POWDER X-RAY DIFFRACTION: STRUCTURAL DETERMINATION OF ALKALI HALIDE SALTS
|
|
|
- Leon Summers
- 10 years ago
- Views:
Transcription
1 EXPERIMENT 4 POWDER X-RAY DIFFRACTION: STRUCTURAL DETERMINATION OF ALKALI HALIDE SALTS I. Introduction The determination of the chemical structure of molecules is indispensable to chemists in their effort to gain insight into chemical problems. Only a few physical methods are capable of determining chemical structure, and amongst these methods, diffraction methods have been the most successful. Diffraction methods are capable of defining bond lengths, bond angles and the spatial proximity of non-bonded atoms for materials capable of forming crystalline solids. For example, the models of myoglobin which were displayed in the fluoride-myoglobin lab are representations of the myoglobin structure found using x-ray diffraction. The importance of structural determination to science has been recognized with several Nobel Prizes for x-ray diffraction related research. Year Prize Awardee Topic 1914 Physics M. Von Laue Discovery of x-ray diffraction 1915 Physics W.H. Bragg Analysis of crystal structure using x-ray W.L. Bragg diffraction 1962 Chemistry J.C. Kendrew M.F. Perutz Structural determination of globular proteins (myoglobin and hemoglobin) 1964 Chemistry D.C. Hodgkin Structure determinations of important biochemical molecules 1962 Medicine F.H. Crick Structure of DNA J.D. Watson M.H. Wilkins 199? Chemistry J. Deisenhofer R. Huber H. Michel Structure of photosynthetic reaction center In this final laboratory exercise, you will determine the structure and unit cell dimension of an alkali halide crystal using powder x-ray diffraction, and use the data to construct a chart of ionic radii.
2 To explain x-ray diffraction it is convenient to think of light as a wave with a wavelength that is related to the energy of the light by the equation, E=hc/λ, where h is Plank s constant, c is the speed of light and λ is the wavelength of light. For light within the x-ray region of the spectrum, the wavelength of the light is within the range 0.1Å to 100Å. One of the fundamental properties of waves is that two waves can interfere with each other if they are in the same spatial region. If the phases of the two waves are coincident they will constructively interfere (increased amplitude) and if their phases are opposed they will destructively interfere (decreased amplitude). Constructive Interference Destructive Interference Wave 1 Wave 2 Sum 1+2 Wave 1 Wave 2 Sum 1+2 The interference patterns formed in x-ray diffraction originates from the x-rays that are scattered by the electrons of atoms within a crystalline solid. A simple picture of a solid (composed of only one type of atom) is a collection of balls packed together to form a crystal lattice. In a crystalline material the packing of the atoms is well defined, and in fact, the entire crystal can be constructed by repeating a pattern of atoms that define the unit cell (see Chang, chapter 11, Kotz, chapter 13). This is analogous to the fact that a wall can be constructed by stacking bricks; the brick is the unit cell of the wall. The simplest case (the only one that we will consider) is when the unit cell is cubic, that is to say that all of the edges of the cube are equal and the corners form right angles. For cubic unit cells the length of the edge is usually given the symbol a. Due to the regular spacing of the atoms, imaginary planes can be pictured which contain the nuclei. For example, each face of the cube in the illustration above is one such plane. The electrons of each of these imaginary planes of atoms scatter the x-rays giving a result that can be interpreted as set of parallel partial mirrors; the light (x-rays) is partially reflected by each plane. Although the x-rays originate in-phase from the x-ray source, the scattered x-rays are not necessarily in phase when they emerge from the crystal and reach the detector. The relative phases of scattered x- rays depend critically on the separation distance between these imaginary planes. This is due to the fact that x-rays that penetrate deeper into the 2 Larsen 4:20 Spring 2000
3 crystal lattice before they are reflected, travel further. Simple geometry demonstrates that for two waves reflected by adjacent planes, the wave that is reflected by the second plane has traveled a distance of (2d sinθ) further than the wave that is reflected by the first plane. In general, the reflected waves from successive planes destructively interfere, however, if the pathlength difference between reflected waves is an integer multiple of the wavelength (λ), constructive interference is observed (a bright spot). This condition (the Bragg s condition for constructive interference) is summarized by Bragg s Law: nλ = 2d sinθ, where λ is the wavelength, d is the interplanar separation, and θ is the Bragg s angle. The n defines the order of the reflection, with n=1 giving first-order, n=2 giving secondorder, etc. From the above equation, it is evident that the structure of the solid (i.e. the distance between the atoms) can be obtained if a monochromatic x-ray source is used (only one λ) and the intensity of x-rays is measured as a function of θ. The constructive interference that results at specific values of theta (θ) map out the interplanar spacing of the solid. Further, the total intensity at a spot, not only depends on the fulfillment of the Bragg s condition, but also on the number of electrons, which depends on the chemical identity of the atoms. There is one further complication however, the planes of atoms can be constructed in several different ways as illustrated below. Each imaginary plane can be indexed using Miller indices. The Miller index is written as three numbers, (h k l), one for each coordinate in three dimensional space. The protocol for writing these indices are given in the reference 1, but for now just consider them as labeling scheme for all possible imaginary planes. Each plane can satisfy the Bragg s condition and give a bright spot due to constructive interference. To account for all possible planes, the Bragg s Law is usually rewritten (for a cubic system) as, ( ) sin 2 λ 2 θ = 4a 2 h 2 + k 2 + l 2 1 Start at the origin of the unit cell, then determine where the point where the plane intersects each of the three axes (a,b,c) of the cell. (abc is used for crystal coordinate rather than xyz) If a plane is parallel to an axis it is assigned an intercept of infinity ( ). The Miller index is form by dividing the cell dimension along each axis (a,b,c), by the intercept to general the Miller indices (h,k,l) respectively. For example, if the intersection occur half way alone a then h=a/(a/2)=2. 3 Larsen 4:20 Spring 2000
4 where a is the unit cell dimension, λ is the wavelength of the radiation, (h, k, l) are the Miller indices, and θ is the Bragg angle. In a powder, composed of a very large number of crystallites, all possible orientations of the crystal are simultaneously present. This means that the Bragg s condition for constructive interference will be fulfilled simultaneously for all possible planes consistent with the given crystalline structure. Therefore, a diffraction pattern, intensity as a function of the scattering angle (2θ), is generated and is directly related to the arrangement of atoms within the unit cell as well as to the distances between the atoms. As you will recall from 4:14 or 4:19 (see Chang, chapter 11, Kotz, chapter 13), ionic solids composed of alkali halides tend to form cubic crystals with either an FCC (face centered cubic) or simple cubic (primative) unit cell. 2 In this laboratory exercise, you will determine the unit cell structure and unit cell dimension of a series of alkali halide salts. By using the cumulative data of the series you will also determine the cation and anion radii in the lattice. To acquire the data needed to determine these radii, you will use the Chemistry Department s powder x-ray diffractometer (powder XRD) which was purchased in 1996 for ~$130,000 (needless to say, each student will not have her/his own instrument). II. Experimental (groups of four) The sample preparation and data acquisition for this lab are almost trivial. Each group member will be responsible for one sample from the following set of alkali halides: NaF, NaCl, NaBr, NaI, KCl, KBr, and CsCl. In addition, a powder diffraction pattern of LiCl, will be provided to each group. Each group member should begin her/his numerical analysis on the LiCl data. Experimental diffraction patterns will be obtained for each of the other samples. The sample preparation consists of grinding the sample in a mortar and pestle and placing a 2 There is one other variant of the cubic unit cell known as BCC (body centered cubic), but the alkali halides do not crystallize with this structure. 4 Larsen 4:20 Spring 2000
5 flattened pile of salt on a glass plate. The plate is then placed into the sample holder and a diffraction pattern is obtained using the wavelength of Å (CuK α, isolated with a Ni foil filter). Your TA or safety monitor will help you will this step. The data will be printed as intensity as a function of scattering angle (2Θ). Each set of reflections will be analyzed to determine the structure (primitive or FCC) and the unit cell dimension. For the simple cubic unit cells, all possible combinations of integer Miller indices are possible. In general for FCC, certain combinations of Miller indices are eliminated by destructive interference caused by the planes formed by the atoms in the faces of the unit cell. The result is that for FCC type lattices reflections occur only if h, k, and l are all even or all odd. There is one caveat though, in FCC systems in which the scattering power of the two types of atoms is nearly identical (i.e. when the two ions have the same number of electrons) a pseudo-primitive pattern (one that looks primitive) is observed; this is the case for KCl. These results can be used to assign the diffraction patterns and extract the lattice type and cell dimensions from the data. Simple Cubic (h k l) Body Centered Cubic (h k l) Face Centered Cubic (h k l) h 2 + k 2 + l , To correlate the observed Bragg s angle with the predicted values the data must be manipulated to resemble the Bragg s law, sin 2 θ = λ2 4a 2 h 2 + k 2 + l 2. This is most easily accomplished using a spreadsheet program such as KaleidaGraph or Excel. First, enter the position of each reflection and label the column 2-theta. Construct a second column that equals sin 2 θ. This is c1=(sin(c0/2))^2 in KaleidaGraph (or =SIN(PI()*A2/360)^2 in Excel then fill down). Realize that Bragg s law has the form sin 2 θ=c*i, where C is a constant equal to λ 2 C = 4a 2 and i is an integer as indicated in the table above. Therefore, divide the experimental values of sin 2 θ by the sin 2 θ value of the first reflection (c2=c1/ sin 2 θ reflection-1 ). If the unit cell is simple cubic, the result of this operation should give an integer series starting with one (see table above). If the unit cell is FCC, the column will need to be multiplied by 3 before the expected integer series is obtained. (There will be some experimental error). Once the unit cell type has been established create a column that contains the expected integer values for the appropriate cell type (e.g. 3,4,8,11,12 for FCC). 5 Larsen 4:20 Spring 2000
6 Graph sin 2 θ vs. the integer column (y vs. x) and fit the result to a straight line to obtain the constant, C. λ 2 Use the wavelength of λ= Å to solve for the constant, a using C = 4a 2. To determine if the constant, a, represents the unit cell dimension, half of the unit cell dimension, or twice the unit cell dimension, requires that the density calculated from the cell volume be compared to the actual (macroscopic) density. The simple cubic unit cell contains 1 molecule (AX) and the FCC contains 4 molecules (4 AX). The volume is the cell length cubed, and density is mass divided by volume. The experimental values for density as well as cell parameter data (for comparison to your own) is presented in the table below. You will find that in the case of KCl and CsI the unit cell may need to be contracted or expanded (why are these special cases?). Salt a o (Å) Density (g/cm 3 ) Molecular Weight (g/mol) Lattice Type NaF Ca FCC LiCl FCC NaCl FCC KCl FCC (pseudosimple) CsCl Simple Cubic NaBr FCC KBr FCC CsI Simple Cubic (pseudobodycentered) Data from F.P. Boer, and T.H. Jordan, J. Chem. Educ., 42, 76 (1965). Ionic radii will be calculated following the seminal work of Landé, 3 which was one of the first determinations of the values for ionic radii. Landé assumed that due to the small size of the lithium cation, the unit cell dimension of lithium halides was determined by anion anion contacts. By inspection of the FCC unit cell, four times the radius of the halide atom is seen to be 4r Cl = 2a o. Once this value is set, r=1.81å in the case of Cl, other radii can be obtained by assuming that cation-anion contacts determine the edge length. Again inspection of the unit cell leads to the conclusion that: a o = 2r cation + 2r anion in the case of FCC and 3a o =2r cation + 2r anion in the case of simple cubic. (Br -, 1.96Å; Na +, 1.01Å; K +, 1.34Å; and Cs +, 1.76Å) 3 A. Landé, Z. Physik, 1, 191 (1920). 6 Larsen 4:20 Spring 2000
7 Prelab Questions (15 points) Answer these questions in your laboratory notebook. 1. Go to the Nobel prize web site at the URL, For one of the Nobel prizes mentioned in this handout, read any one of the following: presentation/speech, press release or a bibliography of one of the scientist. Write a oneparagraph summary of the historic event or person. (10 points) 2. To obtain the powder diffraction patterns of the alkali halides you will use x-rays of wavelength λ=1.54å. The diffraction pattern will be a plot of x-ray intensity vs. the scattering angle, 2Θ. To better see what is going on, go to the Bragg s Law and Diffraction simulation at: Enter a wavelength of λ=1.54å. Choose on salt: NaF, CsCl, or CsI. Use the table in this handout to the unit cell dimension.(e.g. LiCl is a o =5.13Å, do not use this salt, ). Enter the cell dimension into the simulation. Now enter 5 for the beginning angle. Click on the box that says, details. Use the greater than sign (>) to scan the angle. Record the values of theta that give maximum constructive interference and the value of theta that give maximum destructive interference over the range theta=5 to 35. Mathematically determine the value of theta that gives the first order scattering condition for the salt that you used in the simulation. (5 points) No purpose or procedure is needed for the pre-lab this week. Lab Report (35 points) Your lab report should be completed in your laboratory notebook. Hand in the original to your TA. The laboratory report for this lab will consist of all the required calculations, and a brief summary of your results. The report will be turned in at the end of section. There will also be a short exercise that to determine the line spacing of a diffraction pattern that will also be completed while in the laboratory. 7 Larsen 4:20 Spring 2000
Crystal Structure Determination I
 Crystal Structure Determination I Dr. Falak Sher Pakistan Institute of Engineering and Applied Sciences National Workshop on Crystal Structure Determination using Powder XRD, organized by the Khwarzimic
Crystal Structure Determination I Dr. Falak Sher Pakistan Institute of Engineering and Applied Sciences National Workshop on Crystal Structure Determination using Powder XRD, organized by the Khwarzimic
Chapter 3. 1. 3 types of materials- amorphous, crystalline, and polycrystalline. 5. Same as #3 for the ceramic and diamond crystal structures.
 Chapter Highlights: Notes: 1. types of materials- amorphous, crystalline, and polycrystalline.. Understand the meaning of crystallinity, which refers to a regular lattice based on a repeating unit cell..
Chapter Highlights: Notes: 1. types of materials- amorphous, crystalline, and polycrystalline.. Understand the meaning of crystallinity, which refers to a regular lattice based on a repeating unit cell..
X-Ray Diffraction HOW IT WORKS WHAT IT CAN AND WHAT IT CANNOT TELL US. Hanno zur Loye
 X-Ray Diffraction HOW IT WORKS WHAT IT CAN AND WHAT IT CANNOT TELL US Hanno zur Loye X-rays are electromagnetic radiation of wavelength about 1 Å (10-10 m), which is about the same size as an atom. The
X-Ray Diffraction HOW IT WORKS WHAT IT CAN AND WHAT IT CANNOT TELL US Hanno zur Loye X-rays are electromagnetic radiation of wavelength about 1 Å (10-10 m), which is about the same size as an atom. The
Structure Factors 59-553 78
 78 Structure Factors Until now, we have only typically considered reflections arising from planes in a hypothetical lattice containing one atom in the asymmetric unit. In practice we will generally deal
78 Structure Factors Until now, we have only typically considered reflections arising from planes in a hypothetical lattice containing one atom in the asymmetric unit. In practice we will generally deal
Experiment: Crystal Structure Analysis in Engineering Materials
 Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Crystal Structure of High Temperature Superconductors. Marie Nelson East Orange Campus High School NJIT Professor: Trevor Tyson
 Crystal Structure of High Temperature Superconductors Marie Nelson East Orange Campus High School NJIT Professor: Trevor Tyson Introduction History of Superconductors Superconductors are material which
Crystal Structure of High Temperature Superconductors Marie Nelson East Orange Campus High School NJIT Professor: Trevor Tyson Introduction History of Superconductors Superconductors are material which
Chem 106 Thursday Feb. 3, 2011
 Chem 106 Thursday Feb. 3, 2011 Chapter 13: -The Chemistry of Solids -Phase Diagrams - (no Born-Haber cycle) 2/3/2011 1 Approx surface area (Å 2 ) 253 258 Which C 5 H 12 alkane do you think has the highest
Chem 106 Thursday Feb. 3, 2011 Chapter 13: -The Chemistry of Solids -Phase Diagrams - (no Born-Haber cycle) 2/3/2011 1 Approx surface area (Å 2 ) 253 258 Which C 5 H 12 alkane do you think has the highest
X-ray Diffraction and EBSD
 X-ray Diffraction and EBSD Jonathan Cowen Swagelok Center for the Surface Analysis of Materials Case School of Engineering Case Western Reserve University October 27, 2014 Outline X-ray Diffraction (XRD)
X-ray Diffraction and EBSD Jonathan Cowen Swagelok Center for the Surface Analysis of Materials Case School of Engineering Case Western Reserve University October 27, 2014 Outline X-ray Diffraction (XRD)
Introduction to Powder X-Ray Diffraction History Basic Principles
 Introduction to Powder X-Ray Diffraction History Basic Principles Folie.1 History: Wilhelm Conrad Röntgen Wilhelm Conrad Röntgen discovered 1895 the X-rays. 1901 he was honoured by the Noble prize for
Introduction to Powder X-Ray Diffraction History Basic Principles Folie.1 History: Wilhelm Conrad Röntgen Wilhelm Conrad Röntgen discovered 1895 the X-rays. 1901 he was honoured by the Noble prize for
Chapter 7: Basics of X-ray Diffraction
 Providing Solutions To Your Diffraction Needs. Chapter 7: Basics of X-ray Diffraction Scintag has prepared this section for use by customers and authorized personnel. The information contained herein is
Providing Solutions To Your Diffraction Needs. Chapter 7: Basics of X-ray Diffraction Scintag has prepared this section for use by customers and authorized personnel. The information contained herein is
Relevant Reading for this Lecture... Pages 83-87.
 LECTURE #06 Chapter 3: X-ray Diffraction and Crystal Structure Determination Learning Objectives To describe crystals in terms of the stacking of planes. How to use a dot product to solve for the angles
LECTURE #06 Chapter 3: X-ray Diffraction and Crystal Structure Determination Learning Objectives To describe crystals in terms of the stacking of planes. How to use a dot product to solve for the angles
Introduction to X-Ray Powder Diffraction Data Analysis
 Introduction to X-Ray Powder Diffraction Data Analysis Center for Materials Science and Engineering at MIT http://prism.mit.edu/xray An X-ray diffraction pattern is a plot of the intensity of X-rays scattered
Introduction to X-Ray Powder Diffraction Data Analysis Center for Materials Science and Engineering at MIT http://prism.mit.edu/xray An X-ray diffraction pattern is a plot of the intensity of X-rays scattered
Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds.
 Problem 1 Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds. Ionic Bonds Two neutral atoms close to each can undergo an ionization process in order
Problem 1 Explain the ionic bonds, covalent bonds and metallic bonds and give one example for each type of bonds. Ionic Bonds Two neutral atoms close to each can undergo an ionization process in order
X-ray thin-film measurement techniques
 Technical articles X-ray thin-film measurement techniques II. Out-of-plane diffraction measurements Toru Mitsunaga* 1. Introduction A thin-film sample is two-dimensionally formed on the surface of a substrate,
Technical articles X-ray thin-film measurement techniques II. Out-of-plane diffraction measurements Toru Mitsunaga* 1. Introduction A thin-film sample is two-dimensionally formed on the surface of a substrate,
Theory of X-Ray Diffraction. Kingshuk Majumdar
 Theory of X-Ray Diffraction Kingshuk Majumdar Contents Introduction to X-Rays Crystal Structures: Introduction to Lattices Different types of lattices Reciprocal Lattice Index Planes X-Ray Diffraction:
Theory of X-Ray Diffraction Kingshuk Majumdar Contents Introduction to X-Rays Crystal Structures: Introduction to Lattices Different types of lattices Reciprocal Lattice Index Planes X-Ray Diffraction:
LMB Crystallography Course, 2013. Crystals, Symmetry and Space Groups Andrew Leslie
 LMB Crystallography Course, 2013 Crystals, Symmetry and Space Groups Andrew Leslie Many of the slides were kindly provided by Erhard Hohenester (Imperial College), several other illustrations are from
LMB Crystallography Course, 2013 Crystals, Symmetry and Space Groups Andrew Leslie Many of the slides were kindly provided by Erhard Hohenester (Imperial College), several other illustrations are from
12.524 2003 Lec 17: Dislocation Geometry and Fabric Production 1. Crystal Geometry
 12.524 2003 Lec 17: Dislocation Geometry and Fabric Production 1. Bibliography: Crystal Geometry Assigned Reading: [Poirier, 1985]Chapter 2, 4. General References: [Kelly and Groves, 1970] Chapter 1. [Hirth
12.524 2003 Lec 17: Dislocation Geometry and Fabric Production 1. Bibliography: Crystal Geometry Assigned Reading: [Poirier, 1985]Chapter 2, 4. General References: [Kelly and Groves, 1970] Chapter 1. [Hirth
Solid State Theory Physics 545
 Solid State Theory Physics 545 CRYSTAL STRUCTURES Describing periodic structures Terminology Basic Structures Symmetry Operations Ionic crystals often have a definite habit which gives rise to particular
Solid State Theory Physics 545 CRYSTAL STRUCTURES Describing periodic structures Terminology Basic Structures Symmetry Operations Ionic crystals often have a definite habit which gives rise to particular
X-ray Diffraction (XRD)
 X-ray Diffraction (XRD) 1.0 What is X-ray Diffraction 2.0 Basics of Crystallography 3.0 Production of X-rays 4.0 Applications of XRD 5.0 Instrumental Sources of Error 6.0 Conclusions Bragg s Law n l =2dsinq
X-ray Diffraction (XRD) 1.0 What is X-ray Diffraction 2.0 Basics of Crystallography 3.0 Production of X-rays 4.0 Applications of XRD 5.0 Instrumental Sources of Error 6.0 Conclusions Bragg s Law n l =2dsinq
Atomic Force Microscope and Magnetic Force Microscope Background Information
 Atomic Force Microscope and Magnetic Force Microscope Background Information Lego Building Instructions There are several places to find the building instructions for building the Lego models of atomic
Atomic Force Microscope and Magnetic Force Microscope Background Information Lego Building Instructions There are several places to find the building instructions for building the Lego models of atomic
Each grain is a single crystal with a specific orientation. Imperfections
 Crystal Structure / Imperfections Almost all materials crystallize when they solidify; i.e., the atoms are arranged in an ordered, repeating, 3-dimensional pattern. These structures are called crystals
Crystal Structure / Imperfections Almost all materials crystallize when they solidify; i.e., the atoms are arranged in an ordered, repeating, 3-dimensional pattern. These structures are called crystals
Chapter Outline. How do atoms arrange themselves to form solids?
 Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Simple cubic Face-centered cubic Body-centered cubic Hexagonal close-packed
Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Simple cubic Face-centered cubic Body-centered cubic Hexagonal close-packed
We shall first regard the dense sphere packing model. 1.1. Draw a two dimensional pattern of dense packing spheres. Identify the twodimensional
 Set 3: Task 1 and 2 considers many of the examples that are given in the compendium. Crystal structures derived from sphere packing models may be used to describe metals (see task 2), ionical compounds
Set 3: Task 1 and 2 considers many of the examples that are given in the compendium. Crystal structures derived from sphere packing models may be used to describe metals (see task 2), ionical compounds
X-ray diffraction techniques for thin films
 X-ray diffraction techniques for thin films Rigaku Corporation Application Laboratory Takayuki Konya 1 Today s contents (PM) Introduction X-ray diffraction method Out-of-Plane In-Plane Pole figure Reciprocal
X-ray diffraction techniques for thin films Rigaku Corporation Application Laboratory Takayuki Konya 1 Today s contents (PM) Introduction X-ray diffraction method Out-of-Plane In-Plane Pole figure Reciprocal
EXPERIMENT O-6. Michelson Interferometer. Abstract. References. Pre-Lab
 EXPERIMENT O-6 Michelson Interferometer Abstract A Michelson interferometer, constructed by the student, is used to measure the wavelength of He-Ne laser light and the index of refraction of a flat transparent
EXPERIMENT O-6 Michelson Interferometer Abstract A Michelson interferometer, constructed by the student, is used to measure the wavelength of He-Ne laser light and the index of refraction of a flat transparent
Phase determination methods in macromolecular X- ray Crystallography
 Phase determination methods in macromolecular X- ray Crystallography Importance of protein structure determination: Proteins are the life machinery and are very essential for the various functions in the
Phase determination methods in macromolecular X- ray Crystallography Importance of protein structure determination: Proteins are the life machinery and are very essential for the various functions in the
Interference. Physics 102 Workshop #3. General Instructions
 Interference Physics 102 Workshop #3 Name: Lab Partner(s): Instructor: Time of Workshop: General Instructions Workshop exercises are to be carried out in groups of three. One report per group is due by
Interference Physics 102 Workshop #3 Name: Lab Partner(s): Instructor: Time of Workshop: General Instructions Workshop exercises are to be carried out in groups of three. One report per group is due by
Simple vs. True. Simple vs. True. Calculating Empirical and Molecular Formulas
 Calculating Empirical and Molecular Formulas Formula writing is a key component for success in chemistry. How do scientists really know what the true formula for a compound might be? In this lesson we
Calculating Empirical and Molecular Formulas Formula writing is a key component for success in chemistry. How do scientists really know what the true formula for a compound might be? In this lesson we
Modelling Compounds. 242 MHR Unit 2 Atoms, Elements, and Compounds
 6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
CRYSTALLINE SOLIDS IN 3D
 CRYSTALLINE SOLIDS IN 3D Andrew Baczewski PHY 491, October 7th, 2011 OVERVIEW First - are there any questions from the previous lecture? Today, we will answer the following questions: Why should we care
CRYSTALLINE SOLIDS IN 3D Andrew Baczewski PHY 491, October 7th, 2011 OVERVIEW First - are there any questions from the previous lecture? Today, we will answer the following questions: Why should we care
Atoms, Ions and Molecules The Building Blocks of Matter
 Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
Atoms, Ions and Molecules The Building Blocks of Matter Chapter 2 1 Chapter Outline 2.1 The Rutherford Model of Atomic Structure 2.2 Nuclides and Their Symbols 2.3 Navigating the Periodic Table 2.4 The
The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C. = 2(sphere volume) = 2 = V C = 4R
 3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
Austin Peay State University Department of Chemistry Chem 1111. The Use of the Spectrophotometer and Beer's Law
 Purpose To become familiar with using a spectrophotometer and gain an understanding of Beer s law and it s relationship to solution concentration. Introduction Scientists use many methods to determine
Purpose To become familiar with using a spectrophotometer and gain an understanding of Beer s law and it s relationship to solution concentration. Introduction Scientists use many methods to determine
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
X-Ray Diffraction: A Comprehensive Explanation for Multipurpose Research
 X-Ray Diffraction: A Comprehensive Explanation for Multipurpose Research Adree Khondker 1, Shakir Lakhani 2 1 Westdale S.S., Hamilton, ON, Canada 2 Upper Canada College, Toronto, ON, Canada Abstract: The
X-Ray Diffraction: A Comprehensive Explanation for Multipurpose Research Adree Khondker 1, Shakir Lakhani 2 1 Westdale S.S., Hamilton, ON, Canada 2 Upper Canada College, Toronto, ON, Canada Abstract: The
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Chapters 2 and 6 in Waseda. Lesson 8 Lattice Planes and Directions. Suggested Reading
 Analytical Methods for Materials Chapters 2 and 6 in Waseda Lesson 8 Lattice Planes and Directions Suggested Reading 192 Directions and Miller Indices Draw vector and define the tail as the origin. z Determine
Analytical Methods for Materials Chapters 2 and 6 in Waseda Lesson 8 Lattice Planes and Directions Suggested Reading 192 Directions and Miller Indices Draw vector and define the tail as the origin. z Determine
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS
 PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
The Mole. 6.022 x 10 23
 The Mole 6.022 x 10 23 Background: atomic masses Look at the atomic masses on the periodic table. What do these represent? E.g. the atomic mass of Carbon is 12.01 (atomic # is 6) We know there are 6 protons
The Mole 6.022 x 10 23 Background: atomic masses Look at the atomic masses on the periodic table. What do these represent? E.g. the atomic mass of Carbon is 12.01 (atomic # is 6) We know there are 6 protons
FRICTION, WORK, AND THE INCLINED PLANE
 FRICTION, WORK, AND THE INCLINED PLANE Objective: To measure the coefficient of static and inetic friction between a bloc and an inclined plane and to examine the relationship between the plane s angle
FRICTION, WORK, AND THE INCLINED PLANE Objective: To measure the coefficient of static and inetic friction between a bloc and an inclined plane and to examine the relationship between the plane s angle
6) How wide must a narrow slit be if the first diffraction minimum occurs at ±12 with laser light of 633 nm?
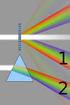 Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
Test IV Name 1) In a single slit diffraction experiment, the width of the slit is 3.1 10-5 m and the distance from the slit to the screen is 2.2 m. If the beam of light of wavelength 600 nm passes through
Sample Exercise 12.1 Calculating Packing Efficiency
 Sample Exercise 12.1 Calculating Packing Efficiency It is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is the fraction of space in a crystal
Sample Exercise 12.1 Calculating Packing Efficiency It is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is the fraction of space in a crystal
Chapter 3: Structure of Metals and Ceramics. Chapter 3: Structure of Metals and Ceramics. Learning Objective
 Chapter 3: Structure of Metals and Ceramics Chapter 3: Structure of Metals and Ceramics Goals Define basic terms and give examples of each: Lattice Basis Atoms (Decorations or Motifs) Crystal Structure
Chapter 3: Structure of Metals and Ceramics Chapter 3: Structure of Metals and Ceramics Goals Define basic terms and give examples of each: Lattice Basis Atoms (Decorations or Motifs) Crystal Structure
EXPERIMENT 11 UV/VIS Spectroscopy and Spectrophotometry: Spectrophotometric Analysis of Potassium Permanganate Solutions.
 EXPERIMENT 11 UV/VIS Spectroscopy and Spectrophotometry: Spectrophotometric Analysis of Potassium Permanganate Solutions. Outcomes After completing this experiment, the student should be able to: 1. Prepare
EXPERIMENT 11 UV/VIS Spectroscopy and Spectrophotometry: Spectrophotometric Analysis of Potassium Permanganate Solutions. Outcomes After completing this experiment, the student should be able to: 1. Prepare
0 Introduction to Data Analysis Using an Excel Spreadsheet
 Experiment 0 Introduction to Data Analysis Using an Excel Spreadsheet I. Purpose The purpose of this introductory lab is to teach you a few basic things about how to use an EXCEL 2010 spreadsheet to do
Experiment 0 Introduction to Data Analysis Using an Excel Spreadsheet I. Purpose The purpose of this introductory lab is to teach you a few basic things about how to use an EXCEL 2010 spreadsheet to do
Laue lens for Nuclear Medicine
 Laue lens for Nuclear Medicine PhD in Physics Gianfranco Paternò Ferrara, 6-11-013 Supervisor: prof. Vincenzo Guidi Sensors and Semiconductors Lab, Department of Physics and Earth Science, University of
Laue lens for Nuclear Medicine PhD in Physics Gianfranco Paternò Ferrara, 6-11-013 Supervisor: prof. Vincenzo Guidi Sensors and Semiconductors Lab, Department of Physics and Earth Science, University of
Chapter 2: Crystal Structures and Symmetry
 Chapter 2: Crystal Structures and Symmetry Laue, ravais December 28, 2001 Contents 1 Lattice Types and Symmetry 3 1.1 Two-Dimensional Lattices................. 3 1.2 Three-Dimensional Lattices................
Chapter 2: Crystal Structures and Symmetry Laue, ravais December 28, 2001 Contents 1 Lattice Types and Symmetry 3 1.1 Two-Dimensional Lattices................. 3 1.2 Three-Dimensional Lattices................
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
Paper 1 (7404/1): Inorganic and Physical Chemistry Mark scheme
 AQA Qualifications AS Chemistry Paper (7404/): Inorganic and Physical Chemistry Mark scheme 7404 Specimen paper Version 0.6 MARK SCHEME AS Chemistry Specimen paper Section A 0. s 2 2s 2 2p 6 3s 2 3p 6
AQA Qualifications AS Chemistry Paper (7404/): Inorganic and Physical Chemistry Mark scheme 7404 Specimen paper Version 0.6 MARK SCHEME AS Chemistry Specimen paper Section A 0. s 2 2s 2 2p 6 3s 2 3p 6
Group Theory and Chemistry
 Group Theory and Chemistry Outline: Raman and infra-red spectroscopy Symmetry operations Point Groups and Schoenflies symbols Function space and matrix representation Reducible and irreducible representation
Group Theory and Chemistry Outline: Raman and infra-red spectroscopy Symmetry operations Point Groups and Schoenflies symbols Function space and matrix representation Reducible and irreducible representation
Math 215 HW #6 Solutions
 Math 5 HW #6 Solutions Problem 34 Show that x y is orthogonal to x + y if and only if x = y Proof First, suppose x y is orthogonal to x + y Then since x, y = y, x In other words, = x y, x + y = (x y) T
Math 5 HW #6 Solutions Problem 34 Show that x y is orthogonal to x + y if and only if x = y Proof First, suppose x y is orthogonal to x + y Then since x, y = y, x In other words, = x y, x + y = (x y) T
For example, estimate the population of the United States as 3 times 10⁸ and the
 CCSS: Mathematics The Number System CCSS: Grade 8 8.NS.A. Know that there are numbers that are not rational, and approximate them by rational numbers. 8.NS.A.1. Understand informally that every number
CCSS: Mathematics The Number System CCSS: Grade 8 8.NS.A. Know that there are numbers that are not rational, and approximate them by rational numbers. 8.NS.A.1. Understand informally that every number
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Waves - Transverse and Longitudinal Waves
 Waves - Transverse and Longitudinal Waves wave may be defined as a periodic disturbance in a medium that carries energy from one point to another. ll waves require a source and a medium of propagation.
Waves - Transverse and Longitudinal Waves wave may be defined as a periodic disturbance in a medium that carries energy from one point to another. ll waves require a source and a medium of propagation.
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL (Student Instructions) Determination of the Formula of a Hydrate A Greener Approach Objectives To experimentally determine the formula of a hydrate salt. To learn to think in terms
SUPPLEMENTARY MATERIAL (Student Instructions) Determination of the Formula of a Hydrate A Greener Approach Objectives To experimentally determine the formula of a hydrate salt. To learn to think in terms
Section 1.1. Introduction to R n
 The Calculus of Functions of Several Variables Section. Introduction to R n Calculus is the study of functional relationships and how related quantities change with each other. In your first exposure to
The Calculus of Functions of Several Variables Section. Introduction to R n Calculus is the study of functional relationships and how related quantities change with each other. In your first exposure to
Below is a very brief tutorial on the basic capabilities of Excel. Refer to the Excel help files for more information.
 Excel Tutorial Below is a very brief tutorial on the basic capabilities of Excel. Refer to the Excel help files for more information. Working with Data Entering and Formatting Data Before entering data
Excel Tutorial Below is a very brief tutorial on the basic capabilities of Excel. Refer to the Excel help files for more information. Working with Data Entering and Formatting Data Before entering data
Sample Exercise 8.1 Magnitudes of Lattice Energies
 Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the ionic compounds NaF, CsI, and CaO in order of increasing lattice energy. Analyze From the formulas for three
Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the ionic compounds NaF, CsI, and CaO in order of increasing lattice energy. Analyze From the formulas for three
Chapter 8 How to Do Chemical Calculations
 Chapter 8 How to Do Chemical Calculations Chemistry is both a qualitative and a quantitative science. In the laboratory, it is important to be able to measure quantities of chemical substances and, as
Chapter 8 How to Do Chemical Calculations Chemistry is both a qualitative and a quantitative science. In the laboratory, it is important to be able to measure quantities of chemical substances and, as
CHAPTER 6 Chemical Bonding
 CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
O6: The Diffraction Grating Spectrometer
 2B30: PRACTICAL ASTROPHYSICS FORMAL REPORT: O6: The Diffraction Grating Spectrometer Adam Hill Lab partner: G. Evans Tutor: Dr. Peter Storey 1 Abstract The calibration of a diffraction grating spectrometer
2B30: PRACTICAL ASTROPHYSICS FORMAL REPORT: O6: The Diffraction Grating Spectrometer Adam Hill Lab partner: G. Evans Tutor: Dr. Peter Storey 1 Abstract The calibration of a diffraction grating spectrometer
The Mole Concept. The Mole. Masses of molecules
 The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
X-ray Powder Diffraction Pattern Indexing for Pharmaceutical Applications
 The published version of this manuscript may be found at the following webpage: http://www.pharmtech.com/pharmtech/peer-reviewed+research/x-ray-powder-diffraction-pattern-indexing-for- Phar/ArticleStandard/Article/detail/800851
The published version of this manuscript may be found at the following webpage: http://www.pharmtech.com/pharmtech/peer-reviewed+research/x-ray-powder-diffraction-pattern-indexing-for- Phar/ArticleStandard/Article/detail/800851
Indiana's Academic Standards 2010 ICP Indiana's Academic Standards 2016 ICP. map) that describe the relationship acceleration, velocity and distance.
 .1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
.1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
ANSWER KEY. Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take!
 ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
Holography 1 HOLOGRAPHY
 Holography 1 HOLOGRAPHY Introduction and Background The aesthetic appeal and commercial usefulness of holography are both related to the ability of a hologram to store a three-dimensional image. Unlike
Holography 1 HOLOGRAPHY Introduction and Background The aesthetic appeal and commercial usefulness of holography are both related to the ability of a hologram to store a three-dimensional image. Unlike
PHYA2. General Certificate of Education Advanced Subsidiary Examination June 2010. Mechanics, Materials and Waves
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 2 For this paper you must have: a ruler a calculator a Data and Formulae Booklet.
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 2 For this paper you must have: a ruler a calculator a Data and Formulae Booklet.
Diffraction Course Series 2015
 Diffraction Course Series 2015 Mark Wainwright Analytical Centre Kensington Campus, Chemical Sciences Building F10, Room G37 The Mark Wainwright Analytical Centre is offering a new series of courses covering
Diffraction Course Series 2015 Mark Wainwright Analytical Centre Kensington Campus, Chemical Sciences Building F10, Room G37 The Mark Wainwright Analytical Centre is offering a new series of courses covering
FURTHER VECTORS (MEI)
 Mathematics Revision Guides Further Vectors (MEI) (column notation) Page of MK HOME TUITION Mathematics Revision Guides Level: AS / A Level - MEI OCR MEI: C FURTHER VECTORS (MEI) Version : Date: -9-7 Mathematics
Mathematics Revision Guides Further Vectors (MEI) (column notation) Page of MK HOME TUITION Mathematics Revision Guides Level: AS / A Level - MEI OCR MEI: C FURTHER VECTORS (MEI) Version : Date: -9-7 Mathematics
Scientific Graphing in Excel 2010
 Scientific Graphing in Excel 2010 When you start Excel, you will see the screen below. Various parts of the display are labelled in red, with arrows, to define the terms used in the remainder of this overview.
Scientific Graphing in Excel 2010 When you start Excel, you will see the screen below. Various parts of the display are labelled in red, with arrows, to define the terms used in the remainder of this overview.
Diffraction of Laser Light
 Diffraction of Laser Light No Prelab Introduction The laser is a unique light source because its light is coherent and monochromatic. Coherent light is made up of waves, which are all in phase. Monochromatic
Diffraction of Laser Light No Prelab Introduction The laser is a unique light source because its light is coherent and monochromatic. Coherent light is made up of waves, which are all in phase. Monochromatic
Thermodynamics of Crystal Formation
 Thermodynamics of Crystal Formation! All stable ionic crystals have negative standard enthalpies of formation, ΔH o f, and negative standard free energies of formation, ΔG o f. Na(s) + ½ Cl 2 (g) NaCl(s)
Thermodynamics of Crystal Formation! All stable ionic crystals have negative standard enthalpies of formation, ΔH o f, and negative standard free energies of formation, ΔG o f. Na(s) + ½ Cl 2 (g) NaCl(s)
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
In mathematics, there are four attainment targets: using and applying mathematics; number and algebra; shape, space and measures, and handling data.
 MATHEMATICS: THE LEVEL DESCRIPTIONS In mathematics, there are four attainment targets: using and applying mathematics; number and algebra; shape, space and measures, and handling data. Attainment target
MATHEMATICS: THE LEVEL DESCRIPTIONS In mathematics, there are four attainment targets: using and applying mathematics; number and algebra; shape, space and measures, and handling data. Attainment target
Chapter 8 Concepts of Chemical Bonding
 Chapter 8 Concepts of Chemical Bonding Chemical Bonds Three types: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other atoms Ionic Bonding
Chapter 8 Concepts of Chemical Bonding Chemical Bonds Three types: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other atoms Ionic Bonding
Calculating Atoms, Ions, or Molecules Using Moles
 TEKS REVIEW 8B Calculating Atoms, Ions, or Molecules Using Moles TEKS 8B READINESS Use the mole concept to calculate the number of atoms, ions, or molecules in a sample TEKS_TXT of material. Vocabulary
TEKS REVIEW 8B Calculating Atoms, Ions, or Molecules Using Moles TEKS 8B READINESS Use the mole concept to calculate the number of atoms, ions, or molecules in a sample TEKS_TXT of material. Vocabulary
Guide to Understanding X-ray Crystallography
 Guide to Understanding X-ray Crystallography What is X-ray Crystallography and why do I need to learn it? X-ray Crystallography is a scientific method of determining the precise positions/arrangements
Guide to Understanding X-ray Crystallography What is X-ray Crystallography and why do I need to learn it? X-ray Crystallography is a scientific method of determining the precise positions/arrangements
One problem often faced in qualitative analysis is to test for one ion in a
 Chemistry 112 Laboratory: Silver Group Analysis Page 11 ANALYSIS OF THE SILVER GROUP CATIONS Ag + Pb Analysis of a Mixture of Cations One problem often faced in qualitative analysis is to test for one
Chemistry 112 Laboratory: Silver Group Analysis Page 11 ANALYSIS OF THE SILVER GROUP CATIONS Ag + Pb Analysis of a Mixture of Cations One problem often faced in qualitative analysis is to test for one
Ionic Bonding Pauling s Rules and the Bond Valence Method
 Ionic Bonding Pauling s Rules and the Bond Valence Method Chemistry 754 Solid State Chemistry Dr. Patrick Woodward Lecture #14 Pauling Rules for Ionic Structures Linus Pauling,, J. Amer. Chem. Soc. 51,,
Ionic Bonding Pauling s Rules and the Bond Valence Method Chemistry 754 Solid State Chemistry Dr. Patrick Woodward Lecture #14 Pauling Rules for Ionic Structures Linus Pauling,, J. Amer. Chem. Soc. 51,,
TWO-DIMENSIONAL TRANSFORMATION
 CHAPTER 2 TWO-DIMENSIONAL TRANSFORMATION 2.1 Introduction As stated earlier, Computer Aided Design consists of three components, namely, Design (Geometric Modeling), Analysis (FEA, etc), and Visualization
CHAPTER 2 TWO-DIMENSIONAL TRANSFORMATION 2.1 Introduction As stated earlier, Computer Aided Design consists of three components, namely, Design (Geometric Modeling), Analysis (FEA, etc), and Visualization
X-ray diffraction in polymer science
 X-ray diffraction in polymer science 1) Identification of semicrystalline polymers and Recognition of crystalline phases (polymorphism) of polymers 2)Polymers are never 100% crystalline. XRD is a primary
X-ray diffraction in polymer science 1) Identification of semicrystalline polymers and Recognition of crystalline phases (polymorphism) of polymers 2)Polymers are never 100% crystalline. XRD is a primary
H 2 + O 2 H 2 O. - Note there is not enough hydrogen to react with oxygen - It is necessary to balance equation.
 CEMICAL REACTIONS 1 ydrogen + Oxygen Water 2 + O 2 2 O reactants product(s) reactant substance before chemical change product substance after chemical change Conservation of Mass During a chemical reaction,
CEMICAL REACTIONS 1 ydrogen + Oxygen Water 2 + O 2 2 O reactants product(s) reactant substance before chemical change product substance after chemical change Conservation of Mass During a chemical reaction,
CHEM 51LB: EXPERIMENT 5 SPECTROSCOPIC METHODS: INFRARED AND NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 CHEM 51LB: EXPERIMENT 5 SPECTROSCOPIC METHODS: INFRARED AND NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY REACTIONS: None TECHNIQUES: IR, NMR Infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy are
CHEM 51LB: EXPERIMENT 5 SPECTROSCOPIC METHODS: INFRARED AND NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY REACTIONS: None TECHNIQUES: IR, NMR Infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy are
Powder diffraction and synchrotron radiation
 Powder diffraction and synchrotron radiation Gilberto Artioli Dip. Geoscienze UNIPD CIRCe Center for Cement Materials single xl diffraction powder diffraction Ideal powder Powder averaging Textured sample
Powder diffraction and synchrotron radiation Gilberto Artioli Dip. Geoscienze UNIPD CIRCe Center for Cement Materials single xl diffraction powder diffraction Ideal powder Powder averaging Textured sample
Find a pair of elements in the periodic table with atomic numbers less than 20 that are an exception to the original periodic law.
 Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Symmetry and group theory
 Symmetry and group theory or How to Describe the Shape of a Molecule with two or three letters Natural symmetry in plants Symmetry in animals 1 Symmetry in the human body The platonic solids Symmetry in
Symmetry and group theory or How to Describe the Shape of a Molecule with two or three letters Natural symmetry in plants Symmetry in animals 1 Symmetry in the human body The platonic solids Symmetry in
LINES AND PLANES IN R 3
 LINES AND PLANES IN R 3 In this handout we will summarize the properties of the dot product and cross product and use them to present arious descriptions of lines and planes in three dimensional space.
LINES AND PLANES IN R 3 In this handout we will summarize the properties of the dot product and cross product and use them to present arious descriptions of lines and planes in three dimensional space.
Electrochemistry - ANSWERS
 Electrochemistry - ANSWERS 1. Using a table of standard electrode potentials, predict if the following reactions will occur spontaneously as written. a) Al 3+ + Ni Ni 2+ + Al Al 3+ + 3e - Al E = -1.68
Electrochemistry - ANSWERS 1. Using a table of standard electrode potentials, predict if the following reactions will occur spontaneously as written. a) Al 3+ + Ni Ni 2+ + Al Al 3+ + 3e - Al E = -1.68
Infrared Spectroscopy: Theory
 u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
Section 9.5: Equations of Lines and Planes
 Lines in 3D Space Section 9.5: Equations of Lines and Planes Practice HW from Stewart Textbook (not to hand in) p. 673 # 3-5 odd, 2-37 odd, 4, 47 Consider the line L through the point P = ( x, y, ) that
Lines in 3D Space Section 9.5: Equations of Lines and Planes Practice HW from Stewart Textbook (not to hand in) p. 673 # 3-5 odd, 2-37 odd, 4, 47 Consider the line L through the point P = ( x, y, ) that
discuss how to describe points, lines and planes in 3 space.
 Chapter 2 3 Space: lines and planes In this chapter we discuss how to describe points, lines and planes in 3 space. introduce the language of vectors. discuss various matters concerning the relative position
Chapter 2 3 Space: lines and planes In this chapter we discuss how to describe points, lines and planes in 3 space. introduce the language of vectors. discuss various matters concerning the relative position
Introduction to single crystal X-ray analysis
 Introduction to single crystal X-ray analysis I. What is X-ray crystallography? Kimiko Hasegawa* 1. Introduction All substances around us consist of atoms. The types of atoms and their three-dimensional
Introduction to single crystal X-ray analysis I. What is X-ray crystallography? Kimiko Hasegawa* 1. Introduction All substances around us consist of atoms. The types of atoms and their three-dimensional
A Guide to Acousto-Optic Modulators
 A Guide to Acousto-Optic Modulators D. J. McCarron December 7, 2007 1 Introduction Acousto-optic modulators (AOMs) are useful devices which allow the frequency, intensity and direction of a laser beam
A Guide to Acousto-Optic Modulators D. J. McCarron December 7, 2007 1 Introduction Acousto-optic modulators (AOMs) are useful devices which allow the frequency, intensity and direction of a laser beam
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
A Beer s Law Experiment
 A Beer s Law Experiment Introduction There are many ways to determine concentrations of a substance in solution. So far, the only experiences you may have are acid-base titrations or possibly determining
A Beer s Law Experiment Introduction There are many ways to determine concentrations of a substance in solution. So far, the only experiences you may have are acid-base titrations or possibly determining
F321 THE STRUCTURE OF ATOMS. ATOMS Atoms consist of a number of fundamental particles, the most important are... in the nucleus of an atom
 Atomic Structure F32 TE STRUCTURE OF ATOMS ATOMS Atoms consist of a number of fundamental particles, the most important are... Mass / kg Charge / C Relative mass Relative Charge PROTON NEUTRON ELECTRON
Atomic Structure F32 TE STRUCTURE OF ATOMS ATOMS Atoms consist of a number of fundamental particles, the most important are... Mass / kg Charge / C Relative mass Relative Charge PROTON NEUTRON ELECTRON
