1 DNA Vaccines An Overview
|
|
|
- Natalie Chapman
- 10 years ago
- Views:
Transcription
1 1 1 DNA Vaccines An Overview Britta Wahren and Margaret Liu 1.1 Rationale for DNA Vaccines Administration of genes via DNA or RNA may be considered the next-generation of scientific development following the use of recombinant proteins for prophylactic vaccines or for therapy. The use of DNA vaccines for the generation of immune responses arose from efforts to find immunogens that would be able to overcome some of the limitations of other modalities of vaccination. With the discovery of the potential widespread applications of DNA plasmids came appreciation of certain of the characteristics of DNA as a product: namely, its advantages, relative to other biologicals, for manufacturing (Chapter 3), product characterization, storage (Chapter 3), and delivery (Chapters 5 12). From the standpoints both of therapeutics and of vaccines, the use of DNA arose from the desire to have a protein be produced in situ. For a variety of applications, ranging from cytokine administration to gene therapy for metabolic and inherited disorders, it was clear that administration of the gene rather than the protein could have multiple advantages: proteins synthesized in situ from DNA could potentially persist locally or systemically for longer periods of time without the toxicities associated with the high levels of intravenously administered proteins, certain proteins such as cytokines could be administered to the desired site (i.e., intratumorally) (Chapter 7) more readily when administered as genes, and a protein synthesized from the gene would have mammalian posttranslational modifications, thus avoiding one of the significant challenges that can arise when making recombinant proteins in nonmammalian hosts. Although vaccines have been considered perhaps the greatest human health achievement, being successful even to the point of eliminating an entire wild-type disease from the planet (smallpox), certain diseases have remained unconquered by vaccination. Two key reasons for this are that the traditional approaches have either simply not worked, or have been considered potentially too risky for a disease such as HIV. As an example, although live attenuated virus vaccines have been extremely effective against a variety of diseases, they have at least the theoretical DNA Pharmaceuticals: Formulation and Delivery in Gene Therapy, DNA Vaccination and Immunotherapy. Edited by Martin Schleef Copyright 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: vch01.pmd 1
2 2 1 DNA Vaccines An Overview risk of reversion to wild type, which in the case of HIV would render the vaccinee infected with a virus that causes what today is still a fatal infection. As understanding of immune responses to disease increased, it became clear that the use of vaccines that induced primarily antibody responses might not be able successfully to target diseases that required a strong CD8+ T cell responses. Proteins that enter the cellular processing pathway resulting in the generation of CD8+ T cell responses generally have to be endogenously synthesized within a cell. Means to deliver the gene for an antigen, rather than the antigen itself, directly into cells were therefore sought, as the latter would generally result in the exogenous protein being taken into the endolysosomal processing pathway, with the resultant generation of MHC Class II-restricted CD4+ T cells rather than CD8+ T cells. The observation that plasmid DNA could directly transfect cells in vivo [1] came as a surprise given the complexity of viral structures that are designed for infecting cells. The process of DNA transfection is very inefficient and, moreover, the best transfected cell type is the muscle cell. Myocytes lack the immune accessory surface molecules needed to activate immune-responding cells appropriately, so it was a surprise to find that direct transfection of myocytes by immunization with unformulated plasmid DNA could indeed result in the generation of CD8+ T cells and protection against a lethal viral challenge [2]. DNA vaccines had further appeal as a product, in additional to their immunologic rationale. The manufacturing process promised to be fairly generic in comparison with those for other biologicals. Traditional live virus vaccines require years of challenging work to attenuate the pathogen properly and to design a cellular production system. Even recombinant proteins can be challenging, because of the need to find the correct producer cell able to make the antigen in the correct form (such as with the correct folding or posttranslational modifications). Because DNA vaccines are bacterial plasmids, the production is quite similar for different vaccines because they differ only in the gene sequence encoding the antigen. The majority of the plasmid, such as the backbone, can be identical or similar. Moreover, DNA vaccines at their simplest, being just plasmids, are potentially more stable (Chapter 3) than live viruses, an attribute that should facilitate their use in resourcepoor settings. 1.2 Preclinical Proof of Concept The initial demonstration that direct immunization with a simple plasmid of DNA encoding a protein from a pathogen could not only result in the generation of both arms of the immune response (cytotoxic T lymphocytes as well as antibodies), but could also protect from an otherwise lethal challenge [2] opened up the field of DNA vaccines. The ability to protect animals from a strain of virus different from the strain from which the gene was cloned generated considerable interest because it offered a potential means to make vaccines for diseases that have multiple strains, such as influenza or HIV. The influenza vaccine, for example, has to contain antigens 1275vch01.pmd 2
3 1.3 Clinical Trials 3 for three strains and needs to be reformulated each year as new strains arise. Not only is this a cumbersome process making the adequate yearly supply of vaccines problematic, but such a vaccine does not protect against the epidemic strains differing from the strain in the vaccine that occasionally arise mid-season. Of even more concern is the fact that such a vaccine will not protect against novel pandemic strains of influenza that periodically may arise, most notably in the 1919 Spanish influenza that killed millions of people worldwide. The demonstration that a DNA vaccine made from the genetic sequence of one strain was able to protect against challenge not just with a slightly different drifted strain, but against a different subtype, raised hopes for the ability of DNA vaccines to be effective against a variety of diseases. From those initial studies, the scientific literature rapidly grew to thousands of publications demonstrating the ability of DNA vaccines to induce immune responses and protective and therapeutic benefits in a variety of preclinical disease models. These models not only included various infectious diseases, including those caused by viruses, bacteria, and parasites, but also encompassed other types of disease, such as cancer, allergy, and autoimmunity (reviewed in [3, 4]). Additional applications for autoimmune diseases and allergies are based upon the ability of the DNA to alter the type of generated T cell help specifically for the particular protein antigen. Autoimmune responses are thought to be due to the inappropriate overproduction of either T helper 1- or T helper 2-type responses. In animal models, DNA vaccines have been shown to be able to alter the form of T cell help, and DNA vaccines have thus been able to prevent or ameliorate the disease in preclinical models of asthma [5] and diabetes [6]. It soon became evident, however, that DNA vaccines, while robust in small animal models, were less immunogenic in nonhuman primates and humans (reviewed in [3, 4]). This has given rise to a variety of approaches for making DNA vaccines of increased potency, as is explored below. 1.3 Clinical Trials Clinical trials have been performed for DNA vaccines encoding antigens from pathogens and tumors. In addition, however, trials have been performed with DNA encoding therapeutic proteins where not an immune response, but rather expression of the therapeutic protein, is desired. Such studies have included the therapeutic administration of a gene encoding a normal growth factor such as Fibroblastic Growth Factor, or other growth factors, the intent being not to replace a defective or missing protein, but rather to administer a supraphysiologic amount of the growth factor to a local site for a period of time more prolonged than would be achievable by administration of the recombinant protein [7, 8]. The factor then induces the growth of new blood vessels to ameliorate the ischemic condition of the limb or myocardium. DNA has also been used for what is more traditionally considered to be the purview of gene therapy: DNA encoding a form of the muscle 1275vch01.pmd 3
4 4 1 DNA Vaccines An Overview protein dystrophin, for example, has been administered to patients with forms of muscular dystrophy who are lacking in the production of any (or any normal) dystrophin ([9], Chapter 11). In both of these types of clinical applications, the hope is that no immune responses against the therapeutic protein will be generated. In the case in which the DNA is intended to provide additional amounts of a therapeutic protein locally, the individual is already tolerized to the protein, so the administration of the gene through the use of a plasmid should not break the tolerance. The use of a DNA plasmid is thought to be potentially less immunogenic for these purposes than the use of viral vectors, another widely studied approach. Of course, the most important observation in all the vaccine and therapeutic clinical trials has been that the vaccines have been safe to administer. Secondly, antibody and cellular immune responses, albeit generally low, have been observed in the patients in clinical trials. Interestingly, in HIV patients with long exposure to high levels of viral antigens (due to their high viral loads), new antibody but particularly T helper and cytolytic T cell responses were seen after DNA immunization [10, 11], the DNA somehow eliciting immune responses that the virus could not. This represents the important observation that different methods of producing an antigen in vivo, or the effects of different vectors, may result in different immune responses, an observation consistent with the results of preclinical prime-boost studies (see below). 1.4 Second-Generation Vaccines Perhaps the simplest approach to increasing the potency of DNA vaccines has been to design the plasmids to produce more protein antigen [12] and/or to increase the doses used in clinical trials, even up to milligram doses per vaccine [13, 14]. Another approach, described more fully in this book, is to formulate the DNA in such a way as to facilitate its uptake into cells, or to protect it from degradation. Alternative delivery modalities, such as combining injection (Chapters 6, 7 and 10) with in vivo electroporation (Chapters 11 and 12) to increase the amount of transfection, are also being explored. The coding sequences of DNA vaccines have also been modified to include genes encoding cytokines or other molecules that may enhance immune responses. Because the bacterial DNA in DNA vaccines has sequences that activate Toll-like receptors, the DNA is not simply an inert carrier of the genes, but itself also activates the innate immune system, which may in turn augment the cognate immune responses (reviewed in [15]). Efforts to increase this innate immune stimulation by increasing the number of CpG motifs in the plasmid have met with limited success, but the principal of harnessing the innate immune response to aid in the antigenspecific response is the focus of considerable attention. DNA vaccines have also been delivered by a variety of routes, variously to increase potency, to generate specific forms of immunity (e.g., mucosal), or to facilitate delivery. The earliest demonstration of the ability of DNA plasmids to generate 1275vch01.pmd 4
5 1.5 Conclusions 5 antibody responses utilized a gene gun to propel DNA-coated gold beads into the cells of the skin (Chapter 10) [16]. This approach has successfully resulted in the generation of antibodies against hepatitis B surface antigen in clinical studies [17]. In these studies, the titers were lower and required more immunizations that with the licensed protein vaccine, but nevertheless demonstrated the desired immune response in humans. Importantly, though, even patients who had not responded well to the traditional recombinant protein vaccine responded to the DNA vaccine [18]. Additional means of delivery have included the production of biodegradable to which the DNA is adhered (reviewed in [19]) or particles containing the DNA for oral delivery [20] (Chapters 5 and 8). Additional devices that propel the free DNA directly into the skin [21] or mucosa [22] have been developed. In vivo electroporation to increase the number of cells that are transfected is also being developed [23] (Chapters 11 and 12). One of the most promising approaches has been the combination of DNA vaccines with viral vectors or recombinant protein [24, 25] (reviewed in [4]). In this approach a DNA plasmid encoding a given antigen is injected, and the subsequent immunizations then utilize a heterologous delivery system such as a viral vector encoding the same antigen, or a different form of the antigen (e.g., a recombinant protein). This has been referred to as the prime-boost approach. While the mechanism for its efficacy has not been completely determined, a variety of different viral vectors, including adenoviruses and pox vectors, have been utilized. Interestingly, it appears that the approach is most effective when the DNA vaccine is given first, rather than the other way around. 1.5 Conclusions Although the second generation of DNA vaccines includes more complex formulations and devices, the inherent simplicity of the core of the vaccine (i.e., the plasmid DNA) nevertheless remains an attraction. For scenarios in which the formulation of final product may be more complex (such as the inclusion of two different vectors), it is felt that if that is what is required to overcome the challenges of making a vaccine for HIV, this will nevertheless be a critical part of the medical armamentarium. The potential for developing a somewhat generic, even if complex, approach to a variety of diseases, including diseases that have hitherto been resistant to prevention or therapy, makes these studies of continued high interest. 1275vch01.pmd 5
6 6 1 DNA Vaccines An Overview References 1 Wolff, J. A., Malone, R. W., Williams, P., et al., Science 1990, 247, Ulmer, J. B, Donnelly, J. J., Parker, S. E., et al., Science 1993, 259, Srivastava, I. K., Liu, M. A., Ann. Int. Med. 2003, 138, Liu, M. A., J. Intern. Med. 2003, 253, Jarman, E. R., Lamb, J. R., Immunology 2004, 112, Prud homme, G. J., Expert Rev. Vaccines 2003, 2, Baumgartner, I., Isner, J. M., Ann. Rev. Physiol. 2001, 63, Comerota, A. J., Throm, R. C., Miller, K. A., et al., J. Vasc. Surg. 2002, 35, Romero, N. B, Braun, S., Benveniste, O., Hum. Gene Ther. 2004, 15, Calarota, S. A., Kjerrstrom, A., Islam, K. B., Wahren, B., Hum. Gene Ther. 2001, 12, Calarota, S., Bratt, G., Nordlund, S., Lancet 1998, 351, zur Megede, J., Chen, M. C., Doe, B., et al., J. Virol. 2000, 74, MacGregor, R. R., Ginsberg, R., Ugen, K. E., et al., AIDS 2002, 16, Le, T. P., Coonan, K. M., Hedstrom, R. C., et al., Vaccine 2000, 18, Klinman, D. M., Yamshchikov, G., Ishigatsubo, Y. J., Immunol. 1997, 158, Tang, D. C., DeVit, M., Johnston, S. A., Nature 1992, 356, Roy, M. J., Wu, M. S., Barr, L. J., et al., Vaccine 2000, 19, Rottinghaus, S. T., Poland, G. A., Jacobson, R. M., et al., Vaccine 2003, 21, O Hagan, D. T., Singh, M., Ulmer, J. B., Immunol. Rev (June), 199, Howard, K. A., Li, X. W., Somavarapu, S., et al., Biochim. Biophys. Acta 2004, 1674, Trimble, C., Lin, C. T., Hung, C. F., et al., Vaccine 2003, 21, Lundholm, P., Leandersson, A. C., Christensson, B., et al., Virus Res. 2002, 82, Otten, G., Schaefer, M., Doe, B., et al., Vaccine 2004 (June 23), 22(19), Moorthy, V. S., Imoukhuede, E. B., Keating, S., et al., J. Infect. Dis. 2004, 189, Epstein, J. E., Charoenvit, Y., Kester, K. E., et al., Vaccine 2004 (April 16), 22(13 14), vch01.pmd 6
specific B cells Humoral immunity lymphocytes antibodies B cells bone marrow Cell-mediated immunity: T cells antibodies proteins
 Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Chapter 18: Applications of Immunology
 Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
The Body s Defenses CHAPTER 24
 CHAPTER 24 The Body s Defenses PowerPoint Lectures for Essential Biology, Third Edition Neil Campbell, Jane Reece, and Eric Simon Essential Biology with Physiology, Second Edition Neil Campbell, Jane Reece,
CHAPTER 24 The Body s Defenses PowerPoint Lectures for Essential Biology, Third Edition Neil Campbell, Jane Reece, and Eric Simon Essential Biology with Physiology, Second Edition Neil Campbell, Jane Reece,
BioMmune Technologies Inc. Corporate Presentation 2015
 BioMmune Technologies Inc Corporate Presentation 2015 * Harnessing the body s own immune system to fight cancer & other autoimmune diseases BioMmune Technologies Inc. (IMU) ABOUT A public biopharmaceutical
BioMmune Technologies Inc Corporate Presentation 2015 * Harnessing the body s own immune system to fight cancer & other autoimmune diseases BioMmune Technologies Inc. (IMU) ABOUT A public biopharmaceutical
Basic Overview of Preclinical Toxicology Animal Models
 Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Hapten - a small molecule that is antigenic but not (by itself) immunogenic.
 Chapter 3. Antigens Terminology: Antigen: Substances that can be recognized by the surface antibody (B cells) or by the TCR (T cells) when associated with MHC molecules Immunogenicity VS Antigenicity:
Chapter 3. Antigens Terminology: Antigen: Substances that can be recognized by the surface antibody (B cells) or by the TCR (T cells) when associated with MHC molecules Immunogenicity VS Antigenicity:
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
Why use passive immunity?
 Vaccines Active vs Passive Immunization Active is longer acting and makes memory and effector cells Passive is shorter acting, no memory and no effector cells Both can be obtained through natural processes:
Vaccines Active vs Passive Immunization Active is longer acting and makes memory and effector cells Passive is shorter acting, no memory and no effector cells Both can be obtained through natural processes:
1) Siderophores are bacterial proteins that compete with animal A) Antibodies. B) Red blood cells. C) Transferrin. D) White blood cells. E) Receptors.
 Prof. Lester s BIOL 210 Practice Exam 4 (There is no answer key. Please do not email or ask me for answers.) Chapters 15, 16, 17, 19, HIV/AIDS, TB, Quorum Sensing 1) Siderophores are bacterial proteins
Prof. Lester s BIOL 210 Practice Exam 4 (There is no answer key. Please do not email or ask me for answers.) Chapters 15, 16, 17, 19, HIV/AIDS, TB, Quorum Sensing 1) Siderophores are bacterial proteins
Chapter 43: The Immune System
 Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
Principles of Vaccination
 Immunology and Vaccine-Preventable Diseases Immunology is a complicated subject, and a detailed discussion of it is beyond the scope of this text. However, an understanding of the basic function of the
Immunology and Vaccine-Preventable Diseases Immunology is a complicated subject, and a detailed discussion of it is beyond the scope of this text. However, an understanding of the basic function of the
The role of IBV proteins in protection: cellular immune responses. COST meeting WG2 + WG3 Budapest, Hungary, 2015
 The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
SYMPOSIUM. June 15, 2013. Photonics Center Colloquium Room (906) 8 Saint Mary's St., Boston, MA 02215 Boston University
 SYMPOSIUM June 15, 2013 Photonics Center Colloquium Room (906) 8 Saint Mary's St., Boston, MA 02215 Bette Korber Los Alamos National Labs HIV evolution and the neutralizing antibody response We have recently
SYMPOSIUM June 15, 2013 Photonics Center Colloquium Room (906) 8 Saint Mary's St., Boston, MA 02215 Bette Korber Los Alamos National Labs HIV evolution and the neutralizing antibody response We have recently
Immunology Ambassador Guide (updated 2014)
 Immunology Ambassador Guide (updated 2014) Immunity and Disease We will talk today about the immune system and how it protects us from disease. Also, we ll learn some unique ways that our immune system
Immunology Ambassador Guide (updated 2014) Immunity and Disease We will talk today about the immune system and how it protects us from disease. Also, we ll learn some unique ways that our immune system
New developments in antigen-specific immunotherapies Novel therapeutic vaccines offer hope in the fight against chronic infectious disease and cancer
 New developments in antigen-specific immunotherapies Novel therapeutic vaccines offer hope in the fight against chronic infectious disease and cancer Vaccination has been used effectively for more than
New developments in antigen-specific immunotherapies Novel therapeutic vaccines offer hope in the fight against chronic infectious disease and cancer Vaccination has been used effectively for more than
Basics of Immunology
 Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
The Immune System: A Tutorial
 The Immune System: A Tutorial Modeling and Simulation of Biological Systems 21-366B Shlomo Ta asan Images taken from http://rex.nci.nih.gov/behindthenews/uis/uisframe.htm http://copewithcytokines.de/ The
The Immune System: A Tutorial Modeling and Simulation of Biological Systems 21-366B Shlomo Ta asan Images taken from http://rex.nci.nih.gov/behindthenews/uis/uisframe.htm http://copewithcytokines.de/ The
Prospects for Vaccines against Hepatitis C Viruses. T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS
 Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
2) Macrophages function to engulf and present antigen to other immune cells.
 Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
Immunity and how vaccines work
 1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
The Immune System and Disease
 Chapter 40 The Immune System and Disease Section 40 1 Infectious Disease (pages 1029 1033) This section describes the causes of disease and explains how infectious diseases are transmitted Introduction
Chapter 40 The Immune System and Disease Section 40 1 Infectious Disease (pages 1029 1033) This section describes the causes of disease and explains how infectious diseases are transmitted Introduction
Modelling and analysis of T-cell epitope screening data.
 Modelling and analysis of T-cell epitope screening data. Tim Beißbarth 2, Jason A. Tye-Din 1, Gordon K. Smyth 1, Robert P. Anderson 1 and Terence P. Speed 1 1 WEHI, 1G Royal Parade, Parkville, VIC 3050,
Modelling and analysis of T-cell epitope screening data. Tim Beißbarth 2, Jason A. Tye-Din 1, Gordon K. Smyth 1, Robert P. Anderson 1 and Terence P. Speed 1 1 WEHI, 1G Royal Parade, Parkville, VIC 3050,
Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines
 FINAL ENGLISH ONLY Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines World Health Organization 2013 All rights reserved. Publications of the World Health Organization
FINAL ENGLISH ONLY Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines World Health Organization 2013 All rights reserved. Publications of the World Health Organization
Overview of Phase 1 Oncology Trials of Biologic Therapeutics
 Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
Guidance. 2. Definitions. 1. Introduction
 Work with naked DNA or RNA (including oligonucleotides, sirna, mirna, sequences that code for highly biologically active molecules and full length viral genomes) Guidance 1. Introduction The following
Work with naked DNA or RNA (including oligonucleotides, sirna, mirna, sequences that code for highly biologically active molecules and full length viral genomes) Guidance 1. Introduction The following
10. T and B cells are types of a. endocrine cells. c. lymphocytes. b. platelets. d. complement cells.
 Virus and Immune System Review Directions: Write your answers on a separate piece of paper. 1. Why does a cut in the skin threaten the body s nonspecific defenses against disease? a. If a cut bleeds, disease-fighting
Virus and Immune System Review Directions: Write your answers on a separate piece of paper. 1. Why does a cut in the skin threaten the body s nonspecific defenses against disease? a. If a cut bleeds, disease-fighting
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY. Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds
 NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
HSA Consumer Guide. Understanding Vaccines, Vaccine Development and Production. www.hsa.gov.sg November 2009. How a Vaccine Works.
 November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
MEDICAL BREAKTHROUGHS RESEARCH SUMMARY
 MEDICAL BREAKTHROUGHS RESEARCH SUMMARY TOPIC: Training the Body to Fight Melanoma REPORT: 3823 BACKGROUND: Melanoma is the most dangerous form of skin cancer that can be hard to treat and fatal if not
MEDICAL BREAKTHROUGHS RESEARCH SUMMARY TOPIC: Training the Body to Fight Melanoma REPORT: 3823 BACKGROUND: Melanoma is the most dangerous form of skin cancer that can be hard to treat and fatal if not
CONTENT. Chapter 1 Review of Literature. List of figures. List of tables
 Abstract Abbreviations List of figures CONTENT I-VI VII-VIII IX-XII List of tables XIII Chapter 1 Review of Literature 1. Vaccination against intracellular pathogens 1-34 1.1 Role of different immune responses
Abstract Abbreviations List of figures CONTENT I-VI VII-VIII IX-XII List of tables XIII Chapter 1 Review of Literature 1. Vaccination against intracellular pathogens 1-34 1.1 Role of different immune responses
Principles of Vaccination
 Immunology and Vaccine-Preventable Diseases Immunology is a complicated subject, and a detailed discussion of it is beyond the scope of this text. However, an understanding of the basic function of the
Immunology and Vaccine-Preventable Diseases Immunology is a complicated subject, and a detailed discussion of it is beyond the scope of this text. However, an understanding of the basic function of the
Name (print) Name (signature) Period. (Total 30 points)
 AP Biology Worksheet Chapter 43 The Immune System Lambdin April 4, 2011 Due Date: Thurs. April 7, 2011 You may use the following: Text Notes Power point Internet One other person in class "On my honor,
AP Biology Worksheet Chapter 43 The Immune System Lambdin April 4, 2011 Due Date: Thurs. April 7, 2011 You may use the following: Text Notes Power point Internet One other person in class "On my honor,
How To Follow Up After Treatment With Gene Therapy
 European Medicines Agency London, 30 May 2008 Doc. Ref. EMEA/CHMP/GTWP/60436/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON FOLLOW-UP OF PATIENTS ADMINISTERED WITH GENE THERAPY
European Medicines Agency London, 30 May 2008 Doc. Ref. EMEA/CHMP/GTWP/60436/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON FOLLOW-UP OF PATIENTS ADMINISTERED WITH GENE THERAPY
Figure 14.2 Overview of Innate and Adaptive Immunity
 I M M U N I T Y Innate (inborn) Immunity does not distinguish one pathogen from another Figure 14.2 Overview of Innate and Adaptive Immunity Our first line of defense includes physical and chemical barriers
I M M U N I T Y Innate (inborn) Immunity does not distinguish one pathogen from another Figure 14.2 Overview of Innate and Adaptive Immunity Our first line of defense includes physical and chemical barriers
Oncos Therapeutics: ONCOS THERAPEUTICS Personalized Cancer Immunotherapy. March 2015. Antti Vuolanto, COO and co-founder
 Oncos Therapeutics: Personalized Cancer Immunotherapy ONCOS THERAPEUTICS Personalized Cancer Immunotherapy March 2015 Antti Vuolanto, COO and co-founder 1 History of Oncos Therapeutics 2002 2007 2009 Research
Oncos Therapeutics: Personalized Cancer Immunotherapy ONCOS THERAPEUTICS Personalized Cancer Immunotherapy March 2015 Antti Vuolanto, COO and co-founder 1 History of Oncos Therapeutics 2002 2007 2009 Research
Chapter 3. Immunity and how vaccines work
 Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
CCR Biology - Chapter 9 Practice Test - Summer 2012
 Name: Class: Date: CCR Biology - Chapter 9 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Genetic engineering is possible
Name: Class: Date: CCR Biology - Chapter 9 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Genetic engineering is possible
Federal Funding for Technological Revolutions: Biotechnology and Healthcare Highlights
 ADVANCED TE CHNOLOGY P ROGRAM The Advanced Technology Program Federal Funding for Technological Revolutions: Biotechnology and Healthcare Highlights April 2006 National Institute of Standards and Technology
ADVANCED TE CHNOLOGY P ROGRAM The Advanced Technology Program Federal Funding for Technological Revolutions: Biotechnology and Healthcare Highlights April 2006 National Institute of Standards and Technology
Transfection-Transfer of non-viral genetic material into eukaryotic cells. Infection/ Transduction- Transfer of viral genetic material into cells.
 Transfection Key words: Transient transfection, Stable transfection, transfection methods, vector, plasmid, origin of replication, reporter gene/ protein, cloning site, promoter and enhancer, signal peptide,
Transfection Key words: Transient transfection, Stable transfection, transfection methods, vector, plasmid, origin of replication, reporter gene/ protein, cloning site, promoter and enhancer, signal peptide,
Non-clinical development of biologics
 Aurigon Life Science GmbH Non-clinical development of biologics Requirements, challenges and case studies Committed to Life. Sigrid Messemer vet. med. M4 Seminar March 10 th 2014 Aurigon - your full service
Aurigon Life Science GmbH Non-clinical development of biologics Requirements, challenges and case studies Committed to Life. Sigrid Messemer vet. med. M4 Seminar March 10 th 2014 Aurigon - your full service
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
JIANGSU CARTMAY INDUSTRIAL CO.,LTD www.labfurniture.asia mail: [email protected]
 The basic layout, the main functions and instrumentation concept of micro Inspection Division laboratory, 1, Virology Laboratory 1. Functions: for the city to monitor the prevalence of HIV disease, dealing
The basic layout, the main functions and instrumentation concept of micro Inspection Division laboratory, 1, Virology Laboratory 1. Functions: for the city to monitor the prevalence of HIV disease, dealing
Gene Therapy. The use of DNA as a drug. Edited by Gavin Brooks. BPharm, PhD, MRPharmS (PP) Pharmaceutical Press
 Gene Therapy The use of DNA as a drug Edited by Gavin Brooks BPharm, PhD, MRPharmS (PP) Pharmaceutical Press Contents Preface xiii Acknowledgements xv About the editor xvi Contributors xvii An introduction
Gene Therapy The use of DNA as a drug Edited by Gavin Brooks BPharm, PhD, MRPharmS (PP) Pharmaceutical Press Contents Preface xiii Acknowledgements xv About the editor xvi Contributors xvii An introduction
CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS
 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS See APPENDIX (1) THE PRECIPITIN CURVE; (2) LABELING OF ANTIBODIES The defining characteristic of HUMORAL immune responses (which distinguishes them from CELL-MEDIATED
CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS See APPENDIX (1) THE PRECIPITIN CURVE; (2) LABELING OF ANTIBODIES The defining characteristic of HUMORAL immune responses (which distinguishes them from CELL-MEDIATED
Masters of Science in Microbiology and Immunology 2013/2014
 Fall semester: Graduate Medical Microbiology (4 credits). This course is designed to introduce graduate students to bacterial, fungal and viral pathogens that are the etiological agents of the most significant
Fall semester: Graduate Medical Microbiology (4 credits). This course is designed to introduce graduate students to bacterial, fungal and viral pathogens that are the etiological agents of the most significant
Asthma (With a little SCID to start) Disclosures Outline Starting with the Immune System The Innate Immune System The Adaptive Immune System
 1 2 3 4 5 6 7 8 9 Asthma (With a little SCID to start) Lauren Smith, MD CHKD Pediatric Allergy/Immunology Disclosures None Will be discussing some medications that are not yet FDA approved Outline SCID
1 2 3 4 5 6 7 8 9 Asthma (With a little SCID to start) Lauren Smith, MD CHKD Pediatric Allergy/Immunology Disclosures None Will be discussing some medications that are not yet FDA approved Outline SCID
Final Review. Aptamers. Making Aptamers: SELEX 6/3/2011. sirna and mirna. Central Dogma. RNAi: A translation regulation mechanism.
 Central Dogma Final Review Section Week 10 DNA RNA Protein DNA DNA replication DNA RNA transcription RNA Protein translation **RNA DNA reverse transcription http://bass.bio.uci.edu/~hudel/bs99a/lecture20/lecture1_1.html
Central Dogma Final Review Section Week 10 DNA RNA Protein DNA DNA replication DNA RNA transcription RNA Protein translation **RNA DNA reverse transcription http://bass.bio.uci.edu/~hudel/bs99a/lecture20/lecture1_1.html
CHAPTER 35 HUMAN IMMUNE SYSTEM STANDARDS:SC.912.L.14.52 & SC.912.L.14.6
 CHAPTER 35 HUMAN IMMUNE SYSTEM STANDARDS:SC.912.L.14.52 & SC.912.L.14.6 SECTION 1 - Infectious Disease 1.Identify the causes of infectious disease. 2.Explain how infectious diseases are spread. Causes
CHAPTER 35 HUMAN IMMUNE SYSTEM STANDARDS:SC.912.L.14.52 & SC.912.L.14.6 SECTION 1 - Infectious Disease 1.Identify the causes of infectious disease. 2.Explain how infectious diseases are spread. Causes
Luca Romagnoli, Ph.D. Business Development Manager
 Modelli innovativi di produzione per lo sviluppo di un processo altamente qualitativo di farmaci biologici Luca Romagnoli, Ph.D. Business Development Manager BIOLOGICAL DRUGS - SOURCES Monoclonal antibodies
Modelli innovativi di produzione per lo sviluppo di un processo altamente qualitativo di farmaci biologici Luca Romagnoli, Ph.D. Business Development Manager BIOLOGICAL DRUGS - SOURCES Monoclonal antibodies
PX Therapeutics : the partner for early stage biotherapeutics development Biotuesday, May 5 2009
 PX Therapeutics : the partner for early stage biotherapeutics development Biotuesday, May 5 2009 Christelle Dagoneau, PhD Business Development Director Company Profile Protein expert incorporated in 2000
PX Therapeutics : the partner for early stage biotherapeutics development Biotuesday, May 5 2009 Christelle Dagoneau, PhD Business Development Director Company Profile Protein expert incorporated in 2000
Human hybridoma technology for the production of monoclonal antibodies
 Human hybridoma technology for the production of monoclonal antibodies A new method of creating cell lines has been developed for the production of "totally human" monoclonal antibodies. Professor Hans
Human hybridoma technology for the production of monoclonal antibodies A new method of creating cell lines has been developed for the production of "totally human" monoclonal antibodies. Professor Hans
Muscular Dystrophy: Stem Cell Therapy
 by Caitlin Pederson Abstract: Genetic disorders affect many people, and muscular dystrophy is a disorder that can greatly decrease the quality of life. Finding treatment to stop or prevent the loss of
by Caitlin Pederson Abstract: Genetic disorders affect many people, and muscular dystrophy is a disorder that can greatly decrease the quality of life. Finding treatment to stop or prevent the loss of
Core Topic 2. The immune system and how vaccines work
 Core Topic 2 The immune system and how vaccines work Learning outcome To be able to describe in outline the immune system and how vaccines work in individuals and populations Learning objectives Explain
Core Topic 2 The immune system and how vaccines work Learning outcome To be able to describe in outline the immune system and how vaccines work in individuals and populations Learning objectives Explain
Hemophilia Care. Will there always be new people in the world with hemophilia? Will hemophilia be treated more effectively and safely in the future?
 Future of This chapter provides answers to these questions: Will there always be new people in the world with hemophilia? Will hemophilia be treated more effectively and safely in the future? Will the
Future of This chapter provides answers to these questions: Will there always be new people in the world with hemophilia? Will hemophilia be treated more effectively and safely in the future? Will the
Immuno-Oncology Therapies to Treat Lung Cancer
 Immuno-Oncology Therapies to Treat Lung Cancer What you need to know ONCHQ14NP07519 Introduction: Immuno-oncology represents an innovative approach to cancer research that seeks to harness the body s own
Immuno-Oncology Therapies to Treat Lung Cancer What you need to know ONCHQ14NP07519 Introduction: Immuno-oncology represents an innovative approach to cancer research that seeks to harness the body s own
DNA Vaccine for Chronic Hepatitis C
 Novel Vaccine Development (3:00PM~3:30PM) DNA Vaccine for Chronic Hepatitis C : Preclinical Evaluation of Immunogenicity and Safety of VGX-6150 Moonsup JEONG, Ph.D. Director of Pharma R&D Division GeneOne
Novel Vaccine Development (3:00PM~3:30PM) DNA Vaccine for Chronic Hepatitis C : Preclinical Evaluation of Immunogenicity and Safety of VGX-6150 Moonsup JEONG, Ph.D. Director of Pharma R&D Division GeneOne
ANIMALS FORM & FUNCTION BODY DEFENSES NONSPECIFIC DEFENSES PHYSICAL BARRIERS PHAGOCYTES. Animals Form & Function Activity #4 page 1
 AP BIOLOGY ANIMALS FORM & FUNCTION ACTIVITY #4 NAME DATE HOUR BODY DEFENSES NONSPECIFIC DEFENSES PHYSICAL BARRIERS PHAGOCYTES Animals Form & Function Activity #4 page 1 INFLAMMATORY RESPONSE ANTIMICROBIAL
AP BIOLOGY ANIMALS FORM & FUNCTION ACTIVITY #4 NAME DATE HOUR BODY DEFENSES NONSPECIFIC DEFENSES PHYSICAL BARRIERS PHAGOCYTES Animals Form & Function Activity #4 page 1 INFLAMMATORY RESPONSE ANTIMICROBIAL
Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO
 Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO Statistically significant difference in progression-free survival continues to be seen in non-squamous
Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO Statistically significant difference in progression-free survival continues to be seen in non-squamous
Report from the visiting commitee
 Section des Unités de recherche Report from the visiting commitee Research unit: Biothérapies hépatiques University of Nantes January 2008 Section des Unités de recherche Report from the visiting committee
Section des Unités de recherche Report from the visiting commitee Research unit: Biothérapies hépatiques University of Nantes January 2008 Section des Unités de recherche Report from the visiting committee
One of the more complex systems we re looking at. An immune response (a response to a pathogen) can be of two types:
 Immune system. One of the more complex systems we re looking at. An immune response (a response to a pathogen) can be of two types: (pathogen - disease causing organism) 1) Non specific. Anything foreign
Immune system. One of the more complex systems we re looking at. An immune response (a response to a pathogen) can be of two types: (pathogen - disease causing organism) 1) Non specific. Anything foreign
Production of antigens and antibodies in plants: alternative technology?
 Production of antigens and antibodies in plants: alternative technology? George Lomonossoff John Innes Centre Norwich, UK ECOPA, Alicante 29 th Sept. 2006 Why use Plants as Biofactories? Produce large
Production of antigens and antibodies in plants: alternative technology? George Lomonossoff John Innes Centre Norwich, UK ECOPA, Alicante 29 th Sept. 2006 Why use Plants as Biofactories? Produce large
ALLIANCE FOR LUPUS RESEARCH AND PFIZER S CENTERS FOR THERAPEUTIC INNOVATION CHALLENGE GRANT PROGRAM PROGRAM GUIDELINES
 ALLIANCE FOR LUPUS RESEARCH AND PFIZER S CENTERS FOR THERAPEUTIC INNOVATION CHALLENGE GRANT PROGRAM PROGRAM GUIDELINES DESCRIPTION OF GRANT MECHANISM The Alliance for Lupus Research (ALR) is an independent,
ALLIANCE FOR LUPUS RESEARCH AND PFIZER S CENTERS FOR THERAPEUTIC INNOVATION CHALLENGE GRANT PROGRAM PROGRAM GUIDELINES DESCRIPTION OF GRANT MECHANISM The Alliance for Lupus Research (ALR) is an independent,
Manufacturing process of biologics
 Manufacturing process of biologics K. Ho Afssaps, France 2011 ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2011 ICH 1 Disclaimer:
Manufacturing process of biologics K. Ho Afssaps, France 2011 ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2011 ICH 1 Disclaimer:
Gene Silencing Oligos (GSOs) Third Generation Antisense
 Gene Silencing Oligos (GSOs) Third Generation Antisense Walter R. Strapps, Ph.D. Executive Director, RNA Therapeutics Idera Pharmaceuticals Cambridge, MA NASDAQ: IDRA www.iderapharma.com Idera is a leader
Gene Silencing Oligos (GSOs) Third Generation Antisense Walter R. Strapps, Ph.D. Executive Director, RNA Therapeutics Idera Pharmaceuticals Cambridge, MA NASDAQ: IDRA www.iderapharma.com Idera is a leader
ELISA BIO 110 Lab 1. Immunity and Disease
 ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
WHY IS THIS IMPORTANT?
 CHAPTER 1 WHAT IS MICROBIOLOGY AND WHY IS IT IMPORTANT? WHO / TDR / Crump WHY IS THIS IMPORTANT? Microbiology is more relevant than ever in today s world. Infectious diseases are a leading health-related
CHAPTER 1 WHAT IS MICROBIOLOGY AND WHY IS IT IMPORTANT? WHO / TDR / Crump WHY IS THIS IMPORTANT? Microbiology is more relevant than ever in today s world. Infectious diseases are a leading health-related
The immune system. Bone marrow. Thymus. Spleen. Bone marrow. NK cell. B-cell. T-cell. Basophil Neutrophil. Eosinophil. Myeloid progenitor
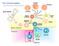 The immune system Basophil Neutrophil Bone marrow Eosinophil Myeloid progenitor Dendritic cell Pluripotent Stem cell Lymphoid progenitor Platelets Bone marrow Thymus NK cell T-cell B-cell Spleen Cancer
The immune system Basophil Neutrophil Bone marrow Eosinophil Myeloid progenitor Dendritic cell Pluripotent Stem cell Lymphoid progenitor Platelets Bone marrow Thymus NK cell T-cell B-cell Spleen Cancer
Stem Cells and Hope for Patients
 Stem Cells and Hope for Patients by Maureen Condic, Ph.D. Most Americans know someone afflicted with an incurable medical condition. The possibility of stem cell cures has given hope to many who face such
Stem Cells and Hope for Patients by Maureen Condic, Ph.D. Most Americans know someone afflicted with an incurable medical condition. The possibility of stem cell cures has given hope to many who face such
Immunity Unit Test Z
 Immunity Unit Test Z Name MB Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the pathogens in Figure 31.1 cause disease by taking over healthy
Immunity Unit Test Z Name MB Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the pathogens in Figure 31.1 cause disease by taking over healthy
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 10 October 2007 Doc. Ref. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON POTENCY TESTING OF CELL BASED IMMUNOTHERAPY
European Medicines Agency Evaluation of Medicines for Human Use London, 10 October 2007 Doc. Ref. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON POTENCY TESTING OF CELL BASED IMMUNOTHERAPY
AAV specific issues pertaining to vector shedding in gene therapy clinical trials. Samuel Wadsworth Genzyme Corporation
 AAV specific issues pertaining to vector shedding in gene therapy clinical trials Samuel Wadsworth Genzyme Corporation Workshop objectives Assess the impact of vector design on shedding (studies) Review
AAV specific issues pertaining to vector shedding in gene therapy clinical trials Samuel Wadsworth Genzyme Corporation Workshop objectives Assess the impact of vector design on shedding (studies) Review
TG1050, A NOVEL IMMUNOTHERAPEUTIC TO TREAT CHRONIC HEPATITIS B, CAN CONTROL HBsAg AND PROVOKE HBsAg SEROCONVERSION IN HBV-PERSISTENT MOUSE MODELS
 TG1050, A NOVEL IMMUNOTHERAPEUTIC TO TREAT CHRONIC HEPATITIS B, CAN CONTROL HBsAg AND PROVOKE HBsAg SEROCONVERSION IN HBV-PERSISTENT MOUSE MODELS Karine Lélu 1, Alexei Evlachev 1, Roland Kratzer 1, Sarah
TG1050, A NOVEL IMMUNOTHERAPEUTIC TO TREAT CHRONIC HEPATITIS B, CAN CONTROL HBsAg AND PROVOKE HBsAg SEROCONVERSION IN HBV-PERSISTENT MOUSE MODELS Karine Lélu 1, Alexei Evlachev 1, Roland Kratzer 1, Sarah
Dendritic Cells: A Basic Review *last updated May 2003
 *last updated May 2003 Prepared by: Eric Wieder, PhD MD Anderson Cancer Center Houston, TX USA What is a dendritic cell? Dendritic cells are antigen-presenting cells (APCs) which play a critical role in
*last updated May 2003 Prepared by: Eric Wieder, PhD MD Anderson Cancer Center Houston, TX USA What is a dendritic cell? Dendritic cells are antigen-presenting cells (APCs) which play a critical role in
Microbiology AN INTRODUCTION EIGHTH EDITION
 TORTORA FUNKE CASE Microbiology AN INTRODUCTION EIGHTH EDITION Differentiate between innate and acquired immunity. Chapter 17 Specific Defenses of the Host: The Immune Response B.E Pruitt & Jane J. Stein
TORTORA FUNKE CASE Microbiology AN INTRODUCTION EIGHTH EDITION Differentiate between innate and acquired immunity. Chapter 17 Specific Defenses of the Host: The Immune Response B.E Pruitt & Jane J. Stein
Custom Antibodies & Recombinant Proteins
 Custom Antibodies & Recombinant Proteins INTRODUCTION Custom services to meet your research and development requirements Improvements in health, medicine and diagnostics over the past century can be largely
Custom Antibodies & Recombinant Proteins INTRODUCTION Custom services to meet your research and development requirements Improvements in health, medicine and diagnostics over the past century can be largely
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College
 Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA
 CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA INTRODUCTION DNA : DNA is deoxyribose nucleic acid. It is made up of a base consisting of sugar, phosphate and one nitrogen base.the
CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA INTRODUCTION DNA : DNA is deoxyribose nucleic acid. It is made up of a base consisting of sugar, phosphate and one nitrogen base.the
Summary of Discussion on Non-clinical Pharmacology Studies on Anticancer Drugs
 Provisional Translation (as of January 27, 2014)* November 15, 2013 Pharmaceuticals and Bio-products Subcommittees, Science Board Summary of Discussion on Non-clinical Pharmacology Studies on Anticancer
Provisional Translation (as of January 27, 2014)* November 15, 2013 Pharmaceuticals and Bio-products Subcommittees, Science Board Summary of Discussion on Non-clinical Pharmacology Studies on Anticancer
The Basics of Drug Resistance:
 CONTACT: Lisa Rossi +1-412-641-8940 +1-412- 916-3315 (mobile) [email protected] The Basics of Drug Resistance: QUESTIONS AND ANSWERS HIV Drug Resistance and ARV-Based Prevention 1. What is drug resistance?
CONTACT: Lisa Rossi +1-412-641-8940 +1-412- 916-3315 (mobile) [email protected] The Basics of Drug Resistance: QUESTIONS AND ANSWERS HIV Drug Resistance and ARV-Based Prevention 1. What is drug resistance?
Welcome to Mini Med School at the Child & Family Research Institute
 Glossary Welcome to Mini Med School at the Child & Family Research Institute On behalf of the Faculty and Staff at the Child & Family Research Institute (CFRI), we would like to welcome you to CFRI s
Glossary Welcome to Mini Med School at the Child & Family Research Institute On behalf of the Faculty and Staff at the Child & Family Research Institute (CFRI), we would like to welcome you to CFRI s
7- Doctoral Degree in Public Health and Public Health Sciences (Majoring Microbiology)
 7- Doctoral Degree in Public Health and Public Health Sciences (Majoring Microbiology) Students should fulfill a total of 44 credit hours: 1- Compulsory courses: 14 credit hours. 1504801, 1504802, 1504803,
7- Doctoral Degree in Public Health and Public Health Sciences (Majoring Microbiology) Students should fulfill a total of 44 credit hours: 1- Compulsory courses: 14 credit hours. 1504801, 1504802, 1504803,
博 士 論 文 ( 要 約 ) A study on enzymatic synthesis of. stable cyclized peptides which. inhibit protein-protein interactions
 博 士 論 文 ( 要 約 ) 論 文 題 目 A study on enzymatic synthesis of stable cyclized peptides which inhibit protein-protein interactions ( 蛋 白 質 間 相 互 作 用 を 阻 害 する 安 定 な 環 状 化 ペプチドの 酵 素 合 成 に 関 する 研 究 ) 氏 名 張 静 1
博 士 論 文 ( 要 約 ) 論 文 題 目 A study on enzymatic synthesis of stable cyclized peptides which inhibit protein-protein interactions ( 蛋 白 質 間 相 互 作 用 を 阻 害 する 安 定 な 環 状 化 ペプチドの 酵 素 合 成 に 関 する 研 究 ) 氏 名 張 静 1
Immunotherapy of Uveal Melanoma
 Eye Am Not Alone (EANA) Patient Retreat Saturday Session - March 3, 2012 Immunotherapy of Uveal Melanoma Do not distribute or copy without the express permission of the Ocular Melanoma Foundation (OMF).
Eye Am Not Alone (EANA) Patient Retreat Saturday Session - March 3, 2012 Immunotherapy of Uveal Melanoma Do not distribute or copy without the express permission of the Ocular Melanoma Foundation (OMF).
Monoclonal Antibody Therapy: Innovations in Cancer Treatment. James Choi ENGL 202C
 Monoclonal Antibody Therapy: Innovations in Cancer Treatment James Choi ENGL 202C Treating Cancer with Monoclonal Antibody Therapy Researchers and scientists have been working for decades to find a cure
Monoclonal Antibody Therapy: Innovations in Cancer Treatment James Choi ENGL 202C Treating Cancer with Monoclonal Antibody Therapy Researchers and scientists have been working for decades to find a cure
2.1.2 Characterization of antiviral effect of cytokine expression on HBV replication in transduced mouse hepatocytes line
 i 1 INTRODUCTION 1.1 Human Hepatitis B virus (HBV) 1 1.1.1 Pathogenesis of Hepatitis B 1 1.1.2 Genome organization of HBV 3 1.1.3 Structure of HBV virion 5 1.1.4 HBV life cycle 5 1.1.5 Experimental models
i 1 INTRODUCTION 1.1 Human Hepatitis B virus (HBV) 1 1.1.1 Pathogenesis of Hepatitis B 1 1.1.2 Genome organization of HBV 3 1.1.3 Structure of HBV virion 5 1.1.4 HBV life cycle 5 1.1.5 Experimental models
Objectives: Immunity Gone Wrong: Autoimmune Diseases in Dental Hygiene Practice
 Objectives: 1) Understand the concept of self- tolerance versus non- self- tolerance. 2) Recognize systemic and oral indicators of autoimmune diseases. 3) Identify various autoimmune diseases and their
Objectives: 1) Understand the concept of self- tolerance versus non- self- tolerance. 2) Recognize systemic and oral indicators of autoimmune diseases. 3) Identify various autoimmune diseases and their
New Directions in Treatment of Ovarian Cancer. Amit M. Oza Princess Margaret Hospital University of Toronto
 New Directions in Treatment of Ovarian Cancer Amit M. Oza Princess Margaret Hospital University of Toronto Newly diagnosed: scenario Ist line Surgery chemotherapy Cure If can t cure can we turn into chronic
New Directions in Treatment of Ovarian Cancer Amit M. Oza Princess Margaret Hospital University of Toronto Newly diagnosed: scenario Ist line Surgery chemotherapy Cure If can t cure can we turn into chronic
KMS-Specialist & Customized Biosimilar Service
 KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
On the origin of giant multinuclear Reed-Sternberg cells and the role of CD4 T cells in Hodgkin lymphoma
 On the origin of giant multinuclear Reed-Sternberg cells and the role of CD4 T cells in Hodgkin lymphoma Uber die Entstehung von multinuklearen Reed-Sternberg Riesenzellen und die Rolle von CD4 T-Zellen
On the origin of giant multinuclear Reed-Sternberg cells and the role of CD4 T cells in Hodgkin lymphoma Uber die Entstehung von multinuklearen Reed-Sternberg Riesenzellen und die Rolle von CD4 T-Zellen
TOWARDS AN HIV VACCINE
 why is it so hard to make an HIV vaccine and where are we now? Neal Nathanson, MD Emeritus Professor Department of Microbiology University of Pennsylvania School of Medicine 1 Estimated number of persons
why is it so hard to make an HIV vaccine and where are we now? Neal Nathanson, MD Emeritus Professor Department of Microbiology University of Pennsylvania School of Medicine 1 Estimated number of persons
Natalia Taborda Vanegas. Doc. Sci. Student Immunovirology Group Universidad de Antioquia
 Pathogenesis of Dengue Natalia Taborda Vanegas Doc. Sci. Student Immunovirology Group Universidad de Antioquia Infection process Epidermis keratinocytes Dermis Archives of Medical Research 36 (2005) 425
Pathogenesis of Dengue Natalia Taborda Vanegas Doc. Sci. Student Immunovirology Group Universidad de Antioquia Infection process Epidermis keratinocytes Dermis Archives of Medical Research 36 (2005) 425
Update in Hematology Oncology Targeted Therapies. Mark Holguin
 Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
