Randomized trial on chest irradiation in extensive disease small cell lung cancer
|
|
|
- Lillian Shepherd
- 8 years ago
- Views:
Transcription
1 Chest Radiotherapy Extensive stage Small cell lung cancer Trial Randomized trial on chest irradiation in extensive disease small cell lung cancer Study coordinator: Prof.dr. B.J. Slotman Dept. of Radiation Oncology VU University medical center Amsterdam, The Netherlands Phone Fax e mail: bj.slotman@vumc.nl Writing committee: Prof.dr. B.J. Slotman Prof.dr. S. Senan C.J.A. Tissing J. Stigt dr. B. Biesma J.O. Praag Prof.dr. P.E. Postmus J.F. Ubbels S.Y. El Sharouni E.J.A. Vonk dr. F.M.N.H. Schramel dr. C. Faivre Finn dr. M. Snee dr. H. van Tinteren dr. C.L. van der Wilt FINAL VERSION 2.0 Study performed within Dutch Lung Cancer Study Group (DLCSG study 3) and Landelijk Platform voor Radiotherapie bij Longtumoren NVRO, The Netherlands. Participation of the UK NCRI Clinical Study Group is under investigation. APRIL
2 Table of contents 1 Background and introduction 4 2 Objectives of the trial General objectives End points 5 3 Patient selection criteria Inclusion criteria Exclusion criteria 5 4 Trial design 6 5 Therapeutic regimens, expected toxicity, dose modifications Radiotherapy Radiation scheme Treatment details Timing of radiotherapy Expected toxicity Treatment in case of relapse or progression 7 6 Clinical evaluation, laboratory tests and follow up Before start of chemotherapy During radiotherapy After randomization / end of radiotherapy 8 7 Criteria of evaluation Primary endpoint Secondary endpoints 8 8 Statistical considerations Statistical design Sample size Randomization and stratification Analysis Interim analyses 9 9 Investigator authorization procedure Patient randomization procedure Forms and procedures for collecting data Case report forms and schedule for completion Data flow Control of data consistency 13 2
3 12 Reporting adverse events Definitions Reporting procedure of Serious adverse events For patients in the complete control arm For patients in the Chest irradiation arm Ethical considerations Patient protection Subject identification Informed consent Administrative responsibilities The study coordinator Coordinating data center Publication policy Financing of datamanagement References 19 Table of appendices: Appendix A : Informed Consent document and Trial insurance 20 Appendix B: WHO performance status scale 23 Appendix C: World Medical Association Declaration of Helsinki 24 Appendix D: Small Cell Cancer VA Classification 28 3
4 1 Background and introduction Chemotherapy is the cornerstone in the treatment of extensive disease small cell lung cancer (ED SCLC), and four to six cycles of chemotherapy without maintenance therapy is current standard [Felip, 2007]. However, survival in patients presenting with ED SCLC is poor and has shown little improvement in the past few decades. An analysis of SEER data revealed the 2 year all cause survival in 2000 was still below 5% [Govindan 2006]. Recent trials using platinum based chemotherapy in ED SCLC show a time to progression of 4 6 months, and a median survival of 9 11 months [Socinski 2006]. Many approaches have been evaluated in an attempt to improve upon survival in SCLC, but most have not shown to be of benefit. These include maintenance chemotherapy [Schiller 2002], new chemotherapeutic agents [Hanna 2006], maintenance therapy using the metalloproteinase inhibitor marimastat [Shepherd 2002] and immunotherapy [Giaccone 2005]. The notable exception was the survival benefit reported in a phase III EORTC trial evaluating prophylactic cranial irradiation (PCI) versus no PCI following any response to induction chemotherapy [Slotman 2007]. Symptomatic brain metastases were significantly more frequent in controls (40% versus 15%), more of whom also died of SCLC (80% versus 68%). More patients in the PCI arm also received salvage chemotherapy at the time of disease recurrence. PCI is the new standard of care in all patients with SCLC who respond to chemotherapy. Intrathoracic tumor control is a major problem in ED SCLC. Over 75% of patients have persisting intra thoracic disease after initial chemotherapy, and about 90% manifest intra thoracic disease progression at 1 year after completing initial chemotherapy [Slotman,2007]. In a trial reported by Jeremic et al., patients with ED SCLC who had a complete response at sites of distant disease, were randomized to thoracic radiotherapy (54 Gy in 18 days) in combination with low dose chemotherapy or an additional four cycles of cisplatin/etoposide chemotherapy only [Jeremic 1999]. A total of 109 patients were randomized after induction chemotherapy, and the reported median (17 versus 11 months) and 5 year survivals (9.1% v 3.7 %,) was far higher than has been reported by any other group for ED SCLC. This study has not yet been repeated. In the absence of promising systemic agents that can improve local response, a logical step would be to evaluate the role of thoracic irradiation in patients with ED SCLC who respond to chemotherapy. 4
5 2 Objectives of the trial 2.1 General objectives The objective of this study is to investigate whether thoracic radiotherapy can improve 1 year survival in patients with extensive disease SCLC, following a response to chemotherapy, from 27% to 37%, as measured from time of randomisation after chemotherapy. 2.2 End points The primary end point of this study is survival. The secondary end points of this study are pattern of failure (thorax, brain, other) and toxicity. 3 Patient selection criteria 3.1 Inclusion criteria Cytologically or histologically proven small cell lung cancer Documented extensive disease (see appendix E) before the start of chemotherapy Any response after 4 to 6 cycles of initial chemotherapy (chemotherapy regimen and response evaluation according to the standard institution policy, provided that none of the existing lesions progressed) Chemotherapy (preferably platinum etoposide; other regimens need approval of study coordinator) completed Maximum delay of 4 weeks between last chemotherapy administration and randomization No evidence of brain metastases or leptomeningeal metastases (A contrast enhanced CT MRI scan of the brain is mandatory in case of clinical suspicion of brain metastases) No evidence of pleural metastases or pleuritis carcinomatosa No prior radiotherapy to the brain No prior radiotherapy to the thorax Age 18 years or older Performance status 0 to 2 (WHO scale, see Appendix B) Patient must be willing to receive chest irradiation Before patient registration/randomization, written informed consent must be given according to ICH/GCP, and national/local regulations Volume should be encompassable in acceptable radiation fields 3.2 Exclusion criteria None 5
6 4 Trial design This is a multicenter phase III randomized trial, aiming at demonstrating a difference in overall survival between the two arms. Patients with cytologically or histologically proven ED small cell lung cancer will be treated with chemotherapy, according to the standard treatment policy of each participating center; chemotherapy is not part of the present protocol treatment. Response to chemotherapy will be evaluated according to the participating center's current practice. Patients with a response, who satisfy the other eligibility criteria listed in paragraph 3, will receive prophylactic cranial irradiation and will be randomized to receive either thoracic irradiation or no further therapy. ED SCLC without brain metastases or pleural involvement Any response to 4 6 cycles chemotherapy RANDOMIZE Thoracic radiotherapy (10x3Gy) No Thoracic radiotherapy All patients will receive PCI 6
7 5 Therapeutic regimens, expected toxicity, dose modifications 5.1 Radiotherapy Radiation scheme For prophylactic cranial irradiation, the following schemes are acceptable: 20 Gy in 5 fractions or 30 Gy in 10 fractions 4 5 times per week. Each investigator should specify the details of the PCI schedule to the study coordinator before randomizing patients to the trial. For thoracic radiotherapy, 30 Gy will be delivered in 10 fractions, 4 5 times per week Treatment details A linear accelerator with a photon energy of 4 10 MV will be used. Higher energies are only allowed in case a treatment planning system with advanced algorithm is being used. The PTV for PCI includes the intracranial contents. Two opposed lateral fields will be used. Each field will be treated daily. The dose will be specified in the midline. The PTV for thoracic radiotherapy includes the post chemotherapy volume (GTV) with a margin of 15 mm. The initially positive hilar nodes and initially positive mediastinal nodal stations are always included, also in case of a complete response (CR). In general, parallel opposed (AP PA) fields can be used. If this will lead to anticipated severe toxicity, a conformal plan using planning CT scan is indicated to keep the V20 below 35%. In all treatment planning, lung correction is required Timing of radiotherapy PCI and thoracic radiotherapy will commence within six weeks after the completion of chemotherapy. However, PCI and thoracic radiotherapy can only start at least 2 weeks after the last administration of chemotherapy, when the acute Grade 2 or higher toxicity of chemotherapy has resolved Expected toxicity Expected acute toxicity using this thoracic radiotherapy schedule includes dysphagia, dyspnea, malaise, and/or cough [Kramer 2006] Treatment in case of relapse or progression In case of relapse or progression, the patient will be treated according to each center s policy. This will not be part of the protocol therapy. Those patients will however continue to be followed for other relapses until death. 7
8 6 Clinical evaluation and follow up 6.1 Before randomization Evaluation of patient eligibility Complete history and physical examination Chest X ray 6.2 During radiotherapy Acute toxicity will be recorded during treatment, and reported on the acute toxicity checklist and at end of treatment, according to CTCAE v 3.0 ( 6.3 After randomization / end of radiotherapy Patients will be followed up at 6 weeks and at 3, 6, 9 and 12 months after randomization in both arms, and, subsequently every 6 months. This follow up schedule must adhere to all patients, in both treatment arms. The following examinations will be performed at each follow up: Medical history and physical evaluation Chest X ray 7 Criteria of evaluation 7.1 Primary endpoint The primary endpoint is survival. Survival will be measured from date of randomisation. 7.2 Secondary endpoints The secondary endpoints are local control, pattern of failure and toxicity (measured on the International Common Toxicity Criteria, version 3.0, see 6.2). Local control is defined as the absence of disease progression in the treated lobe/lung. 8
9 8 Statistical considerations 8.1 Statistical design Sample size It is expected that 1 year survival in this group of patients is 27% from time of randomization [Slotman, 2007]. The primary end point of this trial is to achieve an increase in 1 year survival of 10% (from 27% to 37%; HR=0.76). If we presume a 5% drop between randomisation and end of treatment, a total of 483 patients have to be randomized to obtain a 80% power against this expected difference (two sided alpha=0.05). Based on data from the Dutch Cancer Registry, in the Netherlands there will be about 600 new patients with 25% dying before the end of chemotherapy and about 70% having a good response. As a result, patients will be eligible for this study per year. It is expected that centres from the UK and Belgium will also join the increase accrual Randomization and stratifications It is recommended to refer the patient to the radiation oncologist during chemotherapy to enable timely randomization and start of treatment. Patients will be centrally randomized (for practical details, see chapter on randomization procedure). A minimization technique will be used for random treatment allocation stratifying by institution, and presence of intrathoracic disease. 8.2 Analysis The principal end point is median survival. The Kaplan Meier method will be used to estimate survival at different time points, and the logrank two sided test will be used to compare therapeutic arms according to the intent to treat policy. All randomized patients will be included in this analysis. Compliance to the intended radiotherapy schedule will be summarized by the following parameters: proportion of patients who completed the thoracic radiotherapy, reasons for non completion, proportion of patients for whom thoracic radiotherapy had to be delayed, and reasons for delays. All patients randomized in the radiotherapy arm will be included in this analysis. Toxicity will be reported for patients in both study arms. 8.3 Interim analyses No interim analyses are foreseen in this trial. No independent data monitoring committee has been appointed for this study. Toxicity data will be reported to and evaluated by the study coordinator. 9
10 9 Investigator authorization procedure Investigators will be authorized to register or randomize patients in this trial only when they have returned to the study investigator: a commitment statement / study acknowledgment form, indicating that they will fully comply with the protocol, to include an estimate of their yearly accrual and if any conflict of interest may arrive due to their participation in the trial, a copy of the letter of acceptance of the protocol by their local or national (whichever is applicable) ethics committee, a signed conflict of interest disclosure form: this document will be required only if a possible conflict is declared by the commitment form. Before registering their first patient, investigators also need to return to the IKA trial office a form specifying: the radiotherapy schedule that will be used for PCI, whether it is part of their center policy to perform a contrast enhanced CT and/or MRI scan of the brain before the start of chemotherapy, their policy on the use of CT and/or MRI of the brain after chemotherapy in asymptomatic patients, their policy on the use of CT and/or MRI of the brain at extracranial relapse in asymptomatic patients, their policy on the use of CT and/or PET scanning of the thorax for evaluating thoracic relapse. This form should be appended to the commitment statement / study acknowledgment form. 10
11 10 Patient randomization procedure A patient can be randomized by telephone to the IKA trial office from 8.30 am to5.00 pm (Dutch local time) Monday through Friday. This must be done before the start of the protocol treatment. Telephone: or fax a completed randomisation form to or e mail: trialbureau@ikca.nl; Confirmation of randomisation result will be sent by An exhaustive list of questions to be answered during the randomization procedure is included in the registration checklist, which is part of the case report forms. This checklist should be completed by the responsible investigator before the patient is randomized. Standard questions institution number? name of the responsible investigator? patient's initials (maximum 4 letters)? patient's chart number (if available)? patient's birth date (day/month/year)? Protocol specific questions eligibility criteria? All eligibility criteria will be checked; stratification factors? date of written informed consent? At the end of the procedure, the treatment will be randomly allocated to the patients, as well as a patient sequential identification number. This number and the allocated treatment have to be recorded on the randomization checklist, along with the date of randomization. The completed checklist must be signed by the responsible investigator and returned to the data center with the initial data of the patient. The sequential identification number attributed to the patient at the end of the randomization procedure identifies the patient and must be reported on all case report forms. 11
12 11 Forms and procedures for collecting data 11.1 Case report forms and schedule for completion Data will be reported on the forms and sent to: IKA trial office, P.O. Box 9236, 1006 AE AMSTERDAM, The Netherlands Most likely, an electronic CRF system will also be available to ensure fast data transfer with less mistakes. Case report forms must be completed according to the following schedule: A. Before the treatment starts: the patient must be registered/randomized by the datamanager the following set of forms has to be returned to the datamanager: o the Pre Randomization Form/Registration Check list o the On Study form The optimal way to work is to complete the Pre randomization form, and to register/randomize the patient as soon as data are complete. The date of registration and patient sequential identification number are then completed on the check list, and the whole set can be sent to the datamanager. B. At the end of radiotherapy the End of PCI radiotherapy form (both arms) the End of Chest radiotherapy form (Chest radiotherapy arm only) C. After the end of treatment the follow up form has to be sent at 6 weeks, 3months, 6 months, 9 months, 12 months after randomization and subsequently every 6 months up to three years. D. Upon occurrence of a Serious Adverse Event In the control arm: all Serious Adverse Events occurring within 110 days after randomization must be reported to the IKA trial office In the chest irradiation arm: all Serious Adverse Events at least probably related to the treatment occurring during the treatment period and within 90 days after the end of the last treatment must be reported to the IKA trial office. All serious adverse events must be reported by fax to the IKA trial office within 48 hours. FAX ALL FORMS MUST BE DATED AND SIGNED BY THE RESPONSIBLE INVESTIGATOR OR ONE OF HIS/HER STAFF MEMBERS 11.2 Data flow The case report forms must be completed, dated and signed by the investigator or one of his/her staff members as soon as the requested information is available. 12
13 In all cases, it remains the responsibility of the investigator to check that original case report forms are sent to the IKA trial office and that they are completely and correctly filled out. The original copy must be immediately returned to the IKA trial office and the investigator must keep a copy. Regular consistency checks on the CRFs will be performed and Query Forms will be issued in case of inconsistent data. Those Query Forms must be immediately answered and signed by the investigator and returned to the IKA trial office as soon as possible Control of data consistency Data will be entered in the e CRF database. In case of paper forms this will be done by a double data entry procedure. Computerized and manual consistency checks will be performed on newly entered forms; queries will be issued in case of inconsistencies. Consistent forms will be validated by the Data Manager to be entered on the master database. Inconsistent forms will be kept "pending" until resolution of the inconsistencies. 13
14 12 Reporting adverse events 12.1 Definitions Adverse Events (AE) are any untoward medical occurrence or experience in a patient or clinical investigation subject which occurs following the administration of the trial medication regardless of the dose or causal relationship. This can include any unfavourable and unintended signs (such as rash or enlarged liver), or symptoms (such as nausea or chest pain), an abnormal laboratory finding (including blood tests, x rays or scans) or a disease temporarily associated with the trial treatments. Serious Adverse Events (SAE) are defined as any undesirable experience occurring to a patient, whether or not considered related to the study treatments. Adverse events which are considered as serious are those which result in: Death. REMARK: In this study, death due to progression of disease will not be considered as an SAE and must, therefore, not be reported as an SAE. Life threatening event (i.e. the patient was at immediate risk of death at the time the reaction was observed) Hospitalization or prolongation of hospitalization Persistent or significant disability/incapacity Any other medically important condition (i.e. important adverse reactions that are not immediately life threatening or do not result in death or hospitalization but may jeopardize the patient or may require intervention to prevent one of the other outcomes listed above) Reporting procedure of Serious adverse events For patients in the control arm Only protocol related serious adverse events, occurring within 110 days after randomization, must be reported on an SAE Form. This form must be faxed within 48 hours of the initial observation of the event For patients in the irradiation arm All serious adverse events, occurring during the treatment period and within 90 days after the last protocol treatment, must be reported on an SAE Form to the IKA Trial Office. Any late serious adverse events, occurring after this 90 day period, and at least possibly related to treatment, should follow the same reporting procedure. This SAE Form must be faxed within 24 hours of the initial observation of the event. The investigator will decide if these events are related to the study treatment (i.e. unrelated, unlikely, possible, probable, definitely and not assessable) and the decision will be recorded on the Serious Adverse Event form. The investigator will decide if these events are related to the study treatment (i.e. unrelated, unlikely, possible, probable, definitely and not assessable) and the decision will be recorded on the Serious Adverse Event forms. The assessment of causality is made by the investigator using the following definitions: Relationship Description UNRELATED There is no evidence of any causal relationship 14
15 UNLIKELY There is little evidence to suggest there is a causal relationship (e.g. the event did not occur within a reasonable time after administration of the trial medication). There is another reasonable explanation for the event (e.g. the patient s clinical condition, other concomitant treatments). POSSIBLE There is some evidence to suggest a causal relationship (e.g. because the event occurs within a reasonable time after administration of the trial medication). However, the influence of other factors may have contributed to the event (e.g. the patient s clinical condition, other concomitant treatments). PROBABLE There is evidence to suggest a causal relationship and the influence of other factors is unlikely. DEFINITELY There is clear evidence to suggest a causal relationship and other possible contributing factors can be ruled out. NOT ASSESSABLE There is insufficient or incomplete evidence to make a clinical judgement of the causal relationship. 15
16 13Ethical considerations 13.1 Patient protection The responsible investigator will ensure that this study is conducted in agreement with either the Declaration of Helsinki (Tokyo, Venice, Hong Kong, Somerset West and Edinburgh amendments) or the laws and regulations of the country, whichever provides the greatest protection of the patient. The protocol has been written, and the study will be conducted according to the ICH Harmonized Tripartite Guideline for Good Clinical Practice (ref: The protocol will by approved by the Local, Regional or National Ethics Committees Subject identification The name of the patient will not be asked for nor recorded at the datamanager. A sequential identification number will be automatically attributed to each patient registered in the trial. This number will identify the patient and must be included on all case report forms. In order to avoid identification errors, patient s initials (maximum of 4 letters), date of birth and local chart number (if available) will also be reported on the case report forms Informed consent All patients will be informed of the aims of the study, the possible adverse events, the procedures and possible hazards to which he/she will be exposed, and the mechanism of treatment allocation. They will be informed as to the strict confidentiality of their patient data, but that their medical records may be reviewed for trial purposes by authorized individuals other than their treating physician. An example of a patient informed consent statement is given as an appendix to this protocol. It will be emphasized that the participation is voluntary and that the patient is allowed to refuse further participation in the protocol whenever he/she wants. This will not prejudice the patient s subsequent care. Documented informed consent must be obtained for all patients included in the study before they are registered or randomized. This must be done in accordance with the national and local regulatory requirements. The informed consent procedure must conform to the ICH guidelines on Good Clinical Practice. This implies that the written informed consent form should be signed and personally dated by the patient or by the patient s legally acceptable representative. 16
17 14 Administrative responsibilities 14.1 The study coordinator The Study Coordinator will be responsible for writing the protocol, reviewing all case report forms and documenting his/her review on evaluation forms, discussing the contents of the reports with the datamanager of the IKA Trial Office, and for publishing the study results. He will also generally be responsible for answering all clinical questions concerning eligibility, treatment, and the evaluation of the patients. Study coordinator Prof.dr. BEN J. SLOTMAN VRIJE UNIVERSITEIT MEDICAL CENTER Dept of Radiation Oncology De Boelelaan, 1117 NL 1081 HV AMSTERDAM The Netherlands Tel Fax E mail: bj.slotman@vumc.nl 14.2 Coordination Data center IKA trial office P.O. box 9236 NL 1006 AE Amsterdam The Netherlands Tel Fax trialbureau@ikca.nl 17
18 15 Publication policy The final publication of the trial results will be written by the Study Coordinator on the basis of the final analysis. Draft manuscript(s) will be prepared by the studycoordinator and submitted to the co authors. Authors of the manuscript will include at least the Study Coordinator, the investigators who have included more than 5% of the eligible patients in the trial (by order of inclusion). Authors of abstracts will include at least the Study Coordinator and the investigators who have included more than 10% of the eligible patients in the trial (by order of inclusion). All manuscripts will include an appropriate acknowledgment section, mentioning all investigators and/or centers who have contributed to the trial, as well as supporting bodies. 16 Financing of data management Funding for data management in the Netherlands has been obtained by a grant of the Dutch Cancer Society (KWF) 18
19 17 References Filip E, ESMO Guidelines Working Group. Small cell lung cancer: ESMO Clinical Recommendations for diagnosis, treatment and follow up. Annals of Oncology 18 (Supplement 2), ii32 ii33, Govindan R, Page N, Morgensztern D, et al., Changing Epidemiology of Small Cell Lung Cancer in the United States Over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J Clin Oncol 24, , 2006 Socinski MA, Weissman C, Hart LL, et al. Randomized Phase II Trial of Pemetrexed Combined With Either Cisplatin or Carboplatin in Untreated Extensive Stage Small Cell Lung Cancer. J Clin Oncol 24, , Schiller JH. Small cell lung cancer: defining a role for emerging platinum drugs.oncology 62, , Sundstrøm S, Bremnes RM, Kaasa S, et al. Cisplatin and Etoposide Regimen Is Superior to Cyclophosphamide, Epirubicin, and Vincristine Regimen in Small Cell Lung Cancer: Results From a Randomized Phase III Trial With 5 Years Follow Up. J Clin Oncol 20, ,2002 Hanna N, Bunn Jr. PA, Langer C, et al. Randomized Phase III Trial Comparing Irinotecan/Cisplatin With Etoposide/Cisplatin in Patients With Previously Untreated Extensive Stage Disease Small Cell Lung Cancer. J Clin Oncol 24, , Giaccone G, Debruyne C, Felip E, et al. Phase III Study of Adjuvant Vaccination With Bec2/Bacille Calmette Guerin in Responding Patients With Limited Disease Small Cell Lung Cancer (European Organisation for Research and Treatment of Cancer B; Silva Study). J Clin Oncol 23, , Shepherd FA, Giaccone G, Seymour L, et al. Prospective, randomized, double blind, placebocontrolled trial of marimastat after response to first line chemotherapy in patients with small cell lung cancer: a trial of the National Cancer Institute of Canada Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol 20, , Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined modality treatment of patients with extensive disease small cell lung cancer: A randomized study. J Clin Oncol 17, , Kramer GWPM, Wanders SL, Noordijk EM, et al. Results of the Dutch National Study of the Palliative Effect of Irradiation Using Two Different Treatment Schemes for Non Small Cell Lung Cancer. J Clin Oncol 23, , SlotmanBJ, Faivre Finn C, Kramer GWPM, et al. Prophylactic cranial irradiation in extensive small cell lung cancer. N Engl J Med 357, , Appendix A : Informed Consent document 19
20 This is a clinical trial. Clinical trials include only patients who choose to take part. Please take your time to make your decision. Randomized trial on chest irradiation in extensive disease small cell lung cancer (CREST study) Invitation to participate in the study We have initiated a research study on patients that have a disease similar to yours. The study will be conducted under the supervision of physicians recognized as experts in this field of medicine. Today, you will be invited to take part to this research project after you are given full information about the study. You have received chemotherapy for a lung tumor. After this treatment, a good regression of tumor has been achieved. Patients like you treated for small cell lung cancer have a risk of recurrence or progression of the tumor in the lung. spreading into the brain. In this study, we will investigate whether giving a local irradiation to the tumor area in the lung, can reduce this risk. You are kindly invited to anticipate in this study. Possible side effects include fatigue, dyspnea, coughing, nausea and vomiting, The chest irradiation consists of 10 daily fractions of radiotherapy, given over a period of 2 weeks. In this study, we investigate whether survival can be improved by adding chest irradiation. Should you decide not to take part in this trial this will not effect your further treatment and we advise you to have a standard treatment which is given in your institute. Your doctor will advise you about this. Voluntary participation Your participation in this research trial is entirely voluntary and you will be given sufficient time to decide whether or not you wish to participate. You are free to decide at all times without giving a reason that you no longer wish to participate in the trial. Withdrawal from the trial will not affect your subsequent treatment or relationship with your treating physician or the hospital staff in any way Data protection The trial involves the collection of information contained in your medical records and which relate to your disease. It is very important that the information collected is accurate and from time to time it may be checked against your medical records. Duly authorized persons may have access to your medical records. All information will be strictly confidential and your identity will never be divulged; you have the right to access this information at any time. Insurance Insurance has been taken by the sponsor of the study in accordance with the applicable legislation. If you need to undergo another medical treatment, we advice you to inform the investigator to ensure this will not have any effect on your participation to the trial. Everything has been done and will continue to be done to prevent additional health problems occurring as a result of your taking part in this trial. Ethics Committee 20
21 This research protocol has been submitted to an ethics committee whose mission is to verify all conditions for your safety and respect of your rights are respected. Approval to this research has been given by the Ethics Committee of on Contact persons In case of any problem or question, your doctor will be pleased to answer any further questions and may be contacted as follows: Name of the doctor: Hospital: Telephone: If you consent to join this trial, you will be given a telephone number at the hospital that you can contact at any time if you feel unwell or have further questions. Your family doctor will also be told about your taking part in this trial and what is involved, if you agree. Please take your time to consider this information and do not hesitate to ask further questions of your doctor if anything is not clear. You are entitled to keep a copy of this document after you and your doctor have signed it. Acceptance of participation I have been properly informed of the clinical research that is being proposed to me I have received a copy of the patient information sheet All my rights have been clearly explained I have received a copy of the informed consent document I accept to participate in the research entitled Chest irradiation in extensive disease small cell lung cancer My participation is completely voluntary and I have the possibility to withdraw my consent at anytime without explanation. This will not affect my relationship with my treating physician. The data collected on my behalf will be strictly confidential and treated according to the"directive on the protection of individuals with regard to the processing of personal data and the local applicable laws. My consent does not discharge the organizers of the research from their responsibilities and I keep all my rights guaranteed by the law". have been informed that the data collected may be used in the future for any scientific purpose while confidentiality will be ensured Patient's name: Patient's signature: Date: Person designated by the investigator to participate in the informed consent process: Name: Signature: Date: Investigator's name: Title/Position: Investigator's Signature: Date: This document has been prepared taking into account: 21
22 World Medical Association Declaration of Helsinki, adopted by the 18th World Medical Assembly, Helsinki, Finland June Revised 1975, 1983, 1989, 1996 and on October 6, 2000 in Edinburgh, Scotland ( ICH GCP Guidelines; Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95), Sept International Ethical Guidelines for Biomedical Research involving Human Subjects, Council for International Organizations of Medical Sciences (CIOMS), Geneva WHO: Operating Guidelines for Ethics Committee that Review Biomedical Research, Geneva,
23 Appendix B: WHO performance status scale Grade Performance scale 0 Able to carry out all normal activity without restriction 1 Restricted in physically strenuous activity but ambulatory and able to carry out light work. 2 Ambulatory and capable of all self care but unable to carry out any work; up and about more than 50% of waking hours. 3 Capable of only limited self care; confined to bed or chair more than 50% of waking hours 4 Completely disabled; cannot carry on any self care; totally confined to bed or chair. 23
24 Appendix C: World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th World Medical Assembly Helsinki, Finland, June 1964 and amended by the 29th World Medical Assembly, Tokyo, Japan, October th World Medical Assembly, Venice, Italy, October st World Medical Assembly, Hong Kong, September th General Assembly, Somerset West, Republic of South Africa, October 1996 and the 52nd WMA General Assembly, Edinburgh, Scotland, October 2000 A. INTRODUCTION 1. The World Medical Association has developed the Declaration of Helsinki as a statement of ethical principles to provide guidance to physicians and other participants in medical research involving human subjects. Medical research involving human subjects includes research on identifiable human material or identifiable data. 2. It is the duty of the physician to promote and safeguard the health of the people. The physician s knowledge and conscience are dedicated to the fulfillment of this duty. 3. The Declaration of Geneva of the World Medical Association binds the physician with the words, "The health of my patient will be my first consideration," and the International Code of Medical Ethics declares that, "A physician shall act only in the patient's interest when providing medical care which might have the effect of weakening the physical and mental condition of the patient." 4. Medical progress is based on research which ultimately must rest in part on experimentation involving human subjects. 5. In medical research on human subjects, considerations related to the well being of the human subject should take precedence over the interests of science and society. 6. The primary purpose of medical research involving human subjects is to improve prophylactic, diagnostic and therapeutic procedures and the understanding of the aetiology and pathogenesis of disease. Even the best proven prophylactic, diagnostic, and therapeutic methods must continuously be challenged through research for their effectiveness, efficiency, accessibility and quality. 7. In current medical practice and in medical research, most prophylactic, diagnostic and therapeutic procedures involve risks and burdens. 8. Medical research is subject to ethical standards that promote respect for all human beings and protect their health and rights. Some research populations are vulnerable and need special protection. The particular needs of the economically and medically disadvantaged must be recognized. Special attention is also required for those who cannot give or refuse consent for themselves, for those who may be subject to giving consent under duress, for those who will not benefit personally from the research and for those for whom the research is combined with care. 9. Research Investigators should be aware of the ethical, legal and regulatory requirements for research on human subjects in their own countries as well as applicable international requirements. No national ethical, legal or regulatory requirement should be allowed to reduce or eliminate any of the protections for human subjects set forth in this Declaration. 24
25 B. BASIC PRINCIPLES FOR ALL MEDICAL RESEARCH 10. It is the duty of the physician in medical research to protect the life, health, privacy, and dignity of the human subject. 11. Medical research involving human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and on adequate laboratory and, where appropriate, animal experimentation. 12. Appropriate caution must be exercised in the conduct of research which may affect the environment, and the welfare of animals used for research must be respected. 13. The design and performance of each experimental procedure involving human subjects should be clearly formulated in an experimental protocol. This protocol should be submitted for consideration, comment, guidance, and where appropriate, approval to a specially appointed ethical review committee, which must be independent of the investigator, the sponsor or any other kind of undue influence. This independent committee should be in conformity with the laws and regulations of the country in which the research experiment is performed. The committee has the right to monitor ongoing trials. The researcher has the obligation to provide monitoring information to the committee, especially any serious adverse events. The researcher should also submit to the committee, for review, information regarding funding, sponsors, institutional affiliations, other potential conflicts of interest and incentives for subjects. 14. The research protocol should always contain a statement of the ethical considerations involved and should indicate that there is compliance with the principles enunciated in this Declaration. 15. Medical research involving human subjects should be conducted only by scientifically qualified persons and under the supervision of a clinically competent medical person. The responsibility for the human subject must always rest with a medically qualified person and never rest on the subject of the research, even though the subject has given consent. 16. Every medical research project involving human subjects should be preceded by careful assessment of predictable risks and burdens in comparison with foreseeable benefits to the subject or to others. This does not preclude the participation of healthy volunteers in medical research. The design of all studies should be publicly available. 17. Physicians should abstain from engaging in research projects involving human subjects unless they are confident that the risks involved have been adequately assessed and can be satisfactorily managed. Physicians should cease any investigation if the risks are found to outweigh the potential benefits or if there is conclusive proof of positive and beneficial results. 18. Medical research involving human subjects should only be conducted if the importance of the objective outweighs the inherent risks and burdens to the subject. This is especially important when the human subjects are healthy volunteers. 19. Medical research is only justified if there is a reasonable likelihood that the populations in which the research is carried out stand to benefit from the results of the research. 20. The subjects must be volunteers and informed participants in the research project. 21. The right of research subjects to safeguard their integrity must always be respected. Every precaution should be taken to respect the privacy of the subject, the confidentiality of the patient s information and to minimize the impact of the study on the subject's physical and mental integrity and on the personality of the subject. 25
26 22. In any research on human beings, each potential subject must be adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail. The subject should be informed of the right to abstain from participation in the study or to withdraw consent to participate at any time without reprisal. After ensuring that the subject has understood the information, the physician should then obtain the subject's freely given informed consent, preferably in writing. If the consent cannot be obtained in writing, the non written consent must be formally documented and witnessed. 23. When obtaining informed consent for the research project the physician should be particularly cautious if the subject is in a dependent relationship with the physician or may consent under duress. In that case the informed consent should be obtained by a well informed physician who is not engaged in the investigation and who is completely independent of this relationship. 24. For a research subject who is legally incompetent, physically or mentally incapable of giving consent or is a legally incompetent minor, the investigator must obtain informed consent from the legally authorized representative in accordance with applicable law. These groups should not be included in research unless the research is necessary to promote the health of the population represented and this research cannot instead be performed on legally competent persons. 25. When a subject deemed legally incompetent, such as a minor child, is able to give assent to decisions about participation in research, the investigator must obtain that assent in addition to the consent of the legally authorized representative. 26. Research on individuals from whom it is not possible to obtain consent, including proxy or advance consent, should be done only if the physical/mental condition that prevents obtaining informed consent is a necessary characteristic of the research population. The specific reasons for involving research subjects with a condition that renders them unable to give informed consent should be stated in the experimental protocol for consideration and approval of the review committee. The protocol should state that consent to remain in the research should be obtained as soon as possible from the individual or a legally authorized surrogate. 27. Both authors and publishers have ethical obligations. In publication of the results of research, the investigators are obliged to preserve the accuracy of the results. Negative as well as positive results should be published or otherwise publicly available. Sources of funding, institutional affiliations and any possible conflicts of interest should be declared in the publication. Reports of experimentation not in accordance with the principles laid down in this Declaration should not be accepted for publication. C. ADDITIONAL PRINCIPLES FOR MEDICAL RESEARCH COMBINED WITH MEDICAL CARE 28. The physician may combine medical research with medical care, only to the extent that the research is justified by its potential prophylactic, diagnostic or therapeutic value. When medical research is combined with medical care, additional standards apply to protect the patients who are research subjects. 29. The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. 26
27 This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists. 30. At the conclusion of the study, every patient entered into the study should be assured of access to the best proven prophylactic, diagnostic and therapeutic methods identified by the study. 31. The physician should fully inform the patient which aspects of the care are related to the research. The refusal of a patient to participate in a study must never interfere with the patient physician relationship. 32. In the treatment of a patient, where proven prophylactic, diagnostic and therapeutic methods do not exist or have been ineffective, the physician, with informed consent from the patient, must be free to use unproven or new prophylactic, diagnostic and therapeutic measures, if in the physician s judgement it offers hope of saving life, re establishing health or alleviating suffering. Where possible, these measures should be made the object of research, designed to evaluate their safety and efficacy. In all cases, new information should be recorded and, where appropriate, published. The other relevant guidelines of this Declaration should be followed. 27
28 Appendix D : Small Cell Cancer VA Classification Limited disease: The definition of limited disease is based on the possibility of encompassing all detectable tumor within a tolerable radiotherapy port. Patients with limited disease have tumor deposits restricted to one hemithorax with regional lymph node metastasis (including ipsilateral, mediastinal and supraclavicular nodes. Ipsilateral superior vena cava syndrome and recurrent laryngeal nerve involvement are also considered as limited disease. Contralateral hilar lymph node are also considered as limited disease.) Extensive disease: It represents any tumor beyond the bounds defined above (including ipsilateral lung metastases and malignant pleural effusion). 28
WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI
 WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18 th WMA General Assembly Helsinki, Finland, June 1964 and amended by
WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18 th WMA General Assembly Helsinki, Finland, June 1964 and amended by
WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects
 WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by
WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Vergleich Declaration of Helsinki 2008 vs 2013. Erläuterungen:
 Erläuterungen: Der Vergleich der Deklaration von Helsinki Version 2008 mit der neuen Version 2013 ist in einer simplen Gegenüberstellung nicht möglich. Man hat neue Kapiteltitel erstellt und die Themen
Erläuterungen: Der Vergleich der Deklaration von Helsinki Version 2008 mit der neuen Version 2013 ist in einer simplen Gegenüberstellung nicht möglich. Man hat neue Kapiteltitel erstellt und die Themen
GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE
 ICH E6 GCP: Consolidated Guideline: Investigator 1/7 Institutional Review Board Services ICH HARMONIZED TRIPARTITE GUIDELINE E6: GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE 4. INVESTIGATOR 4.1 Investigator's
ICH E6 GCP: Consolidated Guideline: Investigator 1/7 Institutional Review Board Services ICH HARMONIZED TRIPARTITE GUIDELINE E6: GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE 4. INVESTIGATOR 4.1 Investigator's
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device
 Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device List of Contents Page 1. Introduction 1.1 The Medical Device Administrative Control System and the 3 proposed legislation
Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device List of Contents Page 1. Introduction 1.1 The Medical Device Administrative Control System and the 3 proposed legislation
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Managing Risk in Clinical Research. Dr Martha J Wrigley R&D Manager Senior Visiting Fellow University of Surrey
 Managing Risk in Clinical Research Dr Martha J Wrigley R&D Manager Senior Visiting Fellow University of Surrey Aim of the session To explore the risks associated with clinical research and understand how
Managing Risk in Clinical Research Dr Martha J Wrigley R&D Manager Senior Visiting Fellow University of Surrey Aim of the session To explore the risks associated with clinical research and understand how
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
GENERAL INFORMATION. Adverse Event (AE) Definition (ICH GUIDELINES E6 FOR GCP 1.2):
 Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
Sheffield Kidney Institute. Planning a Clinical Trial
 Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Laurie Shaker-Irwin, Ph.D., M.S. Co-Leader, Regulatory Knowledge and Research Ethics UCLA Clinical and Translational Science Institute
 Laurie Shaker-Irwin, Ph.D., M.S. Co-Leader, Regulatory Knowledge and Research Ethics UCLA Clinical and Translational Science Institute Understand the protocol completely Recognize institutional polices
Laurie Shaker-Irwin, Ph.D., M.S. Co-Leader, Regulatory Knowledge and Research Ethics UCLA Clinical and Translational Science Institute Understand the protocol completely Recognize institutional polices
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
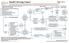 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
EU DIRECTIVE ON GOOD CLINICAL PRACTICE IN CLINICAL TRIALS DH & MHRA BRIEFING NOTE
 EU DIRECTIVE ON GOOD CLINICAL PRACTICE IN CLINICAL TRIALS DH & MHRA BRIEFING NOTE Purpose 1. The Clinical Trials Directive 2001/20/EC heralds certain additional responsibilities for the Medicines and Healthcare
EU DIRECTIVE ON GOOD CLINICAL PRACTICE IN CLINICAL TRIALS DH & MHRA BRIEFING NOTE Purpose 1. The Clinical Trials Directive 2001/20/EC heralds certain additional responsibilities for the Medicines and Healthcare
WHAT ARE THE SPECIFIC DIFFERENCES BETWEEN VERSION 5.5 AND VERSION 5.6 OF THE RESEARCH ETHICS COMMITTEE STANDARD APPLICATION FORM?
 Application Form Cover Sheet - Addition of Application Version No. in Version 5.6 - Addition of Application Date in Version 5.6 - Remove Principal Investigator (deletion) - Remove Applicant s Signature
Application Form Cover Sheet - Addition of Application Version No. in Version 5.6 - Addition of Application Date in Version 5.6 - Remove Principal Investigator (deletion) - Remove Applicant s Signature
Appendix 1 Waiver of the requirement for informed consent for a clinical trial in a medical emergency Page 1 of 2
 Appendix 1 Waiver of the requirement for informed consent for a clinical trial in a medical emergency Page 1 of 2 The Helsinki Committee may approve a clinical trial without the requirement to obtain informed
Appendix 1 Waiver of the requirement for informed consent for a clinical trial in a medical emergency Page 1 of 2 The Helsinki Committee may approve a clinical trial without the requirement to obtain informed
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products
 Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
L Lang-Lazdunski, A Bille, S Marshall, R Lal, D Landau, J Spicer
 Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Revisions on the Declaration of Helsinki
 Revisions on the Declaration of Helsinki Prof. J. Mfutso-Bengo, PhD Center of Bioethics (CEBESA) University of Malawi College of Medicine Cape Town South Africa December 2012 www.medcol.mw Joseph-matthew@gmx.net
Revisions on the Declaration of Helsinki Prof. J. Mfutso-Bengo, PhD Center of Bioethics (CEBESA) University of Malawi College of Medicine Cape Town South Africa December 2012 www.medcol.mw Joseph-matthew@gmx.net
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
CORD BLOOD TRANSPLANTATION STUDY EXPANDED ACCESS PROTOCOL APPENDIX A SAMPLE CONSENT FORM
 APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
Having regard to the Treaty establishing the European Community, and in particular Article 95 thereof,
 L 121/34 DIRECTIVE 2001/20/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to
L 121/34 DIRECTIVE 2001/20/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
CNE Disclosures. To change this title, go to Notes Master
 CNE Disclosures Successful Completion: Participants must complete an evaluation form to receive a certificate of completion Contact Hours: 1 contact hour is available to those who meet the successful completion
CNE Disclosures Successful Completion: Participants must complete an evaluation form to receive a certificate of completion Contact Hours: 1 contact hour is available to those who meet the successful completion
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER
 CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
Clinical research: where are we with the new (Paediatric) RC trial Regulation
 where are we with the new (Paediatric) RC trial Regulation, MD, PhD Ethical Committee DEEP Former member of the PDCO EMA With the aid of Fabio D'Atri European commission and Anabela Marcal of EMA The new
where are we with the new (Paediatric) RC trial Regulation, MD, PhD Ethical Committee DEEP Former member of the PDCO EMA With the aid of Fabio D'Atri European commission and Anabela Marcal of EMA The new
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 2001L0020 EN 07.08.2009 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B DIRECTIVE 2001/20/EC OF THE EUROPEAN PARLIAMENT
2001L0020 EN 07.08.2009 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B DIRECTIVE 2001/20/EC OF THE EUROPEAN PARLIAMENT
PHARMACY AND POISONS ORDINANCE (Cap. 138) APPLICATION FOR A CLINICAL TRIAL/MEDICINAL TEST CERTIFICATE
 PHARMACY AND POISONS ORDINANCE (Cap. 138) APPLICATION FOR A CLINICAL TRIAL/MEDICINAL TEST CERTIFICATE PART A: TRIAL INFORMATION A1. Title of Clinical Trial (as stated in proposed Protocol) Protocol No.
PHARMACY AND POISONS ORDINANCE (Cap. 138) APPLICATION FOR A CLINICAL TRIAL/MEDICINAL TEST CERTIFICATE PART A: TRIAL INFORMATION A1. Title of Clinical Trial (as stated in proposed Protocol) Protocol No.
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products (NIMPs)
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products
PATIENT INFORMATION SHEET
 PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Conduct of clinical Trials Communication of
 PrinciPles on Conduct of clinical Trials Communication of clinical Trial results Table of Contents Preamble...1 Commitment to Protecting Research Participants...5 Conduct of Clinical Trials...7 Ensuring
PrinciPles on Conduct of clinical Trials Communication of clinical Trial results Table of Contents Preamble...1 Commitment to Protecting Research Participants...5 Conduct of Clinical Trials...7 Ensuring
1. Study title Is the title self explanatory to a layperson? If not, a simplified title should be included.
 These guidelines apply to all research projects where human subjects are involved in the study GUIDELINES FOR RESEARCHERS PATIENT INFORMATION SHEET & CONSENT FORM The guidance, which follows, applies primarily
These guidelines apply to all research projects where human subjects are involved in the study GUIDELINES FOR RESEARCHERS PATIENT INFORMATION SHEET & CONSENT FORM The guidance, which follows, applies primarily
Clinical Trial Compensation Guidelines
 Clinical Trial Compensation Guidelines Preface These guidelines contain two distinct sections: Phase I Clinical Trials Compensation Guidelines Phases II, III and IV Clinical Trials Compensation Guidelines
Clinical Trial Compensation Guidelines Preface These guidelines contain two distinct sections: Phase I Clinical Trials Compensation Guidelines Phases II, III and IV Clinical Trials Compensation Guidelines
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Guidance on IRB Continuing Review of Research
 NOTE: THIS GUIDANCE SUPERSEDES OHRP S JANUARY 15, 2007 GUIDANCE ENTITILED GUIDANCE ON CONTINUING REVIEW. CLICK HERE FOR THE JANUARY 15, 2007 GUIDANCE. Office for Human Research Protections Department of
NOTE: THIS GUIDANCE SUPERSEDES OHRP S JANUARY 15, 2007 GUIDANCE ENTITILED GUIDANCE ON CONTINUING REVIEW. CLICK HERE FOR THE JANUARY 15, 2007 GUIDANCE. Office for Human Research Protections Department of
Paris, 15 June 2013 Response to a public consultation
 Paris, 15 June 2013 Response to a public consultation Revision of the World Medical Association Helsinki Declaration: - transparency of clinical trial results must be enhanced (articles 23, 24 & 26) -
Paris, 15 June 2013 Response to a public consultation Revision of the World Medical Association Helsinki Declaration: - transparency of clinical trial results must be enhanced (articles 23, 24 & 26) -
Advancing research: a physician s guide to clinical trials
 Advancing research: a physician s guide to clinical trials Recruiting and retaining trial participants is one of the greatest obstacles to developing the next generation of Alzheimer s treatments Alzheimer
Advancing research: a physician s guide to clinical trials Recruiting and retaining trial participants is one of the greatest obstacles to developing the next generation of Alzheimer s treatments Alzheimer
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009
 Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Cesare Gridelli 1, Francesca Casaluce 2, Assunta Sgambato 2, Fabio Monaco 3, Cesare Guida 4
 Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
Version history Version number Version date Effective date 01 dd-mon-yyyy dd-mon-yyyy 02 dd-mon-yyyy dd-mon-yyyy 03 (current) dd-mon-yyyy dd-mon-yyyy
 Trial name: HOVON xxx yyy Sponsor: HOVON Version history Version number Version date Effective date 01 dd-mon-yyyy dd-mon-yyyy 02 dd-mon-yyyy dd-mon-yyyy 03 (current) dd-mon-yyyy dd-mon-yyyy QRMP authors
Trial name: HOVON xxx yyy Sponsor: HOVON Version history Version number Version date Effective date 01 dd-mon-yyyy dd-mon-yyyy 02 dd-mon-yyyy dd-mon-yyyy 03 (current) dd-mon-yyyy dd-mon-yyyy QRMP authors
Ethics and Scientific Oversight for Phase 1 Clinical Trials in Hong Kong. Sydney TANG Chairman, HKU/HA HKW IRB November 21, 2015
 Ethics and Scientific Oversight for Phase 1 Clinical Trials in Hong Kong Sydney TANG Chairman, HKU/HA HKW IRB November 21, 2015 Clinical Trials at HKU Phase 1 Phase II Phase III Phase IV Conducted on patient
Ethics and Scientific Oversight for Phase 1 Clinical Trials in Hong Kong Sydney TANG Chairman, HKU/HA HKW IRB November 21, 2015 Clinical Trials at HKU Phase 1 Phase II Phase III Phase IV Conducted on patient
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
MOLOGEN AG. Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer. Berlin, 12 May 2015
 Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Cancer Clinical trials:
 Cancer Clinical trials: All you need to know A booklet for patients with cancer The future of cancer therapy F O R E W O R D If you have cancer, clinical trials may offer you additional treatment options.
Cancer Clinical trials: All you need to know A booklet for patients with cancer The future of cancer therapy F O R E W O R D If you have cancer, clinical trials may offer you additional treatment options.
How To Prepare A Meeting For A Health Care Conference
 THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
Data Management in Clinical Trials
 Data Management in Clinical Trials Introduction to the Principles and Practice of Clinical Research February 19, 2013 Diane St. Germain, RN, MS Nurse Consultant Division of Cancer Prevention National Cancer
Data Management in Clinical Trials Introduction to the Principles and Practice of Clinical Research February 19, 2013 Diane St. Germain, RN, MS Nurse Consultant Division of Cancer Prevention National Cancer
Medicals c i e n t i f i c study
 Medicals c i e n t i f i c study design risks medical-ethics review board 2 Table of contents M e d i c a l - s c i e n t i f i c study Preface 2 Introduction 4 Medical-scientific study 5 Why participate?
Medicals c i e n t i f i c study design risks medical-ethics review board 2 Table of contents M e d i c a l - s c i e n t i f i c study Preface 2 Introduction 4 Medical-scientific study 5 Why participate?
Small cell Lung cancer New Chemotherapy options. Nicolas Mach, MD,PD
 Small cell Lung cancer New Chemotherapy options Nicolas Mach, MD,PD > 40 negative Phase III trials List of unsucessful drugs Pemetrexed, imatinib, bevacizumab, bcl-2 antagonist, ASCT, CASE PRESENTATION
Small cell Lung cancer New Chemotherapy options Nicolas Mach, MD,PD > 40 negative Phase III trials List of unsucessful drugs Pemetrexed, imatinib, bevacizumab, bcl-2 antagonist, ASCT, CASE PRESENTATION
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON DATA MONITORING COMMITTEES
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
Malignant Mesothelioma State of the Art
 Malignant Mesothelioma State of the Art Paul Baas The Netherlands Cancer Institute August 12, 2011, Carlsbad, CA Summary Diagnosis; epithelial type subdivided Pleiomorphic vs other Staging: IASLC-IMIG
Malignant Mesothelioma State of the Art Paul Baas The Netherlands Cancer Institute August 12, 2011, Carlsbad, CA Summary Diagnosis; epithelial type subdivided Pleiomorphic vs other Staging: IASLC-IMIG
Nova Southeastern University Institutional Review Board Policies and Procedures
 Page 1 of 14 Purpose: To establish policy with respect to the emergency use of unapproved drugs and biologics. Definitions: 1. IRB approval means the determination of the IRB that the research has been
Page 1 of 14 Purpose: To establish policy with respect to the emergency use of unapproved drugs and biologics. Definitions: 1. IRB approval means the determination of the IRB that the research has been
Goals and Objectives: Breast Cancer Service Department of Radiation Oncology
 Goals and Objectives: Breast Cancer Service Department of Radiation Oncology The breast cancer service provides training in the diagnosis, management, treatment, and follow-up of breast malignancies, including
Goals and Objectives: Breast Cancer Service Department of Radiation Oncology The breast cancer service provides training in the diagnosis, management, treatment, and follow-up of breast malignancies, including
A Outpatient Services A1 AMBULATORY CARE CENTRE A1(d) Cancer Centre Clinical Trials Office
 A1(d) CANCER CENTRE CLINICAL TRIALS OFFICE A1(d).1 SERVICE DESCRIPTION A1(d).1.1 Scope of Clinical Services This section A1(d) sets out the requirements for the centralized facilities for the Clinical
A1(d) CANCER CENTRE CLINICAL TRIALS OFFICE A1(d).1 SERVICE DESCRIPTION A1(d).1.1 Scope of Clinical Services This section A1(d) sets out the requirements for the centralized facilities for the Clinical
Guidance on Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events
 Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events
Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events
AN INTRODUCTION TO ETHICS ISSUES AND PRINCIPLES IN RESEARCH INVOLVING HUMAN AND ANIMAL PARTICIPANTS
 AN INTRODUCTION TO ETHICS ISSUES AND PRINCIPLES IN RESEARCH INVOLVING HUMAN AND ANIMAL PARTICIPANTS Document control Applicable to: All employees and research students Date first approved May 2006 Date
AN INTRODUCTION TO ETHICS ISSUES AND PRINCIPLES IN RESEARCH INVOLVING HUMAN AND ANIMAL PARTICIPANTS Document control Applicable to: All employees and research students Date first approved May 2006 Date
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
DRAFT GUIDELINES ON AUDIO-VISUAL RECORDING OF INFORMED CONSENT PROCESS IN CLINICAL TRIAL
 DRAFT GUIDELINES ON AUDIO-VISUAL RECORDING OF INFORMED CONSENT PROCESS IN CLINICAL TRIAL GUIDANCE DOCUMENT Comments/suggestions, if any, may please be submitted to the office of Drugs Controller General
DRAFT GUIDELINES ON AUDIO-VISUAL RECORDING OF INFORMED CONSENT PROCESS IN CLINICAL TRIAL GUIDANCE DOCUMENT Comments/suggestions, if any, may please be submitted to the office of Drugs Controller General
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
RESEARCH INVOLVING HUMAN SUBJECTS
 RESEARCH INVOLVING HUMAN SUBJECTS GUIDELINES FOR IRBS PART A: INTRODUCTION AND CURRENT FRAMEWORK SECTION I: INTRODUCTION 1. Introduction About these Guidelines 1.1. The Bioethics Advisory Committee (BAC)
RESEARCH INVOLVING HUMAN SUBJECTS GUIDELINES FOR IRBS PART A: INTRODUCTION AND CURRENT FRAMEWORK SECTION I: INTRODUCTION 1. Introduction About these Guidelines 1.1. The Bioethics Advisory Committee (BAC)
NATIONAL HEALTH COUNCIL RESOLUTION Nº 251, DATED 7 AUGUST 1997
 NATIONAL HEALTH COUNCIL RESOLUTION Nº 251, DATED 7 AUGUST 1997 Plenary of the National Health Council in its 15 th Special Meeting, held on 5 August 1997, in the exercise of its competencies, as set forth
NATIONAL HEALTH COUNCIL RESOLUTION Nº 251, DATED 7 AUGUST 1997 Plenary of the National Health Council in its 15 th Special Meeting, held on 5 August 1997, in the exercise of its competencies, as set forth
Operational aspects of a clinical trial
 Operational aspects of a clinical trial Carlo Tomino Pharm.D. Coordinator Pre-authorization Department Head of Research and Clinical Trial Italian Medicines Agency Mwanza (Tanzania), June 11, 2012 1 Declaration
Operational aspects of a clinical trial Carlo Tomino Pharm.D. Coordinator Pre-authorization Department Head of Research and Clinical Trial Italian Medicines Agency Mwanza (Tanzania), June 11, 2012 1 Declaration
Health Care Consent Act
 Briefing Note 2005, 2007 College of Physiotherapists of Ontario 2009 Contents Overview...3 Putting the in Context...3 The HCCA in Brief...4 Key Principles Governing Consent to Treatment...4 Key Aspects
Briefing Note 2005, 2007 College of Physiotherapists of Ontario 2009 Contents Overview...3 Putting the in Context...3 The HCCA in Brief...4 Key Principles Governing Consent to Treatment...4 Key Aspects
International Paralympic Committee Medical Code. December 2011
 International Paralympic Committee Medical Code December 2011 This version of the IPC Medical Code has been approved by the IPC General Assembly December 2011. IPC Medical Code 1 Preamble 1. The Paralympic
International Paralympic Committee Medical Code December 2011 This version of the IPC Medical Code has been approved by the IPC General Assembly December 2011. IPC Medical Code 1 Preamble 1. The Paralympic
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Miami University: Human Subjects Research General Research Application Guidance
 Miami University: Human Subjects Research General Research Application Guidance Use the accompanying Word template for completing the research description. You must provide sufficient information regarding
Miami University: Human Subjects Research General Research Application Guidance Use the accompanying Word template for completing the research description. You must provide sufficient information regarding
Glossary of Clinical Trial Terms
 Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Page 191 TITLE 21 FOOD AND DRUGS 355 1
 Page 191 TITLE 21 FOOD AND DRUGS 355 1 APPEALS TAKEN PRIOR TO OCTOBER 10, 1962 Section 104(d)(3) of Pub. L. 87 781 made amendments to subsec. (h) of this section inapplicable to any appeal taken prior
Page 191 TITLE 21 FOOD AND DRUGS 355 1 APPEALS TAKEN PRIOR TO OCTOBER 10, 1962 Section 104(d)(3) of Pub. L. 87 781 made amendments to subsec. (h) of this section inapplicable to any appeal taken prior
Clinical cases in Malignant Pleural Mesothelioma: Adherence to the ESMO Clinical Practice Guidelines
 Clinical cases in Malignant Pleural Mesothelioma: Adherence to the ESMO Clinical Practice Guidelines Wieneke Buikhuisen The Netherlands Cancer Institute Amsterdam The Netherlands Case (1) Male, 56 year
Clinical cases in Malignant Pleural Mesothelioma: Adherence to the ESMO Clinical Practice Guidelines Wieneke Buikhuisen The Netherlands Cancer Institute Amsterdam The Netherlands Case (1) Male, 56 year
Before, Frank's immune cells could
 Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
12.0 Investigator Responsibilities
 v. 5.13.13 12.0 Investigator Responsibilities 12.1 Policy Investigators are ultimately responsible for the conduct of research. Research must be conducted according to the signed Investigator statement,
v. 5.13.13 12.0 Investigator Responsibilities 12.1 Policy Investigators are ultimately responsible for the conduct of research. Research must be conducted according to the signed Investigator statement,
A Guide to Clinical Trials
 A Guide to Clinical Trials For young people with cancer and their parents Children s Cancer and Leukaemia Group www.cclg.org.uk Original booklet produced in conjunction with the CCLG Patient Advocacy Committee.
A Guide to Clinical Trials For young people with cancer and their parents Children s Cancer and Leukaemia Group www.cclg.org.uk Original booklet produced in conjunction with the CCLG Patient Advocacy Committee.
ETHICS GUIDE FCT INVESTIGATOR PROGRAMME. 25 July 2013
 ETHICS GUIDE FCT INVESTIGATOR PROGRAMME 25 July 2013 ETHICAL ISSUES Ethics is central to scientific integrity, honesty and clarity of science. It is considered essential by FCT in the research activities
ETHICS GUIDE FCT INVESTIGATOR PROGRAMME 25 July 2013 ETHICAL ISSUES Ethics is central to scientific integrity, honesty and clarity of science. It is considered essential by FCT in the research activities
College of Physicians and Surgeons of Saskatchewan POLICY. Physician-Assisted Dying. Background. Introduction
 College of Physicians and Surgeons of Saskatchewan POLICY Physician-Assisted Dying STATUS: APPROVED Approved by Council: November 2015 Amended: n/a To be reviewed: To be determined Background On February
College of Physicians and Surgeons of Saskatchewan POLICY Physician-Assisted Dying STATUS: APPROVED Approved by Council: November 2015 Amended: n/a To be reviewed: To be determined Background On February
MRC. Clinical Trials. Series MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS. Medical Research Council
 MRC Clinical Trials Series MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS 1998 Medical Research Council MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS 1998 MRC GUIDELINES FOR
MRC Clinical Trials Series MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS 1998 Medical Research Council MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS 1998 MRC GUIDELINES FOR
ICH CRA Certification Guide March 2009
 ICH CRA Certification Guide March 2009 ICH CRA CERTIFICATION GUIDE... 1 GENERAL INFORMATION... 2 BENEFITS OF CERTIFICATION... 2 INDUSTRY RECOGNITION... 2 ABOUT THE EXAM... 2 CRA DEFINITION... 2 REQUIREMENTS
ICH CRA Certification Guide March 2009 ICH CRA CERTIFICATION GUIDE... 1 GENERAL INFORMATION... 2 BENEFITS OF CERTIFICATION... 2 INDUSTRY RECOGNITION... 2 ABOUT THE EXAM... 2 CRA DEFINITION... 2 REQUIREMENTS
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
CPSM DRAFT STATEMENT ON PHYSICIAN ASSISTED DYING OCTOBER 15, 2015
 CPSM DRAFT STATEMENT ON PHYSICIAN ASSISTED DYING OCTOBER 15, 2015 BACKGROUND The Supreme Court of Canada (SCC) declared that as of February 6, 2016 it is legal for a physician to assist a competent adult
CPSM DRAFT STATEMENT ON PHYSICIAN ASSISTED DYING OCTOBER 15, 2015 BACKGROUND The Supreme Court of Canada (SCC) declared that as of February 6, 2016 it is legal for a physician to assist a competent adult
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
UK Implementation of the EU Clinical Trial Directive 2001/20/EC:
 UK Implementation of the EU Clinical Trial Directive 2001/20/EC: GCP Aspects. Dr. Colin Wilsher, FRQA. BARQA GCP Committee Chairman; & Pfizer Worldwide Development Quality Assurance. GIQAR, Roma, October
UK Implementation of the EU Clinical Trial Directive 2001/20/EC: GCP Aspects. Dr. Colin Wilsher, FRQA. BARQA GCP Committee Chairman; & Pfizer Worldwide Development Quality Assurance. GIQAR, Roma, October
MEDICAL BENEFITS CLASS ACTION SETTLEMENT NOTICE OF INTENT TO SUE
 MEDICAL BENEFITS CLASS ACTION SETTLEMENT NOTICE OF INTENT TO SUE Complete this form if you are a MEDICAL BENEFITS SETTLEMENT CLASS MEMBER seeking to exercise a BACK END LITIGATION OPTION. In addition to
MEDICAL BENEFITS CLASS ACTION SETTLEMENT NOTICE OF INTENT TO SUE Complete this form if you are a MEDICAL BENEFITS SETTLEMENT CLASS MEMBER seeking to exercise a BACK END LITIGATION OPTION. In addition to
STANDARDS OF PRACTICE (2013)
 STANDARDS OF PRACTICE (2013) COLLEGE OF ALBERTA PSYCHOLOGISTS STANDARDS OF PRACTICE (2013) 1. INTRODUCTION The Health Professions Act (HPA) authorizes and requires the College of Alberta Psychologists
STANDARDS OF PRACTICE (2013) COLLEGE OF ALBERTA PSYCHOLOGISTS STANDARDS OF PRACTICE (2013) 1. INTRODUCTION The Health Professions Act (HPA) authorizes and requires the College of Alberta Psychologists
