Rapid antimicrobial susceptibility testing of clinical isolates by digital time-lapse microscopy
|
|
|
- Antony McDowell
- 7 years ago
- Views:
Transcription
1 Eur J Clin Microbiol Infect Dis (2015) 34: DOI /s ORIGINAL ARTICLE Rapid antimicrobial susceptibility testing of clinical isolates by digital time-lapse microscopy M. Fredborg 1,4 & F. S. Rosenvinge 2 & E. Spillum 3 & S. Kroghsbo 3 & M. Wang 4 & T. E. Sondergaard 5 Received: 19 June 2015 /Accepted: 14 September 2015 /Published online: 25 September 2015 # The Author(s) This article is published with open access at Springerlink.com Abstract Rapid antimicrobial susceptibility testing (AST) is essential for early and appropriate therapy. Methods with short detection time enabling same-day treatment optimisation are highly favourable. In this study, we evaluated the potential of a digital time-lapse microscope system, the ocelloscope system, to perform rapid AST. The ocelloscope system demonstrated a very high accuracy (96 % overall agreement) when determining the resistance profiles of four reference strains, nine clinical isolates, including multi-drug-resistant isolates, and three positive blood cultures. AST of clinical isolates (168 antimicrobial agent organism combinations) demonstrated 3.6 % minor, no major and 1.2 % very major errors of the ocelloscope system compared to conventional susceptibility testing, as well as a rapid and correct phenotypic detection of strains with methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase (ESBL) profiles. The net average time-to-result was 108 min, with 95 % of the results being available within 180 min. In conclusion, this study strongly indicates that the ocelloscope system holds considerable potential as an accurate and sensitive * M. Fredborg Marlene.Fredborg@anis.au.dk Department of Animal Science, Faculty of Science and Technology, Aarhus University, Blichers Allé 20, 8830 Tjele, Denmark Department of Clinical Microbiology, Vejle Hospital, Kabbeltoft 25, 7100 Vejle, Denmark Philips BioCell, Gydevang 42, 3450 Allerød, Denmark Department of Clinical Microbiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus, Denmark Department of Biotechnology, Chemistry and Environmental Engineering, Aalborg University, Sohngaardsholmsvej 49, 9000 Aalborg, Denmark AST method with short time-to-result, enabling same-day targeted antimicrobial therapy, facilitating antibiotic stewardship and better patient management. A full-scale validation of the ocelloscope system including more isolates is necessary to assess the impact of using it for AST. Introduction Bacterial multidrug resistance is emerging worldwide at an alarming rate and is now recognised as a major public health threat [1]. This crisis is not likely to be solved by new antibiotics due to the low rate of antibiotic discovery and by the probability that pathogens will continue to evolve resistance to antibiotics. The initiation of effective antibiotic therapy early in the course of an infection may reduce the probability of pathogens evolving resistance [2]. Especially in the case of sepsis and bloodstream infections, early appropriate antimicrobial therapy is important to decrease mortality [3]. Sepsis management includes empirical antimicrobial therapy started as soon as possible without awaiting results from antimicrobial susceptibility testing (AST) [4, 5]. As empirical antimicrobial therapy is mainly composed of broad-spectrum antibiotics, this can perpetuate the cycle of increasing resistance [6]. Consequently, early determination of antimicrobial susceptibility is pivotal in targeted antimicrobial therapy in order to combat the escalating rates of resistance, as well as to decrease mortality. Today, AST in routine clinical microbiology laboratories is generally performed by conventional methods such as disc diffusion, broth dilution or Etest [7, 8]. During the last decade, several technologies have been suggested as candidates for more accurate AST, e.g. whole-genome sequencing, mass spectrometry, fluorescence-activated cell sorting and microarrays [7]. Technologies relying on genotypic and proteomic
2 2386 Eur J Clin Microbiol Infect Dis (2015) 34: analysis of resistance determinants such as whole-genome sequencing, polymerase chain reaction (PCR) and matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) have the key limitation in that they are unable to detect novel resistance mechanisms [9, 10]. In addition, some of these new techniques are time-consuming and characterised by expensive instruments, high analysis costs, the need for specialised technical personnel and are, in some cases, limited by single-sample analysis. Automated instruments such as Vitek 2 (biomérieux), Phoenix 100 (BD Biosciences) and MicroScan WalkAway (Siemens) are commonly used methods for AST as they are easy to operate and reduce the time-to-result [11 14]. Nevertheless, in clinical practice, even faster AST methods are required if effective treatment with targeted antibiotic is to be achieved within the same working day. In a previous study, we demonstrated the ocelloscope system to be a fast and sensitive, high-throughput AST method capable of detecting antibiotic susceptibility within 6 min for Escherichia coli and within 30 min in complex urine samples from pigs suffering from urinary tract infections [15]. In this study, we performed a preliminary evaluation of the ability of the ocelloscope system to analyse antimicrobial resistance by monitoring bacterial cell growth. The accuracy of the ocelloscope system was examined together with the time required for AST. This initial screening constitutes the proof of concept of the ocelloscope system in relation to AST of clinical isolates. Materials and methods Strains Four quality control (QC) reference strains were included to validate the antimicrobial susceptibility results obtained by the ocelloscope system: Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC and Streptococcus pneumoniae ATCC The QC reference strains used are recommended for quality control by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). In addition, nine clinical isolates, collected in Denmark during the period , were included: two S. epidermidis [one oxacillin resistant (meca-positive) and one oxacillin and penicillin susceptible], one S. pneumoniae (penicillin intermediate and trimethoprim/sulphamethoxazole resistant), two S. aureus [one methicillin-resistant S. aureus (MRSA) (meca-positive) and one methicillin-susceptible S. aureus (MSSA)], two E. faecalis [both gentamicin high-level resistant and one vancomycin resistant (vanb-positive)] and two E. coli [one extended-spectrum β-lactamase (ESBL) and one resistant to ampicillin, gentamicin and ciprofloxacin]. Blood culture instrumentation and media Blood samples from patients (8 10 ml/patient) with suspected bacteraemia were collected in standard aerobe non-charcoal BacT/ALERT FA blood culture bottles (biomérieux, Marcyl Etoile, France) and handled according to the manufacturer s instructions. Blood culture bottles were incubated in the BacT/ ALERT 3D system (biomérieux, Marcy-l Etoile, France) at Aarhus University Hospital (Aarhus, Denmark). Blood cultures detected as positive by the BacT/ALERT 3D system were Gram stained and examined for bacterial morphology by light microscopy. All blood cultures used in this study were recognised as positive during the night and not processed until 8o clock the following morning. Species identification of isolates by the clinical laboratory was provided after the completion of ocelloscope AST measurements. Consequently, only preliminary phenotypic characterisation including Gram staining and examination of cellular morphology by light microscopy was provided prior to ocelloscope AST. Antimicrobial susceptibility testing by manually interpreted Sensititre plates AST was performed on all bacterial isolates using the Sensititre system (TREK Diagnostic Systems, Cleveland, OH, USA), according to the manufacturer s instructions. Three different Sensititre plates were used: NF: E. coli, GPALLIF: Staphylococci and Enterococci, and STP6F: S. pneumoniae. Bacterial isolates were grown overnight on 5 % horse blood agar (Statens Serum Institut, Copenhagen, Denmark). Colony material was emulsified in sterile water (E. coli, Staphylococcus spp. and Enterococcus spp.) or Sensititre cation-adjusted Mueller Hinton broth with TES (CAMHBT, TREK Diagnostic Systems (S. pneumoniae) and the suspension was adjusted to 0.5 McFarland standard using the Vitek DensiChek densitometer (biomérieux). For Staphylococcus spp., Enterococcus spp. and E. coli, 10 μl was transferred into 11 ml CAMHBT and 50 μl of the suspension was inoculated into each well on the Sensititre plate. For S. pneumoniae, 100 μl was transferred into 11 ml Sensititre cation-adjusted Mueller Hinton broth with TES and lysed horse blood (CAMHBT+LHB, TREK Diagnostic Systems) and 100 μl of the suspension was inoculated into each well on the Sensititre plate. Plates were incubated for h at 35 C. For each test, we performed an inoculum check according to the manufacturer s instructions. AST results were read using the Sensititre Manual viewer. Antimicrobial susceptibility testing by the ocelloscope system QC reference strains and clinical isolates were grown under atmospheric conditions at 37 C in CAMHBT, except
3 Eur J Clin Microbiol Infect Dis (2015) 34: S. pneumoniae, which was grown in CAMHBT+LHB. All isolates were grown overnight and transferred to new tubes and incubated for 2 h before AST. Positive blood cultures were prepared by centrifugation at 200 g for 5 min (Sigma 3-18 k, rotor, Buch & Holm, Herlev, Denmark) to remove human blood cells, followed by resuspension of the bacterial pellet in CAMHBT. All broth bacteria suspensions were diluted to McFarland 0.5 based on OD 600 measurements using a UV-3100 PC Spectrophotometer (VWR, Herlev, Denmark) and subsequently diluted in cation-adjusted Mueller Hinton broth to a final bacterial cell suspension of approximately bacteria/ml for all bacteria except for S. pneumoniae, which was diluted to a final concentration of approximately bacteria/ml. To facilitate focus adjustment of the ocelloscope system, beads were added to bacterial cell suspensions ( μm beads/ml, microsphere standard, B7277, Invitrogen, Naerum, Denmark). Broth bacteria bead suspensions were added to Sensititre plates (TREK Diagnostic Systems). NF, GPALL1F and STP6F plates were used as specified above. Bacterial cell suspensions were carefully transferred from the round-bottomed Sensititre plates to flat-bottomed Nunc Edge 96-well plates (Thermo Fisher Scientific, Roskilde, Denmark) in order to be analysed by the ocelloscope system, as it cannot analyse round-bottomed plates. The determination of minimum inhibitory concentration (MIC) values for all experiments on QC reference strains and clinical isolates was carried out in triplicate experiments. The results were reported when at least two out of three MIC results were in agreement. The ocelloscope is a digital time-lapse microscopy technology that scans through a fluid sample generating series of images as described in 2013 by Fredborg et al.[15]. As a result of the tilted imaging plane, the images recorded by the ocelloscope system constitute a parallelepipedum that forms the image stack. The projected z-stack image of a single z-plane was generated by combining the tilted images. Prior to image acquisition, setting the focus manually was performed for each of the 96 wells, taking min. Image acquisition was performed in one scan area in the centre of each well covering approximately 40 % of the well, which was scanned repeatedly every 15 min for 12 h. Figure 1a shows one image plane recorded for the E. coli QC reference strains used for the evaluation of the ocelloscope system. The ocelloscope instrument was placed inside an Innova 44 incubator (New Brunswick Scientific), allowing precise temperature regulation. Time-lapse experiments, digital analysis and image processing were conducted by a custom automation script in MATLAB [MATLAB v (R2012b), The MathWorks Inc., Natick, MA, USA, 2000]. Growth kinetics were determined by image stack processing based on contrastbased segmentation and extraction of surface area (SESA). Data analysis Bacterial susceptibility data for the QC reference strains obtained by the ocelloscope system were compared to QC ranges reported by TREK Diagnostic Systems (version 2.0). The TREK QC ranges were identical to the ranges given by the CLSI (M100-S23, 2013), with penicillin against S. aureus ATCC being the only outlier (Table 1). For clinical and blood culture isolates, antimicrobial susceptibility data obtained by the ocelloscope system were compared to manually read Sensititre plates. The results from manually read Sensititre plates were regarded as the gold standard. The time-to-result was measured from when the ocelloscope initiated image recording; the bacterial inoculum had been in contact with antimicrobials for approximately 10 min at this time point. Essential agreement was defined as MIC agreement within +/ one log 2. All clinical isolates were classified as S (susceptible), I (intermediate) or R (resistant) according to the CLSI breakpoints (M100-S23, 2013). Categorical agreement was defined as agreement within the S-I-R classification. Minor errors were defined as susceptible or resistant isolates misclassified as intermediate or intermediate isolates misclassified as resistant or susceptible, major errors were defined as susceptible isolates misclassified as resistant and very major errors were defined as resistant isolates misclassified as susceptible. GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA, USA) was used for data and statistical analysis, as well as graphing. Fig. 1 Example of an ocelloscope image and graphical output. a Growth curves for S. aureus ATCC29213 treated with vancomycin ( mg/l) and without as a positive control (POS CON). b 2D picture of S. aureus ATCC29213 obtained by the ocelloscope system, with a magnitude comparable to a200 magnification in a standard light microscope
4 2388 Eur J Clin Microbiol Infect Dis (2015) 34: Table 1 Correlation between the ocelloscope system and TREK QC ranges for antimicrobial susceptibility of QC reference strains Antimicrobial susceptibility expressed as MIC values: ocelloscope value (TREK Diagnostic Systems QC range) Antimicrobial agent E. coli ATCC25922 S. pneumoniae ATCC S. aureus ATCC E. faecalis ATCC Amikacin 2 (0.5 4) Ampicillin 1 (0.5 1) Ampicillin/sulbactam 2:1 ratio 4/2 (2/1 8/4) Aztreonam 0.12 ( ) Ceftazidime 0.25 ( ) Cefoperazone 0.25 ( ) Cefoxitin screen 6 ( 6) Cefotaxime 0.25 ( ) 0.25 ( ) Ceftazidime 0.25 ( ) Ceftriaxone 0.25 ( ) 0.06 ( ) Chloramphenicol 4 (2 8) 1 (2 8) 8 (2 16) 4 (4 16) Ciprofloxacin 0.06 ( ) 0.25 ( ) 0.5 (0.25 2) Clindamycin 0.25 ( ) 0.12 ( ) >2 (4 16) Daptomycin 0.06 ( ) 0.5 (0.12 1) 2 (1 4) Erythromycin 0.12 ( ) 0.25 (0.25 1) 1 (1 4) Gentamicin 1 (0.25 1) 0.5 (0.12 1) Imipenem 0.12 ( ) Levofloxacin 0.03 ( ) 0.5 (0.5 2) 0.25 ( ) 1 (0.25 2) Linezolid 0.25 (0.25 2) 2 (1 4) 2 (1 4) Moxifloxacin 0.5 ( ) 0.06 ( ) 0.12 ( ) Nitrofurantoin 16 (8 32) 16 (4 16) Oxacillin 0.25 ( ) >4 (8 32) Penicillin 0.25 (0.25 1) 0.25* (0.12 1) 2 (1 4) Piperacillin 2 (1 4) Piperacillin/tazobactam 4 2/4 (1/4 4/4) Quinupristin/dalfopristin 0.25 (0.25 1) 2 (2 8) Rifampin 0.12 ( ) 0.5 (0.5 4) Tetracycline 2 (0.5 2) 0.5 ( ) 0.5 (0.12 1) 16 (8 32) Ticarcillin 16 (4 16) Ticarcillin/clavulanic acid 2 8/2 (4/2 16/2) Tigecycline 0.06 ( ) 0.06 ( ) 0.12 ( ) Tobramycin 1 (0.25 1) Trimethoprim/sulphamethoxazole 0.12/2.38 ( 0.5/9.5) 0.25/4.75 (0.12/2.4 1/19) 0.12/2.38 ( 0.5/9.5) 0.12/2.38 ( 0.5/9.5) Vancomycin 0.25 ( ) 1 (1 4) 2 (1 4) : the antimicrobial is not present on the TREK Sensititre plates chosen for AST. : MIC value is below the range tested. >: MIC value is above the range tested. *: MIC value is different from those recommended in CLSI M100 Results Antimicrobial susceptibility testing of quality control (QC) reference strains The ability of the ocelloscope system to determine antibiotic susceptibility was assessed by testing four QC reference strains, E. coli ATCC25922, S. aureus ATCC29213, S. pneumoniae ATCC49619 and E. faecalis ATCC29212, using the Sensititre microdilution plates. Figure 1 shows the image output (a) and growth curves (b) determined by the ocelloscope system for S. aureus ATCC Agreements and discrepancies between MIC values obtained by the ocelloscope system and target QC ranges given by TREK Diagnostic Systems are presented in Table 1. Due to the concentration ranges of the antimicrobial panels, it was not possible to determine an exact MIC value for some antimicrobials (e.g. cefotaxime, tetracycline and trimethoprim/ sulphamethoxazole). These values are given as than the lowest concentration in the panel range (all wells without growth)
5 Eur J Clin Microbiol Infect Dis (2015) 34: or>than the highest concentration in the panel range (growth in all wells). A total of 72 bacteria antibiotic combinations were analysed, resulting in 97 % agreement between the results obtained by the ocelloscope system and the TREK target QC range. Similar results were obtained in three separate identical experiments. The average time-to-result for QC reference strains by the ocelloscope system was 102 min, with 93 % of the results being available within 180 min (Table 2). Two discrepancies of the 72 bacteria antibiotic combinations were observed: S. pneumoniae tested against chloramphenicol and clindamycin (Table 1). For chloramphenicol, the MIC value measured by the ocelloscope system was one log 2 concentration lower than the QC range, whereas the MIC value for clindamycin was one log 2 concentration higher than the QC range. Antimicrobial susceptibility testing of clinical isolates Nine clinical isolates representing five different bacterial species with distinctive resistance profiles were included in this study (see the section on the experimental procedures for further information). A total of 168 bacteria antibiotic combinations were tested using the Sensititre microdilution plates. MIC values obtained by the ocelloscope system were compared to MIC values obtained by manual reading of Sensititre plates. The average time-to-result for bacterial isolates by the ocelloscope system was 100 min, with 95 % of the results being available 180 min (Table 2). The average essential and categorical agreement was 93 % (156 of 168) and 93 % (136 of 146), respectively. Bacteria antibiotic combinations that were not in agreement with conventional methods are listed in Table 3. Two major errors (1.2 %) were observed: S. epidermidis tested against nitrofurantoin and E. coli tested against chloramphenicol. Five minor errors (3.6 %) were observed: one for S. pneumoniae and four for E. faecalis, where two isolates were classified as rifampicin susceptible, one isolate was classified as linezolid intermediate, one isolate was classified as vancomycin intermediate and one isolate was classified as erythromycin intermediate. There were no major errors for any of the clinical isolates. Strains with MRSA and ESBL resistance profiles were detected correctly by the ocelloscope system. The vancomycin-resistant enterococci (VRE) strain was detected with a minor error regarding vancomycin: ocelloscope MIC=8 mg/l vs. Sensititre MIC >32 mg/l. Antimicrobial susceptibility testing on positive blood cultures To evaluate the ability of the ocelloscope system to perform AST, three positive blood cultures were included. The selection of appropriate antimicrobial Sensititre panels and growth media was based on morphology and Gram staining. Species identification of the bacteria was performed at Aarhus University Hospital and the results were not available prior to AST by the ocelloscope system. Table 4 shows the AST results obtained by the ocelloscope system compared to susceptibility results obtained by testing the positive blood cultures with the manual Sensititre system. The average timeto-result for susceptibility testing by the ocelloscope system ranged from 1 to 4.2 h, depending on the bacteria antibiotic combination and whether the bacteria had reached stationary Table 2 Time-to-result in minutes required for the ocelloscope system to gain AST results for four QC (ATCC) strains, nine clinical isolates and three positive blood cultures (BC) Strain av. time 25th percentile 75th percentile min max S. aureus ATCC E. coli ATCC E. faecalis ATCC S. pneumoniae ATCC S. aureus MRSA S. aureus MSSA S. epidermidis Pen-R S. epidermidis Pen-S S. pneumoniae E. coli ESBL E. coli Amp-R E. faecalis VRE E. faecalis Gen-R S. aureus BC E. coli BC E. faecalis BC Results are expressed as average time (av. time), 25th percentile, 75th percentile, minimum time-to-result (min) and maximum time-to-result (max)
6 2390 Eur J Clin Microbiol Infect Dis (2015) 34: Table 3 AST of nine clinical isolates by the ocelloscope system and compared to the manually read Sensititre system (gold standard) Organism No. of antimicrobials tested (antimicrobials with CLSI breakpoint) Essential agreement ±1 log 2 (%) Categorical agreement (S-I-R according to CLSI breakpoints) % Minor errors Major errors Very major errors S. epidermidis 19 (17) a S.pneumoniae 20 (18) b 0 0 S. aureus 19 (17) E. faecalis 15 (11) c 0 0 E. coli 22 (19) d Results are expressed as essential agreements ±1 log 2 and categorical agreements according to CLSI breakpoints a S. epidermidis: one isolate classified as nitrofurantoin susceptible b S. pneumoniae: one isolate classified as penicillin susceptible c E. faecalis: two isolates classified as rifampicin susceptible, one isolate classified as linezolid intermediate, one isolate classified as vancomycin intermediate and one isolate classified as erythromycin intermediate d E. coli: one isolate classified as chloramphenicol susceptible growth phase prior to testing (Table 2). The overall essential agreement was 95 % (57 of 60). Two of the discrepancies were S. aureus tested against ampicillin (ocelloscope MIC=1 mg/ L vs. Sensititre MIC=4 mg/l) and S. aureus tested against penicillin (ocelloscope MIC=0.5 mg/l vs. Sensititre MIC=4 mg/l). Both methods categorised the S. aureus isolate as resistant to penicillin. The third discrepancy was observed for E. coli tested against chloramphenicol (ocelloscope MIC 8 mg/l vs. Sensititre MIC>16 mg/l). The categorical agreement was 100 % for S. aureus and E. faecalis. Discussion In this study, we performed an initial screening to evaluate the potential of the ocelloscope system to perform rapid AST. This initial screening constitutes proof-of-concept in relation to bloodstream infections and, although this is not a full validation of the ocelloscope system, the results are compared to commercially available systems. Four QC reference strains were used to evaluate the ability of the ocelloscope system for susceptibility determination, as directed by standards for the assessment of antimicrobial susceptibility [16, 17], with 95 % of the results lying within the QC target range. Subsequently, the evaluation was extended to include 168 bacteria antibiotic combinations of common blood culture pathogens with different resistance profiles, resulting in an overall agreement of 96 %. It has been reported that an overall error rate of <10 % should be obtainable for an accepted performance of susceptibility testing [18 20]. According to guidelines, categorical agreements should be >90 %, major errors should be <3 % and very major errors should be<1.5 % [21]. In this study, the AST results of bacteria antibiotic combinations with CLSI defined breakpoints showed categorical agreement for 93 %, 1.2 % very major errors and no major errors when determined by the ocelloscope system. The AST results obtained in the two multidrug-resistant organisms, an MRSA and an ESBL-producing E. coli, showed no discrepancies in categorical agreement. Evaluation of the Vitek 2 and Phoenix systems for AST on blood cultures demonstrated categorical agreement rates of 94.6 % for Vitek 2 and 100 % for Phoenix when analysing 20 methicillin-resistant staphylococci tested against five antimicrobial agents [13]. Categorical agreements for 23 ESBLpositive E. coli tested against three antimicrobial agents were 97.5 % and 98.1 % for Vitek 2 and Phoenix, respectively [13]. For the VRE strain, ocelloscope determined it as intermediate, while manual reading classified it as resistant to vancomycin. The VRE strain carried the vanb gene. The vanb genotype usually has a vancomycin MIC distribution in the range 8 32 mg/l and generally confers lower MIC to vancomycin than the vana genotype (typical vancomycin MIC> 64 mg/l) [22]. Rapid detection of the vanb genotype has proven to be difficult in automated systems and has led to the development of optimised panels [23, 24]. The disc diffusion test, agar screen and Etest showed 93 %, 100 % and 100 % sensitivity for detecting the vanb genotype, respectively, although agar diffusion tests can be challenging, as they require careful assessment of zone edges (eucast.org, CLSI M100 [23]. Thus, a full validation of the ocelloscope system should include a panel of multidrug-resistant species to determine its potential to rapidly determine the resistance profiles of such strains. VME were observed with nitrofurantoin for S. epidermidis and with chloramphenicol for E. coli, both categorised as susceptible by the ocelloscope system. In a previous study evaluating Vitek 2, essential agreements with nitrofurantoin were 93.7 % for staphylococci and 88.9 % for enterococci [25], indicating that susceptibility testing for nitrofurantoin may
7 Eur J Clin Microbiol Infect Dis (2015) 34: Table 4 Results of AST of positive blood cultures. Positive blood cultures analysed with the ocelloscope system compared to MIC values obtained by testing the blood culture isolates with the manually read Sensititre system ocelloscope MIC value (Sensititre MIC value) Antimicrobial agent E. coli S. aureus E. faecalis Amikacin 4 ( 4) Ampicillin 1(4) 1(1) Ampicillin/sulbactam 2:1 ratio 2/1 ( 2/1) Aztreonam 2 ( 2) Carbenicillin 32 ( 32) Ceftazidime 1 ( 1) Cefepime 2 ( 2) Cefoperazone 4 ( 4) Cefotaxime 4 ( 4) Ceftriaxone 4 ( 4) Chloramphenicol 16 (8) 4 (8) 8 (8) Ciprofloxacin 0.25 ( 0.25) 1 ( 1) 1 ( 1) Clindamycin 0.5 ( 0.5) >2 (>2) Daptomycin 0.5 ( 0.5) 1 (1) Erythromycin 0.25 (0.5) 2 (2) Gentamicin 1 ( 1) 2 ( 2) 16 (16) Imipenem 1 ( 1) Levofloxacin 0.12 ( 0.12) 0.25 ( 0.25) 1 (1) Linezolid 1 (2) 2 (2) Lomefloxacin 0.5 ( 0.5) Moxifloxacin 0.25 ( 0.25) 0.25 ( 0.25) Nitrofurantoin 32 ( 32) 32 ( 32) Oxacillin 0.25 ( 0.25) >4 (>4) Penicillin 0.5 (4) 8 (4) Piperacillin 8 ( 8) Piperacillin/tazobactam 4 8/4 ( 8/4) Quinupristin/dalfopristin 0.5 ( 0.5) >4 (>4) Rifampin 0.5 ( 0.5) 2 (2) Tetracycline 1 ( 1) 2 ( 2) >16 (>16) Ticarcillin 8 ( 8) Ticarcillin/clavulanic acid 2 16/2 ( 16/2) Tigecycline 0.12 (0.06) 0.25 (0.12) Tobramycin 1 ( 1) Trimethoprim/sulphamethoxazole 0.5/9.5 ( 0.5/9.5) 0.5/9.5 ( 0.5/9.5) 0.5/9.5 ( 0.5/9.5) Vancomycin 1(1) 2(2) : the antimicrobial was not included on the chosen TREK Sensititre plate. : MIC value is below the range tested. >: MIC value is above the range tested be difficult for automated instruments. A minor error was observed for E. faecalis when tested against linezolid. Manual reading classified the isolate as susceptible (MIC values 1 mg/l and 2 mg/l), while the ocelloscope system classified both isolates as intermediate (MIC=4 mg/l for both isolates). This discrepancy may be explained by the fact that linezolid MIC is determined at % inhibition of growth (i.e. visible growth in negative wells) for manual reading, while the ocelloscope system measured MIC at 100 % inhibition of growth. The ocelloscope software, however, can be adjusted to allow for % growth. For all other discrepancies, measurements performed by the ocelloscope system resulted in lower MIC values than the manually read Sensititre plates, which were expected, since rapid AST methods generally result in lower MIC values. Based on the promising results from analysis of the clinical isolates, the ability of the ocelloscope system to analyse positive blood cultures was examined. Only one discrepancy was observed in the 60 bacteria antibiotic combinations: E. coli against chloramphenicol. Two possible sources of errors in
8 2392 Eur J Clin Microbiol Infect Dis (2015) 34: AST are direct inoculation of mixed cultures and nonstandardised inoculum size [26 28]. In some cases, mixed blood cultures can be determined by microscopy and Gram staining and, thus, be excluded from the AST method. If the blood culture contains bacteria with different morphology, this would be visible in the ocelloscope system and precautions may be taken. In order to ensure standardised inoculum size, all bacterial cell suspensions were adjusted to McFarland 0.5. Although blood cells and traces of blood culture broth can interfere with measurements, this would be visible in the ocelloscope system when setting up the experiment. For many compounds, the MIC value increases as inoculum increases (e.g. S. aureus tested against ciprofloxacin, oxacillin and vancomycin), while other antimicrobials such as chloramphenicol or the tetracyclines are not associated with inoculum effects [29, 30]. An advantage of the ocelloscope system, as well as other growth-based susceptibility tests, is that it only takes live bacteria into account and that this method, in contrast to agar diffusion tests, is independent of the antibiotic diffusion rate and time to macroscopically visible growth. Taken together, the results from studies on QC reference strains, clinical isolates and positive blood cultures showed an overall high accuracy of the ocelloscope system. A major aim of this study was to assess whether the ocelloscope system could reduce the turnaround time of AST compared to conventional AST methods. If so, the ocelloscope system might serve as an essential screening tool to accelerate appropriate antibiotic therapy. The benefits of timely reporting of susceptibility results have been highlighted previously [31, 32]. Testing of QC reference strains and clinical isolates resulted in an average time-to-result of 100 min, with MIC determination being possible for 43 % of the bacteria antibiotic combinations within 1 h (Table 2). However, it is possible to decrease the time-to-result even further by optimising algorithms and software settings, as the ocelloscope system was set up to analyse every 15 min for 12 h. Naturally, if the ocelloscope system was set up to analyse every 5 min, the time-to-result would have been shorter. Furthermore, a 2-h culture step was included to ensure early to mid-exponential phase of growth of the bacteria prior to analysis by the ocelloscope system. In a clinical setting, this step could be omitted, as the technique of the ocelloscope system could be integrated into automated blood culturing systems and, therefore, perform AST directly on positive blood cultures. For AST on the included positive blood cultures, the average time-to-result ranged from 1 to 4.5 h, depending on the bacteria antibiotic combination and whether the bacteria had reached the stationary growth phase. All blood cultures used in this study became positive during the night and were not processed until 8 o clock the following morning. Consequently, these cultures may have been positive for up to 13 h. A lag phase of 2 h was observed for the S. aureus blood culture, which is the major reason for the prolonged time-to-result compared to that of the QC reference strains and the clinical isolates. According to the manufacturer s instructions, the incubation time of Sensititre MIC plates should be 18 h. This means a reduction in the average timeto-result of approximately 16.5 h by using the ocelloscope system. The fastest commercially available automatic AST method (Vitek 2, biomeriéux, France) requires approximately 8 h to detect antimicrobial resistances in bacteria [33]. Taken together, these results strongly indicate that the ocelloscope system has the potential to decrease the time-to-result for AST. Translating this into daily workflow entails that same-day treatment or adjustments of antibiotic therapy are possible with the ocelloscope system. Only a few methods enable AST under 1 h, and only one method enables the determination of a real MIC value; however, this method is currently not commercially available [7]. AST of blood cultures by ocelloscope using Sensititre microdilution plates may be incorporated into the clinical laboratory workflow, with 95 % of the results being available within 3 h and can be confirmed by inspection of the movies provided for each of the 96 wells. The results from the three different types of samples clearly show that several parameters influence the time-to-result, including bacterial growth phase, bacterial doubling time, mode-of-action of the antibiotic and bacterial expression of resistance genes. However, it is clear that the prolonged time-to-result for the positive blood cultures is due to the growth state of the bacteria. Large variations of the time-toresult were observed for all samples, depending on the antibiotic of concern. As the number of isolates and species tested are limited, these variations might evolve when a greater number are included. However, it is crucial to note that part of these variations may not be clinically relevant, due to, for example, the site of infection, expression of resistance genes or antibiotic preference. To take full advantages of the ocelloscope technique in a clinical setting, it should be incorporated into a fully automatic system, such as the blood culture system, or further developed into a fully automatic AST system of its own, and, most importantly, be able to analyse the bacterial infection as early as possible. However, considering the current version of the ocelloscope system, analysing a 96-well plate of growthoptimised bacteria using autofocus, continuing image acquisition and automated data analysis, the total time-to-result of AST would be around min for 90 % of the analysed antimicrobials. The flexibility of the software makes adaption possible to most laboratory information management systems, even with the integration of movies from each well to ensure confirmation and acceptance of the test results by clinicians. The time-to-result for bacterial identification has been shortened considerably by the use of mass spectrometry techniques, and, therefore, no longer represents the bottleneck of
9 Eur J Clin Microbiol Infect Dis (2015) 34: AST. Consequently, the identification of a given infection is usually available before the results of AST and this information can, therefore, be incorporated into the data analysis part of the software. Conclusion Although our data are preliminary, this study suggests that the ocelloscope system can decrease detection time, making same-day reporting possible and, thus, permitting better patient management. With an overall agreement of 96 % for the nine tested isolates and three positive blood cultures, the rapid detection time does not interfere with sensitivity. Strains with methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase (ESBL) resistance profiles were rapidly detected by the ocelloscope system. A fullscale validation of the ocelloscope system including more isolates is necessary to assess the impact of using it for antimicrobial susceptibility testing (AST). Acknowledgements This work was supported by grants from the Danish National Innovation Foundation and from the Danish Agency for Science We thank Trine Poulsen for the excellent technical assistance. Conflict of interest The authors declare competing financial interests. Declaration: M.F. is employed in a joint research project between Aarhus University, Aalborg University, the Danish National Innovation Foundation and Philips BioCell. E.S. and S.K. are employed at Philips BioCell. Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. References 1. Infectious Diseases Society of America (2011) Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 52:S397 S Deresinski S (2007) Principles of antibiotic therapy in severe infections: optimizing the therapeutic approach by use of laboratory and clinical data. Clin Infect Dis 45:S177 S Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S et al (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34: Cohen J (2009) Sepsis. Medicine 37: Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM et al (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, Intensive Care Med 39: Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM et al (2010) Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother 54: van Belkum A, Durand G, Peyret M, Chatellier S, Zambardi G, Schrenzel J et al (2013) Rapid clinical bacteriology and its future impact. Ann Lab Med 33: Jenkins SG, Schuetz AN (2012) Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin Proc 87: Pulido MR, García-Quintanilla M, Martín-Peña R, Cisneros JM, McConnell MJ (2013) Progress on the development of rapid methods for antimicrobial susceptibility testing. J Antimicrob Chemother 68: Didelot X, Bowden R, Wilson DJ, Peto TE, Crook DW (2012) Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet 13: Chatzigeorgiou KS, Sergentanis TN, Tsiodras S, Hamodrakas SJ, Bagos PG (2011) Phoenix 100 versus Vitek 2 in the identification of gram-positive and gram-negative bacteria: a comprehensive metaanalysis. J Clin Microbiol 49: Duggal S, Gaind R, Tandon N, Deb M, Chugh TD (2012) Comparison of an automated system with conventional identification and antimicrobial susceptibility testing. ISRN Microbiol 2012: doi: /2012/ Gherardi G, Angeletti S, Panitti M, Pompilio A, Di Bonaventura G, Crea F et al (2012) Comparative evaluation of the Vitek-2 Compact and Phoenix systems for rapid identification and antibiotic susceptibility testing directly from blood cultures of Gram-negative and Gram-positive isolates. Diagn Microbiol Infect Dis 72: Mittman SA, Huard RC, Della-Latta P, Whittier S (2009) Comparison of BD Phoenix to Vitek 2, MicroScan MICroSTREP, and Etest for antimicrobial susceptibility testing of Streptococcus pneumoniae. J Clin Microbiol 47: Fredborg M, Andersen KR, Jørgensen E, Droce A, Olesen T, Jensen BB et al (2013) Real-time optical antimicrobial susceptibility testing. J Clin Microbiol 51: Wiegand I, Hilpert K, Hancock RE (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3: Schwarz S, Silley P, Simjee S, Woodford N, van Duijkeren E, Johnson AP et al (2010) Editorial: assessing the antimicrobial susceptibility of bacteria obtained from animals. J Antimicrob Chemother 65: Ferraro MJ, Jorgensen JH (1999) Susceptibility testing instrumentation and computerized expert systems for data analysis and interpretation. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (eds) Manual of clinical microbiology, 7th edn. American Society for Microbiology, Washington, DC, pp Patel JB, Sharp S, Novak-Weekley S (2013) Verification of antimicrobial susceptibility testing methods: a practical approach. Clin Microbiol Newsl 35: Richter SS, Ferraro MJ (2007) Susceptibility testing instrumentation and computerized expert systems for data analysis and interpretation. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (eds) Manual of clinical microbiology, 9th edn. ASM Press, Washington, DC, pp Thornsberry C (1985) Automated procedures for antimicrobial susceptibility tests. In: Lennette EH, Balows A,Hausler WJ Jr, Shadomy HJ (eds) Manual of clinical microbiology, 4th edn. American Society for Microbiology, Washington, DC, pp Rathe M, Kristensen L, Ellermann-Eriksen S, Thomsen MK, Schumacher H (2010) Vancomycin-resistant Enterococcus spp.: validation of susceptibility testing and in vitro activity of vancomycin, linezolid, tigecycline and daptomycin. APMIS 118:66 73
10 2394 Eur J Clin Microbiol Infect Dis (2015) 34: Endtz HP, van den Braak N, van Belkum A, Kluytmans JA, Koeleman JG, Spanjaard L et al (1997) Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in The Netherlands. J Clin Microbiol 35: Winstanley T, Courvalin P (2011) Expert systems in clinical microbiology. Clin Microbiol Rev 24: Barry J, Brown A, Ensor V, Lakhani U, Petts D, Warren C et al (2003) Comparative evaluation of the VITEK 2 Advanced Expert System (AES) in five UK hospitals. J Antimicrob Chemother 51: Trenholme GM, Kaplan RL, Karakusis PH, Stine T, Fuhrer J, Landau W et al (1989) Clinical impact of rapid identification and susceptibility testing of bacterial blood culture isolates. J Clin Microbiol 27: Doern GV, Vautour R, Gaudet M, Levy B (1994) Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol 32: Chapin K, Musgnug M (2003) Evaluation of three rapid methods for the direct identification of Staphylococcus aureus from positive blood cultures. J Clin Microbiol 41: Martinez M, Blondeau J, Cerniglia CE, Fink-Gremmels J, Guenther S, Hunter RP et al (2014) Workshop report: the 2012 antimicrobial agents in veterinary medicine: exploring the consequences of antimicrobial drug use: a 3-D approach. J Vet Pharmacol Ther 37:e1 e Udekwu KI, Parrish N, Ankomah P, Baquero F, Levin BR (2009) Functional relationship between bacterial cell density and the efficacy of antibiotics. J Antimicrob Chemother 63: Daniels R (2011) Surviving the first hours in sepsis: getting the basics right (an intensivist s perspective). J Antimicrob Chemother 66:ii11 ii Gonsalves MD, Sakr Y (2010) Early identification of sepsis. Curr Infect Dis Rep 12: Eigner U, Schmid A, Wild U, Bertsch D, Fahr AM (2005) Analysis of the comparative workflow and performance characteristics of the VITEK 2 and Phoenix systems. J Clin Microbiol 43:
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013. Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M.
 The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2008 (part I)
 ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2008 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus bacteraemia annual report, part I The format
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2008 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus bacteraemia annual report, part I The format
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2009 (part I)
 ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2009 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus Bacteraemia Annual Report, Part I The annual
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2009 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus Bacteraemia Annual Report, Part I The annual
ANTIBIOTICS IN SEPSIS
 ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
Disc Diffusion Susceptibility Methods
 Disc Diffusion Susceptibility Methods Introduction When a filter paper disc impregnated with a chemical is placed on agar the chemical will diffuse from the disc into the agar. This diffusion will place
Disc Diffusion Susceptibility Methods Introduction When a filter paper disc impregnated with a chemical is placed on agar the chemical will diffuse from the disc into the agar. This diffusion will place
The Antimicrobial Susceptibility Testing Device Manufacturer: Development Process, Timelines, and Challenges
 The Antimicrobial Susceptibility Testing Device Manufacturer: Development Process, Timelines, and Challenges Sheila Farnham, MT(ASCP) President Susceptibility Testing Manufacturers Association January
The Antimicrobial Susceptibility Testing Device Manufacturer: Development Process, Timelines, and Challenges Sheila Farnham, MT(ASCP) President Susceptibility Testing Manufacturers Association January
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital
 Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
BD Phoenix Automated Microbiology System
 BD Phoenix Automated Microbiology System Resistance Detection Workflow Efficiency Analysis and Communication BD Diagnostics 7 Loveton Circle Sparks, MD 115-0999 800.638.8663 www.bd.com/ds CHROMagar is
BD Phoenix Automated Microbiology System Resistance Detection Workflow Efficiency Analysis and Communication BD Diagnostics 7 Loveton Circle Sparks, MD 115-0999 800.638.8663 www.bd.com/ds CHROMagar is
Biological Sciences Initiative
 Biological Sciences Initiative HHMI Student Activities Measuring Antibiotic Resistance Introduction: You might be aware that antibiotics were once thought of as a magic bullet; a nearly perfect drug for
Biological Sciences Initiative HHMI Student Activities Measuring Antibiotic Resistance Introduction: You might be aware that antibiotics were once thought of as a magic bullet; a nearly perfect drug for
Liofilchem - Antibiotic Disk Interpretative Criteria and Quality Control - F14013 - Rev.7 / 20.02.2013
 Liofilchem Antibiotic Disk Interpretative Criteria and Quality Control F0 Rev.7 /.02. Amikacin AK Amoxicillin + Clavulanic acid AUG (+) ATCC 352 Coagulasenegative staphylococci Amoxicillin + Clavulanic
Liofilchem Antibiotic Disk Interpretative Criteria and Quality Control F0 Rev.7 /.02. Amikacin AK Amoxicillin + Clavulanic acid AUG (+) ATCC 352 Coagulasenegative staphylococci Amoxicillin + Clavulanic
GLYKOPEPTID- RESISTENS HOS ENTEROKOKKER
 GLYKOPEPTID- RESISTENS HOS ENTEROKOKKER KRISTIN HEGSTAD Wirth 1994 Eur J Biochem 222:235-46 ENTEROCOCCI (1) Gram+, normal inhabitants of the gastrointestinal tract (GIT) Arias 2012 Nat Rev Microbiol 16:266-78
GLYKOPEPTID- RESISTENS HOS ENTEROKOKKER KRISTIN HEGSTAD Wirth 1994 Eur J Biochem 222:235-46 ENTEROCOCCI (1) Gram+, normal inhabitants of the gastrointestinal tract (GIT) Arias 2012 Nat Rev Microbiol 16:266-78
A baseline survey of antimicrobial resistance in bacteria from selected New Zealand foods, 2009 2010
 A baseline survey of antimicrobial resistance in bacteria from New Zealand foods, 2009 2010 MAF Technical Paper No: 2011/53 Helen Heffernan 1, Teck Lok Wong 2, Jennifer Lindsay 2, Barbara Bowen1 and Rosemary
A baseline survey of antimicrobial resistance in bacteria from New Zealand foods, 2009 2010 MAF Technical Paper No: 2011/53 Helen Heffernan 1, Teck Lok Wong 2, Jennifer Lindsay 2, Barbara Bowen1 and Rosemary
Introduction. Introduction. Why do we need microbiological diagnostics of udder infections? Microbiological diagnostics How is it done?
 Introduction Microbiological diagnostics of udder infections Karin Persson Waller National Veterinary Institute (SVA) Swedish University of Agricultural Sciences Uppsala, Sweden Mastitis = in most cases
Introduction Microbiological diagnostics of udder infections Karin Persson Waller National Veterinary Institute (SVA) Swedish University of Agricultural Sciences Uppsala, Sweden Mastitis = in most cases
Urinary Tract Infections
 Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Date: November 30, 2010
 Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Date: November 30, 2010 To: Through: From: Subject: Drug Name(s): Application
Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Date: November 30, 2010 To: Through: From: Subject: Drug Name(s): Application
Welcome to Implementing Inquirybased Microbial Project. Veronica Ardi, PhD
 Welcome to Implementing Inquirybased Microbial Project Veronica Ardi, PhD Microbiology Laboratory Courses CourseSmart: ebook resources http://instructors.coursesmart.com/ Microbiology Laboratory Courses
Welcome to Implementing Inquirybased Microbial Project Veronica Ardi, PhD Microbiology Laboratory Courses CourseSmart: ebook resources http://instructors.coursesmart.com/ Microbiology Laboratory Courses
Annual Report on Staphylococcus aureus Bacteraemia Cases 2006
 Annual Report on Staphylococcus aureus Bacteraemia Cases 2006 STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT, 2006 Members of the Danish Staphylococcus aureus bacteremia group Thomas Benfield, Dr. med.
Annual Report on Staphylococcus aureus Bacteraemia Cases 2006 STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT, 2006 Members of the Danish Staphylococcus aureus bacteremia group Thomas Benfield, Dr. med.
Thermo Scientific Sensititre. Susceptibility and Identification System. Customizable testing solutions. maximum performance
 Thermo Scientific Sensititre Susceptibility and Identification System Customizable testing solutions maximum performance True MIC results the key to battling resistance The Thermo Scientific Sensititre
Thermo Scientific Sensititre Susceptibility and Identification System Customizable testing solutions maximum performance True MIC results the key to battling resistance The Thermo Scientific Sensititre
BD Modified CNA Agar BD Modified CNA Agar with Crystal Violet
 INSTRUCTIONS FOR USE READY-TO-USE PLATED MEDIA PA-255082.02 Rev.: June 2003 BD Modified CNA Agar BD Modified CNA Agar with Crystal Violet INTENDED USE BD Modified CNA Agar is a selective medium for the
INSTRUCTIONS FOR USE READY-TO-USE PLATED MEDIA PA-255082.02 Rev.: June 2003 BD Modified CNA Agar BD Modified CNA Agar with Crystal Violet INTENDED USE BD Modified CNA Agar is a selective medium for the
British Society for Antimicrobial Chemotherapy
 British Society for Antimicrobial Chemotherapy BSAC to actively support the EUCAST Disc Diffusion Method for Antimicrobial Susceptibility Testing in preference to the current BSAC Disc Diffusion Method
British Society for Antimicrobial Chemotherapy BSAC to actively support the EUCAST Disc Diffusion Method for Antimicrobial Susceptibility Testing in preference to the current BSAC Disc Diffusion Method
BACTERIAL ENUMERATION
 BACTERIAL ENUMERATION In the study of microbiology, there are numerous occasions when it is necessary to either estimate or determine the number of bacterial cells in a broth culture or liquid medium.
BACTERIAL ENUMERATION In the study of microbiology, there are numerous occasions when it is necessary to either estimate or determine the number of bacterial cells in a broth culture or liquid medium.
Practice Guidelines. Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections
 Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections CARRIE ARMSTRONG Guideline source: Surgical Infection Society, Infectious Diseases Society of America Literature search described?
Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections CARRIE ARMSTRONG Guideline source: Surgical Infection Society, Infectious Diseases Society of America Literature search described?
Staphylococcus aureus Bloodstream Infection Treatment Guideline
 Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
Optimizing Antimicrobial Susceptibility Test Reporting
 JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
Short Report: Failure of Burkholderia pseudomallei to Grow in an Automated Blood Culture System
 Accepted for Publication, Published online October 13, 2014; doi:10.4269/ajtmh.14-0018. The latest version is at http://ajtmh.org/cgi/doi/10.4269/ajtmh.14-0018 In order to provide our readers with timely
Accepted for Publication, Published online October 13, 2014; doi:10.4269/ajtmh.14-0018. The latest version is at http://ajtmh.org/cgi/doi/10.4269/ajtmh.14-0018 In order to provide our readers with timely
ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES
 ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES FOR USE WITH CLSI DOCUMENTS M2 AND M7 Beta-lactams: penicillins e penicillin a penicillin beta-lactam/beta-lactamase inhibitor combinations aminopenicillin a
ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES FOR USE WITH CLSI DOCUMENTS M2 AND M7 Beta-lactams: penicillins e penicillin a penicillin beta-lactam/beta-lactamase inhibitor combinations aminopenicillin a
BBL Mueller Hinton II Agar L007393 Rev. 11 July 2006
 ! I II BBL Mueller Hinton II Agar L007393 Rev. 11 July 2006 QUALITY CONTROL PROCEDURES INTRODUCTION Mueller Hinton II Agar is used in the standardized disc diffusion procedure for determining the susceptibility
! I II BBL Mueller Hinton II Agar L007393 Rev. 11 July 2006 QUALITY CONTROL PROCEDURES INTRODUCTION Mueller Hinton II Agar is used in the standardized disc diffusion procedure for determining the susceptibility
Drug Use Review. Edward Cox, M.D. Director Office of Antimicrobial Products
 Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Drug Use Review Date: April 5, 2012 To: Through: Edward Cox, M.D. Director
Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Drug Use Review Date: April 5, 2012 To: Through: Edward Cox, M.D. Director
Streptococcus pneumoniae IgG AB (13 Serotypes), MAID... 7
 Volume 12 December 2011 Table of Contents Summary of Test Changes... 3 Test Changes... 6 Human Anti-Mouse AB (HAMA), ELISA... 6 Hepatitis E Antibody (IgG, IgM)... 6 Hepatitis E Antibody (IgG)... 6 Hepatitis
Volume 12 December 2011 Table of Contents Summary of Test Changes... 3 Test Changes... 6 Human Anti-Mouse AB (HAMA), ELISA... 6 Hepatitis E Antibody (IgG, IgM)... 6 Hepatitis E Antibody (IgG)... 6 Hepatitis
Laboratory Exercise # 11: Differentiation of the Species Staphylococcus and Streptococcus
 Laboratory Exercise # 11: Differentiation of the Species Staphylococcus and Streptococcus Purpose: The purpose of this laboratory exercise is to explore the differences between Staphylococcal species and
Laboratory Exercise # 11: Differentiation of the Species Staphylococcus and Streptococcus Purpose: The purpose of this laboratory exercise is to explore the differences between Staphylococcal species and
FACULTY OF MEDICAL SCIENCE
 Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Medical Microbiology Microscopic slides and media
 Medical Microbiology Microscopic slides and media Head of Microbiology Department and Laboratory Medical Immunology : Janina Grzegorczyk MD, PhD, professor Implementators: Małgorzata Brauncajs MD Zbigniew
Medical Microbiology Microscopic slides and media Head of Microbiology Department and Laboratory Medical Immunology : Janina Grzegorczyk MD, PhD, professor Implementators: Małgorzata Brauncajs MD Zbigniew
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
Version 11.1 May 2012
 BSAC Methods for Antimicrobial Susceptibility Testing Version 11.1 May 2012 All enquiries to: Jenny Andrews at: + 44 (0) 121 507 5693 Email: jenny.andrews1@nhs.net Contents Page Working Party members 5
BSAC Methods for Antimicrobial Susceptibility Testing Version 11.1 May 2012 All enquiries to: Jenny Andrews at: + 44 (0) 121 507 5693 Email: jenny.andrews1@nhs.net Contents Page Working Party members 5
Received 22 March 2006/Returned for modification 2 May 2006/Accepted 14 September 2006
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2006, p. 4085 4094 Vol. 44, No. 11 0095-1137/06/$08.00 0 doi:10.1128/jcm.00614-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Two-Center
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2006, p. 4085 4094 Vol. 44, No. 11 0095-1137/06/$08.00 0 doi:10.1128/jcm.00614-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Two-Center
BD Sensi-Disc Susceptibility Test Discs
 INSTRUCTIONS FOR USE BD SENSI-DISCS SD-BD.01 Rev.: May 2005 BD Sensi-Disc Susceptibility Test Discs INTENDED USE Sensi-Disc susceptibility test discs are used for semi-quantitative in vitro susceptibility
INSTRUCTIONS FOR USE BD SENSI-DISCS SD-BD.01 Rev.: May 2005 BD Sensi-Disc Susceptibility Test Discs INTENDED USE Sensi-Disc susceptibility test discs are used for semi-quantitative in vitro susceptibility
Urinary tract infections caused by extended spectrum β-lactamase (ESBL) producing Escherichia coli and Klebsiella pneumoniae
 African Journal of Biotechnology Vol. 10(73), pp. 16661-16666, 21 November, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB11.2449 ISSN 1684 5315 2011 Academic Journals Full
African Journal of Biotechnology Vol. 10(73), pp. 16661-16666, 21 November, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB11.2449 ISSN 1684 5315 2011 Academic Journals Full
Effects of Antibiotics on Bacterial Growth and Protein Synthesis: Student Laboratory Manual
 Effects of Antibiotics on Bacterial Growth and Protein Synthesis: Student Laboratory Manual I. Purpose...1 II. Introduction...1 III. Inhibition of Bacterial Growth Protocol...2 IV. Inhibition of in vitro
Effects of Antibiotics on Bacterial Growth and Protein Synthesis: Student Laboratory Manual I. Purpose...1 II. Introduction...1 III. Inhibition of Bacterial Growth Protocol...2 IV. Inhibition of in vitro
Medical Microbiology Culture Media :
 Lecture 3 Dr. Ismail I. Daood Medical Microbiology Culture Media : Culture media are used for recognition and identification (diagnosis) of microorganisms. The media are contained in plates (Petri dishes),
Lecture 3 Dr. Ismail I. Daood Medical Microbiology Culture Media : Culture media are used for recognition and identification (diagnosis) of microorganisms. The media are contained in plates (Petri dishes),
The 11 th EURL-AR Proficiency Testing Salmonella and Campylobacter 2011. Susanne Karlsmose Lina M. Cavaco Rene S. Hendriksen Frank M.
 The 11 th EURL-AR Proficiency Testing Salmonella and Campylobacter 2011 Susanne Karlsmose Lina M. Cavaco Rene S. Hendriksen Frank M. Aarestrup European Union Reference Laboratory - Antimicrobial Resistance
The 11 th EURL-AR Proficiency Testing Salmonella and Campylobacter 2011 Susanne Karlsmose Lina M. Cavaco Rene S. Hendriksen Frank M. Aarestrup European Union Reference Laboratory - Antimicrobial Resistance
How To Treat Mrsa From A Dead Body
 HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
Global Spread of Carbapenemase- Producing Klebsiella pneumoniae
 Global Spread of Carbapenemase- Producing Klebsiella pneumoniae These pathogens arose in the mid-1990s and continue to spread, leaving few options for treating infected patients John Quale John Quale is
Global Spread of Carbapenemase- Producing Klebsiella pneumoniae These pathogens arose in the mid-1990s and continue to spread, leaving few options for treating infected patients John Quale John Quale is
Tigecycline and intravenous fosfomycin zone breakpoints equivalent to the EUCAST MIC criteria for Enterobacteriaceae
 Brief Original Article Tigecycline and intravenous fosfomycin zone breakpoints equivalent to the EUCAST MIC criteria for Enterobacteriaceae Fernando Pasteran, Celeste Lucero, Melina Rapoport, Leonor Guerriero,
Brief Original Article Tigecycline and intravenous fosfomycin zone breakpoints equivalent to the EUCAST MIC criteria for Enterobacteriaceae Fernando Pasteran, Celeste Lucero, Melina Rapoport, Leonor Guerriero,
Venkatadri Babu. et al. / International Journal of Biological & Pharmaceutical Research. 2014; 5(1): 66-70.
 66 e- ISSN 0976-3651 Print ISSN 2229-7480 International Journal of Biological & Pharmaceutical Research Journal homepage: www.ijbpr.com IJBPR MULTIDRUG RESISTANT ESCHERICHIA COLI AND KLEBSIELLA PNEUMONIAE
66 e- ISSN 0976-3651 Print ISSN 2229-7480 International Journal of Biological & Pharmaceutical Research Journal homepage: www.ijbpr.com IJBPR MULTIDRUG RESISTANT ESCHERICHIA COLI AND KLEBSIELLA PNEUMONIAE
Version 12 May 2013. BSAC Methods for Antimicrobial Susceptibility Testing. All enquiries to: Mandy Wootton. Email: Mandy.Wootton@wales.nhs.
 BSAC Methods for Antimicrobial Susceptibility Testing Version 12 May 2013 All enquiries to: Mandy Wootton Email: Mandy.Wootton@wales.nhs.uk Telephone: +44 (0) 2920 746581 Contents Page Working Party members
BSAC Methods for Antimicrobial Susceptibility Testing Version 12 May 2013 All enquiries to: Mandy Wootton Email: Mandy.Wootton@wales.nhs.uk Telephone: +44 (0) 2920 746581 Contents Page Working Party members
Determination of minimum inhibitory concentrations. Jennifer M. Andrews* Department of Microbiology, City Hospital NHS Trust, Birmingham B18 7QH, UK
 Journal of Antimicrobial Chemotherapy (2001) 48, Suppl. S1, 5 16 Determination of minimum inhibitory concentrations JAC Jennifer M. Andrews* Department of Microbiology, City Hospital NHS Trust, Birmingham
Journal of Antimicrobial Chemotherapy (2001) 48, Suppl. S1, 5 16 Determination of minimum inhibitory concentrations JAC Jennifer M. Andrews* Department of Microbiology, City Hospital NHS Trust, Birmingham
HUSRES Annual Report 2008 Martti Vaara. www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2009
 ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 29 Aalborg Viborg Herning Aarhus Hillerød Esbjerg Vejle Herlev København Hvidovre Odense Slagelse Næstved Sønderborg DEAR COLLEAGUE
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 29 Aalborg Viborg Herning Aarhus Hillerød Esbjerg Vejle Herlev København Hvidovre Odense Slagelse Næstved Sønderborg DEAR COLLEAGUE
Open Access Article pissn 0976 3325 eissn 2229 6816
 Original Article A STUDY OF EXTENDED SPECTRUM β-lactamase (ESBL) AND AmpC β-lactamase PRODUCING KLEBSIELLA PNEUMONIAE IN NEONATAL INTENSIVE CARE UNIT AT TERTIARY CARE HOSPITAL, AHMEDABAD Modi Dhara J 1,
Original Article A STUDY OF EXTENDED SPECTRUM β-lactamase (ESBL) AND AmpC β-lactamase PRODUCING KLEBSIELLA PNEUMONIAE IN NEONATAL INTENSIVE CARE UNIT AT TERTIARY CARE HOSPITAL, AHMEDABAD Modi Dhara J 1,
Intra-abdominal abdominal Infections
 Intra-abdominal abdominal Infections Marnie Peterson, Pharm.D., BCPS Dept. of Pediatric Infectious Diseases Medical School University of Minnesota Intra-abdominal abdominal Infections Intra-abdominal abdominal
Intra-abdominal abdominal Infections Marnie Peterson, Pharm.D., BCPS Dept. of Pediatric Infectious Diseases Medical School University of Minnesota Intra-abdominal abdominal Infections Intra-abdominal abdominal
SEA-HLM-415 Distribution: General. Establishment of national laboratory-based surveillance of antimicrobial resistance
 SEA-HLM-415 Distribution: General Establishment of national laboratory-based surveillance of antimicrobial resistance World Health Organization 2011 All rights reserved. Requests for publications, or for
SEA-HLM-415 Distribution: General Establishment of national laboratory-based surveillance of antimicrobial resistance World Health Organization 2011 All rights reserved. Requests for publications, or for
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES
 APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View.
 Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View. Samir K Saha, Ph.D. Department of Microbiology Dhaka Shishu Hospital Bangladesh Typhoid Fever:Salmonella
Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View. Samir K Saha, Ph.D. Department of Microbiology Dhaka Shishu Hospital Bangladesh Typhoid Fever:Salmonella
CEFA-DROPS AND CEFA-TABS
 Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
UTILIZATION of PLASMA ACTIVATED WATER in Biotechnology, Pharmacology and Medicine. JSC TECHNOSYSTEM-ECO Moscow, Russia April, 2009
 UTILIZATION of PLASMA ACTIVATED WATER in Biotechnology, Pharmacology and Medicine JSC TECHNOSYSTEM-ECO Moscow, Russia April, 2009 METHOD of WATER ACTIVATION with PLASMA of GAS DISCHARGE ANODE VACUUM WATER
UTILIZATION of PLASMA ACTIVATED WATER in Biotechnology, Pharmacology and Medicine JSC TECHNOSYSTEM-ECO Moscow, Russia April, 2009 METHOD of WATER ACTIVATION with PLASMA of GAS DISCHARGE ANODE VACUUM WATER
Flow Cytometry Analysis of the Activity of Disinfecting Agents Tested with Staphylococcus aureus and Escherichia coli
 Polish Journal of Microbiology 2005, Vol. 54, No 1, 21 26 Flow Cytometry Analysis of the Activity of Disinfecting Agents Tested with Staphylococcus aureus and Escherichia coli A. WO NIAK-KOSEK and J. KAWIAK*
Polish Journal of Microbiology 2005, Vol. 54, No 1, 21 26 Flow Cytometry Analysis of the Activity of Disinfecting Agents Tested with Staphylococcus aureus and Escherichia coli A. WO NIAK-KOSEK and J. KAWIAK*
Antibiotic Lock Therapy Guideline
 Antibiotic Lock Therapy Guideline I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition, chemotherapy, or hemodialysis,
Antibiotic Lock Therapy Guideline I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition, chemotherapy, or hemodialysis,
TREATMENT OF PERITONEAL DIALYSIS (PD) RELATED PERITONITIS. General Principles
 WA HOME DIALYSIS PROGRAM (WAHDIP) GUIDELINES General Principles 1. PD related peritonitis is an EMERGENCY early empiric treatment followed by close review is essential 2. When culture results and sensitivities
WA HOME DIALYSIS PROGRAM (WAHDIP) GUIDELINES General Principles 1. PD related peritonitis is an EMERGENCY early empiric treatment followed by close review is essential 2. When culture results and sensitivities
HUSRES Annual Report 2015
 HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
Testing Waste Water for Fecal Coliforms and/or E.coli using Colilert and Colilert -18 & Quanti-Tray
 Testing Waste Water for Fecal Coliforms and/or E.coli using Colilert and Colilert -18 & Quanti-Tray Gil Dichter World Wide Technical Support Manager, Water www.idexx.com/water 1 FOR ALL OF YOU WHO ARE
Testing Waste Water for Fecal Coliforms and/or E.coli using Colilert and Colilert -18 & Quanti-Tray Gil Dichter World Wide Technical Support Manager, Water www.idexx.com/water 1 FOR ALL OF YOU WHO ARE
Test Method for the Continuous Reduction of Bacterial Contamination on Copper Alloy Surfaces
 Test Method for the Continuous Reduction of Bacterial Contamination on Copper Alloy Surfaces Test Organisms: Staphylococcus aureus (ATCC 6538) Enterobacter aerogenes (ATCC 13048) Pseudomonas aeruginosa
Test Method for the Continuous Reduction of Bacterial Contamination on Copper Alloy Surfaces Test Organisms: Staphylococcus aureus (ATCC 6538) Enterobacter aerogenes (ATCC 13048) Pseudomonas aeruginosa
Microbiology BIOL 275 DILUTIONS
 DILUTIONS Occasionally a solution is too concentrated to be used as is. For example, when one is performing manual blood counts, the blood contains too many cells to be counted as such. Or when performing
DILUTIONS Occasionally a solution is too concentrated to be used as is. For example, when one is performing manual blood counts, the blood contains too many cells to be counted as such. Or when performing
Adapted from Biology 15 Laboratory Supplemental Manual: Wrightsman, Ininns and Cannon- Moloznic.
 Biology 3B Laboratory Cultural Characteristics of Bacteria Objectives: Describe bacterial structure: colony morphology, cell shape, growth patterns. To distinguish how various growth media will affect
Biology 3B Laboratory Cultural Characteristics of Bacteria Objectives: Describe bacterial structure: colony morphology, cell shape, growth patterns. To distinguish how various growth media will affect
TEST METHOD VERIFICATION AND VALIDATION
 1 TEST METHOD VERIFICATION AND VALIDATION SWACM 2014 MICHAEL LOEFFELHOLZ, PH.D., ABMM DEPT. PATHOLOGY UNIV TEXAS MEDICAL BRANCH GALVESTON, TX 2 OBJECTIVES Define validation and verification Describe components
1 TEST METHOD VERIFICATION AND VALIDATION SWACM 2014 MICHAEL LOEFFELHOLZ, PH.D., ABMM DEPT. PATHOLOGY UNIV TEXAS MEDICAL BRANCH GALVESTON, TX 2 OBJECTIVES Define validation and verification Describe components
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings
 Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Enumerating Chromogenic Agar Plates Using the Color QCOUNT Automated Colony Counter
 P3-4 IAFP, 06 Enumerating Chromogenic Agar Plates Using the Color QCOUNT Automated Colony Counter EILEEN GARRY, GRACE OUATTARA, PATRICK WILLIAMS, AND MEREDITH PESTA SPIRAL BIOTECH, INC., NORWOOD, MA Two
P3-4 IAFP, 06 Enumerating Chromogenic Agar Plates Using the Color QCOUNT Automated Colony Counter EILEEN GARRY, GRACE OUATTARA, PATRICK WILLIAMS, AND MEREDITH PESTA SPIRAL BIOTECH, INC., NORWOOD, MA Two
SELECTIVE AND DIFFERENTIAL MEDIA
 SELECTIVE AND DIFFERENTIAL MEDIA Selective and differential media are used to isolate or identify particular organisms. Selective media allow certain types of organisms to grow, and inhibit the growth
SELECTIVE AND DIFFERENTIAL MEDIA Selective and differential media are used to isolate or identify particular organisms. Selective media allow certain types of organisms to grow, and inhibit the growth
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
Antimicrobial Resistance and Human Health
 Antimicrobial Resistance and Human Health Dearbháile Morris, Discipline of Bacteriology, School of Medicine, National University of Ireland, Galway The microbial world The is a gene Talk cloud in a The
Antimicrobial Resistance and Human Health Dearbháile Morris, Discipline of Bacteriology, School of Medicine, National University of Ireland, Galway The microbial world The is a gene Talk cloud in a The
Sampling of the surface contamination using sterile cotton swabs from toys obtained from
 RESULTS Sampling of the surface contamination using sterile cotton swabs from toys obtained from the Nursery at Queen Mary, University of London showed diverse microorganism growth. A variety of species
RESULTS Sampling of the surface contamination using sterile cotton swabs from toys obtained from the Nursery at Queen Mary, University of London showed diverse microorganism growth. A variety of species
Oscar E. Guzman, PharmD, BCPS
 P Chapter 32 Antibiotic Streamlining Oscar E. Guzman, PharmD, BCPS Introduction Antibiotic streamlining is one of the most important methods pharmacists can use to encourage appropriate antibiotic use,
P Chapter 32 Antibiotic Streamlining Oscar E. Guzman, PharmD, BCPS Introduction Antibiotic streamlining is one of the most important methods pharmacists can use to encourage appropriate antibiotic use,
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity
 Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2012
 ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2012 Aalborg Viborg Herning Aarhus Hillerød Esbjerg Vejle Herlev København Hvidovre Odense Slagelse Sønderborg Nykøbing Falster DEAR
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2012 Aalborg Viborg Herning Aarhus Hillerød Esbjerg Vejle Herlev København Hvidovre Odense Slagelse Sønderborg Nykøbing Falster DEAR
URINE CULTURES GENERAL PROCEDURE
 University of Nebraska Medical Center Division of Laboratory Science Clinical Laboratory Science Program CLS 418/CLS 419 URINE CULTURES GENERAL PROCEDURE I. Principle Urine cultures are performed to detect
University of Nebraska Medical Center Division of Laboratory Science Clinical Laboratory Science Program CLS 418/CLS 419 URINE CULTURES GENERAL PROCEDURE I. Principle Urine cultures are performed to detect
Decreased Mortality Associated With Prompt Gram Staining of Blood Cultures
 Microbiology and Infectious Disease / Timely Gram Staining of Blood Cultures Decreased Mortality Associated With Prompt Gram Staining of Blood Cultures Joan Barenfanger, MD, Donald R. Graham, MD, 2 Lavanya
Microbiology and Infectious Disease / Timely Gram Staining of Blood Cultures Decreased Mortality Associated With Prompt Gram Staining of Blood Cultures Joan Barenfanger, MD, Donald R. Graham, MD, 2 Lavanya
Vancomycin. Beta-lactams. Beta-lactams. Vancomycin (Glycopeptide) Rifamycins (rifampin) MID 4
 Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
Prevalence of Metallo-β-lactamases Encoding Genes Among Pseudomonas
 Avicenna J Clin Microb Infec. 2014 May; 1(1): e19216. Published online 2014 April 28. DOI: 10.17795/ajcmi-19216 Research Article Prevalence of Metallo-β-lactamases Encoding Genes Among Pseudomonas aeruginosa
Avicenna J Clin Microb Infec. 2014 May; 1(1): e19216. Published online 2014 April 28. DOI: 10.17795/ajcmi-19216 Research Article Prevalence of Metallo-β-lactamases Encoding Genes Among Pseudomonas aeruginosa
Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults
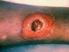 1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
How To Treat Mrsa In Finnish
 HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
IDENTIFICATION OF OTHER UNKNOWN BACTERIAL SPECIES: OU
 IDENTIFICATION OF OTHER UNKNOWN BACTERIAL SPECIES: OU I. OBJECTIVES To demonstrate the capacity to utilize previous laboratory experiences to accurately interpret tests conducted to identify a certain
IDENTIFICATION OF OTHER UNKNOWN BACTERIAL SPECIES: OU I. OBJECTIVES To demonstrate the capacity to utilize previous laboratory experiences to accurately interpret tests conducted to identify a certain
Quantifying Bacterial Concentration using a Calibrated Growth Curve
 BTEC 4200 Lab 2. Quantifying Bacterial Concentration using a Calibrated Growth Curve Background and References Bacterial concentration can be measured by several methods, all of which you have studied
BTEC 4200 Lab 2. Quantifying Bacterial Concentration using a Calibrated Growth Curve Background and References Bacterial concentration can be measured by several methods, all of which you have studied
Antimicrobial Susceptibility Patterns of Common and Unusual Enterococcus species Isolated from Clinical Specimens
 Original Article Vol. 24 No. 2 Isolation and sensitivity pattern of enterococci:- Chaudhary U, et al. 55 Antimicrobial Susceptibility Patterns of Common and Unusual Enterococcus species Isolated from Clinical
Original Article Vol. 24 No. 2 Isolation and sensitivity pattern of enterococci:- Chaudhary U, et al. 55 Antimicrobial Susceptibility Patterns of Common and Unusual Enterococcus species Isolated from Clinical
Antimicrobial Activity and Spectrum of PPI-0903M (T-91825), a Novel Cephalosporin, Tested against a Worldwide Collection of Clinical Strains
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein
 Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
Guidelines for Antimicrobial Stewardship in Hospitals in Ireland. A Strategy for the Control of Antimicrobial Resistance in Ireland
 A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
MagExtractor -Genome-
 Instruction manual MagExtractor-Genome-0810 F0981K MagExtractor -Genome- NPK-101 100 preparations Store at 4 C Contents [1] Introduction [2] Components [3] Materials required [4] Protocol 1. Purification
Instruction manual MagExtractor-Genome-0810 F0981K MagExtractor -Genome- NPK-101 100 preparations Store at 4 C Contents [1] Introduction [2] Components [3] Materials required [4] Protocol 1. Purification
General Trends in Infectious Disease
 General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
MTT Cell Proliferation Assay
 ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad
 Indian J Med Res 134, November 2011, pp 704-708 Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad Venubabu
Indian J Med Res 134, November 2011, pp 704-708 Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad Venubabu
Johns Hopkins Pathology Informatics
 Johns Hopkins Pathology Informatics Joel Saltz, MD, PhD - Director Support to Johns Hopkins Pathology Research in Medical Informatics Research in Computer Science Development of External Software Products
Johns Hopkins Pathology Informatics Joel Saltz, MD, PhD - Director Support to Johns Hopkins Pathology Research in Medical Informatics Research in Computer Science Development of External Software Products
SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS
 Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
Antimicrobial Susceptibility of Propionibacterium acnes Isolates from Shoulder Surgery
 AAC Accepts, published online ahead of print on 29 April 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.00463-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6
AAC Accepts, published online ahead of print on 29 April 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.00463-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6
CHAPTER 13. Quality Control/Quality Assurance
 CHAPTER 13 Quality Control/Quality Assurance Quality Control/Quality Assurance (QC/QA) can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements
CHAPTER 13 Quality Control/Quality Assurance Quality Control/Quality Assurance (QC/QA) can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements
VANCOMYCIN-RESISTANT ENTEROCOCCI INFECTIONS IN CANADIAN ACUTE-CARE HOSPITALS
 INFECTIOUS DISEASE PREVENTION AND CONTROL VANCOMYCIN-RESISTANT ENTEROCOCCI INFECTIONS IN CANADIAN ACUTE-CARE HOSPITALS SURVEILLANCE REPORT JANUARY 1, 1999 TO DECEMBER 31, 2011 PROTECTI NG C ANADIANS FROM
INFECTIOUS DISEASE PREVENTION AND CONTROL VANCOMYCIN-RESISTANT ENTEROCOCCI INFECTIONS IN CANADIAN ACUTE-CARE HOSPITALS SURVEILLANCE REPORT JANUARY 1, 1999 TO DECEMBER 31, 2011 PROTECTI NG C ANADIANS FROM
Susceptibility Testing of Clinical Isolates of Enterococcus faecium
 JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 1992, p. 41-45 0095-1137/92/010041-05$02.00/0 Copyright 1992, American Society for Microbiology Vol. 30, No. 1 Susceptibility Testing of Clinical Isolates of Enterococcus
JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 1992, p. 41-45 0095-1137/92/010041-05$02.00/0 Copyright 1992, American Society for Microbiology Vol. 30, No. 1 Susceptibility Testing of Clinical Isolates of Enterococcus
Metabolism Dr.kareema Amine Al-Khafaji Assistant professor in microbiology, and dermatologist Babylon University, College of Medicine, Department of
 Metabolism Dr.kareema Amine Al-Khafaji Assistant professor in microbiology, and dermatologist Babylon University, College of Medicine, Department of Microbiology. Metabolism sum of all chemical processes
Metabolism Dr.kareema Amine Al-Khafaji Assistant professor in microbiology, and dermatologist Babylon University, College of Medicine, Department of Microbiology. Metabolism sum of all chemical processes
Microbiological Testing of the Sawyer Mini Filter. 16 December 2013. Summary
 Microbiological Testing of the Sawyer Mini Filter 16 December 2013 Summary The Sawyer Mini Filter was tested for its ability to remove three microorganisms Raoultella terrigena, Bacillus subtilis, and
Microbiological Testing of the Sawyer Mini Filter 16 December 2013 Summary The Sawyer Mini Filter was tested for its ability to remove three microorganisms Raoultella terrigena, Bacillus subtilis, and
