III. BACKGROUND KNOWLEDGE
|
|
|
- Toby Walton
- 10 years ago
- Views:
Transcription
1 Chemical Bonds and Reactions (Water) Grade Level: 7 (Applied Technology) Presented by: James Rubright, Three Oaks Middle School, Fort Myers, Florida Length of Unit: Ten Daily Lessons and Activities I. ABSTRACT Students will be able to test and evaluate water samples for hardness and the effects it has on drinking water. Students will apply the principles of the Scientific Method. This addresses the Core Knowledge topic, Chemical Bonds and Reactions. II. OVERVIEW A. Concept Objectives 1. Students will develop an awareness of chemical bonds and reactions, and their impact on the Earth s water supply.. 2. Students will develop an awareness of the limited fresh water supply available on Earth B. Content from the Core Knowledge Sequence (List specific content from the Core Knowledge Sequence that will be covered in the unit.) 1. Chemical Bonds and Reactions 2. The water cycle (Review from Grade 4) a. Acid rain C. Skill Objectives (List specific skills to be taught in each lesson.) 1. Read physical maps. 2. Use note taking to record scientific observationsl 4. Identify the reasons and sources for water contamination 5. Apply knowledge of the scientific method. 6. Test, observe and record findings on various water samples III. BACKGROUND KNOWLEDGE A. For Teachers 1. The Tap Water Tour. 2. Environment and Ecology (Both available through Paxton Patterson Company, Chicago, IL.) B. For Students 1. Geography: Locations for fresh water on earth 2. Science: Fresh water/acid rain 3. Language Arts: Writing reports 4. Mathematics: Weight and measurement IV. RESOURCES 1. The Tap Water Tour. Activity Book Paxton-Patterson Company. 2. Environment and Ecology. Activity Book Paxton-Patterson Company. 3. Video: Down The Drain. 4. Where Does Water Come From? McArthur-Burney Falls Park publication V. LESSONS Lesson One: Introduction to tap water, tap water sources and the water cycle A. Daily Objectives: 1.Concept Objective(s) a. Students will develop an awareness of chemical bonds and reactions, and their impact on the Earth s water supply Chemical Bonds and Reactions, Grade Conference 1
2 b. Students will develop an awareness of the limited fresh water supply available on Earth 2. Lesson Content a. Acids, ph, reactions with acids and bases 3. Skill Objective(s) a. Read physical maps. b. Use note taking to record scientific observations c. Apply knowledge of the scientific method by practicing observation and reporting B. Materials 1. Individual maps, or paper to draw 2. Crayons, or markers 3. Pencils 4. Ruler 5. Notebook or journal 6. Water Facts sheet 7. Research Contract (Appendix A) 8. Water Facts (Appendix B) 9. Water Words ( Appendix C) 10. Where Do We Get Our Water? (Appendix E) 11. Pretest (Appendix S) C. Key Vocabulary 1. Drinking Water Fresh water for everyday use by all living things 2. Evaporation The change of state of water from liquid to gas, part of the hydrological cycle 3. Acid and acidic a condition that causes water to be corrosive. 4. Hardness, as in hard water, the amount of minerals found in some fresh water 5. ph a scale used to measure the content of chemicals (hardness) in water 6. Neutral a ph of 7 D. Procedures/Activities 1. Administer pretest to students 2. Watch the video Down the Drain 3. Observe, and note from the video, the concept of the water cycle 4. Observe, and note from the video, the statistics for our daily consumption of water 5. Using knowledge gained from the video have students hypothesize where used water goes E. Assessment/Evaluation 1. Review the student s journals for accurate notes 2. Students write short essays using the four vocabulary words for this lesson 3. Students will develop a chart that shows the understanding of water consumption Lesson Two: Testing for Chlorine A. Daily Objectives: 1. Concept Objective(s) a. Students will develop an awareness of chemical bonds and reactions,a nd their impact on the Earth s water supply. b. Students will develop an awareness of the limited fresh water supply available on Earth. 2. Lesson Content a. Reactions with acids and bases. 3. Skill Objectives a. Use note taking to record scientific observations. b. Apply knowledge of the scientific method by practicing observation Chemical Bonds and Reactions, Grade Conference 2
3 B. Materials 1. Chlorine bleach 2. Distilled water 4. DPD #4 Tablets 5. Sample bags 6. Pre Mixed Water Samples a. Chlorine sample #1 i. In a cup, mix one teaspoon of liquid bleach with three teaspoons of water ii. Use an eyedropper to add one drop of the solution you made in step i to one quart of distilled water. This represents the amount of chlorine found in the average drinking water b. Chlorine sample #2 i. Use an eyedropper to add ten drops of the solution in ii to one quart of distilled water c. Student Data Sheet (Appendix P) d. Scramble Game (Appendix M) C. Key Vocabulary 1. Chlorine A toxic, corrosive chemical element used in water supplies to disinfect and in bleaching applications. 2. Disinfect To remove, or reduce, harmful bacteria from areas used by, or items consumed by, living organisms. 3. Distilled the process of removing minerals and other impurities from water by evaporation. D. Procedures/Activities 1. Watch the video Down The Drain a. Instruct students to take notes about key terms. b. Instruct students to be alert for the explained water cycle, the technical term for this (hydrological cycle) and the importance of this cycle. c. Suggest that students begin to understand the importance of the theory that all the water that was on Earth when it formed is still here, and how our use is causing shortages. (Nature cannot recycle as fast as our current needs) E. Assessment/Evaluation 1. Have students write, in their journals, short essays using each vocabulary word, an essay about how we can reduce the amount of fresh water we use, and one essay describing the hydrological cycle. 2. Have students read their work product to the class. Lesson Three: Testing For Iron A. Daily Objectives 1. Concept Objective(s) a. Students will develop an awareness of chemical bonds and reactions, and their impact on the Earth s water supply. b.. 2. Lesson Content a. Oxidation b. Reactions with Acids and Bases 3. Skill Objective(s) a. Use note taking to record scientific observations. b. Apply knowledge of the scientific method. c. Test, observe and record findings on various water samples. Chemical Bonds and Reactions, Grade Conference 3
4 d. B. Materials 1. Two quart containers 2. Iron supplement tablets 3. One cup 4. Three quarts distilled water 5. ½ cup measuring cup 6. Sample bags 7. Iron LR tablets 8. Student Data Sheet (Appendix O) 9. Word Search Game (Appendix D) C. Key Vocabulary 1. React To change chemically 2. Indicator A method or chemical that will allow you see the reaction of one chemical to another. In this case, a purple reaction. D. Procedures/Activities 1. Fill one-quart container with distilled water. Leave one empty. 2. Place one iron supplement tablet in a cup; add three teaspoons (15ml) of distilled water. 3. Swirl to dissolve the tablet coating. Ask students why they think we do this. 4. Remove the now uncoated tablet into one quart, or one liter of distilled water. Mix for 30 seconds. 5. Promptly pour the solution into an empty container. Be careful not to pour in the undissolved portion of the tablet. 6. Now, pour out ½ cup of this solution into a clean one-quart container. 7. Fill this container with distilled water. 8. Hand out Iron Data sheets 9. Fill two sample bags to line C. 10. Add an Iron LR tablet to each bag. 11. Have students hypothesize what reaction they will observe. 12. Have students record their observations. 13. Have students hypothesize the result of a test on tap water. Do the experiment and compare the results to the samples. Record their observations and results. E. Assessment/Evaluation 1. Check student journals for proper entries 2. Observe student laboratory procedures 3. Have students write an essay explaining the steps taken to do this experiment and the purpose of the reactant. Lesson Four: Testing for Copper A. Daily Objectives 1. Concept Objective(s) a. To develop an awareness of chemical bonds and reactions, and their impact on our water supply. 2. Lesson Content a. Kinds of reactions 3. Skill Objective(s) a. Apply knowledge of scientific method by practicing observation and recording. b. Use note taking to record scientific observations. B. Materials 1. Copper HR tablets 2. Sample bags Chemical Bonds and Reactions, Grade Conference 4
5 3. Tap water 4. Copper Data Sheet (Appendix Q) C. Key Vocabulary 1. Acidic A corrosive solution 2. Indicator A system for measuring, observing, the condition of items. 3. Blue/green stains The result of high copper content water 4. Dissolve The ability of chemicals and minerals in water to cause pipes to dissolve. D. Procedures/Activities 1. Fill a sample bag to line C with tap water 2. Add a copper HR tablet. Close bag and shake 3. Write on the board: a. Orange no copper in the water b. Red-brown Water may taste bitter and stain c. Blue Water WILL taste bitter and stain 4. Students complete data sheet 5. Students write about the effects of excessive copper in drinking water. 6. Pour a small amount of vinegar in a jar. Place a penny in the vinegar and close the lid. Have students check the penny over the next few days. Have them record their findings. Keep the jar in a conspicuous place throughout the year for continued observation of the reaction-taking place. E. Assessment/Evaluation 1. Review student journals for accuracy and completeness. 2. Have students hypothesize about what other materials might be affected by vinegar (acid). Have them design their own experiment. Lesson Five: Testing for hardness (ph) using home water samples A. Daily Objectives: 1. Concept Objective(s) a. Students will develop an awareness of chemical bonds and reactions, and their impact on the Earth s water supply 2. Lesson Content a. ph scale b. Kinds of reactions 3. Skill Objective(s) a. Identify the reasons and sources for water contamination. b. Use note taking to record scientific observations. c. Apply knowledge of the scientific method. B. Materials 1. Wide range ph tablets 2. Sample bags 3. Baking soda 4. Vinegar 5. Aspirin 6. Tap water 7. Straws 8. Distilled water (4 Quarts) 9. Quart containers (3) 10. Data sheet (Appendix H) 11. Paper and materials for class result chart 12. Bubblegram Game (Appendix K) 13. Crossword Puzzle (Appendix N) 14. Water Pipe Diagram (Appendix G) Chemical Bonds and Reactions, Grade Conference 5
6 15. ph Scale (Appendix F) 16. Water Quality Report (Appendix I) 17. Hardness Data Sheet (Appendix J) 18. Matching Game (Appendix L) C. Key Vocabulary 1. Basic- Referring to ph of water 2. Hardness Referring to ph of water 3. Neutral Referring to the ph of water 4. Minerals- Referring to the content of minerals in water. 5. Scale- Deposits left behind by evaporated water that contained minerals D. Procedures/Activities 1. Hand out ph scale ( Alternate project from your overhead projector and have students create their own ph scale) 2. Hand out sample bags 3. Hand out ph tablets (Alternate teacher places liquid ph test material in each bag) 4. Hand out ph data sheet 5. Divide class into teams 6. Test tap water 7. Write on board a. pink ph 4 b. orange ph 5 c. yellow ph6 d. apple-green ph7 e. blue-green ph8 f. blue ph9 g. purple ph10 8. Locate results for water sample, place on chart 9. Place straw in bag, blow gently record results. Have students explain (Added carbon dioxide) Write a paragraph explaining what happened. 10. Make water samples a. Sample #1. Dissolve one teaspoon of baking soda in one quart of distilled water b. Sample #2 Mix 5 drops of vinegar in one quart of distilled water c. Sample #3 Dissolve one aspirin in one quart of distilled water 11. Have students fill sample bags to line C. Place a ph tablet in each F. Assessment/evaluation 1. Review student journals 2. Examine student s ph chart 3. Students write an essay using key vocabulary words to explain the concept of ph ranging from basic to acidic. VI. CULMINATING ACTIVITY A. Daily Objectives: 1. Concept Objective(s) a. Students will develop an awareness of chemical bonds and reactions, and their impact on the Earth s water supply b. Students will develop an awareness of the limited fresh water supply available on Earth. 2. Lesson Content a. Chemical bonds and reactions b. The water cycle (review from Grade 4) 3. Skill Objective(s) a. Read physical maps. Chemical Bonds and Reactions, Grade Conference 6
7 b. Use note taking to record scientific observations. c. Identify the reasons and sources for water contamination d. Apply knowledge of the scientific method. e. Test, observe and record findings on various water samples. B. Materials: (To be included in Final Report ) 1. Student designed cover sheet 2. Table of Contents 3. Daily student notes organized by lesson 4. All hand out sheets organized by lesson. These should include: a. Appendix A: Research Contract b. Appendix B: Water Facts c. Appendix C: Water Words d. Appendix D: Word Search e. Appendix E: Where Do We Get Our Water? f. Appendix F: ph Scale g. Appendix G: Water Pipe Diagram h. Appendix H: Data Sheet (ph Test) i. Appendix I: Water Quality Report (For ph) j. Appendix J: Data Sheet (Hardness) k. Appendix K: Bubblegram Game l. Appendix L: Matching Game 5. Pre and post test paper 6. Final essay describing the water cycle and why, if there is the same amount of water on Earth since the beginning of time, there is a danger of serious shortages. C. Key Vocabulary 1. no new vocabulary D. Procedures/Activities 1. Administer post test 2. Pass out Where Does The Water Come From? Have students read this to reinforce understanding of the water cycle. 3 Help students set up their journals for final publication. This includes instructions for the cover page, table of contents, graphics, work sheets, test papers and essay. VII. HANDOUTS/WORKSHEETS A. Research Contract B. Water Facts C. Water Words (to be used as needed) D. Word Search E. Where Do We Get Our Water? F. ph Scale G. Water Pipe Diagram H. Data Sheet ph I. Water Quality Report J. Data Sheet Hardness K. Bubblegram Game L. Matching Game M. Scramble Game N. Crossword Game O. Data Sheet Iron P. Data Sheet Chloring Q. Data Sheet Copper R. Shopping List Chemical Bonds and Reactions, Grade Conference 7
8 S. Pre/Post Test T. Pre/Post Test Answer Key VIII. BIBLIOGRAPHY A. Where Does The Water Come From? Newspaper article from McArthur-Burney Falls Memorial State Park publication, fifth edition Published by McArthur- Burney Falls Interpretive Association, P.O. Box 777, Burney CA B. Publication rights for all other materials used in this unit are owned by the Paxton- Patterson Company, Chicago, IL. Chemical Bonds and Reactions, Grade Conference 8
9 Chemical Bonds and Reactions, Grade Conference 9
10 Chemical Bonds and Reactions, Grade Conference 10
11 Chemical Bonds and Reactions, Grade Conference 11
12 Chemical Bonds and Reactions, Grade Conference 12
13 Chemical Bonds and Reactions, Grade Conference 13
14 Chemical Bonds and Reactions, Grade Conference 14
15 Chemical Bonds and Reactions, Grade Conference 15
16 Chemical Bonds and Reactions, Grade Conference 16
17 Chemical Bonds and Reactions, Grade Conference 17
18 Chemical Bonds and Reactions, Grade Conference 18
19 Chemical Bonds and Reactions, Grade Conference 19
20 Chemical Bonds and Reactions, Grade Conference 20
21 Chemical Bonds and Reactions, Grade Conference 21
22 Chemical Bonds and Reactions, Grade Conference 22
23 Chemical Bonds and Reactions, Grade Conference 23
24 Chemical Bonds and Reactions, Grade Conference 24
25 Chemical Bonds and Reactions, Grade Conference 25
26 Appendix R CHEMICAL BONDS AND REACTIONS James Rubright Presenter [email protected] 941/ Shopping List Supplies Needed 7 quart containers Down The Drain VHS video tape 1 box plastic drinking straws 1 eye dropper 3 quarts distilled water water sample bags teaspoon 1 box baking soda 1 bottle chlorine bleach 1 bottle white vinegar 1 bottle liquid soap ph wide range tablets iron LR tablets DPD tablets Copper tablets hardness tablets Pre Mix Solutions Label quart bottles: ph sample #1 ph sample #2 ph sample #3 Chemical Bonds and Reactions, Grade Conference 26
27 Appendix S Pre/ Post Test For Chemical Bonds and Reaction Name: Date: Period 1. T F 2/3 of your body is water 2. T F It takes 10 gallons on water, on average, to take a shower 3. T F Three states of water are liquid, solid and gas 4. T F Without water, the planet Earth would become too hot and too cold 5. T F About half of the water moving through the water cycle is drinkable 6. T F About half of all things that pollute water, seep down through the ground water supply 7. T F We personally average about 50 gallons a day in use of water 8. T F In an average American city, about 20% of the usable water supply is wasted due to leaks 9. T F Evaporation is the process of water changing from liquid to solid 10. T F Condensation is the process of water turning from gas to a liquid 11. T F Ground Water is water that has gone through a grinding process 12. T F By blowing into a water sample the carbon dioxide you exhale will change the ph of the sample to being more acidic 13. T F Chlorine is added to our drinking water to help with the color 14. T F Filtration of water removes impurities 15. T F A reacted sample refers to a visible change that has taken place in a sample Chemical Bonds and Reactions, Grade Conference 27
28 Appendix T Answer Sheet For Pre and Post Test For Chemical Bonds and Reactions 1. True 2. False, 50 gallons 3. True 4. True 5. False About 1% 6. True 7. False 120 gallons 8. True 9. False Changing liquid to gas 10. True 11. False It is the water that is returned to Earth that seeps into the ground for our reuse 12. True 13. False It is added to kill unwanted bacteria 14. True 15. True Chemical Bonds and Reactions, Grade Conference 28
Lesson Plan: How Do We Know What is Healthy Water?
 Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
CLEANING WATER. Student Section
 National Aeronautics and Space Administration CLEANING WATER Student Section Student Name This lesson challenges you to create and test a water filtration system. During this lesson, you will design and
National Aeronautics and Space Administration CLEANING WATER Student Section Student Name This lesson challenges you to create and test a water filtration system. During this lesson, you will design and
Physical and Chemical Changes
 Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 [email protected] Eighth Grade Integrated Science Standard I: Students will understand the
Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 [email protected] Eighth Grade Integrated Science Standard I: Students will understand the
Acids and Bases. AND a widemouth container of the following solids:
 Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Corrosivity of Water Supplies
 WD-DWGB-3-4 2009 Corrosivity of Water Supplies What is meant by Corrosivity? Corrosive water can be defined as a condition of water quality which will dissolve metals from metallic plumbing at an excessive
WD-DWGB-3-4 2009 Corrosivity of Water Supplies What is meant by Corrosivity? Corrosive water can be defined as a condition of water quality which will dissolve metals from metallic plumbing at an excessive
Household Acids and Bases
 Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Wherever chemical solutions are involved, ph matters. Some
 47 Acids, Bases, and the ph Scale r e a d i n g Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
47 Acids, Bases, and the ph Scale r e a d i n g Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
Dry Ice Color Show Dry Ice Demonstrations
 elearning 2009 Introduction Dry Ice Color Show Dry Ice Demonstrations Publication No. 95016 Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately
elearning 2009 Introduction Dry Ice Color Show Dry Ice Demonstrations Publication No. 95016 Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately
Chemquest: Physical Changes or Chemical Reactions
 Chemquest: Physical Changes or Chemical Reactions Erik Misner May 9, 2005 Background: This lesson is designed to be an interactive and fun way to learn the difference between physical changes and chemical
Chemquest: Physical Changes or Chemical Reactions Erik Misner May 9, 2005 Background: This lesson is designed to be an interactive and fun way to learn the difference between physical changes and chemical
ACIDS AND BASES SAFETY PRECAUTIONS
 ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
II. III. IV. RESOURCES A. Best Buy Bargain Books, Social Studies K-1 by Frank Schaffer ISBN 0867344512
 What on Earth is...a Map?...a Globe? Grade Level: Kindergarten Written by: Lisa Fagan Cross County Elementary, Teressa Davis Vanndale Elementary, Amy Searcy, and Cheryl Green Hickory Ridge Elementary,
What on Earth is...a Map?...a Globe? Grade Level: Kindergarten Written by: Lisa Fagan Cross County Elementary, Teressa Davis Vanndale Elementary, Amy Searcy, and Cheryl Green Hickory Ridge Elementary,
Neutralizing an Acid and a Base
 Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Letter to the Student... 5 Test-Taking Checklist... 6 Next Generation Sunshine State Standards Correlation Chart... 7
 Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE. Acids and Bases. Fall 2012
 VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
Acids & Bases Around the House Use a ph indicator to find acids and bases
 Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
NAILING RUST. Jim Lowry, fourth-grade teacher Webster Elementary School Pemberville, OH
 Jim Lowry, fourth-grade teacher Webster Elementary School Pemberville, OH NAILING RUST Lesson Summary for Grades 3 8 The students are grouped in pairs and go on a walking tour of the school grounds. They
Jim Lowry, fourth-grade teacher Webster Elementary School Pemberville, OH NAILING RUST Lesson Summary for Grades 3 8 The students are grouped in pairs and go on a walking tour of the school grounds. They
Properties of Acids and Bases
 Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
FIRST GRADE CHEMISTRY
 FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
Water Treatment Filtration Lab. discharged into an aquatic ecosystem? We had to build a water filtration system with
 Water Treatment Filtration Lab Brandon Lyons P.5 APES Abstract: How could polluted water be remediated so that it could support life when it is discharged into an aquatic ecosystem? We had to build a water
Water Treatment Filtration Lab Brandon Lyons P.5 APES Abstract: How could polluted water be remediated so that it could support life when it is discharged into an aquatic ecosystem? We had to build a water
Review and apply Investigation 5. Let s review Pages 311-312
 Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
Syllabus OC18 Use litmus or a universal indicator to test a variety of solutions, and classify these as acidic, basic or neutral
 Chemistry: 9. Acids and Bases Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC18 Use litmus or a universal indicator to test a variety
Chemistry: 9. Acids and Bases Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC18 Use litmus or a universal indicator to test a variety
ph Measurements of Common Substances
 Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Reaction in a Bag. Scientific Method Demonstrations
 elearning 2009 Introduction Reaction in a Bag Scientific Method Demonstrations Publication No. 91419 Careful observation is the foundation of science, leading to questions about what we have observed how,
elearning 2009 Introduction Reaction in a Bag Scientific Method Demonstrations Publication No. 91419 Careful observation is the foundation of science, leading to questions about what we have observed how,
Lesson 4. Temperature change
 54 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
54 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction
 Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
Kool Demo for Acid-Base Reactions
 Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
WHAT S NEW, CO? Thanks for the opportunity to work with your students. Our goal is to teach developmentally TEACHER S GUIDE
 TEACHER S GUIDE WHAT S NEW, CO? GET TO KNOW A CHEMICAL REACTION 2 Thanks for the opportunity to work with your students. Our goal is to teach developmentally appropriate chemistry concepts that support
TEACHER S GUIDE WHAT S NEW, CO? GET TO KNOW A CHEMICAL REACTION 2 Thanks for the opportunity to work with your students. Our goal is to teach developmentally appropriate chemistry concepts that support
Experiment 16-Acids, Bases and ph
 Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
III. BACKGROUND KNOWLEDGE
 Chemistry: Phase Changes: Heat and Matter Grade Level: 5 Presented by: Gail Scott-Taylor, R.N. Harris Integrated Arts/Core Knowledge School Length of Unit: Seven lessons I. ABSTRACT Roll up your sleeves
Chemistry: Phase Changes: Heat and Matter Grade Level: 5 Presented by: Gail Scott-Taylor, R.N. Harris Integrated Arts/Core Knowledge School Length of Unit: Seven lessons I. ABSTRACT Roll up your sleeves
Drain to Drinking Water
 Drain to Drinking Water Adapted from Project WET Curriculum and Activity Guide Subject: Science, Social Studies Target Grades: 6-8 Duration: one class period Materials Per group (2-3 students) 1 set of
Drain to Drinking Water Adapted from Project WET Curriculum and Activity Guide Subject: Science, Social Studies Target Grades: 6-8 Duration: one class period Materials Per group (2-3 students) 1 set of
Water Treatment & Purification Chemicals
 Lime-Out Extra Water Treatment Cleaning Chemicals Dissolves tough lime, calcium & rust stains Non-abrasive thick jelly like liquid clings to vertical surfaces Cleans tubs, sinks, shower doors, dishwashers
Lime-Out Extra Water Treatment Cleaning Chemicals Dissolves tough lime, calcium & rust stains Non-abrasive thick jelly like liquid clings to vertical surfaces Cleans tubs, sinks, shower doors, dishwashers
Introduction. ph = log [H + ]
![Introduction. ph = log [H + ] Introduction. ph = log [H + ]](/thumbs/40/21282159.jpg) Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
Water Cycle. DELTA SCIENCE READER Overview... 123 Before Reading... 124 Guide the Reading... 125 After Reading... 130
 Water Cycle T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and Materials
Water Cycle T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and Materials
Leavener Lineup. Getting started. How do we use chemical reactions in the kitchen? Hands-on experiment. Year levels 4 5. Curriculum Links.
 rise and Shine: what Makes Bread Rise? Lesson 2 Leavener Lineup Year levels 4 5 Curriculum Links Science Science knowledge helps people to understand the effect of their actions (Yr 4, ACSHE062). Solids,
rise and Shine: what Makes Bread Rise? Lesson 2 Leavener Lineup Year levels 4 5 Curriculum Links Science Science knowledge helps people to understand the effect of their actions (Yr 4, ACSHE062). Solids,
Lesson Plan: How Do We Clean Polluted Water?
 Lesson Plan: How Do We Clean Polluted Water? Oil Spill Cleanup / Phosphate Cleanup / Groundwater Contamination / Water Treatment Simulation Estimated Time: 2-4 days State Standards taught and addressed
Lesson Plan: How Do We Clean Polluted Water? Oil Spill Cleanup / Phosphate Cleanup / Groundwater Contamination / Water Treatment Simulation Estimated Time: 2-4 days State Standards taught and addressed
Balloon Inside a Bottle
 Balloon Inside a Bottle What is Needed * One small party balloon * One small bottle. A 16 ounce pop bottle works well. What to Do Put approximately 1 tablespoon of water into the empty pop bottle. Then
Balloon Inside a Bottle What is Needed * One small party balloon * One small bottle. A 16 ounce pop bottle works well. What to Do Put approximately 1 tablespoon of water into the empty pop bottle. Then
Solids, Liquids, and Gases
 Solids, Liquids, and Gases nd Intended for Grade: 2 Grade Subject: Science Description: Activities to help students understand solids, liquids, gases, and the changes between these states. Objective: The
Solids, Liquids, and Gases nd Intended for Grade: 2 Grade Subject: Science Description: Activities to help students understand solids, liquids, gases, and the changes between these states. Objective: The
Dissolving of sodium hydroxide generates heat. Take care in handling the dilution container.
 TITRATION: STANDARDIZATION OF A BASE AND ANALYSIS OF STOMACH ANTACID TABLETS 2009, 1996, 1973 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright
TITRATION: STANDARDIZATION OF A BASE AND ANALYSIS OF STOMACH ANTACID TABLETS 2009, 1996, 1973 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright
Teacher Demo: Turning Water into Wine into Milk into Beer
 SNC2D/2P Chemical Reactions/Chemical Reactions and their Practical Applications Teacher Demo: Turning Water into Wine into Milk into Beer Topics evidence of chemical change types of chemical reactions
SNC2D/2P Chemical Reactions/Chemical Reactions and their Practical Applications Teacher Demo: Turning Water into Wine into Milk into Beer Topics evidence of chemical change types of chemical reactions
Chemistry 101. Chemistry Experiments for the Home Acidity Determination Using Indicators
 Chemistry 101 Chemistry Experiments for the Home Acidity Determination Using Indicators I. Objective: To determine the acidity of a variety of common substances by the use of indicators. To prepare your
Chemistry 101 Chemistry Experiments for the Home Acidity Determination Using Indicators I. Objective: To determine the acidity of a variety of common substances by the use of indicators. To prepare your
Lab 25. Acid-Base Titration and Neutralization Reactions: What Is the Concentration of Acetic Acid in Each Sample of Vinegar?
 Lab 25. Acid-Base Titration and Neutralization Reactions: What Is the Concentration of Acetic Acid in Each Sample of Vinegar? Introduction Vinegar is basically a solution of acetic acid (CH3COOH). It is
Lab 25. Acid-Base Titration and Neutralization Reactions: What Is the Concentration of Acetic Acid in Each Sample of Vinegar? Introduction Vinegar is basically a solution of acetic acid (CH3COOH). It is
Taking Apart the Pieces
 Lab 4 Taking Apart the Pieces How does starting your morning out right relate to relief from a headache? I t is a lazy Saturday morning and you ve just awakened to your favorite cereal Morning Trails and
Lab 4 Taking Apart the Pieces How does starting your morning out right relate to relief from a headache? I t is a lazy Saturday morning and you ve just awakened to your favorite cereal Morning Trails and
Unit A: Studying Materials Scientifically
 ITEM BANKS Unit A: Studying Materials Scientifically Multiple choice: Circle the best answer. 1. What safety rules should you always follow while doing a science laboratory? a. Wear safety goggles at all
ITEM BANKS Unit A: Studying Materials Scientifically Multiple choice: Circle the best answer. 1. What safety rules should you always follow while doing a science laboratory? a. Wear safety goggles at all
ANSWER KEY. Acids, Bases, and Solutions. Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries,
 Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Elements of Chemistry Acids and Bases Teacher s Guide
 Teacher s Guide oneone oee Grade Level: 9 12 Curriculum Focus: Physical Science Lesson Duration: Two class periods Program Description Explore the chemistry of acids and bases to see how fundamental they
Teacher s Guide oneone oee Grade Level: 9 12 Curriculum Focus: Physical Science Lesson Duration: Two class periods Program Description Explore the chemistry of acids and bases to see how fundamental they
Of Cabbages and Kings
 Of Cabbages and Kings Learning Objectives: Students will learn about indicators, acids, bases, and the ph scale. GRADE LEVEL K 8 SCIENCE TOPICS Physical Properties Techniques Chemical Reactions PROCESS
Of Cabbages and Kings Learning Objectives: Students will learn about indicators, acids, bases, and the ph scale. GRADE LEVEL K 8 SCIENCE TOPICS Physical Properties Techniques Chemical Reactions PROCESS
Recovery of Elemental Copper from Copper (II) Nitrate
 Recovery of Elemental Copper from Copper (II) Nitrate Objectives: Challenge: Students should be able to - recognize evidence(s) of a chemical change - convert word equations into formula equations - perform
Recovery of Elemental Copper from Copper (II) Nitrate Objectives: Challenge: Students should be able to - recognize evidence(s) of a chemical change - convert word equations into formula equations - perform
Hardness Comparisons
 Hardness Comparisons Hardness Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania 16335. Grade Level: all Duration: 50 minutes Setting:
Hardness Comparisons Hardness Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania 16335. Grade Level: all Duration: 50 minutes Setting:
Return to Lab Menu. Acids and Bases in Your House
 Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
ENVIRONMENTAL MANAGEMENT: CLEAN WATER RESEARCH AS RELATED TO ACID MINE DRAINAGE
 ENVIRONMENTAL MANAGEMENT: CLEAN WATER RESEARCH AS RELATED TO ACID MINE DRAINAGE LESSON PLAN CREATED BY: Todd Mills (Grades 9 12) Lois Morris (Grades 6-8) Terry Rostcheck (Grades 6 8) Susan Tegi (Grades
ENVIRONMENTAL MANAGEMENT: CLEAN WATER RESEARCH AS RELATED TO ACID MINE DRAINAGE LESSON PLAN CREATED BY: Todd Mills (Grades 9 12) Lois Morris (Grades 6-8) Terry Rostcheck (Grades 6 8) Susan Tegi (Grades
Private Water Supplies Sampling Manual. A Field Guide
 Private Water Supplies Sampling Manual A Field Guide Foreword This sampling manual details standard procedures for the collection, storage and transportation of samples at private water supplies in accordance
Private Water Supplies Sampling Manual A Field Guide Foreword This sampling manual details standard procedures for the collection, storage and transportation of samples at private water supplies in accordance
Acids, Bases, and ph
 CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
ph Test #1: Scaling Common Liquids (From a series of 5)
 ph ph Test #1: Scaling Common Liquids (From a series of 5) Adapted from: Acid Tests in Environmental Education in the Schools. Braus, Judy and David Wood. Peace Corps, 1993. Grade Level: basic Duration:
ph ph Test #1: Scaling Common Liquids (From a series of 5) Adapted from: Acid Tests in Environmental Education in the Schools. Braus, Judy and David Wood. Peace Corps, 1993. Grade Level: basic Duration:
SNEAK PEAK inside ACTIVITY. ADVANCE PREPARATION see next page for more details Dilute alcohol with water Set out plastic cups, etc.
 Reaction: Yes or No? Learning Objectives: Students will list and identify three characteristics of chemical reactions. GRADE LEVEL 3 8 SCIENCE TOPICS Solutions and Mixtures Chemical Reactions PROCESS SKILLS
Reaction: Yes or No? Learning Objectives: Students will list and identify three characteristics of chemical reactions. GRADE LEVEL 3 8 SCIENCE TOPICS Solutions and Mixtures Chemical Reactions PROCESS SKILLS
Enzyme Pre-Lab. Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab.
 Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
Making Biodiesel from Virgin Vegetable Oil: Teacher Manual
 Making Biodiesel from Virgin Vegetable Oil: Teacher Manual Learning Goals: Students will understand how to produce biodiesel from virgin vegetable oil. Students will understand the effect of an exothermic
Making Biodiesel from Virgin Vegetable Oil: Teacher Manual Learning Goals: Students will understand how to produce biodiesel from virgin vegetable oil. Students will understand the effect of an exothermic
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5
 First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
A PRIMER ON ph. A Presentation for ASTA Conference September 30-October 1, 2008
 A PRIMER ON ph A Presentation for ASTA Conference September 30-October 1, 2008 Rebecca Richardson Martha Gothard Director Science Specialist Regional In-Service Education Center AMSTI University of Montevallo
A PRIMER ON ph A Presentation for ASTA Conference September 30-October 1, 2008 Rebecca Richardson Martha Gothard Director Science Specialist Regional In-Service Education Center AMSTI University of Montevallo
Improving your water quality and answering questions about your water
 Improving your water quality and answering questions about your water Developed and Prepared by 2013 Reynolds Water Conditioning Co. Water Filtration & Purification Guide Including answers to common water
Improving your water quality and answering questions about your water Developed and Prepared by 2013 Reynolds Water Conditioning Co. Water Filtration & Purification Guide Including answers to common water
This lesson introduces students to decimals.
 NATIONAL MATH + SCIENCE INITIATIVE Elementary Math Introduction to Decimals LEVEL Grade Five OBJECTIVES Students will compare fractions to decimals. explore and build decimal models. MATERIALS AND RESOURCES
NATIONAL MATH + SCIENCE INITIATIVE Elementary Math Introduction to Decimals LEVEL Grade Five OBJECTIVES Students will compare fractions to decimals. explore and build decimal models. MATERIALS AND RESOURCES
CHM 130LL: ph, Buffers, and Indicators
 CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
Luminol Test PROCESS SKILLS SCIENCE TOPICS VOCABULARY
 EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
TEACHER ACTIVITY GUIDE
 Page 1/5 EXPECTED OUTCOMES TEACHER ACTIVITY GUIDE ROOT BEER PRODUCTION Taken from IFT Experiments in Food Science Series This activity will allow student an opportunity to explore yeast fermentation by
Page 1/5 EXPECTED OUTCOMES TEACHER ACTIVITY GUIDE ROOT BEER PRODUCTION Taken from IFT Experiments in Food Science Series This activity will allow student an opportunity to explore yeast fermentation by
PART I: PREPARATION OF SOLUTIONS AND STANDARDIZATION OF A BASE
 TITRATION: STANDARDIZATION OF A BASE AND ANALYSIS OF STOMACH ANTACID TABLETS 2009, 1996, 1973 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright
TITRATION: STANDARDIZATION OF A BASE AND ANALYSIS OF STOMACH ANTACID TABLETS 2009, 1996, 1973 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright
Treatment options for hydrogen sulfide. Testing for hydrogen sulfide
 Sometimes hot water will have a sour smell, similar to that of an old damp rag. This smell often develops when the thermostat has been lowered to save energy or reduce the potential for scalding. Odor-causing
Sometimes hot water will have a sour smell, similar to that of an old damp rag. This smell often develops when the thermostat has been lowered to save energy or reduce the potential for scalding. Odor-causing
Reduce Reduce Reduce. Reuse. Reuse. Recycle. Recycle. Lesson: Plastic Polymers. Background: Procedures:
 Lesson: Plastic Polymers Grade: 4-5 Subject: Science Objectives: Students will: conduct a series of tests to determine the properties of different types of plastics audit the plastic waste generated in
Lesson: Plastic Polymers Grade: 4-5 Subject: Science Objectives: Students will: conduct a series of tests to determine the properties of different types of plastics audit the plastic waste generated in
The Digestive System Grade 5
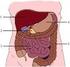 TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
Water Remediation Lab Worksheet
 Name: Date: Class: Water Remediation Lab Worksheet Earlier we talked about how contaminants and pollutants enter groundwater. We also talked about how engineers design ways to clean groundwater and make
Name: Date: Class: Water Remediation Lab Worksheet Earlier we talked about how contaminants and pollutants enter groundwater. We also talked about how engineers design ways to clean groundwater and make
Chemical Weathering Lab. 4th grade PSI
 Chemical Weathering Lab 4th grade PSI Teacher s Notes: Having students conduct Mohs Hardness Test requires a lot of preparation. Rock samples and the materials used to test them need to be collected and
Chemical Weathering Lab 4th grade PSI Teacher s Notes: Having students conduct Mohs Hardness Test requires a lot of preparation. Rock samples and the materials used to test them need to be collected and
II. 2007 Core Knowledge National Conference, First Graders Measure Up!, Grade 1 1
 First Graders Measure Up! Grade Level or Special Area: First Grade Written by: Cindy Todd and Nancy Sanchez, Roscoe Wilson Elementary Magnet School, Lubbock, TX Length of Unit: Ten Lessons (10 days) I.
First Graders Measure Up! Grade Level or Special Area: First Grade Written by: Cindy Todd and Nancy Sanchez, Roscoe Wilson Elementary Magnet School, Lubbock, TX Length of Unit: Ten Lessons (10 days) I.
Acids, Bases and Salts
 Acids, Bases and Salts 2 HAPTER Tips and Tricks Acids are sour in taste and bases are bitter in taste. Indicators are chemical substances which give different colours in acidic and basic solutions. If
Acids, Bases and Salts 2 HAPTER Tips and Tricks Acids are sour in taste and bases are bitter in taste. Indicators are chemical substances which give different colours in acidic and basic solutions. If
Solutions and Suspensions
 Science Unit: Lesson 11: Matter Solutions and Suspensions School year: 2005/2006 Developed for: Developed by: Grade level: Duration of Lesson McBride Elementary School, Vancouver School District Catriona
Science Unit: Lesson 11: Matter Solutions and Suspensions School year: 2005/2006 Developed for: Developed by: Grade level: Duration of Lesson McBride Elementary School, Vancouver School District Catriona
Danielle Abrahamson and Susan Michalek, Academy of Charter Schools, Denver, Colorado Four lessons over a period of five to seven days
 MATTER: Grade Level: Presented by: Length of Unit: ITS STATES AND PROPERTIES First Grade Danielle Abrahamson and Susan Michalek, Academy of Charter Schools, Denver, Colorado Four lessons over a period
MATTER: Grade Level: Presented by: Length of Unit: ITS STATES AND PROPERTIES First Grade Danielle Abrahamson and Susan Michalek, Academy of Charter Schools, Denver, Colorado Four lessons over a period
Summary This lesson will introduce the concept of the water cycle by using a simple demonstration.
 Partnerships Implementing Engineering Education Worcester Polytechnic Institute Worcester Public Schools Supported by: National Science Foundation Weather: 4.H.3 Water Cycle Grade Level 4 Sessions Seasonality
Partnerships Implementing Engineering Education Worcester Polytechnic Institute Worcester Public Schools Supported by: National Science Foundation Weather: 4.H.3 Water Cycle Grade Level 4 Sessions Seasonality
Acid 7 Base. 1. Describe two things hydrochloric acid does in your body system. 2. What does sodium hydrogencarbonate do in your body system?
 Acids and Bases acid: a compound that, when dissolved in water, forms a solution with a ph less than 7 base: a compound that, when dissolved in water, forms a solution with a ph greater than 7 ph: the
Acids and Bases acid: a compound that, when dissolved in water, forms a solution with a ph less than 7 base: a compound that, when dissolved in water, forms a solution with a ph greater than 7 ph: the
Eighth Grade, Density To Float or Not to Float? 2004 Colorado Unit Writing Project 1
 Density To Float or Not to Float? That is the Question! Grade Level or Special Area: Eighth Grade Science Written by: Aida Peterson, Clear Lake Middle School, Denver, Colorado Length of Unit: Twelve lessons
Density To Float or Not to Float? That is the Question! Grade Level or Special Area: Eighth Grade Science Written by: Aida Peterson, Clear Lake Middle School, Denver, Colorado Length of Unit: Twelve lessons
COMMON LABORATORY APPARATUS
 COMMON LABORATORY APPARATUS Beakers are useful as a reaction container or to hold liquid or solid samples. They are also used to catch liquids from titrations and filtrates from filtering operations. Bunsen
COMMON LABORATORY APPARATUS Beakers are useful as a reaction container or to hold liquid or solid samples. They are also used to catch liquids from titrations and filtrates from filtering operations. Bunsen
The Co-operative s Green Schools Revolution. LESSON PLAN KS3: Creating the world s finest drink clean water. SUGGESTED TIME: 60 MINS
 Water The Co-operative s Green Schools Revolution LESSON PLAN KS3: Creating the world s finest drink clean water. SUGGESTED TIME: 60 MINS Age group No. of pupils in cohort Classroom support (to be completed
Water The Co-operative s Green Schools Revolution LESSON PLAN KS3: Creating the world s finest drink clean water. SUGGESTED TIME: 60 MINS Age group No. of pupils in cohort Classroom support (to be completed
WATER CHEMISTRY AND POOL WATER BALANCE
 C R6 H A PT E WATER CHEMISTRY AND POOL WATER BALANCE LEARNING OBJECTIVES After completely studying this chapter, you should be able to: Understand and list the parameters upon which water balance is based.
C R6 H A PT E WATER CHEMISTRY AND POOL WATER BALANCE LEARNING OBJECTIVES After completely studying this chapter, you should be able to: Understand and list the parameters upon which water balance is based.
Remember the best arguments are based on the strongest evidence and can explain why opposing arguments are incorrect.
 Magnesium and carbon dioxide Student sheet Burning magnesium in carbon dioxide what will happen? Either the magnesium will go out or it will continue to burn. Which will it be? You will use the evidence
Magnesium and carbon dioxide Student sheet Burning magnesium in carbon dioxide what will happen? Either the magnesium will go out or it will continue to burn. Which will it be? You will use the evidence
Stoichiometry: Baking Soda and Vinegar Reactions
 Stoichiometry: Baking Soda and Vinegar Reactions California Science Content Standards: Teacher Version 3. Conservation of Matter and Stoichiometry: The conservation of atoms in chemical reactions leads
Stoichiometry: Baking Soda and Vinegar Reactions California Science Content Standards: Teacher Version 3. Conservation of Matter and Stoichiometry: The conservation of atoms in chemical reactions leads
Chemical versus Physical Changes
 Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS
 Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
Experiment 9: Acids and Bases Adapted from: Chemistry, Experimental Foundations, 4th Ed. Laboratory Manual, by Merrill, Parry & Bassow.
 Chem 121 Lab Clark College Experiment 9: Acids and Bases Adapted from: Chemistry, Experimental Foundations, 4th Ed. Laboratory Manual, by Merrill, Parry & Bassow. Content Goals: Increase understanding
Chem 121 Lab Clark College Experiment 9: Acids and Bases Adapted from: Chemistry, Experimental Foundations, 4th Ed. Laboratory Manual, by Merrill, Parry & Bassow. Content Goals: Increase understanding
A Study On Fire And Global Warming
 A Study On Fire And Global Warming By Nicole Curran,Doireann Lynam and Alesi Horan Introduction Our project is a study on fire and global warming. Nicole Curran, Doireann Lynam and Alesi Horan from Colaiste
A Study On Fire And Global Warming By Nicole Curran,Doireann Lynam and Alesi Horan Introduction Our project is a study on fire and global warming. Nicole Curran, Doireann Lynam and Alesi Horan from Colaiste
Adjusting Chemical Levels in a Swimming Pool
 Adjusting Chemical Levels in a Swimming Pool When adding chemicals, there are three types of chemical adjustments that can be performed: product label chemical dosage, product label chemical adjustment,
Adjusting Chemical Levels in a Swimming Pool When adding chemicals, there are three types of chemical adjustments that can be performed: product label chemical dosage, product label chemical adjustment,
Healthy Drinking Waters
 Healthy Drinking Waters for M A S S A C H U S E T T S S a f e a n d h e a l t h y l i v e s i n s a f e a n d h e a l t h y c o m m u n i t i e s Copper in Private Drinking Water Wells Private well owners
Healthy Drinking Waters for M A S S A C H U S E T T S S a f e a n d h e a l t h y l i v e s i n s a f e a n d h e a l t h y c o m m u n i t i e s Copper in Private Drinking Water Wells Private well owners
Wallingford Public Schools - HIGH SCHOOL COURSE OUTLINE
 Wallingford Public Schools - HIGH SCHOOL COURSE OUTLINE Course Title: Applied Chemistry Course Number: G 2614 Department: Science Grade(s): 11-12 Level(s): General Credit: 1 Course Description This is
Wallingford Public Schools - HIGH SCHOOL COURSE OUTLINE Course Title: Applied Chemistry Course Number: G 2614 Department: Science Grade(s): 11-12 Level(s): General Credit: 1 Course Description This is
6.8 Measuring the Acidity of Solutions Page 160
 6.8 Measuring the Acidity of Solutions Page 160 PRESCRIBED LEARNING OUTCOMES measure substances and solutions according to ph, solubility, and concentration KNOWLEDGE ph is the measure of the tendency
6.8 Measuring the Acidity of Solutions Page 160 PRESCRIBED LEARNING OUTCOMES measure substances and solutions according to ph, solubility, and concentration KNOWLEDGE ph is the measure of the tendency
The Acid Test Grade Nine
 Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Separation by Solvent Extraction
 Experiment 3 Separation by Solvent Extraction Objectives To separate a mixture consisting of a carboxylic acid and a neutral compound by using solvent extraction techniques. Introduction Frequently, organic
Experiment 3 Separation by Solvent Extraction Objectives To separate a mixture consisting of a carboxylic acid and a neutral compound by using solvent extraction techniques. Introduction Frequently, organic
We use in our daily life a large
 5 Acids, Bases and Salts We use in our daily life a large number of substances such as lemon, tamarind, common salt, sugar and vinegar. Do they have the same taste? Let us recall tastes of some edible
5 Acids, Bases and Salts We use in our daily life a large number of substances such as lemon, tamarind, common salt, sugar and vinegar. Do they have the same taste? Let us recall tastes of some edible
Dukes of Hazard Activity Time:
 Dukes of Hazard Activity Time: TEACHERS: Read Populution and Water Source Protection on page 80 of Peel Water Story book. Objectives: Students will: Identify household hazardous waste (HHW) materials around
Dukes of Hazard Activity Time: TEACHERS: Read Populution and Water Source Protection on page 80 of Peel Water Story book. Objectives: Students will: Identify household hazardous waste (HHW) materials around
Disinfecting Your Private Well
 TCEQ GENERAL INFORMATION Water Supply Division GI-432 August 2013 Disinfecting Your Private Well Is Your Well Flooded? Disinfect It Before You Drink It! If your private well is flooded, do not use water
TCEQ GENERAL INFORMATION Water Supply Division GI-432 August 2013 Disinfecting Your Private Well Is Your Well Flooded? Disinfect It Before You Drink It! If your private well is flooded, do not use water
SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES
 SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SECOND GRADE WATER WEEK 1. PRE: Exploring the properties of water. LAB: Experimenting with different soap mixtures. POST: Analyzing
SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SECOND GRADE WATER WEEK 1. PRE: Exploring the properties of water. LAB: Experimenting with different soap mixtures. POST: Analyzing
Chemists use the ph value to measure how acidic or basic a solution is. The ph scale runs from 0 to 14:
 The ph Value Chemists use the ph value to measure how acidic or basic a solution is. The ph scale runs from 0 to 14: If the ph value is lower than 7 (0 to 6.99) the solution contains more H + ions than
The ph Value Chemists use the ph value to measure how acidic or basic a solution is. The ph scale runs from 0 to 14: If the ph value is lower than 7 (0 to 6.99) the solution contains more H + ions than
experiment5 Understanding and applying the concept of limiting reagents. Learning how to perform a vacuum filtration.
 81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb
 Document: AND Lead 100 7 2013 Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb July, 2013 Edition 1 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of Contents
Document: AND Lead 100 7 2013 Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb July, 2013 Edition 1 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of Contents
Determination of Vitamin C of Citrus Juices. by Dr. Walter Scharf and Dr. Charles Malerich Natural Sciences, Baruch College New York, NY 10010
 Determination of Vitamin C of Citrus Juices by Dr. Walter Scharf and Dr. Charles Malerich Natural Sciences, Baruch College New York, NY 10010 Introduction--Vitamin C is a water-soluble vitamin that is
Determination of Vitamin C of Citrus Juices by Dr. Walter Scharf and Dr. Charles Malerich Natural Sciences, Baruch College New York, NY 10010 Introduction--Vitamin C is a water-soluble vitamin that is
