In the previous lecture we have learnt the quantum mechanical treatment of hydrogen atom.
|
|
|
- Leo Chandler
- 7 years ago
- Views:
Transcription
1 Lecture : Title : ALKALI SPCTA Page- In the previous lecture we have learnt the quantum mechanical treatment of hydrogen atom. The similar picture is not able to explain the alkali atoms, the other elements in the first group of periodic table. Here, we will discuss the development of the theory to explain the alkali spectra. We will also elaborate the concept behind the modification of the potential required to explain the observation in alkali atoms.
2 Page- The absorption spectra of alkali vapors (Such as lithium, sodium) appear quite similar in many respects to the absorption spectrum of H atom. They are only displaced to a considerable extent, toward longer wavelengths. These spectra also consist of a series of lines with regularly decreasing separation and decreasing intensity. It cannot, however, be represented by a formula completely analogous to the Bohr formula. On the other hand, since the lines converge to a limit, we must be able to represent them as differences between two terms. ydberg formula, ν = TPS where m =,3 ( m+ p) p is a constant, known as Quantum Defect. T PS is known as series limit. This series is known as Principal series. Other series, in addition to this, may be observed for the alkalis. They are diffuse, sharp and Bergmann series. Sharp Series ν = TSS m =,3 m+ s Diffuse Series ν = T m = 3, 4 ( m+ d) Bergmann Series ν = T m = 4,5 SS BS ( m+ f ) n =,,,3 Selection rules: =± As an example, sodium energy levels and transitions are given in figure-. Bergmann series Sharp series Diffuse series Principal series Sodium atom energy levels and transitions Figure-.
3 Page- As a specific example, we consider the alkali metals such as lithium, sodium and potassium, which come from group I of the periodic table. They have one valence electron outside filled inner shells. They are therefore approximately one-electron systems, and can be understood by introducing a phenomenological number called the quantum defect to describe the energies. Let us consider the sodium atom. The optical spectra are determined by excitations of the outermost 3s electron. The energy of each (n; l) term of the valence electron is given by: nl, =.. ( n δ ( l)) Where n 3, δ ( l) is the quantum defect. 3s Nucleus +e s s valence electron p Sodium Atom Z = Figure-. The quantum defect δ () l was introduced empirically to account for the optical spectra. In principle it should depend on both n and l, but it was found experimentally to depend mainly on l as given in the following table. Values of quantum defect for sodium l n = 3 n = 4 n = 5 n =
4 Page 3 The dependence of the quantum defect on l can be understood with reference to the figure where the radial probability densities for the 3s and 3p orbitals of a hydrogenic atom with Z = are plotted with respect to normalized radial distance. An individual electron in sodium atom experiences an electrostatic potential due to the Coulomb repulsion from all the other electrons in the atom. Ten out of eleven electrons are in closed sub-shells, which have spherically-symmetric charge clouds. The off-radial forces from electrons in these closed shells cancel because of the spherical symmetry. Hydrogen radial probability distribution (figure-.3) is expected to be a reasonable approximation for the single valence electron of sodium. We see that both the 3s and 3p orbitals penetrate the inner shells, and that this penetration is much greater for the 3s electron. The electron will therefore see a larger effective nuclear charge for part of its orbit, and this will have the effect of reducing the energies. The energy reduction is largest for the 3s electron due to its larger core penetration. Probability density radial probability density r a normalized radial distance adial Probability densities s s 3s 3S 3P 3D. r a normalized radial distance 3 35 Figure-.3
5 Page 4 The effect of this penetration results in the shift of energy levels. A comparison with the hydrogen energy level is shown in figure-.4. Hydrogen energy levels Sodium energy levels Figure-.4 We can draw the conclusions that, nergy levels with different have different energies. In other words degeneracy removed. From the hydrogen atom energy levels, it cannot be described, because energy depends on n only.
6 Page 5 Classical xplanation Penetrating and Non-Penetrating Orbits as shown in figure-.5: ) Non-Penetrating orbits The first is the case when the outer electron has a non penetrating orbit, as in the figure. If its accepted that the mean symmetry of the cloud formed by ( Z ) electrons is similar, the electron experiences the electrostatic potential of the nuclear charge of Ze and of the spherical distribution of charge ( Z ). The discussion presented for the hydrogen atom remains valid. (Z-)e -e ) Penetrating orbit On the other hand, if the orbit of the outer electron penetrates inside the core of the atom, the problem is much more complex, simple solution by Somerfield, is this, e Vext = r " r" Large -e Ze V in = + Const. r " r " Small (Z-)e Figure-.5
7 Page 6 Quantum Mechanical Calculation e b Form of the potential energy, V ( r) = + r r.. This form represents the potential energy requirement at large distance, V r e = r and at small distance, V ( r) Ze = r This potential with respect to radial distance is shown in figure-.6 e b + r r e r Potential V r 4 6 Ze r 8 r 3 adial distance 4 5 Figure-.6
8 Page 7 Since this is radial dependence and we need to solve only radial equation of the Schrödinger equation of hydrogen atom problem e b V ( r) = + r r c b = + where, c = e r r Now, the Hamiltonian for one electron atom, H µ = + µ = + + µ V r c r b r c b ψ ψ ψ r r + + = ψ µ c b ψ r r + + =...3 The radial equation: d χ µ µ c b ( + ) + () + r = dr r r r d χ µ µ c µ cb ( + ) r () = d r r r r.4 µ µ c Taking A= ; B= and substituting in equation.4 d χ B Bb+ + A χ + = dr r r Let ( + ) = B b + ( + ) * * B Bb+ ( + ) * *, d χ So, A + χ = dr r r
9 Page 8 Same radial equation as in hydrogen atom, solution with = = * * ( n ) ( + p+ ) n= + p+, = + + = + * * * So n p n = = * * n n n * * * n n p where = + + Now, * * + = + Bb ( * )( * ) ( ) * ( * ) + = Bb + + = Bb Bb Bb = = * B b b = = + a + hc = b n a + n, Note that : This energy expression is dependent on both n and l Maximum l -> small correction Small l -> correction term is large
10 Page-9 hc = Bb n + n, Now we know = hν c ν = ν = λ λ hc = ( cm ) = λ λ hc n, = cm Bb n + Where = 9, 78.7 cm Conversion ergs / molecule Cal / molecule electron volts 6 4 cm Take an example: Lithium : ionization potential : 43, 486cm or, 5.39eV = Bb ( Bb) =.533 Bb=.588 Bb= , = = 363.8cm , = = cm 3.4 = = cm ,
11 Page = = 853.9cm 4, ( 4.4) = = 735.8cm = = 748.cm , 4, = = 763.4cm ,3 l = l = l = l = 3 5 cm - cm - 4p 4p 4s 4f 5 cm - cm - 3s 3p 3d 5 cm - 3 cm - p 35 cm - 4 cm - 45 cm - s Figure-.7
12 Page- ecap In this lecture, we have learnt that The departure in the spectra of alkali atom from hydrogen such as lithium, sodium is that the energies are not only dependent on n but also l. The modification of the potential of electron in such multielectronic atoms is that the energy correction term is large of small l values.
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
Multi-electron atoms
 Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
Lecture 3: Optical Properties of Bulk and Nano. 5 nm
 Lecture 3: Optical Properties of Bulk and Nano 5 nm First H/W#1 is due Sept. 10 Course Info The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model)
Lecture 3: Optical Properties of Bulk and Nano 5 nm First H/W#1 is due Sept. 10 Course Info The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model)
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW
 CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
Question: Do all electrons in the same level have the same energy?
 Question: Do all electrons in the same level have the same energy? From the Shells Activity, one important conclusion we reached based on the first ionization energy experimental data is that electrons
Question: Do all electrons in the same level have the same energy? From the Shells Activity, one important conclusion we reached based on the first ionization energy experimental data is that electrons
Bohr s Model of the Atom
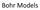 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Lecture 3: Optical Properties of Bulk and Nano. 5 nm
 Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
CHEM6085: Density Functional Theory Lecture 2. Hamiltonian operators for molecules
 CHEM6085: Density Functional Theory Lecture 2 Hamiltonian operators for molecules C.-K. Skylaris 1 The (time-independent) Schrödinger equation is an eigenvalue equation operator for property A eigenfunction
CHEM6085: Density Functional Theory Lecture 2 Hamiltonian operators for molecules C.-K. Skylaris 1 The (time-independent) Schrödinger equation is an eigenvalue equation operator for property A eigenfunction
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Chapter 7. Electron Structure of the Atom. Chapter 7 Topics
 Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
IONISATION ENERGY CONTENTS
 IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
IONISATION ENERGY IONISATION ENERGY CONTENTS What is Ionisation Energy? Definition of t Ionisation Energy What affects Ionisation Energy? General variation across periods Variation down groups Variation
Chemistry 102 Summary June 24 th. Properties of Light
 Chemistry 102 Summary June 24 th Properties of Light - Energy travels through space in the form of electromagnetic radiation (EMR). - Examples of types of EMR: radio waves, x-rays, microwaves, visible
Chemistry 102 Summary June 24 th Properties of Light - Energy travels through space in the form of electromagnetic radiation (EMR). - Examples of types of EMR: radio waves, x-rays, microwaves, visible
Lecture 12 Atomic structure
 Lecture 12 Atomic structure Atomic structure: background Our studies of hydrogen-like atoms revealed that the spectrum of the Hamiltonian, Ĥ 0 = ˆp2 2m 1 Ze 2 4πɛ 0 r is characterized by large n 2 -fold
Lecture 12 Atomic structure Atomic structure: background Our studies of hydrogen-like atoms revealed that the spectrum of the Hamiltonian, Ĥ 0 = ˆp2 2m 1 Ze 2 4πɛ 0 r is characterized by large n 2 -fold
AP* Atomic Structure & Periodicity Free Response Questions KEY page 1
 AP* Atomic Structure & Periodicity ree Response Questions KEY page 1 1980 a) points 1s s p 6 3s 3p 6 4s 3d 10 4p 3 b) points for the two electrons in the 4s: 4, 0, 0, +1/ and 4, 0, 0, - 1/ for the three
AP* Atomic Structure & Periodicity ree Response Questions KEY page 1 1980 a) points 1s s p 6 3s 3p 6 4s 3d 10 4p 3 b) points for the two electrons in the 4s: 4, 0, 0, +1/ and 4, 0, 0, - 1/ for the three
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES. PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points
 TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
The Phenomenon of Photoelectric Emission:
 The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Unit 2 Periodic Behavior and Ionic Bonding
 Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Abstract. 1. Introduction
 An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr 1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. mohdabubakr@hotmail.com Abstract Periodic table of chemical
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr 1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. mohdabubakr@hotmail.com Abstract Periodic table of chemical
The Advanced Placement Examination in Chemistry. Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010
 The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
Be (g) Be + (g) + e - O (g) O + (g) + e -
 2.13 Ionisation Energies Definition :First ionisation energy The first ionisation energy is the energy required when one mole of gaseous atoms forms one mole of gaseous ions with a single positive charge
2.13 Ionisation Energies Definition :First ionisation energy The first ionisation energy is the energy required when one mole of gaseous atoms forms one mole of gaseous ions with a single positive charge
13- What is the maximum number of electrons that can occupy the subshell 3d? a) 1 b) 3 c) 5 d) 2
 Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
Note: Please use the actual date you accessed this material in your citation.
 MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science, Fall 2005 Please use the following citation format: Sylvia Ceyer and Catherine Drennan, 5.111 Principles of Chemical Science,
MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science, Fall 2005 Please use the following citation format: Sylvia Ceyer and Catherine Drennan, 5.111 Principles of Chemical Science,
Models of the Atom and periodic Trends Exam Study Guide
 Name 1. What is the term for the weighted average mass of all the naturally occurring isotopes of an element? ans: atomic mass 2. Which is exactly equal to 1/12 the mass of a carbon -12 atom? ans: atomic
Name 1. What is the term for the weighted average mass of all the naturally occurring isotopes of an element? ans: atomic mass 2. Which is exactly equal to 1/12 the mass of a carbon -12 atom? ans: atomic
Flame Tests & Electron Configuration
 Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
THE BOHR QUANTUM MODEL
 THE BOHR QUANTUM MODEL INTRODUCTION When light from a low-pressure gas is subject to an electric discharge, a discrete line spectrum is emitted. When light from such a low-pressure gas is examined with
THE BOHR QUANTUM MODEL INTRODUCTION When light from a low-pressure gas is subject to an electric discharge, a discrete line spectrum is emitted. When light from such a low-pressure gas is examined with
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Part I: Principal Energy Levels and Sublevels
 Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
Part I: Principal Energy Levels and Sublevels As you already know, all atoms are made of subatomic particles, including protons, neutrons, and electrons. Positive protons and neutral neutrons are found
SAMPLE EXAM 2 FALL 2012 SOLUTIONS Chemistry 11, Fall 2007 Exam II November 15, 2007 7:30 PM 9:30 PM
 Name: SOLUTIONS III, IV, and V Section (circle): 1 2 3 4 5 SAMPLE EXAM 2 FALL 2012 SOLUTIONS Chemistry 11, Fall 2007 Exam II November 15, 2007 7:30 PM 9:30 PM As always, full credit will not be given unless
Name: SOLUTIONS III, IV, and V Section (circle): 1 2 3 4 5 SAMPLE EXAM 2 FALL 2012 SOLUTIONS Chemistry 11, Fall 2007 Exam II November 15, 2007 7:30 PM 9:30 PM As always, full credit will not be given unless
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS
 DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
Review of the isotope effect in the hydrogen spectrum
 Review of the isotope effect in the hydrogen spectrum 1 Balmer and Rydberg Formulas By the middle of the 19th century it was well established that atoms emitted light at discrete wavelengths. This is in
Review of the isotope effect in the hydrogen spectrum 1 Balmer and Rydberg Formulas By the middle of the 19th century it was well established that atoms emitted light at discrete wavelengths. This is in
Atoms Absorb & Emit Light
 Atoms Absorb & Emit Light Spectra The wavelength of the light that an element emits or absorbs is its fingerprint. Atoms emit and absorb light First Test is Thurs, Feb 1 st About 30 multiple choice questions
Atoms Absorb & Emit Light Spectra The wavelength of the light that an element emits or absorbs is its fingerprint. Atoms emit and absorb light First Test is Thurs, Feb 1 st About 30 multiple choice questions
Electron Orbits. Binding Energy. centrifugal force: electrostatic force: stability criterion: kinetic energy of the electron on its orbit:
 Electron Orbits In an atom model in which negatively charged electrons move around a small positively charged nucleus stable orbits are possible. Consider the simple example of an atom with a nucleus of
Electron Orbits In an atom model in which negatively charged electrons move around a small positively charged nucleus stable orbits are possible. Consider the simple example of an atom with a nucleus of
Copyrighted by Gabriel Tang B.Ed., B.Sc.
 Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
Chapter 8: The Periodic Table 8.1: Development of the Periodic Table Johann Dobereiner: - first to discover a pattern of a group of elements like Cl, Br, and I (called triads). John Newland: - suggested
Chapter 7 Periodic Properties of the Elements
 Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Unit 1, Lesson 03: Answers to Homework 1, 0, +1 2, 1, 0, +1, +2 1, 0, +1 2, 1, 0, +1, +2 3, 2, 1, 0, +1, +2, +3. n = 3 l = 2 m l = -2 m s = -½
 Unit, Lesson : Answers to Homework Summary: The allowed values for quantum numbers for each principal quantum level n : n l m l m s corresponding sub-level number of orbitals in this sub-level n = s n
Unit, Lesson : Answers to Homework Summary: The allowed values for quantum numbers for each principal quantum level n : n l m l m s corresponding sub-level number of orbitals in this sub-level n = s n
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
The Atom and the Periodic Table. Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams
 The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
The Atom and the Periodic Table Electron Cloud Structure Energy Levels Rows on the Periodic Table Bohr Models Electron Dot Diagrams Review The vertical columns in the periodic table are called groups.
Bohr's Theory of the Hydrogen Atom
 OpenStax-CNX module: m42596 1 Bohr's Theory of the Hydrogen Atom OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Describe
OpenStax-CNX module: m42596 1 Bohr's Theory of the Hydrogen Atom OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Describe
Test Review Periodic Trends and The Mole
 Test Review Periodic Trends and The Mole The Mole SHOW ALL WORK ON YOUR OWN PAPER FOR CREDIT!! 1 2 (NH42SO2 %N 24.1 %H 6.9 %S 27.6 %O 41.3 % Al %C 35.3 %H 4.4 %O 47.1 Al(C2H3O23 13.2 3 How many moles are
Test Review Periodic Trends and The Mole The Mole SHOW ALL WORK ON YOUR OWN PAPER FOR CREDIT!! 1 2 (NH42SO2 %N 24.1 %H 6.9 %S 27.6 %O 41.3 % Al %C 35.3 %H 4.4 %O 47.1 Al(C2H3O23 13.2 3 How many moles are
WAVES AND ELECTROMAGNETIC RADIATION
 WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
WAVES AND ELECTROMAGNETIC RADIATION All waves are characterized by their wavelength, frequency and speed. Wavelength (lambda, ): the distance between any 2 successive crests or troughs. Frequency (nu,):
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
2, 8, 20, 28, 50, 82, 126.
 Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of magic nuclei for which either the number of neutrons
Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of magic nuclei for which either the number of neutrons
CHAPTER 9 THE PERIODIC TABLE AND SOME ATOMIC PROPERTIES
 CHAPTER 9 THE PERIODIC TABLE AND SOME ATOMIC PROPERTIES PRACTICE EXAMPLES 1A 1B A B A Atomic size decreases from left to right across a period, and from bottom to top in a family. We expect the smallest
CHAPTER 9 THE PERIODIC TABLE AND SOME ATOMIC PROPERTIES PRACTICE EXAMPLES 1A 1B A B A Atomic size decreases from left to right across a period, and from bottom to top in a family. We expect the smallest
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Practice Questions - Chapter 7 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following represents an impossible set of
Practice Questions - Chapter 7 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following represents an impossible set of
Section 11.3 Atomic Orbitals Objectives
 Objectives 1. To learn about the shapes of the s, p and d orbitals 2. To review the energy levels and orbitals of the wave mechanical model of the atom 3. To learn about electron spin A. Electron Location
Objectives 1. To learn about the shapes of the s, p and d orbitals 2. To review the energy levels and orbitals of the wave mechanical model of the atom 3. To learn about electron spin A. Electron Location
Unit 2: Chemical Bonding and Organic Chemistry
 Chemistry AP Unit : Chemical Bonding and Organic Chemistry Unit : Chemical Bonding and Organic Chemistry Chapter 7: Atomic Structure and Periodicity 7.1: Electromagnetic Radiation Electromagnetic (EM)
Chemistry AP Unit : Chemical Bonding and Organic Chemistry Unit : Chemical Bonding and Organic Chemistry Chapter 7: Atomic Structure and Periodicity 7.1: Electromagnetic Radiation Electromagnetic (EM)
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Sample Exercise 6.1 Concepts of Wavelength and Frequency
 Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Wave Function, ψ. Chapter 28 Atomic Physics. The Heisenberg Uncertainty Principle. Line Spectrum
 Wave Function, ψ Chapter 28 Atomic Physics The Hydrogen Atom The Bohr Model Electron Waves in the Atom The value of Ψ 2 for a particular object at a certain place and time is proportional to the probability
Wave Function, ψ Chapter 28 Atomic Physics The Hydrogen Atom The Bohr Model Electron Waves in the Atom The value of Ψ 2 for a particular object at a certain place and time is proportional to the probability
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
2 Absorbing Solar Energy
 2 Absorbing Solar Energy 2.1 Air Mass and the Solar Spectrum Now that we have introduced the solar cell, it is time to introduce the source of the energy the sun. The sun has many properties that could
2 Absorbing Solar Energy 2.1 Air Mass and the Solar Spectrum Now that we have introduced the solar cell, it is time to introduce the source of the energy the sun. The sun has many properties that could
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
neutrons are present?
 AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
Periodic Table. 1. In the modern Periodic Table, the elements are arranged in order of increasing. A. atomic number B. mass number
 Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
CHAPTER 11: MODERN ATOMIC THEORY
 CHAPTER 11: MODERN ATOMIC THEORY Active Learning Questions: 1-2, 8-10, 14-18; End-of-Chapter Problems: 3-9, 11-13, 16, 18, 20-36, 45-54, 56-64, 66b, 67, 69-91, 98, 101-102, 108, 110, 113, 116, 11.2 ELECTROMAGNETIC
CHAPTER 11: MODERN ATOMIC THEORY Active Learning Questions: 1-2, 8-10, 14-18; End-of-Chapter Problems: 3-9, 11-13, 16, 18, 20-36, 45-54, 56-64, 66b, 67, 69-91, 98, 101-102, 108, 110, 113, 116, 11.2 ELECTROMAGNETIC
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Molecular Models & Lewis Dot Structures
 Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Molecular Models & Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
CHAPTER 8 PRACTICE TEST QUESTIONS (END OF CHAPTER 7 TOO)
 CHAPTER 8 PRACTICE TEST QUESTIONS (END OF CHAPTER 7 TOO) Information that most likely will be on the front cover of your exam: h i Z 2 ΔE = @ 2.18 x 10 @ 18 f Z 2 f J j @ k n f 2 n i 2 1. Which of the
CHAPTER 8 PRACTICE TEST QUESTIONS (END OF CHAPTER 7 TOO) Information that most likely will be on the front cover of your exam: h i Z 2 ΔE = @ 2.18 x 10 @ 18 f Z 2 f J j @ k n f 2 n i 2 1. Which of the
Chapter 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE
 Chapter 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE Problems: 1-3, 13-15, 19, 23-25, 31-32, 43, 45-46, 49c, 50a, 50b, 57c, 58 (b,c,d), 61-62, 69, 71-74, 77-88, 91-94 9.5 LIGHT: Electromagnetic Radiation
Chapter 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE Problems: 1-3, 13-15, 19, 23-25, 31-32, 43, 45-46, 49c, 50a, 50b, 57c, 58 (b,c,d), 61-62, 69, 71-74, 77-88, 91-94 9.5 LIGHT: Electromagnetic Radiation
P. Table & E Configuration Practice TEST
 P. Table & E Configuration Practice TEST Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A line spectrum is produced when an electron moves from one energy
P. Table & E Configuration Practice TEST Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A line spectrum is produced when an electron moves from one energy
Level spacing 1 ev 0.1 mev Ionization energy Typical magnetic field 10 4 T 1-10 T
 Quantum dots Quantum dot (QD) is a conducting island of a size comparable to the Fermi wavelength in all spatial directions. Often called the artificial atoms, however the size is much bigger (100 nm for
Quantum dots Quantum dot (QD) is a conducting island of a size comparable to the Fermi wavelength in all spatial directions. Often called the artificial atoms, however the size is much bigger (100 nm for
CHEMSITRY NOTES Chapter 13. Electrons in Atoms
 CHEMSITRY NOTES Chapter 13 Electrons in Atoms Goals : To gain an understanding of : 1. Atoms and their structure. 2. The development of the atomic theory. 3. The quantum mechanical model of the atom. 4.
CHEMSITRY NOTES Chapter 13 Electrons in Atoms Goals : To gain an understanding of : 1. Atoms and their structure. 2. The development of the atomic theory. 3. The quantum mechanical model of the atom. 4.
Chapter Outline. Review of Atomic Structure Electrons, Protons, Neutrons, Quantum mechanics of atoms, Electron states, The Periodic Table
 Review of Atomic Structure Electrons, Protons, Neutrons, Quantum mechanics of atoms, Electron states, The Periodic Table Atomic Bonding in Solids Bonding Energies and Forces Periodic Table Chapter Outline
Review of Atomic Structure Electrons, Protons, Neutrons, Quantum mechanics of atoms, Electron states, The Periodic Table Atomic Bonding in Solids Bonding Energies and Forces Periodic Table Chapter Outline
GCE. Chemistry A. Mark Scheme for June 2012. Advanced Subsidiary GCE Unit F321: Atoms, Bonds and Groups. Oxford Cambridge and RSA Examinations
 GCE Chemistry A Advanced Subsidiary GCE Unit F321: Atoms, Bonds and Groups Mark Scheme for June 2012 Oxford Cambridge and RSA Examinations OCR (Oxford Cambridge and RSA) is a leading UK awarding body,
GCE Chemistry A Advanced Subsidiary GCE Unit F321: Atoms, Bonds and Groups Mark Scheme for June 2012 Oxford Cambridge and RSA Examinations OCR (Oxford Cambridge and RSA) is a leading UK awarding body,
Electron spectroscopy Lecture 1-21. Kai M. Siegbahn (1918 - ) Nobel Price 1981 High resolution Electron Spectroscopy
 Electron spectroscopy Lecture 1-21 Kai M. Siegbahn (1918 - ) Nobel Price 1981 High resolution Electron Spectroscopy 653: Electron Spectroscopy urse structure cture 1. Introduction to electron spectroscopies
Electron spectroscopy Lecture 1-21 Kai M. Siegbahn (1918 - ) Nobel Price 1981 High resolution Electron Spectroscopy 653: Electron Spectroscopy urse structure cture 1. Introduction to electron spectroscopies
Instructors Guide: Atoms and Their Isotopes
 Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
ELECTRON CONFIGURATION (SHORT FORM) # of electrons in the subshell. valence electrons Valence electrons have the largest value for "n"!
 179 ELECTRON CONFIGURATION (SHORT FORM) - We can represent the electron configuration without drawing a diagram or writing down pages of quantum numbers every time. We write the "electron configuration".
179 ELECTRON CONFIGURATION (SHORT FORM) - We can represent the electron configuration without drawing a diagram or writing down pages of quantum numbers every time. We write the "electron configuration".
Lecture 3 Properties and Evolution of Molecular Clouds. Spitzer space telescope image of Snake molecular cloud (IRDC G11.11-0.11
 Lecture 3 Properties and Evolution of Molecular Clouds Spitzer space telescope image of Snake molecular cloud (IRDC G11.11-0.11 From slide from Annie Hughes Review CO t in clouds HI: Atomic Hydrogen http://www.atnf.csiro.au/research/lvmeeting/magsys_pres/
Lecture 3 Properties and Evolution of Molecular Clouds Spitzer space telescope image of Snake molecular cloud (IRDC G11.11-0.11 From slide from Annie Hughes Review CO t in clouds HI: Atomic Hydrogen http://www.atnf.csiro.au/research/lvmeeting/magsys_pres/
3) Of the following, radiation has the shortest wavelength. A) X-ray B) radio C) microwave D) ultraviolet E) infrared Answer: A
 1) Which one of the following is correct? A) ν + λ = c B) ν λ = c C) ν = cλ D) λ = c ν E) νλ = c Answer: E 2) The wavelength of light emitted from a traffic light having a frequency of 5.75 1014 Hz is.
1) Which one of the following is correct? A) ν + λ = c B) ν λ = c C) ν = cλ D) λ = c ν E) νλ = c Answer: E 2) The wavelength of light emitted from a traffic light having a frequency of 5.75 1014 Hz is.
3.091 Fall Term 2002 Homework #4 Solutions
 3.091 all Term 2002 omework #4 olutions 5-5. We imply that sodium is a better electron donor than lithium. Evidence for this can be found in the lower value of AVEE which for these two elements is equivalent
3.091 all Term 2002 omework #4 olutions 5-5. We imply that sodium is a better electron donor than lithium. Evidence for this can be found in the lower value of AVEE which for these two elements is equivalent
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Photons. ConcepTest 27.1. 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy. Which has more energy, a photon of:
 ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
THE PERIODIC TABLE O F T H E E L E M E N T S. The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27)
 THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Atomic structure. Chapter 9
 Chapter 9 Atomic structure Previously, we have seen that the quantum mechanics of atomic hydrogen, and hydrogen-like atoms is characterized by a large degeneracy with eigenvalues separating into multiplets
Chapter 9 Atomic structure Previously, we have seen that the quantum mechanics of atomic hydrogen, and hydrogen-like atoms is characterized by a large degeneracy with eigenvalues separating into multiplets
MASS DEFECT AND BINDING ENERGY
 MASS DEFECT AND BINDING ENERGY The separate laws of Conservation of Mass and Conservation of Energy are not applied strictly on the nuclear level. It is possible to convert between mass and energy. Instead
MASS DEFECT AND BINDING ENERGY The separate laws of Conservation of Mass and Conservation of Energy are not applied strictly on the nuclear level. It is possible to convert between mass and energy. Instead
The Periodic Table; Chapter 5: Section 1 - History of the Periodic Table Objectives: Explain the roles of Mendeleev and Moseley in the development of
 The Periodic Table; Chapter 5: Section 1 - History of the Periodic Table Objectives: Explain the roles of Mendeleev and Moseley in the development of the periodic table. Describe the modern periodic table.
The Periodic Table; Chapter 5: Section 1 - History of the Periodic Table Objectives: Explain the roles of Mendeleev and Moseley in the development of the periodic table. Describe the modern periodic table.
Ernest Rutherford Atomic Model 1911. Plum Pudding Model J.J. Thomson 1897
 1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
1 The arrangement of electrons in an atom determine most of the chemical properties of that atom. Electrons are what actually do the reacting. Plum Pudding Model J.J. Thomson 1897 Ernest Rutherford Atomic
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Experiment #5: Qualitative Absorption Spectroscopy
 Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D
 Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
Electrons In Atoms Mr. O Brien (SFHS) Chapter 5 Standard 1D Electrons in Atoms (std.1d) What are Bohr Models? planetary model in which the negatively-charged electrons orbit a small, positively-charged
5.61 Physical Chemistry 25 Helium Atom page 1 HELIUM ATOM
 5.6 Physical Chemistry 5 Helium Atom page HELIUM ATOM Now that we have treated the Hydrogen like atoms in some detail, we now proceed to discuss the next simplest system: the Helium atom. In this situation,
5.6 Physical Chemistry 5 Helium Atom page HELIUM ATOM Now that we have treated the Hydrogen like atoms in some detail, we now proceed to discuss the next simplest system: the Helium atom. In this situation,
NMR and IR spectra & vibrational analysis
 Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
electron configuration
 electron configuration Electron Configuration Knowing the arrangement of electrons in atoms will better help you understand chemical reactivity and predict an atom s reaction behavior. We know when n=1
electron configuration Electron Configuration Knowing the arrangement of electrons in atoms will better help you understand chemical reactivity and predict an atom s reaction behavior. We know when n=1
Austin Peay State University Department of Chemistry Chem 1111. The Use of the Spectrophotometer and Beer's Law
 Purpose To become familiar with using a spectrophotometer and gain an understanding of Beer s law and it s relationship to solution concentration. Introduction Scientists use many methods to determine
Purpose To become familiar with using a spectrophotometer and gain an understanding of Beer s law and it s relationship to solution concentration. Introduction Scientists use many methods to determine
Chapter Test. Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a.
 Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Chapter 3. Elements, Atoms, Ions, and the Periodic Table
 Chapter 3. Elements, Atoms, Ions, and the Periodic Table The Periodic Law and the Periodic Table In the early 1800's many elements had been discovered and found to have different properties. In 1817 Döbreiner's
Chapter 3. Elements, Atoms, Ions, and the Periodic Table The Periodic Law and the Periodic Table In the early 1800's many elements had been discovered and found to have different properties. In 1817 Döbreiner's
AP CHEMISTRY 2009 SCORING GUIDELINES
 AP CHEMISTRY 2009 SCORING GUIDELINES Question 6 (8 points) Answer the following questions related to sulfur and one of its compounds. (a) Consider the two chemical species S and S 2. (i) Write the electron
AP CHEMISTRY 2009 SCORING GUIDELINES Question 6 (8 points) Answer the following questions related to sulfur and one of its compounds. (a) Consider the two chemical species S and S 2. (i) Write the electron
