7.61, PCO2: 44, HCO3-: ph: 7.64, PCO2: 45, HCO3-: ph: 7.65, PCO2: 44, HCO3-: ph: 7.71, PCO2: 55, HCO3-: ph: 7.59, PCO2: 42, HCO3-: ph: 7.61, PCO2: 24, HCO3-: ph: 7.33, PCO2: 27, HCO3-: 15 Additional Practice ABGs showing full compensation 1. ph: 7.36, PCO2: 30, HCO3-: ph: 7.36, PCO2: 75, HCO3-: ph: 7.44, PCO2: 27, HCO3-: ph: 7.43, PCO2: 49, HCO3-: 31 Video Tutorials: There are four video tutorials on my web site for Arterial Blood Gas (ABG) Tic-Tac- Toe method. Look for the links in the Student Resources section, Anatomy and Physiology, and then Blood Test Information. Answers to Practice ABGs 1. ph: 7.32, PCO2: 21, HCO3-: 21 - Partially Compensated Metabolic Acidosis 2. ph: 7.27, PCO2: 49, HCO3-: 35 - Partially Compensated Respiratory Acidosis 3. ph: 7.29, PCO2: 50, HCO3-: 23 - Uncompensated Respiratory Acidosis 4. ph: 7.61, PCO2: 44, HCO3-: 37 - Uncompensated Metabolic Alkalosis 5. ph: 7.64, PCO2: 45, HCO3-: 32 - Uncompensated Metabolic Alkalosis 6. ph: 7.65, PCO2: 44, HCO3-: 33 - Uncompensated Metabolic Alkalosis 7. ph: 7.71, PCO2: 55, HCO3-: 28 - Partially Compensated Metabolic Alkalosis 8. ph: 7.59, PCO2: 42, HCO3-: 28 - Uncompensated Metabolic Alkalosis 9. ph: 7.61, PCO2: 24, HCO3-: 23 - Uncompensated Respiratory Alkalosis 10. ph: 7.33, PCO2: 27, HCO3-: 15 - Partially Compensated Metabolic Acidosis Answers to Additional Practice ABGs showing full compensation 5. ph: 7.36, PCO2: 30, HCO3-: 15 Fully compensated metabolic acidosis 6. ph: 7.36, PCO2: 75, HCO3-: 40 Fully compensated respiratory acidosis 7. ph: 7.44, PCO2: 27, HCO3-: 18 Fully compensated respiratory alkalosis 8. ph: 7.43, PCO2: 49, HCO3-: 31 Fully compensated metabolic alkalosis
16 Terminology Explained Arterial Blood Gas (ABG) include the following. Since the first word is arterial in ABG often the small a is left off. For example PaO2 vs. PO2. Alveolar partial pressure of oxygen (PAO2) Venous partial pressure of oxygen (PvO2) Venous hemoglobin oxygen saturation (SvO2) Arterial partial pressure of oxygen (PaO2) Arterial hemoglobin oxygen saturation (SaO2) Arterial Oxygen content (CaO2) OXYGEN PRESSURE: PaO2. PaO2, the partial pressure of oxygen in the plasma phase of arterial blood, is registered by an electrode that senses randomlymoving, dissolved oxygen molecules. Oxygen molecules dissolved in plasma (i.e., not bound to hemoglobin) are free to impinge on the measuring oxygen electrode. This "impingement" of free O2 molecules is reflected as the partial pressure of oxygen; if the sample being tested is arterial blood, then it is the PaO2. The amount of dissolved oxygen in the plasma phase -- and hence the PaO2 -- is determined by alveolar PO2 and lung architecture only, and is unrelated to anything about hemoglobin. Oxygen molecules that pass through the thin alveolar-capillary membrane enter the plasma phase as dissolved (free) molecules; most of these molecules quickly enter the red blood cell and bind with hemoglobin. There is a dynamic equilibrium between the freely dissolved and the hemoglobin-bound oxygen molecules. However, the more dissolved molecules there are (i.e., the greater the PaO2) the more will bind to available hemoglobin; thus SaO2 always depends, 16
17 to a large degree, on the concentration of dissolved oxygen molecules (i.e., on the PaO2). Since PaO2 reflects only free oxygen molecules dissolved in plasma and not those bound to hemoglobin, PaO2 cannot tell us "how much" oxygen is in the blood; for that you need to know how much oxygen is also bound to hemoglobin, information given by the SaO2 and hemoglobin content. Once bound, oxygen no longer exerts a gas pressure. Thus hemoglobin is like an efficient sponge that soaks up oxygen so more can enter the blood. Hemoglobin continues to soak up oxygen molecules until it becomes saturated with the maximum amount it can hold - an amount that is largely determined by the PaO2. Of course this whole process is near instantaneous and dynamic; at any given moment a given O2 molecule could be bound or dissolved. However, depending on the PaO2 and other factors, a certain percentage of all O2 molecules will be dissolved and a certain percentage will be bound. OXYGEN SATURATION: SaO2. The percentage of all the available heme binding sites saturated with oxygen is the hemoglobin oxygen saturation (in arterial blood, the SaO2). Note that SaO2 alone doesn't reveal how much oxygen is in the blood; for that we also need to know the hemoglobin content. An SaO2 of 97% simply means that of every 100 hemoglobin binding sites, 97 are occupied with an oxygen molecule and the other three are either bound to something else or are unbound. Binding sites for oxygen are the heme groups, the Fe ++ - porphyrin portions of the hemoglobin molecule. There are four heme sites, and hence four oxygen binding sites, per hemoglobin molecule. Heme sites occupied by oxygen molecules are said to be "saturated" with oxygen. Each hemoglobin molecule has four Fe ++ heme sites for binding oxygen. If there is no interference (as from carbon monoxide, for example), the free O2 molecules bind to these sites with great affinity. The total percentage of sites actually bound with O2 is constant for a given set of conditions, and is the 'saturation of blood with oxygen'. This is called SvO2 and SaO2 in the venous and arterial circulations, respectively. 17
18 OXYGEN CONTENT: CaO2. Tissues need a requisite amount of O2 molecules for metabolism. Neither the PaO2 nor the SaO2 provide information on the number of oxygen molecules, i.e., of how much oxygen is in the blood. (Note that neither PaO2 nor SaO2 have units that denote any quantity.) Of the three values used for assessing blood oxygen levels, how much is provided only by the oxygen content, CaO2 (units ml O2/dl). This is because CaO2 is the only value that incorporates the hemoglobin content. Oxygen content can be measured directly or calculated by the oxygen content equation: CaO2 = Hb (gm/dl) x 1.34 ml O2/gm Hb x SaO2 + PaO2 x (.003 ml O2/mm Hg/dl) 18 Summary of PaO2, SaO2, CaO2 PaO2 is determined by alveolar PO2 (PAO2) and the state of the alveolar-capillary interface, not by the amount of hemoglobin available to soak them up. PaO2, in turn, determines the oxygen saturation of hemoglobin (along with other factors that affect the position of the oxyhemoglobin dissociation curve. The SaO2, plus the concentration of hemoglobin (15 gm/dl in this example), determine the total amount of oxygen in the blood or CaO2 (see equation for CaO2). Clinical Problem 1 At 10 a.m. a patient has a PaO2 of 85 mm Hg, an SaO2 of 98%, and a hemoglobin of 14 gm/dl. At 10:05 a.m. she suffers a severe hemolytic reaction that suddenly leaves her with a hemoglobin of only 7 gm/dl. Assuming no lung disease occurs from the hemolytic reaction, what will be her new PaO2, SaO2, and CaO2? a) PaO2 unchanged, SaO2 unchanged, CaO2 unchanged b) PaO2 unchanged, SaO2 unchanged, CaO2 reduced c) PaO2 reduced, SaO2 unchanged, CaO2 reduced d) PaO2 reduced, SaO2 reduced, CaO2 reduced Answer: Clinical Problem 1 b) PaO2 unchanged, SaO2 unchanged, CaO2 reduced. Hemoglobin content is suddenly reduced by half, which will lower CaO2 by half. However, the PaO2 and SaO2 will be unaffected, since their values are independent of the content of hemoglobin present. Neither the amount of hemoglobin, nor the binding characteristics of hemoglobin, should affect the amount of dissolved oxygen, and hence should not affect the PaO2). Stated another way, the number of dissolved oxygen molecules is independent of the amount of hemoglobin or what is bound to it. To repeat one more time (because it is so important), PaO2 is not a function of hemoglobin content or of its characteristics, but only of the alveolar PO2 and the lung architecture (alveolar-capillary interface). This explains why, for example, patients with severe anemia or carbon monoxide poisoning or methemoglobinemia can (and often do) have a normal PaO2.
19 19 Clinical Problem 2 State which of the following situations would be expected to lower PaO2. a) anemia. b) carbon monoxide toxicity. c) an abnormal hemoglobin that holds oxygen with half the affinity of normal hemoglobin. d) an abnormal hemoglobin that holds oxygen with twice the affinity of normal hemoglobin. e) lung disease with intra-pulmonary shunting. Note: A pulmonary shunt is a physiological condition which results when the alveoli of the lungs are perfused with blood as normal, but ventilation (the supply of air) fails to supply the perfused region. A pulmonary shunt often occurs when the alveoli fill with fluid, causing parts of the lung to be unventilated although they are still perfused. An Example would be pulmonary edema and conditions such as pneumonia. Answer to Clinical Problem 2. Of the choices given only answer e), lung disease with intra-pulmonary shunting, would be expected to lower PaO2. The other choices represent changes in hemoglobin content and binding and should not (by themselves) lower PaO2. Clinical Problem 3 Test your understanding by answering the following statements a-h as either True or False. a. If the lungs and heart are normal, then PaO2 is affected only by the alveolar PO2. b. In a person with normal heart and lungs, anemia should not lower the PaO2. c. PaO2 will go up in a patient with hemolysis of red blood cells, as dissolved oxygen is given off when the cells lyse. d. As the oxygen dissociation curve shifts to the right, PaO2 rises since less oxygen is bound to hemoglobin. e. An anemic patient who receives a blood transfusion should experience a rise in both SaO2 and CaO2. f. The PaO2 in a cup of water is zero since there is no blood perfusing the water. g. The SaO2 in a cup of water is zero since there is no hemoglobin present. h. The CaO2 in a cup of water is zero since there is no hemoglobin present. Answers to Clinical Problem 3 Test your understanding by answering the following statements a-h as either True or False. a. If the lungs and heart are normal, then PaO2 is affected only by the alveolar PO2. True b. In a person with normal heart and lungs, anemia should not lower the PaO2. True c. PaO2 will go up in a patient with hemolysis of red blood cells, as dissolved oxygen is given off when the cells lyse. False d. As the oxyhemoglobin dissociation curve shifts to the right, PaO2 rises since less oxygen is bound to hemoglobin. False e. An anemic patient who receives a blood transfusion should experience a rise in both SaO2 and CaO2. False. SaO2 not affected but the CaO2 will be increased. f. The PaO2 in a cup of water is zero since there is no blood perfusing the water. False g. The SaO2 in a cup of water is zero since there is no hemoglobin present. True h. The CaO2 in a cup of water is zero since there is no hemoglobin present. False
Homeostasis. The body must maintain a delicate balance of acids and bases.
 Homeostasis The body must maintain a delicate balance of acids and bases. Metabolic and respiratory processes must work together to keep hydrogen ion (H+) levels normal and stable. ph of Blood The ph of
Homeostasis The body must maintain a delicate balance of acids and bases. Metabolic and respiratory processes must work together to keep hydrogen ion (H+) levels normal and stable. ph of Blood The ph of
Acid/Base Homeostasis (Part 4)
 Acid/Base Homeostasis (Part 4) Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 5. The newly formed bicarbonate moves into the plasma.
Acid/Base Homeostasis (Part 4) Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 5. The newly formed bicarbonate moves into the plasma.
6 Easy Steps to ABG Analysis
 6 Easy Steps to ABG Analysis E-Booklet David W. Woodruff, MSN, RN- BC, CNS, CMSRN, CEN 571 Ledge Road, Macedonia, OH 44056 Telephone (800) 990-2629 Fax (800) 990-2585 1997-2012 Ed4Nurses, Inc. All rights
6 Easy Steps to ABG Analysis E-Booklet David W. Woodruff, MSN, RN- BC, CNS, CMSRN, CEN 571 Ledge Road, Macedonia, OH 44056 Telephone (800) 990-2629 Fax (800) 990-2585 1997-2012 Ed4Nurses, Inc. All rights
ACID- BASE and ELECTROLYTE BALANCE. MGHS School of EMT-Paramedic Program 2011
 ACID- BASE and ELECTROLYTE BALANCE MGHS School of EMT-Paramedic Program 2011 ACID- BASE BALANCE Ions balance themselves like a see-saw. Solutions turn into acids when concentration of hydrogen ions rises
ACID- BASE and ELECTROLYTE BALANCE MGHS School of EMT-Paramedic Program 2011 ACID- BASE BALANCE Ions balance themselves like a see-saw. Solutions turn into acids when concentration of hydrogen ions rises
Eileen Whitehead 2010 East Lancashire HC NHS Trust
 Eileen Whitehead 2010 East Lancashire HC NHS Trust 1 Introduction: Arterial blood gas analysis is an essential part of diagnosing and managing a patient s oxygenation status and acid-base balance However,
Eileen Whitehead 2010 East Lancashire HC NHS Trust 1 Introduction: Arterial blood gas analysis is an essential part of diagnosing and managing a patient s oxygenation status and acid-base balance However,
Acid-Base Balance and the Anion Gap
 Acid-Base Balance and the Anion Gap 1. The body strives for electrical neutrality. a. Cations = Anions b. One of the cations is very special, H +, and its concentration is monitored and regulated very
Acid-Base Balance and the Anion Gap 1. The body strives for electrical neutrality. a. Cations = Anions b. One of the cations is very special, H +, and its concentration is monitored and regulated very
Interpretation of the Arterial Blood Gas Self-Learning Packet
 Interpretation of the Arterial Blood Gas Self-Learning Packet * See SWIFT for list of qualifying boards for continuing education hours. Table of Contents Purpose... 3 Objectives... 3 Instructions... 4
Interpretation of the Arterial Blood Gas Self-Learning Packet * See SWIFT for list of qualifying boards for continuing education hours. Table of Contents Purpose... 3 Objectives... 3 Instructions... 4
Determinants of Blood Oxygen Content Instructor s Guide
 Determinants of Blood Oxygen Content Instructor s Guide Time to Complete This activity will take approximately 75 minutes, but can be shortened depending on how much time the instructor takes to review
Determinants of Blood Oxygen Content Instructor s Guide Time to Complete This activity will take approximately 75 minutes, but can be shortened depending on how much time the instructor takes to review
Ventilation Perfusion Relationships
 Ventilation Perfusion Relationships VENTILATION PERFUSION RATIO Ideally, each alveolus in the lungs would receive the same amount of ventilation and pulmonary capillary blood flow (perfusion). In reality,
Ventilation Perfusion Relationships VENTILATION PERFUSION RATIO Ideally, each alveolus in the lungs would receive the same amount of ventilation and pulmonary capillary blood flow (perfusion). In reality,
BLOOD GAS VARIATIONS. Respiratory Values PCO2 35-45 mmhg Normal range. PCO2 ( > 45) ph ( < 7.35) Respiratory Acidosis
 BLOOD GAS VARIATIONS 1 BLOOD ph Normal range 7.35 7.45 Think of 7.40 as your new 0 or neutral If the reading is below 7.4 it is acid. Below 7.35 it is acid out of range or Acidosis If the reading is above
BLOOD GAS VARIATIONS 1 BLOOD ph Normal range 7.35 7.45 Think of 7.40 as your new 0 or neutral If the reading is below 7.4 it is acid. Below 7.35 it is acid out of range or Acidosis If the reading is above
ACID-BASE BALANCE AND ACID-BASE DISORDERS. I. Concept of Balance A. Determination of Acid-Base status 1. Specimens used - what they represent
 ACID-BASE BALANCE AND ACID-BASE DISORDERS I. Concept of Balance A. Determination of Acid-Base status 1. Specimens used - what they represent II. Electrolyte Composition of Body Fluids A. Extracellular
ACID-BASE BALANCE AND ACID-BASE DISORDERS I. Concept of Balance A. Determination of Acid-Base status 1. Specimens used - what they represent II. Electrolyte Composition of Body Fluids A. Extracellular
TRANSPORT OF BLOOD GASES From The Lungs To The Tissues & Back
 TRANSPORT OF BLOOD GASES From The Lungs To The Tissues & Back Dr. Sally Osborne Department of Cellular & Physiological Sciences University of British Columbia Room 3602, D.H Copp Building 604 822-3421
TRANSPORT OF BLOOD GASES From The Lungs To The Tissues & Back Dr. Sally Osborne Department of Cellular & Physiological Sciences University of British Columbia Room 3602, D.H Copp Building 604 822-3421
Acid/Base Homeostasis (Part 3)
 Acid/Base Homeostasis (Part 3) Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 27. Effect of Hypoventilation Now let's look at how the
Acid/Base Homeostasis (Part 3) Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 27. Effect of Hypoventilation Now let's look at how the
Fluid, Electrolyte & ph Balance
 , Electrolyte & ph Balance / Electrolyte / AcidBase Balance Body s: Cell function depends not only on continuous nutrient supply / waste removal, but also on the physical / chemical homeostasis of surrounding
, Electrolyte & ph Balance / Electrolyte / AcidBase Balance Body s: Cell function depends not only on continuous nutrient supply / waste removal, but also on the physical / chemical homeostasis of surrounding
Understanding Hypoventilation and Its Treatment by Susan Agrawal
 www.complexchild.com Understanding Hypoventilation and Its Treatment by Susan Agrawal Most of us have a general understanding of what the term hyperventilation means, since hyperventilation, also called
www.complexchild.com Understanding Hypoventilation and Its Treatment by Susan Agrawal Most of us have a general understanding of what the term hyperventilation means, since hyperventilation, also called
Arterial Blood Gas Case Questions and Answers
 Arterial Blood Gas Case Questions and Answers In the space that follows you will find a series of cases that include arterial blood gases. Each case is then followed by an explanation of the acid-base
Arterial Blood Gas Case Questions and Answers In the space that follows you will find a series of cases that include arterial blood gases. Each case is then followed by an explanation of the acid-base
What, roughly, is the dividing line between the upper and lower respiratory tract? The larynx. What s the difference between the conducting zone and
 What, roughly, is the dividing line between the upper and lower respiratory tract? The larynx. What s the difference between the conducting zone and the respiratory zone? Conducting zone is passageways
What, roughly, is the dividing line between the upper and lower respiratory tract? The larynx. What s the difference between the conducting zone and the respiratory zone? Conducting zone is passageways
Acid-Base Balance and Renal Acid Excretion
 AcidBase Balance and Renal Acid Excretion Objectives By the end of this chapter, you should be able to: 1. Cite the basic principles of acidbase physiology. 2. Understand the bicarbonatecarbon dioxide
AcidBase Balance and Renal Acid Excretion Objectives By the end of this chapter, you should be able to: 1. Cite the basic principles of acidbase physiology. 2. Understand the bicarbonatecarbon dioxide
Select the one that is the best answer:
 MQ Kidney 1 Select the one that is the best answer: 1) n increase in the concentration of plasma potassium causes increase in: a) release of renin b) secretion of aldosterone c) secretion of H d) release
MQ Kidney 1 Select the one that is the best answer: 1) n increase in the concentration of plasma potassium causes increase in: a) release of renin b) secretion of aldosterone c) secretion of H d) release
PULMONARY PHYSIOLOGY
 I. Lung volumes PULMONARY PHYSIOLOGY American College of Surgeons SCC Review Course Christopher P. Michetti, MD, FACS and Forrest O. Moore, MD, FACS A. Tidal volume (TV) is the volume of air entering and
I. Lung volumes PULMONARY PHYSIOLOGY American College of Surgeons SCC Review Course Christopher P. Michetti, MD, FACS and Forrest O. Moore, MD, FACS A. Tidal volume (TV) is the volume of air entering and
Gas Exchange Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.
 Gas Exchange Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.com/bc) Page 1. Introduction Oxygen and carbon dioxide diffuse between
Gas Exchange Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.com/bc) Page 1. Introduction Oxygen and carbon dioxide diffuse between
IMPAIRED BLOOD-GAS EXCHANGE. Intraoperative blood gas analysis
 IMPAIRED BLOOD-GAS EXCHANGE Intraoperative blood gas analysis When do you perform BGA Intraoperatively? Informe actual NEVER Routine:Thoracic Thoracic, Cardiac,Neurosurgery Emergency situation Drop in
IMPAIRED BLOOD-GAS EXCHANGE Intraoperative blood gas analysis When do you perform BGA Intraoperatively? Informe actual NEVER Routine:Thoracic Thoracic, Cardiac,Neurosurgery Emergency situation Drop in
Rules on Oxygen Therapy:
 Rules on Oxygen Therapy: Physiology: 1. PO 2, SaO 2, CaO 2 are all related but different. 2. PaO2 is a sensitive and non-specific indicator of the lungs ability to exchange gases with the atmosphere. 3.
Rules on Oxygen Therapy: Physiology: 1. PO 2, SaO 2, CaO 2 are all related but different. 2. PaO2 is a sensitive and non-specific indicator of the lungs ability to exchange gases with the atmosphere. 3.
Oxygenation. Chapter 21. Anatomy and Physiology of Breathing. Anatomy and Physiology of Breathing*
 Oxygenation Chapter 21 Anatomy and Physiology of Breathing Inspiration ~ breathing in Expiration ~ breathing out Ventilation ~ Movement of air in & out of the lungs Respiration ~ exchange of O2 & carbon
Oxygenation Chapter 21 Anatomy and Physiology of Breathing Inspiration ~ breathing in Expiration ~ breathing out Ventilation ~ Movement of air in & out of the lungs Respiration ~ exchange of O2 & carbon
Approach to the Patient with Acid-Base Problems. Maintenance of Normal ph. Henderson - Hasselbach Equation. normal ph = 7.40 --> [H + ] = 40 neq / L
![Approach to the Patient with Acid-Base Problems. Maintenance of Normal ph. Henderson - Hasselbach Equation. normal ph = 7.40 --> [H + ] = 40 neq / L Approach to the Patient with Acid-Base Problems. Maintenance of Normal ph. Henderson - Hasselbach Equation. normal ph = 7.40 --> [H + ] = 40 neq / L](/thumbs/26/8280150.jpg) Approach to the Patient with Acid-Base Problems Maintenance of Normal ph normal ph = 7.40 --> [H + ] = 40 neq / L H 2 O + CO 2 H 2 CO 3 H + + HCO 3 - dietary breakdown of protein (about 80 meq
Approach to the Patient with Acid-Base Problems Maintenance of Normal ph normal ph = 7.40 --> [H + ] = 40 neq / L H 2 O + CO 2 H 2 CO 3 H + + HCO 3 - dietary breakdown of protein (about 80 meq
ACID-BASE DISORDER. Presenter: NURUL ATIQAH AWANG LAH Preceptor: PN. KHAIRUL BARIAH JOHAN
 ACID-BASE DISORDER Presenter: NURUL ATIQAH AWANG LAH Preceptor: PN. KHAIRUL BARIAH JOHAN OBJECTIVES OF PRESENTATION 1. To refresh knowledge of acid-base disorders 2. To evaluate acid-base disorders using
ACID-BASE DISORDER Presenter: NURUL ATIQAH AWANG LAH Preceptor: PN. KHAIRUL BARIAH JOHAN OBJECTIVES OF PRESENTATION 1. To refresh knowledge of acid-base disorders 2. To evaluate acid-base disorders using
Gas Exchange. Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com)
 Gas Exchange Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Oxygen and carbon dioxide diffuse between the alveoli
Gas Exchange Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Oxygen and carbon dioxide diffuse between the alveoli
Oxygenation and Oxygen Therapy Michael Billow, D.O.
 Oxygenation and Oxygen Therapy Michael Billow, D.O. The delivery of oxygen to all body tissues is the essence of critical care. Patients in respiratory distress/failure come easily to mind as the ones
Oxygenation and Oxygen Therapy Michael Billow, D.O. The delivery of oxygen to all body tissues is the essence of critical care. Patients in respiratory distress/failure come easily to mind as the ones
Chapter 1 Dissolved Oxygen in the Blood
 Chapter 1 Dissolved Oxygen in the Blood Say we have a volume of blood, which we ll represent as a beaker of fluid. Now let s include oxygen in the gas above the blood (represented by the green circles).
Chapter 1 Dissolved Oxygen in the Blood Say we have a volume of blood, which we ll represent as a beaker of fluid. Now let s include oxygen in the gas above the blood (represented by the green circles).
TRANSPORT OF OXYGEN AND CARBON DIOXIDE IN BLOOD
 TRANSPORT OF OXYGEN AND CARBON DIOXIDE IN BLOOD CONTENTS INTRODUCTION OXYGEN CASCADE OXYGEN DELIVERY DURING EXERCISE OXYGEN DELIVERY DURING CRITICAL ILLNESS CARBON DIOXIDE TRANSPORT O2 TRANSPORT REQUIREMENTS
TRANSPORT OF OXYGEN AND CARBON DIOXIDE IN BLOOD CONTENTS INTRODUCTION OXYGEN CASCADE OXYGEN DELIVERY DURING EXERCISE OXYGEN DELIVERY DURING CRITICAL ILLNESS CARBON DIOXIDE TRANSPORT O2 TRANSPORT REQUIREMENTS
Essentials of Human Anatomy & Physiology. Chapter 15. The Urinary System. Slides 15.1 15.20. Lecture Slides in PowerPoint by Jerry L.
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 15 The Urinary System Slides 15.1 15.20 Lecture Slides in PowerPoint by Jerry L. Cook Functions of the Urinary System Elimination
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 15 The Urinary System Slides 15.1 15.20 Lecture Slides in PowerPoint by Jerry L. Cook Functions of the Urinary System Elimination
2161-1 - Page 1. Name: 1) Choose the disease that is most closely related to the given phrase. Questions 10 and 11 refer to the following:
 Name: 2161-1 - Page 1 1) Choose the disease that is most closely related to the given phrase. a disease of the bone marrow characterized by uncontrolled production of white blood cells A) meningitis B)
Name: 2161-1 - Page 1 1) Choose the disease that is most closely related to the given phrase. a disease of the bone marrow characterized by uncontrolled production of white blood cells A) meningitis B)
Acid-Base Disorders. Jai Radhakrishnan, MD, MS. Objectives. Diagnostic Considerations. Step 1: Primary Disorder. Formulae. Step 2: Compensation
 Objectives Diagnostic approach to acid base disorders Common clinical examples of acidoses and alkaloses Acid-Base Disorders Jai Radhakrishnan 1 2 Diagnostic Considerations Data points required: ABG: ph,
Objectives Diagnostic approach to acid base disorders Common clinical examples of acidoses and alkaloses Acid-Base Disorders Jai Radhakrishnan 1 2 Diagnostic Considerations Data points required: ABG: ph,
Lab #11: Respiratory Physiology
 Lab #11: Respiratory Physiology Background The respiratory system enables the exchange of O 2 and CO 2 between the cells and the atmosphere, thus enabling the intake of O 2 into the body for aerobic respiration
Lab #11: Respiratory Physiology Background The respiratory system enables the exchange of O 2 and CO 2 between the cells and the atmosphere, thus enabling the intake of O 2 into the body for aerobic respiration
Acid/Base and ABG Interpretation Made Simple
 Acid/Base and ABG Interpretation Made Simple A-a a Gradient FIO2 = PA O2 + (5/4) PaCO2 FIO2 = 713 x O2% A-a a gradient = PA O2 - PaO2 Normal is 0-100 mm Hg 2.5 + 0.21 x age in years With higher inspired
Acid/Base and ABG Interpretation Made Simple A-a a Gradient FIO2 = PA O2 + (5/4) PaCO2 FIO2 = 713 x O2% A-a a gradient = PA O2 - PaO2 Normal is 0-100 mm Hg 2.5 + 0.21 x age in years With higher inspired
Hypoxia and Oxygenation Hypoxia is a serious threat to patients and escorts alike when
 Chapter 4 2 71 Hypoxia and Oxygenation Hypoxia is a serious threat to patients and escorts alike when they fly. Air medical escorts need to understand what causes hypoxia, why some people are more likely
Chapter 4 2 71 Hypoxia and Oxygenation Hypoxia is a serious threat to patients and escorts alike when they fly. Air medical escorts need to understand what causes hypoxia, why some people are more likely
Chapter 26. Assisting With Oxygen Needs. Elsevier items and derived items 2014, 2010 by Mosby, an imprint of Elsevier Inc. All rights reserved.
 Chapter 26 Assisting With Oxygen Needs Oxygen (O 2 ) is a gas. Oxygen It has no taste, odor, or color. It is a basic need required for life. Death occurs within minutes if breathing stops. Brain damage
Chapter 26 Assisting With Oxygen Needs Oxygen (O 2 ) is a gas. Oxygen It has no taste, odor, or color. It is a basic need required for life. Death occurs within minutes if breathing stops. Brain damage
LECTURE 1 RENAL FUNCTION
 LECTURE 1 RENAL FUNCTION Components of the Urinary System 2 Kidneys 2 Ureters Bladder Urethra Refer to Renal System Vocabulary in your notes Figure 2-1,page10 Kidney Composition Cortex Outer region Contains
LECTURE 1 RENAL FUNCTION Components of the Urinary System 2 Kidneys 2 Ureters Bladder Urethra Refer to Renal System Vocabulary in your notes Figure 2-1,page10 Kidney Composition Cortex Outer region Contains
ACID-BASE DISORDERS MADE SO EASY EVEN A CAVEMAN CAN DO IT
 ACIDBASE DISORDERS MADE SO EASY EVEN A CAVEMAN CAN DO IT Lorraine R Franzi, MS/HSM, RD, LDN, CNSD Nutrition Support Specialist Pittsburgh, PA I. LEARNING OBJECTIVES The clinician after participating in
ACIDBASE DISORDERS MADE SO EASY EVEN A CAVEMAN CAN DO IT Lorraine R Franzi, MS/HSM, RD, LDN, CNSD Nutrition Support Specialist Pittsburgh, PA I. LEARNING OBJECTIVES The clinician after participating in
Goals Upon completion of this course, one should be able to do the following:
 Blood Gas Analysis WWW.RN.ORG Reviewed August 2014, Expires August 2016 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2014 RN.ORG, S.A., RN.ORG, LLC By
Blood Gas Analysis WWW.RN.ORG Reviewed August 2014, Expires August 2016 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2014 RN.ORG, S.A., RN.ORG, LLC By
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Pathophysiology of hypercapnic and hypoxic respiratory failure and V/Q relationships. Dr.Alok Nath Department of Pulmonary Medicine PGIMER Chandigarh
 Pathophysiology of hypercapnic and hypoxic respiratory failure and V/Q relationships Dr.Alok Nath Department of Pulmonary Medicine PGIMER Chandigarh Jan 2006 Respiratory Failure inadequate blood oxygenation
Pathophysiology of hypercapnic and hypoxic respiratory failure and V/Q relationships Dr.Alok Nath Department of Pulmonary Medicine PGIMER Chandigarh Jan 2006 Respiratory Failure inadequate blood oxygenation
CHAPTER 1: THE LUNGS AND RESPIRATORY SYSTEM
 CHAPTER 1: THE LUNGS AND RESPIRATORY SYSTEM INTRODUCTION Lung cancer affects a life-sustaining system of the body, the respiratory system. The respiratory system is responsible for one of the essential
CHAPTER 1: THE LUNGS AND RESPIRATORY SYSTEM INTRODUCTION Lung cancer affects a life-sustaining system of the body, the respiratory system. The respiratory system is responsible for one of the essential
Quiz Urinary System. 1. The kidneys help regulate blood volume. help control blood pressure. help control ph. All of the above are correct.
 Quiz Urinary System 1. The kidneys help regulate blood volume. help control blood pressure. help control ph. All of the above are correct. 2. The location of the kidneys in relationship to the peritoneal
Quiz Urinary System 1. The kidneys help regulate blood volume. help control blood pressure. help control ph. All of the above are correct. 2. The location of the kidneys in relationship to the peritoneal
Circulatory System Review
 Circulatory System Review 1. Draw a table to describe the similarities and differences between arteries and veins? Anatomy Direction of blood flow: Oxygen concentration: Arteries Thick, elastic smooth
Circulatory System Review 1. Draw a table to describe the similarities and differences between arteries and veins? Anatomy Direction of blood flow: Oxygen concentration: Arteries Thick, elastic smooth
Renal Acid/Base. Acid Base Homeostasis... 2 H+ Balance... 2
 Renal Acid/Base By Adam Hollingworth Table of Contents Acid Base Homeostasis... 2 H+ Balance... 2 Acid Base Homeostasis... 2 Role of Kidneys in Acid- Base Homeostasis... 3 Renal H+ Secretion... 3 Proximal
Renal Acid/Base By Adam Hollingworth Table of Contents Acid Base Homeostasis... 2 H+ Balance... 2 Acid Base Homeostasis... 2 Role of Kidneys in Acid- Base Homeostasis... 3 Renal H+ Secretion... 3 Proximal
1. DEFINITION OF PHYSIOLOGY. Study of the functions of the healthy human body. How the body works. Focus on mechanisms of action.
 1. DEFINITION OF PHYSIOLOGY Study of the functions of the healthy human body. How the body works. Focus on mechanisms of action. Anatomy & Physiology: inseparable & complementary They are complementary
1. DEFINITION OF PHYSIOLOGY Study of the functions of the healthy human body. How the body works. Focus on mechanisms of action. Anatomy & Physiology: inseparable & complementary They are complementary
Chemistry 201. Practical aspects of buffers. NC State University. Lecture 15
 Chemistry 201 Lecture 15 Practical aspects of buffers NC State University The everyday ph scale To review what ph means in practice, we consider the ph of everyday substances that we know from experience.
Chemistry 201 Lecture 15 Practical aspects of buffers NC State University The everyday ph scale To review what ph means in practice, we consider the ph of everyday substances that we know from experience.
Chemical equilibria Buffer solutions
 Chemical equilibria Buffer solutions Definition The buffer solutions have the ability to resist changes in ph when smaller amounts of acid or base is added. Importance They are applied in the chemical
Chemical equilibria Buffer solutions Definition The buffer solutions have the ability to resist changes in ph when smaller amounts of acid or base is added. Importance They are applied in the chemical
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Metabolic alkalosis. ICU Fellowship Training Radboudumc
 Metabolic alkalosis ICU Fellowship Training Radboudumc Case History 28-year-old male Discovered by roommate at home in bewildering state During transport by EMS possible tonicclonic seizure Arrival in
Metabolic alkalosis ICU Fellowship Training Radboudumc Case History 28-year-old male Discovered by roommate at home in bewildering state During transport by EMS possible tonicclonic seizure Arrival in
- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.
![- Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells. - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - lungs provide oxygen to blood, blood brings oxygen to the cells.](/thumbs/40/21014327.jpg) Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Cellular respiration - how cells make energy - Oxygen is needed for cellular respiration [OVERHEAD, fig. 6.2, p. 90 / 4th: 6.1] - ATP - this is provided by the lungs - lungs provide oxygen to blood, blood
Dr. Johnson PA Renal Winter 2010
 1 Renal Control of Acid/Base Balance Dr. Johnson PA Renal Winter 2010 Acid/Base refers to anything having to do with the concentrations of H + ions in aqueous solutions. In medical physiology, we are concerned
1 Renal Control of Acid/Base Balance Dr. Johnson PA Renal Winter 2010 Acid/Base refers to anything having to do with the concentrations of H + ions in aqueous solutions. In medical physiology, we are concerned
Return to Lab Menu. Stoichiometry Exploring the Reaction between Baking Soda and Vinegar
 Return to Lab Menu Stoichiometry Exploring the Reaction between Baking Soda and Vinegar Objectives -to observe and measure mass loss in a gas forming reaction -to calculate CO 2 loss and correlate to a
Return to Lab Menu Stoichiometry Exploring the Reaction between Baking Soda and Vinegar Objectives -to observe and measure mass loss in a gas forming reaction -to calculate CO 2 loss and correlate to a
Pulmonary Diseases. Lung Disease: Pathophysiology, Medical and Exercise Programming. Overview of Pathophysiology
 Lung Disease: Pathophysiology, Medical and Exercise Programming Overview of Pathophysiology Ventilatory Impairments Increased airway resistance Reduced compliance Increased work of breathing Ventilatory
Lung Disease: Pathophysiology, Medical and Exercise Programming Overview of Pathophysiology Ventilatory Impairments Increased airway resistance Reduced compliance Increased work of breathing Ventilatory
Metabolism: Cellular Respiration, Fermentation and Photosynthesis
 Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Case Study. Objectives
 Case Study One in a series of case studies developed to stimulate enhancement of problem-solving techniques for physicians and nurses and paramedical personnel when challenged by patients who present with
Case Study One in a series of case studies developed to stimulate enhancement of problem-solving techniques for physicians and nurses and paramedical personnel when challenged by patients who present with
Functions of Blood System. Blood Cells
 Functions of Blood System Transport: to and from tissue cells Nutrients to cells: amino acids, glucose, vitamins, minerals, lipids (as lipoproteins). Oxygen: by red blood corpuscles (oxyhaemoglobin - 4
Functions of Blood System Transport: to and from tissue cells Nutrients to cells: amino acids, glucose, vitamins, minerals, lipids (as lipoproteins). Oxygen: by red blood corpuscles (oxyhaemoglobin - 4
Paramedic Program Anatomy and Physiology Study Guide
 Paramedic Program Anatomy and Physiology Study Guide Define the terms anatomy and physiology. List and discuss in order of increasing complexity, the body from the cell to the whole organism. Define the
Paramedic Program Anatomy and Physiology Study Guide Define the terms anatomy and physiology. List and discuss in order of increasing complexity, the body from the cell to the whole organism. Define the
Name Date Period. Keystone Review Enzymes
 Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
Name Date Period Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells
UNIT 3 : MAINTAINING DYNAMIC EQUILIBRIUM
 BIOLOGY - 2201 UNIT 3 : MAINTAINING DYNAMIC EQUILIBRIUM What happens to your body as you run? Breathing, heart rate, temperature, muscle pain, thirsty... Homeotasis Homeostasis is the process of maintaining
BIOLOGY - 2201 UNIT 3 : MAINTAINING DYNAMIC EQUILIBRIUM What happens to your body as you run? Breathing, heart rate, temperature, muscle pain, thirsty... Homeotasis Homeostasis is the process of maintaining
STAGE 5: Interacting Systems
 Stage 5: In this stage, students will explore how systems interact with each other to maintain healthy and optimal body functioning, including how the body responds to changes in the environment such as
Stage 5: In this stage, students will explore how systems interact with each other to maintain healthy and optimal body functioning, including how the body responds to changes in the environment such as
Our Human Body On-site student activities Years 5 6
 Our Human Body On-site student activities Years 5 6 Our Human Body On-site student activities: Years 5-6 Student activity (and record) sheets have been developed with alternative themes for students to
Our Human Body On-site student activities Years 5 6 Our Human Body On-site student activities: Years 5-6 Student activity (and record) sheets have been developed with alternative themes for students to
MECHINICAL VENTILATION S. Kache, MD
 MECHINICAL VENTILATION S. Kache, MD Spontaneous respiration vs. Mechanical ventilation Natural spontaneous ventilation occurs when the respiratory muscles, diaphragm and intercostal muscles pull on the
MECHINICAL VENTILATION S. Kache, MD Spontaneous respiration vs. Mechanical ventilation Natural spontaneous ventilation occurs when the respiratory muscles, diaphragm and intercostal muscles pull on the
Enzymes. A. a lipid B. a protein C. a carbohydrate D. a mineral
 Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Enzymes 1. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following
Water Homeostasis. Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Water Homeostasis Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 1. Water Homeostasis The body maintains a balance of water intake
Water Homeostasis Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 1. Water Homeostasis The body maintains a balance of water intake
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
HUMAN ANATOMY & PHYSIOLOGY MAINTENANCE 30
 Curriculum Development In the Fairfield Public Schools FAIRFIELD PUBLIC SCHOOLS FAIRFIELD, CONNECTICUT HUMAN ANATOMY & PHYSIOLOGY MAINTENANCE 30 Board of Education Approved 05/22/2007 HUMAN ANATOMY & PHYSIOLOGY
Curriculum Development In the Fairfield Public Schools FAIRFIELD PUBLIC SCHOOLS FAIRFIELD, CONNECTICUT HUMAN ANATOMY & PHYSIOLOGY MAINTENANCE 30 Board of Education Approved 05/22/2007 HUMAN ANATOMY & PHYSIOLOGY
Oxygen Dissociation Curve
 122 Visit http://www.anaesthesiamcq.com for details Chapter 4 Dissociation Curve Can you draw the oxygen dissociation curve of normal adult haemoglobin? How many points on the curve can you indicate with
122 Visit http://www.anaesthesiamcq.com for details Chapter 4 Dissociation Curve Can you draw the oxygen dissociation curve of normal adult haemoglobin? How many points on the curve can you indicate with
Acid-Base Disorders. Jai Radhakrishnan, MD, MS
 Acid-Base Disorders Jai Radhakrishnan, MD, MS 1 Diagnostic Considerations Data points required: ABG: ph, pco 2, HCO 3 Chem-7 panel: anion gap Step 1: Acidemia/alkalemia (Primary disorder) Step 2: Compensation
Acid-Base Disorders Jai Radhakrishnan, MD, MS 1 Diagnostic Considerations Data points required: ABG: ph, pco 2, HCO 3 Chem-7 panel: anion gap Step 1: Acidemia/alkalemia (Primary disorder) Step 2: Compensation
Syllabus for Biology 2402 Human Anatomy & Physiology 2 [This is a generic syllabus. Each instructor will give a syllabus customized for their course.
 Syllabus for Biology 2402 Human Anatomy & Physiology 2 [This is a generic syllabus. Each instructor will give a syllabus customized for their course.] Course Description Human Anatomy and Physiology II
Syllabus for Biology 2402 Human Anatomy & Physiology 2 [This is a generic syllabus. Each instructor will give a syllabus customized for their course.] Course Description Human Anatomy and Physiology II
240- PROBLEM SET INSERTION OF SWAN-GANZ SYSTEMIC VASCULAR RESISTANCE. Blood pressure = f(cardiac output and peripheral resistance)
 240- PROBLEM SET INSERTION OF SWAN-GANZ 50 kg Pig Rt Jugular 0 cm Rt Atrium 10 cm Rt ventricle 15 cm Wedge 20-25 cm SYSTEMIC VASCULAR RESISTANCE Blood pressure = f(cardiac output and peripheral resistance)
240- PROBLEM SET INSERTION OF SWAN-GANZ 50 kg Pig Rt Jugular 0 cm Rt Atrium 10 cm Rt ventricle 15 cm Wedge 20-25 cm SYSTEMIC VASCULAR RESISTANCE Blood pressure = f(cardiac output and peripheral resistance)
Understanding Pulmonary Function Testing. PFTs, Blood Gases and Oximetry Skinny Little Reference Guide
 Understanding Pulmonary Function Testing PFTs, Blood Gases and Oximetry Skinny Little Reference Guide INTRODUCTION This brochure is intended to help you understand the meaning of Pulmonary Function Testing,
Understanding Pulmonary Function Testing PFTs, Blood Gases and Oximetry Skinny Little Reference Guide INTRODUCTION This brochure is intended to help you understand the meaning of Pulmonary Function Testing,
Respiratory failure and Oxygen Therapy
 Respiratory failure and Oxygen Therapy A patient with Hb 15 G % will carry 3X more O2 in his blood than someone with Hb 5G % Give Controlled O2 treatment in acute pulmonary oedema to avoid CO2 retention
Respiratory failure and Oxygen Therapy A patient with Hb 15 G % will carry 3X more O2 in his blood than someone with Hb 5G % Give Controlled O2 treatment in acute pulmonary oedema to avoid CO2 retention
Anatomy and Physiology: Understanding the Importance of CPR
 Anatomy and Physiology: Understanding the Importance of CPR Overview This document gives you more information about the body s structure (anatomy) and function (physiology). This information will help
Anatomy and Physiology: Understanding the Importance of CPR Overview This document gives you more information about the body s structure (anatomy) and function (physiology). This information will help
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Renal Topics 1) renal function 2) renal system 3) urine formation 4) urine & urination 5) renal diseases
 Renal Topics 1) renal function 2) renal system 3) urine formation 4) urine & urination 5) renal diseases 1/9/2015 Renal Biology - Sandra Hsu 1 Renal Functions 1) excrete metabolic wastes (blood cleaning)
Renal Topics 1) renal function 2) renal system 3) urine formation 4) urine & urination 5) renal diseases 1/9/2015 Renal Biology - Sandra Hsu 1 Renal Functions 1) excrete metabolic wastes (blood cleaning)
12.1: The Function of Circulation page 478
 12.1: The Function of Circulation page 478 Key Terms: Circulatory system, heart, blood vessel, blood, open circulatory system, closed circulatory system, pulmonary artery, pulmonary vein, aorta, atrioventricular
12.1: The Function of Circulation page 478 Key Terms: Circulatory system, heart, blood vessel, blood, open circulatory system, closed circulatory system, pulmonary artery, pulmonary vein, aorta, atrioventricular
FIGURE 2.18. A. The phosphate end of the molecule is polar (charged) and hydrophilic (attracted to water).
 PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
Respiration occurs in the mitochondria in cells.
 B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
B3 Question Which process occurs in the mitochondria in cells? Why do the liver and muscle cells have large number of mitochondria? What is the function of the ribosomes? Answer Respiration occurs in the
Chapter 23. Composition and Properties of Urine
 Chapter 23 Composition and Properties of Urine Composition and Properties of Urine urinalysis the examination of the physical and chemical properties of urine appearance - clear, almost colorless to deep
Chapter 23 Composition and Properties of Urine Composition and Properties of Urine urinalysis the examination of the physical and chemical properties of urine appearance - clear, almost colorless to deep
2.06 Understand the functions and disorders of the respiratory system
 2.06 Understand the functions and disorders of the respiratory system 2.06 Understand the functions and disorders of the respiratory system Essential questions What are the functions of the respiratory
2.06 Understand the functions and disorders of the respiratory system 2.06 Understand the functions and disorders of the respiratory system Essential questions What are the functions of the respiratory
Human Anatomy & Physiology I with Dr. Hubley. Practice Exam 1
 Human Anatomy & Physiology I with Dr. Hubley Practice Exam 1 1. Which definition is the best definition of the term gross anatomy? a. The study of cells. b. The study of tissues. c. The study of structures
Human Anatomy & Physiology I with Dr. Hubley Practice Exam 1 1. Which definition is the best definition of the term gross anatomy? a. The study of cells. b. The study of tissues. c. The study of structures
1. Enzymes. Biochemical Reactions. Chapter 5: Microbial Metabolism. 1. Enzymes. 2. ATP Production. 3. Autotrophic Processes
 Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
1. Give the name and functions of the structure labeled A on the diagram. 2. Give the name and functions of the structure labeled B on the diagram.
 2013 ANATOMY & PHYSIOLOGY Sample Tournament Station A: Use the diagram in answering Questions 1-5. 1. Give the name and functions of the structure labeled A on the diagram. 2. Give the name and functions
2013 ANATOMY & PHYSIOLOGY Sample Tournament Station A: Use the diagram in answering Questions 1-5. 1. Give the name and functions of the structure labeled A on the diagram. 2. Give the name and functions
Objectives COPD. Chronic Obstructive Pulmonary Disease (COPD) 4/19/2011
 Objectives Discuss assessment findings and treatment for: Chronic Obstructive Pulmonary Disease Bronchitis Emphysema Asthma Anaphylaxis Other respiratory issues Provide some definitions Chronic Obstructive
Objectives Discuss assessment findings and treatment for: Chronic Obstructive Pulmonary Disease Bronchitis Emphysema Asthma Anaphylaxis Other respiratory issues Provide some definitions Chronic Obstructive
1 The diagram shows blood as seen under a microscope. Which identifies parts P, Q, R and S of the blood?
 1 1 The diagram shows blood as seen under a microscope. Which identifies parts P, Q, R and S of the blood? 2 The plan shows the blood system of a mammal. What does the part labelled X represent? A heart
1 1 The diagram shows blood as seen under a microscope. Which identifies parts P, Q, R and S of the blood? 2 The plan shows the blood system of a mammal. What does the part labelled X represent? A heart
The digestive system eliminated waste from the digestive tract. But we also need a way to eliminate waste from the rest of the body.
 Outline Urinary System Urinary System and Excretion Bio105 Lecture 20 Chapter 16 I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system 1
Outline Urinary System Urinary System and Excretion Bio105 Lecture 20 Chapter 16 I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system 1
Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems
 Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems Myoglobin and Hemoglobin Oxygen is required for oxidative metabolism
Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems Myoglobin and Hemoglobin Oxygen is required for oxidative metabolism
Chapter 16: Circulation
 Section 1 (The Body s Transport System) Chapter 16: Circulation 7 th Grade Cardiovascular system (the circulatory system) includes the heart, blood vessels, and blood carries needed substances to the cells
Section 1 (The Body s Transport System) Chapter 16: Circulation 7 th Grade Cardiovascular system (the circulatory system) includes the heart, blood vessels, and blood carries needed substances to the cells
Department of Surgery
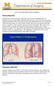 What is emphysema? 2004 Regents of the University of Michigan Emphysema is a chronic disease of the lungs characterized by thinning and overexpansion of the lung-like blisters (bullae) in the lung tissue.
What is emphysema? 2004 Regents of the University of Michigan Emphysema is a chronic disease of the lungs characterized by thinning and overexpansion of the lung-like blisters (bullae) in the lung tissue.
Interpretation of Laboratory Values
 Interpretation of Laboratory Values Konrad J. Dias PT, DPT, CCS Overview Electrolyte imbalances Renal Function Tests Complete Blood Count Coagulation Profile Fluid imbalance Sodium Electrolyte Imbalances
Interpretation of Laboratory Values Konrad J. Dias PT, DPT, CCS Overview Electrolyte imbalances Renal Function Tests Complete Blood Count Coagulation Profile Fluid imbalance Sodium Electrolyte Imbalances
Vtial sign #1: PULSE. Vital Signs: Assessment and Interpretation. Factors that influence pulse rate: Importance of Vital Signs
 Vital Signs: Assessment and Interpretation Elma I. LeDoux, MD, FACP, FACC Associate Professor of Medicine Vtial sign #1: PULSE Reflects heart rate (resting 60-90/min) Should be strong and regular Use 2
Vital Signs: Assessment and Interpretation Elma I. LeDoux, MD, FACP, FACC Associate Professor of Medicine Vtial sign #1: PULSE Reflects heart rate (resting 60-90/min) Should be strong and regular Use 2
Diabetic Emergencies. David Hill, D.O.
 Diabetic Emergencies David Hill, D.O. Class Outline Diabetic emergency/glucometer training Identify the different signs of insulin shock Diabetic coma, and HHNK Participants will understand the treatment
Diabetic Emergencies David Hill, D.O. Class Outline Diabetic emergency/glucometer training Identify the different signs of insulin shock Diabetic coma, and HHNK Participants will understand the treatment
Blood Pressure Regulation
 Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Dehydration & Overhydration. Waseem Jerjes
 Dehydration & Overhydration Waseem Jerjes Dehydration 3 Major Types Isotonic - Fluid has the same osmolarity as plasma Hypotonic -Fluid has fewer solutes than plasma Hypertonic-Fluid has more solutes than
Dehydration & Overhydration Waseem Jerjes Dehydration 3 Major Types Isotonic - Fluid has the same osmolarity as plasma Hypotonic -Fluid has fewer solutes than plasma Hypertonic-Fluid has more solutes than
Work and Energy in Muscles
 Work and Energy in Muscles Why can't I sprint forever? I'll start this section with that silly question. What lies behind the undisputable observation that we must reduce speed if we want to run longer
Work and Energy in Muscles Why can't I sprint forever? I'll start this section with that silly question. What lies behind the undisputable observation that we must reduce speed if we want to run longer
ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND
 #3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
#3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
GLUCOSE HOMEOSTASIS-II: An Overview
 GLUCOSE HOMEOSTASIS-II: An Overview University of Papua New Guinea School of Medicine & Health Sciences, Division of Basic Medical Sciences Discipline of Biochemistry & Molecular Biology, M Med Part I
GLUCOSE HOMEOSTASIS-II: An Overview University of Papua New Guinea School of Medicine & Health Sciences, Division of Basic Medical Sciences Discipline of Biochemistry & Molecular Biology, M Med Part I
