Learning Guide for Chapter 6 - Stereochemistry
|
|
|
- Brice Franklin
- 7 years ago
- Views:
Transcription
1 Learning Guide for Chapter 6 - tereochemistry I. tereoisomers containing CC p 1 II. Introduction to ity - compounds with one stereoisomer - p 5 Chirality Recognizing compounds Labeling stereocenters as R or III. Compounds with two or more stereoisomers - p 9 Diastereomers and meso compounds Fischer projections IV. Physical properties of compounds - p 14 Melting point, boiling point, etc Biological environments ptical activity eparation of Determination of stereochemical correlation I. tereoisomers containing CC What do two compounds which are constitutional isomers have in common? ow are they different? same molecular formula different bonding sequence Give the molecular formula and one constitutional isomer for each of the following compounds. C 7 14 C 7 16 Are these compounds constitutional isomers of each other? no - different formula What do two compounds which are stereoisomers have in common? ow are the different? same molecular formula same bonding sequence different shape stereoisomers can only occur under certain conditions Draw line structures for two different molecules with the condensed structure shown. C 3 CCC 3 make models!
2 What keeps these two compounds from being the same? LG Ch 6 p 2 they can't rotate around the CC draw a picture of sigma and pi bonds Why aren't these two structures the same as the ones above? What relationship do they have? staggered eclipsed can rotate around C-C bond two conformations of the same compound Draw a constitutional isomer, a stereoisomer, and a conformation of the following compound. constitutional stereoisomer conformation isomer same formula? same bonding sequence? same shape? same compound? yes yes yes no yes yes no no no no no yes ow many stereoisomers are possible for a compound containing a CC? two max Do all compounds containing a CC have stereoisomers? no ow many stereoisomers does the following compound have? Why? only one 's on one side are the same ow can you tell if a compound with a CC will have a stereoisomer? look at both sides of the CC; both groups must be different from each other
3 LG Ch 6 p 3 Determine whether or not the compounds below will have a stereoisomer, and for those that will, draw it. yes no same! no yes C 3 's the same yes yes no 's the same, ring the same also no can't switch because of the ring! no What is a stereocenter? carbon - switch two substituents, get a new compound new compound! #2 carbon is a stereocenter #2 and #3 are both stereocenters same thing - not an isomer (can just turn over) Can a compound containing a CC have only one stereocenter? no - it must either have 2, or none
4 When can we use cis and trans to label the two stereoisomers? LG Ch 6 p 4 when there is only one substituent on each side of the CC Which of these is cis, and which is trans? trans cis opposite sides same side this is an older system Why can't cis and trans be used on the following compound? more than one group on each side - can't tell what to compare In the following examples, the #1 substituent has priority over the #2 substituent. Which is E and which is Z? E Z Z same side 2 2 cis - Z trans - E Label the following as E or Z and explain why. C > C > C C C > C C E Z C > CC CCC If they were the same all the way out, then what? no stereochemistry C C CC C C Z Z C > C C CC Z CCC CC E C
5 II. Introduction to Chirality LG Ch 6 p 5 What does it mean to say that an object is? What about a? - its mirror image is different from the original object a - its mirror image is the same as the original object Many everyday objects are. Consider the following. Are they or a? Is the mirror image of the object the same, or different? hand scissors spoon a gloves fork a nail a knife a (unless is has an edge) screw socks a DNA helix shoes folding student desk Tetris shapes a a a striped quilt triangles ow can a molecule be? a most common way - asymmetric carbon: 4 different groups Using models, make the compound below. Then construct its mirror image mirror image - different compound Are they the same (are they superimposable)? no
6 What is the relationship between these two compounds? LG Ch 6 p 6 same molecular formula same bonding sequence different shape non-superimposable mirror images Are the central carbon atoms stereocenters? stereoisomers Construct the compound below, and its mirror image. (type of stereoisomer) yes - switching any two substituents gives the other enantiomer mirror image - same compound Are these compounds superimposable? yes Is the original compound? no Does the original compound have an enantiomer? no ow can you determine if a molecule is? 1) Is the mirror image superimposable? only completely reliable test yes - a no - same compound a
7 2) Is there a plane of symmetry in the molecule? Yes - molecule is a. No - molecule is probably. LG Ch 6 p 7 There are other, rarer kinds of symmetry that can also make the molecule a. no plane of symmetry plane of symmetry a 3) ow many asymmetric carbons are in the molecule? None - molecule is probably not. ne - molecule is. Two or more - molecule is unless there is a plane of symmetry. one asymmetric C no asymmetric C's a more about molecules with more than one stereocenter shortly 2 asymmetric C's no plane of symmetry 2 asymmetric C's plane of symmetry a Which of the following molecules are? Which have planes of symmetry? Where are the asymmetric carbons? Where are the stereocenters? a a only one compound same! two compounds different
8 ow are labeled? LG Ch 6 p 8 1) Prioritize the groups 2) Rotate so that the last priority group is pointed away. 3) Look at the direction. clockwise - R counterclockwise - when last group is already back, you're set R use overhead of clock! when last group is forward, you're looking at it from behind go 1-2-3, then reverse when last group is in the plane of the page, use models! at 1st glance it looks R Practice: N 2 R R R ow is this label included in the name? ()-3-methylhexane 1 2 (1,2R)-1-ethyl-2-methylcyclopentane 3 4 could be or (cis)-1,4-dimethylcyclohexane (or a mixture of both!)
9 III. Compounds with two or more stereocenters LG Ch 6 p 9 Consider the following compound. ow many stereocenters does it have? ow many stereoisomers could it have? R e 2 stereocenters 4 possible stereoisomers d R d d R d Which stereoisomers are? Which stereoisomers are diastereomers? R all stereocenters are opposite e R-R RR- one stereocenter is the same, the other opposite non-superimposable mirror images non-superimposable, not mirror images RR-R RR-R -R -R Next, consider the following compound. ow many stereocenters does it have? ow many stereoisomers does it have? R same! meso 2 stereocenters 4 possible stereoisomers only 3 are different d R d Enantiomers? R e RR- Diastereomers? R-RR R-
10 What is the difference between the following compounds? LG Ch 6 p 10 a (plain of symmetry) no stereocenters - not meso a (plain of symmetry) 2 stereocenters - meso Consider the following compound. ow many stereocenters does it have? ow many stereoisomers does it have? cis meso 2 stereocenters not asymmetric - can't assign R or 4 possible stereoisomers only 2 are different d trans meso Enantiomers? none Diastereomers? cis-trans
11 LG Ch 6 p 11 All of the isomers we have seen so far can be related as follows: Isomers same molecular formula constitutional isomers different connectivity stereoisomers same connectivity, different shape non-superimposable mirror images diastereomers not mirror images 2 or more stereocenters or cis/trans assify the following compounds as identical,, diastereomers, meso, or constitutional isomers. or identical identical meso diastereomers enant. diast. diastereomers meso
12 Fischer Projections LG Ch 6 p 12 When are Fischer projections useful? showing multiple stereocenters biological molecules like sugars Draw a Fischer projection of the following molecule: lactic acid model C means C C C 3 C 3 right/left coming forward up/down going away 1) main C chain up and down most oxidized C at the top 2) substituents left or right Convert the following Fischer projection to a line structure. ribose C Why are the 's all on the right in the Fischer projection, but some up and some down on the line structure? line structure - staggered Fischer projection - eclipsed have to rotate to change between them Which shows the relationship between the stereocenters better? Fischer projection Which is the more stable conformation? line structure What is the relationship between the following molecules? C C 2 C C 2 C C 2 C C 2 diastereomers all stereocenters opposite some opposite some the same
13 LG Ch 6 p 13 C 2 C 2 same - meso plane of symmetry two stereocenters C 2 C 2 C 2 C 2 C 2 C 2 all stereocenters opposite Label each stereocenter as R or : 2 N C C 3 C 3 R R C 3 Label each of the following molecules as D or L: C C C C C 2 C 2 C 2 C 2 D-glucose L-glucose D-mannose L-mannose diastereomers What is the relationship between the first two molecules? What about the second two? What about the middle two? ince D and L only give the stereochemistry of one stereocenter, how can you tell what the rest are? each set of has a different name D, L just tells you which of the pair it is
14 IV. Physical Properties of Chiral Compounds LG Ch 6 p 14 Regular physical properties What are some of the physical properties that we regularly talk about with organic compounds? melting point, boiling point, solubility, density, color, etc ow are the properties of related? they are the same ow are these properties of diastereomers related? they are different What is the relationship of the pairs of compounds below? What would you predict about their physical properties? - same constitutional isomers - different bp 175 o C bp 175 o C bp 196 o C bp 176 o C diastereomers - different diastereomers - different mp -105 o C mp -139 o C C C C 2 C 2 mp 158 o C mp 256 o C Why is the odor of a compound an exception to this? receptors in your nose are - can detect different -carvone R-carvone (caraway seed) (spearmint) What areas of chemistry are strongly influenced by stereochemistry? Why? biochemistry, pharmaceuticals biological molecules are often - amino acids, nucleotides, carbohydrates, neurotransmitters, hormones
15 ptical Activity LG Ch 6 p 15 ow is plane polarized light different from ordinary light? ordinary light - light waves in all planes polarized light - light waves in only one plane ow do you obtain plane polarized light? put ordinary light through a filter What would happen if you put two polarizing filters together and then turned them at right angles? no light would get through Why do polarizing sunglasses work? reflected light (glare) is polarized - filter blocks it out What happens when polarized light passes through a solution containing molecules? it rotates the plane of the light waves show overhead What is this called? ow is it measured? optical rotation - angle that the plane is rotated using a polarimeter What factors affect the optical rotation? Which is the most useful? ow do we eliminate the effect of other factors? structure this is what we are interested in concentration standard concentration - 1 g/ml distance traveled - path length standard path length - 1 dm (10 cm) temperature always measure at 25 o C wavelength of light always use the same wavelength (D line of sodium) The optical rotation of a compound measured under these conditions is called: specific rotation - [!] there are tables where you can look these up
16 We can use a different concentration or path length and correct for them using what equation? Why does this work?! [!] x c x l optical rotation is directly proportional to c and l LG Ch 6 p 16 The specific rotation of ()-2-butanol is o. If a sample is made by dissolving 6 g of the compound in 40 ml of solvent, and a 2 cm cell was used to measure the optical activity, what angle of rotation will be observed? [!] o c 6 g / 40 ml! (-13.5 o )(6/40)(0.2) o l 0.2 dm If given an observed rotation, how could you figure out the specific rotation? What is the relationship between the following molecules? ow would their melting points and specific rotations be related? C [!]!/ cl C 2 same mp opposite [!] C C 2 diastereomers different mp different [!] C C 2 same mp opposite [!] C C 2 D-erythrose L-erythrose D-threose L-threose [!] o [!] o [!] o [!] o mp 164 o C mp 164 o C mp 137 o C mp 137 o C What can you tell about R and vs. + and - rotations? What can't you tell? can tell: if R is + is -; if R is -, is + why? can't tell: if R will be + or -
17 What does it mean to say a solution is optically active? LG Ch 6 p 17 it rotates the plane of polarized light - has an! Would a solution of the following compound(s) be optically active? a) molecule - yes b) a molecule - no c) meso compound, a - no d) e) f) 5 mol 5 mol 5 mol 3 mol 5 mol 5 mol racemic mixture - no (exactly cancel) could we use grams? yes - same MW 3 mol of racemic - yes some left over diastereomers, won't cancel - yes Why are racemic mixtures common? both compounds have the same energy, formed in the same amounts. If a solution has 40% optical purity, what does this mean? the optical rotation has been reduced by 40% by having some of the other enantiomer If a pure enantiomer has an optical rotation of 20 o, what rotation will a solution have which has a 40% optical purity? 8 o What equation describes this? %o.p. rotation of solution rotation of pure enantiomer
18 What proportion of will give you a 40% optical purity? LG Ch 6 p 18 % o.p. % e.e. %R - % Take 3 mol of R, 7 mol of % - 30% 40%) 40% e.e. 6g of racemic mixture 4g of excess R enantiomer If the specific rotation of ()-2-butanol is o, and a mixture of (R)- and ()-2-butanol has a rotation of -6.75, what is the optical purity of the solution? What is the % e.e.? What percentage of the two isomers is present? o 13.5 o.5 % o.p. 50% % e.e. 50% 75% - 25% 50% since is + and mixture is -, must be 75% R, 25% eparation of Enantiomers Where do enantiomerically pure compounds come from? 1. many found in nature 2. separation of (resolution) 3. stereoselective reactions (Ch 7) Why can't be separated by operations like chromatography, distillation, etc? same physical properties What must be done to separate them? turn them into diastereomers - now they will have different properties
19 Consider the following example: LG Ch 6 p 19 same properties found in nature (R)-2-butanol ()-2-butanol (R, R)-tartaric acid 2 4 react with R,R compound (R)-2-butyl (R, R)-tartrate ()-2-butyl (R, R)-tartrate diastereomers different properties! 3. separate using physical properties (R)-2-butyl (R, R)-tartrate ()-2-butyl (R, R)-tartrate 3 +, heat reverse original reaction 3 +, heat (R)-2-butanol + (R, R)-tartaric acid ()-2-butanol + (R, R)-tartaric acid remove tartaric acid 5. enantiomerically pure compounds!
20 Determining the stereochemical correlation LG Ch 6 p 20 What does it mean to determine the stereochemical correlation for a pair of? match up R and structures with + and - rotations Why is this difficult? you can't tell by looking at the structure whether it is + or - + tartaric acid - tartaric acid C C C C 2R, 3R 2, 3 What method has been developed? 1 - get a pure sample 2 - measure its rotation 3 - evaporate to get a crystal 4 - do x-ray crystallography to get 3D structure 5 - see if structure is R or nce the stereochemical correlation of one compound has been discovered, how can it be used to determine another without x-ray crystallography? change molecule in a predictable way (structure still known), measure rotation 1. B
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Molecule Projections
 Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Stereochemistry Tutorial: Drawing Enantiomers and Diastereomers
 tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Sugar Identification using Polarimetry
 Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
ORGANIC COMPOUNDS IN THREE DIMENSIONS
 (adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
(adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride
 CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
Alkanes. Chapter 1.1
 Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391)
 NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
Name Lab #3: Solubility of Organic Compounds Objectives: Introduction: soluble insoluble partially soluble miscible immiscible
 Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
1. What is the hybridization of the indicated atom in the following molecule?
 Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
IR Applied to Isomer Analysis
 DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
Molecular Models Experiment #1
 Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
1 Introduction The Scientific Method (1 of 20) 1 Introduction Observations and Measurements Qualitative, Quantitative, Inferences (2 of 20)
 The Scientific Method (1 of 20) This is an attempt to state how scientists do science. It is necessarily artificial. Here are MY five steps: Make observations the leaves on my plant are turning yellow
The Scientific Method (1 of 20) This is an attempt to state how scientists do science. It is necessarily artificial. Here are MY five steps: Make observations the leaves on my plant are turning yellow
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
experiment5 Understanding and applying the concept of limiting reagents. Learning how to perform a vacuum filtration.
 81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
Properties of a molecule. Absolute configuration, Cahn-Ingold Prelog. Planar, axial, topological chirality and chirality at atoms other than carbon
 Key stereochemical terminology Stereochemical terminology in organic chemistry can refer to structure of a molecule or to properties of a physical sample of the molecule. It is very important to recognize
Key stereochemical terminology Stereochemical terminology in organic chemistry can refer to structure of a molecule or to properties of a physical sample of the molecule. It is very important to recognize
Solid Phase Peptide Synthesis
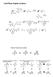 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Part B 2. Allow a total of 15 credits for this part. The student must answer all questions in this part.
 Part B 2 Allow a total of 15 credits for this part. The student must answer all questions in this part. 51 [1] Allow 1 credit for 3 Mg(s) N 2 (g) Mg 3 N 2 (s). Allow credit even if the coefficient 1 is
Part B 2 Allow a total of 15 credits for this part. The student must answer all questions in this part. 51 [1] Allow 1 credit for 3 Mg(s) N 2 (g) Mg 3 N 2 (s). Allow credit even if the coefficient 1 is
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Performing Calculatons
 Performing Calculatons There are three basic units for measurement in the organic laboratory mass, volume, and number, measured in moles. Most of the other types of measurements are combinations of them,
Performing Calculatons There are three basic units for measurement in the organic laboratory mass, volume, and number, measured in moles. Most of the other types of measurements are combinations of them,
cyclohexane cyclopentane Nomenclature Follows same rules as for stright-chain alkanes. Examples: name the following
 Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-10- 3-16 Study Problems: 3-43, 3-44, 3-45, 3-46 Key oncepts and Skills: ompare the energies of cycloalkanes, and explain ring
Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-10- 3-16 Study Problems: 3-43, 3-44, 3-45, 3-46 Key oncepts and Skills: ompare the energies of cycloalkanes, and explain ring
EXPERIMENT 2 (Organic Chemistry II) Pahlavan/Cherif Diels-Alder Reaction Preparation of ENDO-NORBORNENE-5, 6-CIS-CARBOXYLIC ANHYDRIDE
 EXPERIMENT 2 (rganic Chemistry II) Pahlavan/Cherif Diels-Alder Reaction Preparation of END-NRBRNENE-5, 6-CIS-CARBXYLIC ANYDRIDE Purpose a) Study conjugated dienes b) Study diene and dienophile c) Study
EXPERIMENT 2 (rganic Chemistry II) Pahlavan/Cherif Diels-Alder Reaction Preparation of END-NRBRNENE-5, 6-CIS-CARBXYLIC ANYDRIDE Purpose a) Study conjugated dienes b) Study diene and dienophile c) Study
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Chemistry B11 Chapter 4 Chemical reactions
 Chemistry B11 Chapter 4 Chemical reactions Chemical reactions are classified into five groups: A + B AB Synthesis reactions (Combination) H + O H O AB A + B Decomposition reactions (Analysis) NaCl Na +Cl
Chemistry B11 Chapter 4 Chemical reactions Chemical reactions are classified into five groups: A + B AB Synthesis reactions (Combination) H + O H O AB A + B Decomposition reactions (Analysis) NaCl Na +Cl
Conjugation is broken completely by the introduction of saturated (sp3) carbon:
 Chapter 16 Conjugation, resonance, and dienes Conjugation relies on the partial overlap of p-orbitals on adjacent double or triple bonds. A common conjugated system involves 1,3-dienes, such as 1,3-butadiene.
Chapter 16 Conjugation, resonance, and dienes Conjugation relies on the partial overlap of p-orbitals on adjacent double or triple bonds. A common conjugated system involves 1,3-dienes, such as 1,3-butadiene.
EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT
 EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT Pre-Lab Questions: None. 64 I. Background Information DIPEPTIDE RESEARCH PROJECT Methods developed by organic chemists for the synthesis of biopolymers have had
EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT Pre-Lab Questions: None. 64 I. Background Information DIPEPTIDE RESEARCH PROJECT Methods developed by organic chemists for the synthesis of biopolymers have had
Chemistry 1110 Organic Chemistry IUPAC Nomenclature
 hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
Chapter 12 Organic Compounds with Oxygen and Sulfur
 Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 4 Lecture Notes
 Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Green Principles Atom Economy Solventless Reactions Catalysis
 Lab 5: The Aldol Reaction Solventless vs Traditional Reactions: (Melting Point Study & Recrystallization) (adapted from Doxsee, K.M. and Hutchison, J.E., Green Organic Chemistry and John Thompson; Lane
Lab 5: The Aldol Reaction Solventless vs Traditional Reactions: (Melting Point Study & Recrystallization) (adapted from Doxsee, K.M. and Hutchison, J.E., Green Organic Chemistry and John Thompson; Lane
2.4 Enantiomeric Isomers
 2.4 Enantiomeric Isomers There is an additional type of isomer that can exist when we have an atom with 4 different groups attached to that atom in tetrahedral geometry. Consider a methane molecule with
2.4 Enantiomeric Isomers There is an additional type of isomer that can exist when we have an atom with 4 different groups attached to that atom in tetrahedral geometry. Consider a methane molecule with
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
2/10/2011. Stability of Cycloalkanes: Ring Strain. Stability of Cycloalkanes: Ring Strain. 4.3 Stability of Cycloalkanes: Ring Strain
 4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models
 EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 1: Chemistry: Measurements and Methods
 Chapter 1: Chemistry: Measurements and Methods 1.1 The Discovery Process o Chemistry - The study of matter o Matter - Anything that has mass and occupies space, the stuff that things are made of. This
Chapter 1: Chemistry: Measurements and Methods 1.1 The Discovery Process o Chemistry - The study of matter o Matter - Anything that has mass and occupies space, the stuff that things are made of. This
Freezing Point Depression: Why Don t Oceans Freeze? Teacher Advanced Version
 Freezing Point Depression: Why Don t Oceans Freeze? Teacher Advanced Version Freezing point depression describes the process where the temperature at which a liquid freezes is lowered by adding another
Freezing Point Depression: Why Don t Oceans Freeze? Teacher Advanced Version Freezing point depression describes the process where the temperature at which a liquid freezes is lowered by adding another
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Chapter 5, Lesson 3 Why Does Water Dissolve Salt?
 Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Organic Chemistry Calculations
 Organic Chemistry Calculations There are three basic units for measurement in the organic laboratory mass, volume, and number, measured in moles. Most of the other types of measurements are combinations
Organic Chemistry Calculations There are three basic units for measurement in the organic laboratory mass, volume, and number, measured in moles. Most of the other types of measurements are combinations
 2814 hains, Rings and Spectroscopy June 2003 Mark Scheme 2814 Mark Scheme June 2003 The following annotations may be used when marking: X = incorrect response (errors may also be underlined) ^ = omission
2814 hains, Rings and Spectroscopy June 2003 Mark Scheme 2814 Mark Scheme June 2003 The following annotations may be used when marking: X = incorrect response (errors may also be underlined) ^ = omission
Compounds vs mixtures. Physics and Chemistry IES Jaume Salvador i Pedrol February 2009
 Compounds vs mixtures Physics and Chemistry IES Jaume Salvador i Pedrol February 2009 Compounds Remember that a compound is a substance made up from two or more elements, chemically joined together. This
Compounds vs mixtures Physics and Chemistry IES Jaume Salvador i Pedrol February 2009 Compounds Remember that a compound is a substance made up from two or more elements, chemically joined together. This
F322: Chains, Energy and Resources 2.2.4 Alcohols
 F322: hains, Energy and Resources 2.2.4 Alcohols 167 marks 1. This question is about the six alcohols below. butan-2-ol 2-methylpentan-3-ol propan-1-ol ethane-1,2-diol 2-methylpropan-2-ol propan-2-ol Which
F322: hains, Energy and Resources 2.2.4 Alcohols 167 marks 1. This question is about the six alcohols below. butan-2-ol 2-methylpentan-3-ol propan-1-ol ethane-1,2-diol 2-methylpropan-2-ol propan-2-ol Which
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Chem 11 Oa-Section 2. Exam #2. 1 October 2004
 " Chem 11 Oa-Section 2 Exam #2 1 October 2004 Name: K~ Instructions Please read each question carefully and answer it completely and clearly. Do the problems in the order that is easiest for you. Point
" Chem 11 Oa-Section 2 Exam #2 1 October 2004 Name: K~ Instructions Please read each question carefully and answer it completely and clearly. Do the problems in the order that is easiest for you. Point
4.5 Physical Properties: Solubility
 4.5 Physical Properties: Solubility When a solid, liquid or gaseous solute is placed in a solvent and it seems to disappear, mix or become part of the solvent, we say that it dissolved. The solute is said
4.5 Physical Properties: Solubility When a solid, liquid or gaseous solute is placed in a solvent and it seems to disappear, mix or become part of the solvent, we say that it dissolved. The solute is said
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Warm-Up 9/9. 1. Define the term matter. 2. Name something in this room that is not matter.
 Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
17.2 REACTIONS INVOLVING ALLYLIC AND BENZYLIC RADICALS
 17. REACTINS INVLVING ALLYLIC AND BENZYLIC RADICALS 793 As Eq. 17. shows, the products derived from the reaction of water at the ring carbons are not formed. The reason is that these products are not aromatic
17. REACTINS INVLVING ALLYLIC AND BENZYLIC RADICALS 793 As Eq. 17. shows, the products derived from the reaction of water at the ring carbons are not formed. The reason is that these products are not aromatic
methyl RX example primary RX example secondary RX example secondary RX example tertiary RX example
 ucleophilic Substitution & Elimination hemistry 1 eginning patterns to knowfor S and E eactions - horizontal and vertical templates for practice Example 1 - two possible perspectives (deuterium and tritium
ucleophilic Substitution & Elimination hemistry 1 eginning patterns to knowfor S and E eactions - horizontal and vertical templates for practice Example 1 - two possible perspectives (deuterium and tritium
Prentice Hall. Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition. High School. High School
 Prentice Hall Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition High School C O R R E L A T E D T O High School C-1.1 Apply established rules for significant digits,
Prentice Hall Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition High School C O R R E L A T E D T O High School C-1.1 Apply established rules for significant digits,
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Chapter 6 An Overview of Organic Reactions
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Why this chapter? To understand organic and/or biochemistry, it is necessary to know: -What occurs -Why and
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Why this chapter? To understand organic and/or biochemistry, it is necessary to know: -What occurs -Why and
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
Saturated NaCl solution rubber tubing (2) Glass adaptor (2) thermometer adaptor heating mantle
 EXPERIMENT 5 (Organic Chemistry II) Pahlavan/Cherif Dehydration of Alcohols - Dehydration of Cyclohexanol Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination
EXPERIMENT 5 (Organic Chemistry II) Pahlavan/Cherif Dehydration of Alcohols - Dehydration of Cyclohexanol Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination
Experiment 8: Chemical Moles: Converting Baking Soda to Table Salt
 Experiment 8: Chemical Moles: Converting Baking Soda to Table Salt What is the purpose of this lab? We want to develop a model that shows in a simple way the relationship between the amounts of reactants
Experiment 8: Chemical Moles: Converting Baking Soda to Table Salt What is the purpose of this lab? We want to develop a model that shows in a simple way the relationship between the amounts of reactants
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
H 2O gas: molecules are very far apart
 Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
Separation of Amino Acids by Paper Chromatography
 Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS
 CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS Purpose: It is important for chemists to be able to determine the composition of unknown chemicals. This can often be done by way of chemical tests.
CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS Purpose: It is important for chemists to be able to determine the composition of unknown chemicals. This can often be done by way of chemical tests.
Liquid phase. Balance equation Moles A Stoic. coefficient. Aqueous phase
 STOICHIOMETRY Objective The purpose of this exercise is to give you some practice on some Stoichiometry calculations. Discussion The molecular mass of a compound is the sum of the atomic masses of all
STOICHIOMETRY Objective The purpose of this exercise is to give you some practice on some Stoichiometry calculations. Discussion The molecular mass of a compound is the sum of the atomic masses of all
EXPERIMENT 3 (Organic Chemistry II) Nitration of Aromatic Compounds: Preparation of methyl-m-nitrobenzoate
 EXPERIMENT 3 (Organic Chemistry II) Nitration of Aromatic Compounds: Preparation of methyl-m-nitrobenzoate Pahlavan/Cherif Purpose a) Study electrophilic aromatic substitution reaction (EAS) b) Study regioselectivity
EXPERIMENT 3 (Organic Chemistry II) Nitration of Aromatic Compounds: Preparation of methyl-m-nitrobenzoate Pahlavan/Cherif Purpose a) Study electrophilic aromatic substitution reaction (EAS) b) Study regioselectivity
Making Biodiesel from Virgin Vegetable Oil: Teacher Manual
 Making Biodiesel from Virgin Vegetable Oil: Teacher Manual Learning Goals: Students will understand how to produce biodiesel from virgin vegetable oil. Students will understand the effect of an exothermic
Making Biodiesel from Virgin Vegetable Oil: Teacher Manual Learning Goals: Students will understand how to produce biodiesel from virgin vegetable oil. Students will understand the effect of an exothermic
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.
 CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
Chapter Test A. Elements, Compounds, and Mixtures MULTIPLE CHOICE. chemically combined? MIXs2 a. element b. compound c. mixture d.
 Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
An Adventure in Stereochemistry: Alice in Mirror Image Land*
 An Adventure in Stereochemistry: Alice in Mirror Image Land* by Frank J. Dinan Department of Chemistry and Biochemistry Canisius College, Buffalo, NY Gordon T. Yee Department of Chemistry Virginia Polytechnic
An Adventure in Stereochemistry: Alice in Mirror Image Land* by Frank J. Dinan Department of Chemistry and Biochemistry Canisius College, Buffalo, NY Gordon T. Yee Department of Chemistry Virginia Polytechnic
LMB Crystallography Course, 2013. Crystals, Symmetry and Space Groups Andrew Leslie
 LMB Crystallography Course, 2013 Crystals, Symmetry and Space Groups Andrew Leslie Many of the slides were kindly provided by Erhard Hohenester (Imperial College), several other illustrations are from
LMB Crystallography Course, 2013 Crystals, Symmetry and Space Groups Andrew Leslie Many of the slides were kindly provided by Erhard Hohenester (Imperial College), several other illustrations are from
Chemistry B11 Chapter 6 Solutions and Colloids
 Chemistry B11 Chapter 6 Solutions and Colloids Solutions: solutions have some properties: 1. The distribution of particles in a solution is uniform. Every part of the solution has exactly the same composition
Chemistry B11 Chapter 6 Solutions and Colloids Solutions: solutions have some properties: 1. The distribution of particles in a solution is uniform. Every part of the solution has exactly the same composition
CHEM 2423 Recrystallization of Benzoic Acid EXPERIMENT 4 - Purification - Recrystallization of Benzoic acid
 EXPERIMENT 4 - Purification - Recrystallization of Benzoic acid Purpose: a) To purify samples of organic compounds that are solids at room temperature b) To dissociate the impure sample in the minimum
EXPERIMENT 4 - Purification - Recrystallization of Benzoic acid Purpose: a) To purify samples of organic compounds that are solids at room temperature b) To dissociate the impure sample in the minimum
Chemical Changes. Measuring a Chemical Reaction. Name(s)
 Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
The Mole. 6.022 x 10 23
 The Mole 6.022 x 10 23 Background: atomic masses Look at the atomic masses on the periodic table. What do these represent? E.g. the atomic mass of Carbon is 12.01 (atomic # is 6) We know there are 6 protons
The Mole 6.022 x 10 23 Background: atomic masses Look at the atomic masses on the periodic table. What do these represent? E.g. the atomic mass of Carbon is 12.01 (atomic # is 6) We know there are 6 protons
Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding
 Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding Key Concepts The attractions between the protons and electrons of atoms can cause an electron to move completely from one atom to the other.
Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding Key Concepts The attractions between the protons and electrons of atoms can cause an electron to move completely from one atom to the other.
EXPERIMENT 12: Empirical Formula of a Compound
 EXPERIMENT 12: Empirical Formula of a Compound INTRODUCTION Chemical formulas indicate the composition of compounds. A formula that gives only the simplest ratio of the relative number of atoms in a compound
EXPERIMENT 12: Empirical Formula of a Compound INTRODUCTION Chemical formulas indicate the composition of compounds. A formula that gives only the simplest ratio of the relative number of atoms in a compound
Chemical Calculations: Formula Masses, Moles, and Chemical Equations
 Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
MOLES, MOLECULES, FORMULAS. Part I: What Is a Mole And Why Are Chemists Interested in It?
 NAME PARTNERS SECTION DATE_ MOLES, MOLECULES, FORMULAS This activity is designed to introduce a convenient unit used by chemists and to illustrate uses of the unit. Part I: What Is a Mole And Why Are Chemists
NAME PARTNERS SECTION DATE_ MOLES, MOLECULES, FORMULAS This activity is designed to introduce a convenient unit used by chemists and to illustrate uses of the unit. Part I: What Is a Mole And Why Are Chemists
In this experiment, we will use three properties to identify a liquid substance: solubility, density and boiling point..
 Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
Chemistry Assessment Unit AS 1
 Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2011 Chemistry Assessment Unit AS 1 assessing Basic Concepts in Physical and Inorganic Chemistry [AC111]
Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2011 Chemistry Assessment Unit AS 1 assessing Basic Concepts in Physical and Inorganic Chemistry [AC111]
An Introduction to Organic Chemistry
 An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
Absolute Structure Absolute Configuration
 Absolute Structure Absolute Configuration Some definitions Absolute Configuration -> spatial arrangement of the atoms for a chiral molecule (R/S, P/M or D/L assignment). Absolute Structure -> spatial arrangement
Absolute Structure Absolute Configuration Some definitions Absolute Configuration -> spatial arrangement of the atoms for a chiral molecule (R/S, P/M or D/L assignment). Absolute Structure -> spatial arrangement
ANALYSIS OF ASPIRIN INFRARED (IR) SPECTROSCOPY AND MELTING POINT DETERMINATION
 Chem 306 Section (Circle) M Tu W Th Name Partners Date ANALYSIS OF ASPIRIN INFRARED (IR) SPECTROSCOPY AND MELTING POINT DETERMINATION Materials: prepared acetylsalicylic acid (aspirin), stockroom samples
Chem 306 Section (Circle) M Tu W Th Name Partners Date ANALYSIS OF ASPIRIN INFRARED (IR) SPECTROSCOPY AND MELTING POINT DETERMINATION Materials: prepared acetylsalicylic acid (aspirin), stockroom samples
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
Chemical Calculations: The Mole Concept and Chemical Formulas. AW Atomic weight (mass of the atom of an element) was determined by relative weights.
 1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
Alcohols. Copyright 2009 by Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. CH 3 CH 2 CH 2 OH 1-propanol OH
 Chapter 12 rganic Compounds with xygen and Sulfur 12.1 Alcohols, Thiols, and Ethers Alcohols An alcohol contains a hydroxyl group ( ) attached to a carbon chain. A phenol contains a hydroxyl group ( )
Chapter 12 rganic Compounds with xygen and Sulfur 12.1 Alcohols, Thiols, and Ethers Alcohols An alcohol contains a hydroxyl group ( ) attached to a carbon chain. A phenol contains a hydroxyl group ( )
Replication Study Guide
 Replication Study Guide This study guide is a written version of the material you have seen presented in the replication unit. Self-reproduction is a function of life that human-engineered systems have
Replication Study Guide This study guide is a written version of the material you have seen presented in the replication unit. Self-reproduction is a function of life that human-engineered systems have
3/5/2014. iclicker Participation Question: A. MgS < AlP < NaCl B. MgS < NaCl < AlP C. NaCl < AlP < MgS D. NaCl < MgS < AlP
 Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Infrared Spectroscopy: Theory
 u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
STANDARD ANSWERS AND DEFINITIONS
 Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
Experiment #8 properties of Alcohols and Phenols
 Introduction Experiment #8 properties of Alcohols and Phenols As has been mentioned before, over 20 million organic compounds have been identified. If each substance had to be studied as an entity completely
Introduction Experiment #8 properties of Alcohols and Phenols As has been mentioned before, over 20 million organic compounds have been identified. If each substance had to be studied as an entity completely
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Properties and Classifications of Matter
 PS-3.1 Distinguish chemical properties of matter (including reactivity) from physical properties of matter (including boiling point, freezing/melting point, density [with density calculations], solubility,
PS-3.1 Distinguish chemical properties of matter (including reactivity) from physical properties of matter (including boiling point, freezing/melting point, density [with density calculations], solubility,
