clinical practice guidelines
|
|
|
- Lesley Cross
- 8 years ago
- Views:
Transcription
1 clinical practice guidelines Annals of Oncology 21 (Supplement 5): v120 v125, 2010 doi: /annonc/mdq172 Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up M. Sørensen 1, M. Pijls-Johannesma 2 & E. Felip 3 On behalf of the ESMO Guidelines Working Group* 1 Department of Oncology, The Finsen Centre, Copenhagen, Denmark; 2 Department of Radiation Oncology, MAASTRO Clinic, GROW Research Institute, Maastricht University Medical Center, Maastricht, The Netherlands; 3 Medical Oncology Service, Vall d Hebron University Hospital, Barcelona, Spain incidence The most common cause of cancer-related deaths in Europe in 2006 is lung cancer (estimated deaths). After prostate cancer, lung cancer is the most frequent type of cancer in men. Age-standardized incidence and mortality rates in 2006 are estimated to be 75.3 and 64.8/ /year, respectively, in men, and 18.3 and 15.1/ /year in women. Small-cell lung cancer (SCLC) accounts for 15% 18% of all cases. In recent years the incidence of SCLC has decreased. SCLC is strongly associated with tobacco smoking. diagnosis Pathological diagnosis should be made according to the WHO classification. Biopsies can be obtained by flexible bronchoscopy, mediastinoscopy, endoscopic ultrasound, transthoracic needle aspiration and thoracoscopy depending on the localization of the tumour. A biopsy from a metastatic lesion can substitute for a biopsy from the primary tumour. The least invasive approach should be used [V, D]. staging and risk assessment Staging procedures should include medical history, physical examination, chest X-ray, complete blood count including differential count, liver, lung and renal function tests, lactate dehydrogenase (LDH) and sodium levels, and a CT scan of the chest and abdomen including the liver and adrenal glands. In patients with symptoms or abnormal physical examination suggesting metastasis, additional tests may include bone scintigraphy, CT scan with intravenous contrast or MRI *Correspondence to: ESMO Guidelines Working Group, ESMO Head Office, Via L. Taddei 4, CH-6962 Viganello-Lugano, Switzerland; clinicalrecommendations@esmo.org Approved by the ESMO Guidelines Working Group: February 2002, last update December This publication supercedes the previously published version Ann Oncol 2009; 20 (Suppl 4): iv71 iv72. Conflict of interest: Dr Sørensen has reported that he is conducting clinical research sponsored by GSK; Dr Pijls-Johannesma and Dr Felip have reported no conflicts of interest. of the brain, and bone marrow aspiration and biopsy. If extensive disease is detected by one test, further staging can be omitted. Brain CT/MRI is recommended if chemoradiation with curative intent is under consideration [V, D]. The role of combined FDG-PET/CT scanning is yet to be completely defined but if available, it may be useful in certain cases that require accurate staging. Staging has been performed according to a two-stage system developed by the Veteran s Administration Lung Cancer Study Group (VALSG) in the USA dividing patients into limited and extensive disease. Limited disease was defined as tumour tissue that could be encompassed in a single radiation port and extensive disease was defined as any tumour that extended beyond the boundaries of a single radiation port. In 1989, the International Association for the Study of Lung Cancer (IASLC) revised the VALSG staging system and defined limited disease as tumour tissue confined to one hemithorax with regional lymph node metastasis including both ipsilateral and contralateral hilar, supraclavicular and mediastinal nodes, as well as ipsilateral pleural effusion. In most clinical trials with limited disease, patients with contralateral hilar or supraclavicular lymphadenopathy as well as malignant pleural or pericardial effusions have been excluded. The relevance of disease involvement at these sites remains controversial with respect to treatment planning. The IASLC proposed to apply the seventh edition of the TNM classification in the staging of SCLC. This proposal is based on analysis showing that survival of limited-disease patients with N2 and N3 involvement differs significantly from N0 and N1 disease. Patients with pleural effusion have an intermediate prognosis between limited and extensive disease patients with haematogenous spread. Furthermore, conformal radiation techniques and IMRT require more precise nodal staging. first-line treatment limited disease Limited-disease patients should be treated with etoposide/ platinum [I, C], preferably etoposide/cisplatin, in combination with thoracic radiotherapy [I, A]. ª The Author Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please journals.permissions@oxfordjournals.org
2 Annals of Oncology clinical practice guidelines Patients with limited disease are potentially curable as evidenced by a 5-year survival rate of between 20% and 25% in large meta-analyses and randomized trials using early concurrent platinum-based chemoradiotherapy. The evidence regarding the use of chemotherapy is addressed in the section concerning extensive disease as most trials investigating chemotherapy tend to include both limited and extensive disease. Thoracic radiotherapy increases local control and survival in limited-disease patients. A meta-analysis of 13 randomized trials including individual data from 2140 patients concluded that thoracic radiotherapy increased 3-year survival from 8.9% to 14.3%. timing of radiotherapy. Timing of radiotherapy has been addressed in at least eight individual trials and in a number of meta-analyses. The analyses differ with respect to the definition of early and late radiotherapy. Thirty days or 9 weeks after the initiation of chemotherapy have been the most common time points for the discrimination between early and late radiotherapy. Fried et al. reported a significant survival benefit at 2 years, which disappeared at the 3-year time point. In a Cochrane meta-analysis, 2- and 5-year survival rates were not significantly different when all trials were taken into account. However, when excluding one trial using non-platinum-based chemotherapy the odds ratio of survival at 5 years significantly favoured early chest radiotherapy with 5-year survival rates of 20.2% for early versus 13.8% for late radiotherapy. One meta-analysis suggested that early radiotherapy only increases survival when delivery of the intended doses of chemotherapy is achieved. Finally, completing radiotherapy in <30 days after initiating chemotherapy was associated with a significantly higher 5-year survival (RR: 0.62, 95% CI , P = ) in one meta-analysis. In conclusion, the bulk of the evidence suggests that early radiotherapy concurrent with platinum-based chemotherapy is superior to the delivery of late radiotherapy [II, B]. fractionation of radiotherapy. Fractionation and the overall treatment time of chest radiotherapy have been explored in the North American intergroup trial 0096 comparing twice-daily with once-daily radiotherapy. This trial achieved the longest 5- year survival ever reported in a large randomized study, i.e. 26% using 45 Gy in twice-daily fractions delivered over 3 weeks compared with 16% in patients receiving once-daily fractions to a total of 45 Gy in 5 weeks. However, twice-daily fractionation has not been uniformly implemented as standard treatment presumably because of its inconvenience. The nominal dose of 45 Gy on the once-daily arm corresponds to a lower biologic effective dose and the two treatment arms were not equitoxic as reflected by a rate of severe oesophagitis of 27% compared with 11% on the once-daily arm. Indeed, the maximal tolerable dose of twice-daily and once-daily concurrent radiochemoherapy has been determined to be 45 Gy in 30 fractions over 3 weeks and 70 Gy in 35 fractions over 7 weeks, respectively. A trial performed by the North Central Cancer Group failed to show any survival benefit of twice-daily radiotherapy in 32 fractions to a total of 48 Gy compared with once-daily radiotherapy in 28 fractions to a total of 50.4 Gy with an identical overall treatment time of 5.6 weeks in both arms. However, the late delivery of radiotherapy and the introduction of a 2.5 week split on the twice-daily arm might compromise the effectiveness of the twice-daily regimen. In conclusion, it remains to be determined whether twice-daily fractionation is superior to once-daily using biologically equivalent doses. Phase III studies that compare 45 Gy in twicedaily fractions over 3 weeks with once-daily schedules with a higher total dose (e.g. 66 Gy in 33 fractions in 6.6 weeks) are ongoing. The North American intergroup trial 0096 suggests that the overall treatment time of chest radiotherapy may be important for long-term survival. dose of radiotherapy. The optimal dose of radiotherapy is still to be established as no direct comparisons of delivered dose have been performed in randomized trials. However, retrospective analyses suggest that increasing dose is associated with increased local control. Consequently, doses in the range Gy delivered in 6 7 weeks have been explored in recent feasibility studies. Phase III trials investigating both the total dose and the overall treatment time are ongoing, both in Europe and in the USA, but presently, no data support the use of high-dose thoracic radiotherapy outside a clinical trial. target volume of radiotherapy. The optimal target volume remains to be defined as mainly retrospective studies are available making definitive recommendations inappropriate. Omission of elective node irradiation based on CT scans should be used with caution [III, C] as this strategy resulted in three isolated nodal failures in 27 patients. In contrast, recent prospective data from the same group indicate that selective node irradiation based on pretreatment FDG-PET scans resulted in a low rate of isolated nodal failures, i.e. two nodal failures in 60 patients. surgery. In patients with very limited disease (i.e. T1 2, N0), surgical resection may be considered followed by adjuvant chemotherapy and prophylactic cranial irradiation. Preoperative staging should include mediastinoscopy [III, D]. No randomized trials have compared this strategy with concurrent chemo-radiotherapy. extensive disease Extensive disease patients should be treated with cisplatin or carboplatin in combination with etoposide [I, C]. The prognosis of extensive disease is poor with a median survival of 10 months and a 2-year survival rate of 10%. Longterm survivors are extremely rare. One of the largest and most recent randomized trials including both limited and extensive disease patients favours cisplatin and etoposide with respect to survival. However, meta-analyses including studies with both limited and extensive disease patients from the last three decades have reached conflicting results. A meta-analysis of 19 randomized trials with a total of 4054 patients showed that patients treated with a cisplatin-containing regimen had higher response rates and prolonged survival. In contrast, a recent Cochrane review of 29 randomized studies reported no statistically significant differences in 6-, 12- and 24-month survival rates when comparing platinum- with non-platinum-based chemotherapy although the risk ratios numerically favoured the platinumbased regimens. The rate of complete response was significantly higher following platinum-containing treatments. A meta- Volume 21 Supplement 5 May 2010 doi: /annonc/mdq172 v121
3 clinical practice guidelines Annals of Oncology analysis of 36 trials compared etoposide- and/or cisplatincontaining regimens with regimens that did not contain one of the two drugs. A survival benefit in favour of etoposide alone or in combination with cisplatin was reported. Thus, most evidence supports the recommendation of etoposide/platinum as the standard of care although inconsistence exists [I, C]. Carboplatin is an acceptable option in the non-curative setting of extensive disease, whereas cisplatin is recommended when cure is intented in limited disease [II, C]. Trials comparing etoposide with either topotecan or irinotecan in combination with platinum have reached conflicting results. A study performed by the Japanese Cooperative Oncology Group (JCOG) was prematurely stopped due to a pre-planned interim analysis reporting an encouraging 3.4-month survival benefit favouring irinotecan/ cisplatin compared with etoposide/cisplatin. However, two confirmative studies failed to reproduce the Japanese data. Hanna et al. used a slightly modified schedule, whereas the SWOG study was a true replicate of the JCOG study using the exact same schedule. Both confirmative studies were substantially larger (n = 331 and n = 651) than the Japanese study (n = 152). No significant differences were seen in overall survival, time to progression or response rates. Etoposide induced more myelotoxicity and irinotecan caused more gastrointestinal toxicity. A Norwegian trial of 210 patients using oral etoposide in combination with carboplatin as the comparator arm reported a slight but significant increase in survival from 7.1 to 8.5 months favouring irinotecan/ carboplatin. Both oral and intravenous topotecan have been compared with etoposide in combination with cisplatin in two recent large randomized trials. No increase in survival was reported in either study but both oral and intravenous topotecan were deemed non-inferior to etoposide with respect to survival according to pre-specified non-inferiority criteria. Time to progression in the intravenous topotecan arm was superior to etoposide. However, the opposite was true in the oral study, where time to progression was inferior to etoposide. Neither irinotecan nor topotecan are recommended as part of first-line therapy [II, C]. A substantial number of frail patients with extensive disease do not tolerate aggressive chemotherapy due to low performance status and high incidence of co-morbidity. Therefore, less intensive regimens have been developed including oral etoposide. However, two randomized trials comparing single-agent oral etoposide with standard multidrug intravenous chemotherapy both found that oral etoposide was inferior with respect to survival, symptom control and quality of life. First-line oral single-agent etoposide is not recommended [I, A]. The addition of a third drug to a standard two-drug platinum-based regimen has not uniformly been proved beneficial in either limited or extensive disease. Two randomized studies evaluating the addition of ifosfamide reached conflicting results. One study reported a survival benefit while the other study was negative. In both studies, ifosfamide increased myelotoxicity. The addition of paclitaxel to cisplatin and etoposide failed to improve survival in a large randomized study (n = 587). Paclitaxel resulted in more non-haematological toxicity and increased the toxic death rate. Another randomized study using a similar schedule was prematurely closed after 133 patients were enrolled due to a high toxic death rate of 13%. duration of chemotherapy. Two trials have shown that maintenance chemotherapy beyond six cycles of induction therapy was unable to increase survival in patients responding to induction therapy. Likewise, seven additional cycles of maintenance chemotherapy in non-progressing patients after five cycles of induction chemotherapy did not improve survival. A modest and non-significant survival benefit has been reported after six cycles compared with three cycles. In a fourarm randomized study, four cycles of chemotherapy was inferior to eight cycles in terms of survival. However, this disadvantage in survival disappeared for those randomized to second-line therapy at relapse. Four cycles of topotecan maintenance did not increase survival after four cycles of cisplatin and etoposide induction therapy. Maintenance therapy improved progression-free survival in some trials. However, the clinical relevance of this improvement is questionable. Targeted maintenance treatment has failed to improve survival including anti-gd3 immunization, antiangiogenic treatment with thalidomide and metalloproteinase inhibition with marimastat. Four to six cycles of chemotherapy are recommended in extensive as well as in limited disease [II, B]. Maintenance therapy has failed to achieve a relevant clinical advantage and is not recommended in either extensive or limited disease [II, B]. dose intensity. The role of increased dose intensity remains unresolved. A number of studies have evaluated dose-dense therapy by the use of colony stimulating factors and bloodprogenitor-cell support. In most trials, dose intensification was achieved by decreasing intervals between cycles. A decade ago, two large randomized trials (n = 300 and n = 403) reported a survival benefit favouring dose intensification. However, the two most recent larger trials (n = 318 and n = 244) were unable to confirm these results in good-prognosis patients despite the achievement of a 1.7- to 1.8-fold increase in dose intensity compared with the standard dose arm. In contrast, a very recent trial with an identical study design and schedule did in fact show an impressive survival benefit of 1 year. However, this study was a single institution study of only 83 patients. Of note, one study was prematurely terminated due to decremental survival in the dose-dense arm. Dose-intense treatment is not recommended outside a clinical trial in extensive as well as in limited disease [II, C]. prophylactic cranial irradiation. Patients with any response to first-line treatment irrespective of stage should be offered prophylactic cranial irradiation (PCI) after the completion of first-line treatment [I, A]. A meta-analysis based on individual data from 987 patients with mainly limited disease in complete remission showed that PCI increased 3-year survival from 15.3% to 20.7%. The risk reduction for brain metastases was 54%. Increasing doses of radiation from 8 to 40 Gy was associated with a decreased risk of brain metastases. Recently, the benefit of PCI in extensive disease was demonstrated in a randomized trial. PCI resulted in a 73% reduction in the risk of brain metastases and prolongation of survival in patients with extensive disease v122 Sørensen et al. Volume 21 Supplement 5 May 2010
4 Annals of Oncology clinical practice guidelines responding to chemotherapy. The most frequent schedules used in this trial were either 20 Gy in five fractions or 30 Gy in 10 fractions, which were delivered to 85% of the patients. A recent trial randomized 720 limited-disease patients in complete remission to receive PCI either at a dose of 25 Gy in 10 fractions or 36 Gy in 18 or 24 fractions. Increased mortality and no reduction in brain metastases were observed in the high-dose and a number of different PCI schedules can be used provided that the dose is <36 Gy. The recommendation of a specific PCI schedule is not justified. No safety data exist on PCI concurrent with chemotherapy and therefore this combination is not recommended outside a clinical trial. second-line treatment Relapsing patients should be considered for second-line chemotherapy as survival benefit and maintenance of quality of life are achievable in selected patients [II, B]. Candidates for second-line chemotherapy should be selected on the basis of response to first-line therapy, time interval since the discontinuation of first-line therapy, residual toxicity to first-line therapy and performance status, as the likelihood of response to second-line chemotherapy is dependent on these factors [III, C]. Symptomatic patients with a low likelihood of benefit from second-line chemotherapy should be considered for palliative radiotherapy [III, C]. Recently, a survival benefit was demonstrated for patients treated with second-line chemotherapy in a small randomized study (n = 141). Oral topotecan extended median survival from 14 to 26 weeks compared with best supportive care. Of note, significant survival benefit was maintained in the subgroup of patients with a treatment-free interval of <60 days. There were fewer early deaths (<30 days from randomization), better symptom control and slower deterioration of quality of life in the chemotherapy arm. In two randomized studies no differences in overall survival were seen comparing oral and intravenous topotecan. Furthermore, in a randomized phase III trial, equivalent survival rates were observed when comparing singleagent intravenous topotecan with combination chemotherapy with cyclophosphamide, adriamycin and vincristine. The available data from randomized studies do not justify the recommendation of a specific regimen of chemotherapy as no regimen has proved superior to others. With the lack of evidence with regard to efficacy, the choice of second-line therapy should be based on patient preference and convenience as well as toxicity patterns. response evaluation Response evaluation is recommended during and at the completion of therapy. Initial positive imaging should be repeated [V, D]. follow-up There is no evidence that follow-up of asymptomatic patients is needed. Specific examinations should be as clinically indicated. For patients who achieve long-term survival, monitoring for development of a second primary cancer may be considered. Smoking cessation is recommended. note Levels of Evidence [I V] and Grades of Recommendation [A D] as used by the American Society of Clinical Oncology are given in square brackets. Statements without grading were considered justified standard clinical practice by the experts and the ESMO faculty. literature 1. Ferlay J, Autier P, Boniol M et al. Estimates of the cancer incidence and mortality in Europe in Ann Oncol 2007; 18: Fischer BM, Mortensen J, Langer SW et al. A prospective study of PET/CT in initial staging of small-cell lung cancer: comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol 2007; 18(2): Stahel R, Ginsberg R, Havemann K et al. Staging and prognostic factors in small cell lung cancer: a consensus report. Lung Cancer 1989; 5: Shepherd FA, Crowley J, Van Houtte P et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007; 2: Pignon JP, Arriagada R, Ihde DC et al. A meta-analysis of thoracic radiotherapy for small cell lung cancer. N Engl J Med 1992; 327: De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 2006; 17: Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J et al. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev 2007; 33: Pijls-Johannesma MC, De Ruysscher D, Lambin P et al. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev 2005; issue 1: CD Fried DB, Morris DE, Poole C et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage smallcell lung cancer. J Clin Oncol 2004; 22: Spiro SG, James LE, Rudd RM et al. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trials and metaanalysis. J Clin Oncol 2006; 24: De Ruysscher D, Pijls-Johannesma M, Bentzen SM et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol 2006; 24: Turrisi AT, Kyungmann K, Blum R et al. Twice-daily compared with once-daily thoratic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999; 17: Choi NC, Herndon JE 2nd, Rosenman J et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol 1998; 16: Bonner JA, Sloan JA, Shanahan TG et al. Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J Clin Oncol 1999; 17: Choi NC, Carey RW. Importance of radiation dose in achieving improved locoregional tumor control in limited stage small-cell lung carcinoma: an update. Int J Radiat Oncol Biol Phys 1989; 17: Bogart JA, Herndon JE 2nd, Lyss AP et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis Volume 21 Supplement 5 May 2010 doi: /annonc/mdq172 v123
5 clinical practice guidelines Annals of Oncology of Cancer and Leukemia Group B study Int J Radiat Oncol Biol Phys 2004; 59: Komaki R, Swann RS, Ettinger DS et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: Report of Radiation Therapy Oncology Group (RTOG) protocol Int J Radiat Oncol Biol Phys 2005; 62: De Ruysscher D, Bremer RH, Koppe F et al. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: a phase II trial. Radiother Oncol 2006; 80: van Loon J, De Ruysscher D, Wanders R et al. Selective nodal irradiation on basis of 18FDG-PET scan in limited disease small cell lung cancer: A prospective study. Int J Radiat Oncol Biol Phys 2009 Sep 24 [Epub ahead of print]. 20. Sundstrom S, Bremnes RM, Kaasas S et al. Cisplatin and etoposide regimen is superior to cyclophosphanide, epirubicin, and vincristine regimen in small cell lung cancer: results from a randomized phase III trial with 5 years follow-up. J Clin Oncol 2002; 20: Pujol JL, Carestia L, Daures JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatincontaining regimen versus a regimen without this alkylating agent. Br J Cancer 2000; 83: Amarasena IU, Walters JA, Wood-Baker R, Fong K. Platinum versus nonplatinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev 2008; issue 8: CD Mascaux C, Paesmans M, Berghmans T et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. European Lung Cancer Working Party (ELCWP). Lung Cancer 2000; 30: Noda K, Nishiwaki Y, Kawahara M et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: Hanna N, Bunn PA Jr, Langer C et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006; 24: Lara PN Jr, Natale R, Crowley J et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009; 27: Hermes A, Bergman B, Bremnes R et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol 2008; 26: Eckardt JR, von Pawel J, Papai Z et al. Open-label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small-cell lung cancer. J Clin Oncol 2006; 24: Heigener DF, Freitag L, Eschbach C et al. Topotecan/cisplatin (TP) compared to cisplatin/etoposide (PE) for patients with extensive disease-small cell lung cancer (ED-SCLC): Final results of a randomised phase III trial. J Clin Oncol 2008; 26(20 Suppl): Abstr Souhami RL, Spiro SG, Rudd RM et al. Five-day oral etoposide treatment for advanced small-cell lung cancer: randomized comparison with intravenous chemotherapy. J Natl Cancer Inst 1997; 89: Girling DJ. Comparison of oral etoposide and standard intravenous multidrug chemotherapy for small-cell lung cancer: a stopped multicentre randomised trial. Medical Research Council Lung Cancer Working Party. Lancet 1996; 348: Loehrer PJ Sr, Ansari R, Gonin R et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol 1995; 13: Miyamoto H, Nakabayashi T, Isobe H et al. A phase III comparison of etoposide/ cisplatin with or without added ifosfamide in small-cell lung cancer. Oncology 1992; 49: Niell HB, Herndon JE, Miller AA et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colonystimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial J Clin Oncol 2005; 23: Mavroudis D, Papadakis E, Veslemes M et al. Greek Lung Cancer Cooperative Group. A multicenter randomized clinical trial comparing paclitaxel-cisplatinetoposide versus cisplatin-etoposide as first-line treatment in patients with smallcell lung cancer. Ann Oncol 2001; 12: Sculier JP, Paesmans M, Bureau G et al. Randomized trial comparing induction chemotherapy versus induction chemotherapy followed by maintenance chemotherapy in small-cell lung cancer. European Lung Cancer Working Party. J Clin Oncol 1996; 14: Controlled trial of twelve versus six courses of chemotherapy in the treatment of small-cell lung cancer. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer 1989; 59: Giaccone G, Dalesio O, McVie GJ et al. Maintenance chemotherapy in small-cell lung cancer: long-term results of a randomized trial. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 1993; 11: Bleehen NM, Girling DJ, Machin D et al. A randomised trial of three or six courses of etoposide cyclophosphamide methotrexate and vincristine or six courses of etoposide and ifosfamide in small cell lung cancer (SCLC). I: survival and prognostic factors. Medical Research Council Lung Cancer Working Party. Br J Cancer 1993; 68: Spiro SG, Souhami RL, Geddes DM et al. Duration of chemotherapy in small cell lung cancer: a Cancer Research Campaign trial. Br J Cancer 1989; 59: Schiller JH, Adak S, Cella D et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593 a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2001; 19: Giaccone G, Debruyne C, Felip E et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer B; Silva Study). J Clin Oncol 2005; 23: Lee SM, Woll PJ, Rudd R et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized doubleblind placebo-controlled trial. J Natl Cancer Inst 2009; 101: Shepherd FA, Giaccone G, Seymour L et al. Prospective, randomized, doubleblind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol 2002; 20: Lorigan P, Woll PJ, O Brien ME et al. Randomized phase III trial of dose-dense chemotherapy supported by whole-blood hematopoietic progenitors in betterprognosis small-cell lung cancer. J Natl Cancer Inst 2005; 97: Buchholz E, Manegold C, Pilz L et al. Standard versus dose-intensified chemotherapy with sequential reinfusion of hematopoietic progenitor cells in small cell lung cancer patients with favorable prognosis. J Thorac Oncol 2007; 2: Steward WP, von Pawel J, Gatzemeier U et al. Effects of granulocytemacrophage colony-stimulating factor and dose intensification of V-ICE chemotherapy in small-cell lung cancer: a prospective randomized study of 300 patients. J Clin Oncol 1998; 16: Thatcher N, Girling DJ, Hopwood P et al. Improving survival without reducing quality of life in small-cell lung cancer patients by increasing the dose-intensity of chemotherapy with granulocyte colony-stimulating factor support: results of a British Medical Research Council Multicenter Randomized Trial. Medical Research Council Lung Cancer Working Party. J Clin Oncol 2000; 18: Ardizzoni A, Tjan-Heijnen VC, Postmus PE et al. Standard versus intensified chemotherapy with granulocyte colony-stimulating factor support in small-cell lung cancer: a prospective European Organization for Research and Treatment of Cancer-Lung Cancer Group Phase III Trial J Clin Oncol 2002; 20: Pujol JL, Douillard JY, Rivière A et al. Dose-intensity of a four-drug chemotherapy regimen with or without recombinant human granulocytemacrophage colony-stimulating factor in extensive-stage small-cell lung cancer: a multicenter randomized phase III study. J Clin Oncol 1997; 15: Auperin A, Arriagada R, Pignon JP et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999; 341: Slotman B, Faivre-Finn C, Kramer G et al. Prophylactic cranial irradiation in extensive samll-cell lung cancer. N Engl J Med 2007; 357: v124 Sørensen et al. Volume 21 Supplement 5 May 2010
6 Annals of Oncology clinical practice guidelines 53. Le Péchoux C, Dunant A, Senan S et al. Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99 01, EORTC , RTOG 0212, and IFCT 99 01): a randomised clinical trial. Lancet Oncol 2009; 10: O Brien ME, Ciuleanu TE, Tsekov H et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006; 24: Eckardt JR, von Pawel J, Pujol JL et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. Clin Oncol 2007; 25: von Pawel J, Gatzemeier U, Pujol JL et al. Phase II comparator study of oral versus intravenous topotecan in patients with chemosensitive small-cell lung cancer. J Clin Oncol 2001; 19: von Pawel J, Schiller JH, Shepherd FA et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999; 17: Volume 21 Supplement 5 May 2010 doi: /annonc/mdq172 v125
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
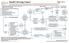 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
4.8 Lung cancer. 4.8.5 In the UK, three fractionation regimens are most commonly used:
 4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
Moving towards novel treatments in mesothelioma. Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich
 Moving towards novel treatments in mesothelioma Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich Malignant pleural mesothelioma (MPM) has proven to be quite resistant to
Moving towards novel treatments in mesothelioma Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich Malignant pleural mesothelioma (MPM) has proven to be quite resistant to
Small-Cell Lung Cancer: Is There a Standard Therapy?
 Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Cesare Gridelli 1, Francesca Casaluce 2, Assunta Sgambato 2, Fabio Monaco 3, Cesare Guida 4
 Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
American College of Radiology ACR Appropriateness Criteria RADIATION THERAPY FOR SMALL-CELL LUNG CANCER
 Date of origin: 2012 American College of Radiology ACR Appropriateness Criteria RADIATION THERAPY FOR SMALL-CELL LUNG CANCER Expert Panel on Radiation Oncology Lung: Feng-Ming (Spring) Kong, MD, PhD, MPH
Date of origin: 2012 American College of Radiology ACR Appropriateness Criteria RADIATION THERAPY FOR SMALL-CELL LUNG CANCER Expert Panel on Radiation Oncology Lung: Feng-Ming (Spring) Kong, MD, PhD, MPH
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy on limited small cell lung cancer
 [Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
[Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
Treatment of Small Cell Lung Cancer
 Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology. Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Small Cell Lung Cancer* State-of-the-Art Therapy 1994
 Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER
 CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
LUNG CANCER INTRODUCTION
 LUNG CANCER INTRODUCTION Lung cancer remains the leading cause of cancer death in the UK. Approximately 50% of those diagnosed present with stage IV disease. Significant advances in the management of lung
LUNG CANCER INTRODUCTION Lung cancer remains the leading cause of cancer death in the UK. Approximately 50% of those diagnosed present with stage IV disease. Significant advances in the management of lung
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Lung Cancer - Combination Chemotherapy and the Risk of Failure
 Evidence-based Series 7-13-1 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Role of Combination Chemotherapy in the Initial Management of Limited-Stage
Evidence-based Series 7-13-1 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Role of Combination Chemotherapy in the Initial Management of Limited-Stage
2.2.5 Target Volumes in Small Cell Lung Cancer
 Target Volumes in Small Cell Lung Cancer 111 2.2.5 Target Volumes in Small Cell Lung Cancer Yolanda I. Garces and James A. Bonner CONTENTS 2.2.5.1 Introduction 111 2.2.5.2 Tumor Volume Definitions 111
Target Volumes in Small Cell Lung Cancer 111 2.2.5 Target Volumes in Small Cell Lung Cancer Yolanda I. Garces and James A. Bonner CONTENTS 2.2.5.1 Introduction 111 2.2.5.2 Tumor Volume Definitions 111
Avastin: Glossary of key terms
 Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
RadioTherapy Timing in Small Cell Lung Cancer
 RadioTherapy Timing in Small Cell Lung Cancer A meta-analysis of randomised trials using individual patient data on the timing of chest radiotherapy in patients with limited stage small cell lung cancer
RadioTherapy Timing in Small Cell Lung Cancer A meta-analysis of randomised trials using individual patient data on the timing of chest radiotherapy in patients with limited stage small cell lung cancer
The Need for Accurate Lung Cancer Staging
 The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
New Approaches for Small-Cell Lung Cancer: Local Treatments
 The current management of limited-disease small-cell lung cancer is reviewed, and strategies to improve local control are discussed. Gary Ernest Smith. Sorting Old Bales. Oil on canvas, 18 24. Courtesy
The current management of limited-disease small-cell lung cancer is reviewed, and strategies to improve local control are discussed. Gary Ernest Smith. Sorting Old Bales. Oil on canvas, 18 24. Courtesy
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
INTRODUCTION. liver neoplasms, adrenal gland neoplasms, non small-cell lung cancer, radionuclide imaging, bisphosphonates,
 VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER
 GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER Author: Dr Susan O Reilly On behalf of the Breast CNG Written: December 2008 Agreed at CNG: June 2009 & June 2010 Review due: June 2011 Guidelines Adjuvant Systemic
GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER Author: Dr Susan O Reilly On behalf of the Breast CNG Written: December 2008 Agreed at CNG: June 2009 & June 2010 Review due: June 2011 Guidelines Adjuvant Systemic
PET/CT in Lung Cancer
 PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
Management of Small Cell Lung Cancer
 Evidence Report/Technology Assessment Number 143 Management of Small Cell Lung Cancer Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road
Evidence Report/Technology Assessment Number 143 Management of Small Cell Lung Cancer Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road
Emerging Drug List GEFITINIB
 Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Duration of chemotherapy in small cell lung cancer: a Cancer Research Campaign trial
 Br. J. Cancer (1989), 59, 578-583 (B8 The Macmillan Press Ltd., 1989 Duration of chemotherapy in small cell lung cancer: a Cancer Research Campaign trial S.G. Spiro1, R.L. Souhami2, D.M. Geddes3, C.M.
Br. J. Cancer (1989), 59, 578-583 (B8 The Macmillan Press Ltd., 1989 Duration of chemotherapy in small cell lung cancer: a Cancer Research Campaign trial S.G. Spiro1, R.L. Souhami2, D.M. Geddes3, C.M.
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board
 Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Lung Cancer and Mesothelioma
 Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Randomized trial on chest irradiation in extensive disease small cell lung cancer
 Chest Radiotherapy Extensive stage Small cell lung cancer Trial Randomized trial on chest irradiation in extensive disease small cell lung cancer Study coordinator: Prof.dr. B.J. Slotman Dept. of Radiation
Chest Radiotherapy Extensive stage Small cell lung cancer Trial Randomized trial on chest irradiation in extensive disease small cell lung cancer Study coordinator: Prof.dr. B.J. Slotman Dept. of Radiation
Mesothelioma. 1. Introduction. 1.1 General Information and Aetiology
 Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
L Lang-Lazdunski, A Bille, S Marshall, R Lal, D Landau, J Spicer
 Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
3.0 With final Comments for presentation at Sub Group Meeting 24. 24.11.10
 Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
Out-Patient Chemotherapy for Lung Cancer
 Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Small Cell Lung Cancer
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Small Cell Lung Cancer Version 2.2014 NCCN.org Continue Version 2.2014 09/17/13 National Comprehensive Cancer Network, Inc. 2013, All rights
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Small Cell Lung Cancer Version 2.2014 NCCN.org Continue Version 2.2014 09/17/13 National Comprehensive Cancer Network, Inc. 2013, All rights
Chemotherapy in Ovarian Cancer. Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group
 Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Small Cell Lung Cancer: An Update on Therapeutic Aspects
 Review Article Small Cell Lung Cancer: An Update on Therapeutic Aspects Manisha Bhutani, Ashutosh Kumar Pathak, Anant Mohan 1, Randeep Guleria 1 and Vinod Kochupillai Department of Medical Oncology, Institute
Review Article Small Cell Lung Cancer: An Update on Therapeutic Aspects Manisha Bhutani, Ashutosh Kumar Pathak, Anant Mohan 1, Randeep Guleria 1 and Vinod Kochupillai Department of Medical Oncology, Institute
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka
 Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
Metastatic Breast Cancer 201. Carolyn B. Hendricks, MD October 29, 2011
 Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer
 Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Small cell lung cancer
 Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy
 17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro. Joon H. Lee 9/17/2012
 Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro Joon H. Lee 9/17/2012 Malignant Pleural Mesothelioma (Epidemiology) Incidence: 7/mil (Japan) to 40/mil (Australia) Attributed secondary to asbestos
Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro Joon H. Lee 9/17/2012 Malignant Pleural Mesothelioma (Epidemiology) Incidence: 7/mil (Japan) to 40/mil (Australia) Attributed secondary to asbestos
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Lung cancer LUNG CANCER. Box 1 Clinical signs
 22 LUNG CANCER Lung cancer Bronchial carcinoma refers to two distinct clinical entities small cell and non-small cell carcinoma. Although these conditions have much in common, with broadly similar presenting
22 LUNG CANCER Lung cancer Bronchial carcinoma refers to two distinct clinical entities small cell and non-small cell carcinoma. Although these conditions have much in common, with broadly similar presenting
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Mesothelioma. Malignant Pleural Mesothelioma
 Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
 September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
People Living with Cancer
 Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)?
 Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Combination Chemotherapy for Small Cell Lung Cancer
 ORIGINAL ARTICLE Combination Chemotherapy for Small Cell Lung Cancer A W. Sufarlan, MRCP B.M.Z. Zainudin, MRCP Respiratory Unit, Department of Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia,
ORIGINAL ARTICLE Combination Chemotherapy for Small Cell Lung Cancer A W. Sufarlan, MRCP B.M.Z. Zainudin, MRCP Respiratory Unit, Department of Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia,
In the mid 1950s Roger Altounyan began work Small cell lung cancer
 598 Thorax 1997;52:598 Altounyan address Clinical trials in lung cancer: nihilism versus enthusiasm Stephen G Spiro In the mid 1950s Roger Altounyan began work Small cell lung cancer on a natural compound,
598 Thorax 1997;52:598 Altounyan address Clinical trials in lung cancer: nihilism versus enthusiasm Stephen G Spiro In the mid 1950s Roger Altounyan began work Small cell lung cancer on a natural compound,
ACUTE MYELOID LEUKEMIA (AML),
 1 ACUTE MYELOID LEUKEMIA (AML), ALSO KNOWN AS ACUTE MYELOGENOUS LEUKEMIA WHAT IS CANCER? The body is made up of hundreds of millions of living cells. Normal body cells grow, divide, and die in an orderly
1 ACUTE MYELOID LEUKEMIA (AML), ALSO KNOWN AS ACUTE MYELOGENOUS LEUKEMIA WHAT IS CANCER? The body is made up of hundreds of millions of living cells. Normal body cells grow, divide, and die in an orderly
Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence
 Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Treatment of Stage III Non-small Cell Lung Cancer
 CHEST Supplement DIAGNOSIS AND MANAGEMENT OF LUNG CANCER, 3RD ED: ACCP GUIDELINES Treatment of Stage III Non-small Cell Lung Cancer Diagnosis and Management of Lung Cancer, 3rd ed: American College of
CHEST Supplement DIAGNOSIS AND MANAGEMENT OF LUNG CANCER, 3RD ED: ACCP GUIDELINES Treatment of Stage III Non-small Cell Lung Cancer Diagnosis and Management of Lung Cancer, 3rd ed: American College of
Small Cell Lung Cancer
 78 NCCN Small Cell Lung Cancer Clinical Practice Guidelines in Oncology Gregory P. Kalemkerian, MD; Wallace Akerley, MD; Paul Bogner, MD; Hossein Borghaei, DO, MS; Laura QM Chow, MD; Robert J. Downey,
78 NCCN Small Cell Lung Cancer Clinical Practice Guidelines in Oncology Gregory P. Kalemkerian, MD; Wallace Akerley, MD; Paul Bogner, MD; Hossein Borghaei, DO, MS; Laura QM Chow, MD; Robert J. Downey,
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Cetuximab (Erbitux) MM.04.005 05/10/2005. HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s) of Service: Office: Outpatient
 Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
ADJUVANT TREATMENT CLINICAL EVALUATION NEOADJUVANT TREATMENT
 te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Lung cancer is the number one cancer cause of mortality
 ORIGINAL ARTICLE Prognostic Factors for Survival in Extensive Stage Small Cell Lung Cancer (ED-SCLC) The Importance of Smoking History, Socioeconomic and Marital Statuses, and Sai-Hong Ignatius Ou, MD,
ORIGINAL ARTICLE Prognostic Factors for Survival in Extensive Stage Small Cell Lung Cancer (ED-SCLC) The Importance of Smoking History, Socioeconomic and Marital Statuses, and Sai-Hong Ignatius Ou, MD,
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Small cell Lung cancer New Chemotherapy options. Nicolas Mach, MD,PD
 Small cell Lung cancer New Chemotherapy options Nicolas Mach, MD,PD > 40 negative Phase III trials List of unsucessful drugs Pemetrexed, imatinib, bevacizumab, bcl-2 antagonist, ASCT, CASE PRESENTATION
Small cell Lung cancer New Chemotherapy options Nicolas Mach, MD,PD > 40 negative Phase III trials List of unsucessful drugs Pemetrexed, imatinib, bevacizumab, bcl-2 antagonist, ASCT, CASE PRESENTATION
Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
 JBUON 2013; 18(2): 453-458 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
JBUON 2013; 18(2): 453-458 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
Adjuvant cisplatin-based chemotherapy in non-small-cell lung cancer: new insights into
 Annals of Oncology Advance Access published September 5, 2014 1 Adjuvant cisplatin-based chemotherapy in non-small-cell lung cancer: new insights into the effect on failure type via a multistate approach
Annals of Oncology Advance Access published September 5, 2014 1 Adjuvant cisplatin-based chemotherapy in non-small-cell lung cancer: new insights into the effect on failure type via a multistate approach
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Lung Cancer: More than meets the eye
 Lung Cancer Education Program November 23, 2013 Lung Cancer: More than meets the eye Shantanu Banerji MD, FRCPC Presenter Disclosure Faculty: Shantanu Banerji Relationships with commercial interests: Grants/Research
Lung Cancer Education Program November 23, 2013 Lung Cancer: More than meets the eye Shantanu Banerji MD, FRCPC Presenter Disclosure Faculty: Shantanu Banerji Relationships with commercial interests: Grants/Research
LUNG CANCER TREATMENT REGIMENS (Part 1 of 7)
 LUNG CANCER TREATMENT S (Part 1 of 7) Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration,
LUNG CANCER TREATMENT S (Part 1 of 7) Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration,
What is neuroendocrine cervical cancer?
 Key Points: 1. Neuroendocrine cancer of the uterine cervix is a rare and aggressive disease. 2. Treatment for neuroendocrine cervical cancer is usually more intensive than that for most other types of
Key Points: 1. Neuroendocrine cancer of the uterine cervix is a rare and aggressive disease. 2. Treatment for neuroendocrine cervical cancer is usually more intensive than that for most other types of
