Determine the polarity of several molecules and their solubility.
|
|
|
- Merilyn Wheeler
- 7 years ago
- Views:
Transcription
1 Exploring Polarity & Solubility Using WebMO The purpose of this activity is to compare the polarity of solute and solvent molecules to determine if their polarity affects how well they mix. Solute and solvent molecules that readily mix are said to form a solution. A common measure of how well they mix is referred to as solubility. Solubility measures the amount of solute that can mix in a given amount of solute. You will be using the Internet to access a program called WebMO. Using the program will allow you to view the geometry of a molecule and determine the polarity. During the activity students will import the following molecules and determine the polarity direction and strength of each molecule: C 6 H 14 hexane CH 3 CH 2 CH 3 -- propane H 2 O water CH 3 CH 2 -OH ethanol H 3 C-O-CH 3 dimethyl ether CO 2 -- carbon dioxide HF hydrogen fluoride Polarity is a measure of how symmetrically electrons are distributed relative to the proton distribution in the atoms of a molecule. Molecules in which the electrons are not symmetrically distributed are said to possess a dipole moment. The dipole moment is expressed in the unit Debyes and can be represented by an arrow. The head of the arrow points in the direction of greater electron concentration. The tail of the arrow points to the region of the molecule with a lower electron concentration. You will examine the molecules to determine the direction of the dipole arrow (polarity direction) and identify the strength of the dipole moment (calculated value in Debyes). To begin the activity, access the Internet and Go to Wait for the site to redirect to the login page. Sign on with the username and password provided by your teacher. Exercise 1 Determine the polarity of several molecules and their solubility. The molecules for this activity have been assembled and saved as Jmol files (*.mol) for import. You will need to import the files one at a time and process them as indicated below in order to observe the molecular geometries and dipole moments. Importing the Molecules: Your instructor has saved the Jmol files in a directory to which you have access. You will be able to access the files using the browse control during import. Preparing to import a molecule: This part assumes you have logged onto WebMO and are at the Job Manager" screen. Click the New Job menu option at the top left of the WebMO viewing screen and select the "Create New Job" option on the drop down menu list shown below University of Illinois Board of Trustees
2 1 A Build Molecule" window will open as shown below. Click on the "Import Molecule" button at the bottom right of the window. This will open the "Import Molecule" window shown below. Click on the "Browse" button at the center right to locate the file for import. Double click on the file name to upload it to the import molecule window. With the file selected, click on the "Import Molecule" button at the bottom left and the file will be imported into the "Build Molecule" window as shown below.
3 2 The next step is to process the molecule through the calculation engine of the software. To begin this process, click on the blue arrow at the bottom right of the Build Molecule screen. This will open the "Choose Computational Engine" screen shown below. A list of five selectable engines can be seen at the center left of the window. Select the engine titled "Mopac" by clicking on the circle to the left of the engine name. Once the engine has been selected, click on the blue arrow at the bottom right of the window. This will open the "Configure Mopac Job Options" window shown below. Modify the job name by appending your initials or other identifier after the chemical formula (C2H6O in the example). Use the pull down tab in the "Calculations menu to view the drop down list. Select "Geometry Optimization" as shown in the example. Finally, click on the blue
4 arrow at the bottom right of the screen to submit the job. This will return you to the main Job Manager" screen as show below. 3 You will see your job listed as a line entry (Number, Name, Description, Date, Status, Time, and Actions). When the computer is finished with its computations, the job status will be shown as "complete" (as shown in the example above). You are now ready to access the job, view the molecule, and determine the direction and magnitude of the dipole moment. Click on the job name to open the window shown below. The molecule viewing window is located at the top of the screen. If you scroll down, you will find the "Calculated Quantities" window. Locate the dipole moment listing in the calculated quantities. Record the value given in the table provided within the lab handout. If you click on the magnifying glass icon, the molecule in the viewing window will display a blue arrow showing the location and direction of the dipole moment. You can rotate the molecule using your mouse to view the dipole moment from different vantage points. Repeat the process until you have observed all of the molecules. Gathering solubility and experimental dipole moments for your molecules: Information on the solubility of the molecules included in this activity can be found at a variety of websites. To search for information of the solubilities in water or hexane, one might use a search query such as: ethanol solubility in water
5 In a similar manner, one might search for experimentally measured dipole moments using a query such as: ethanol dipole moment To completely fill in the chart below, you will need to do a search on the Internet for the experimental dipole moments and solubilities of each of the molecules in water and hexane. Record the dipole moments obtained from WebMO (calculated) and the experimental dipole moments from the Internet search. Use parentheses to set off the experimental value. The polarity of a molecule increases as its dipole moment increases. 4 Formula Name Dipole (Debye) C 6 H 14 H 2 O CH 3 OCH 3 CH 3 CH 2 OH HF CH 3 CH 2 CH 3 CO 2 Polarity Solubility Predictions H 2 O C 6 H 14 xxxxxxxxxx xxxxxxxxxxx A completely nonpolar molecule is expected to have a dipole moment at zero. The dipole moment is a measure of the force of attraction or repulsion per unit of mass on the affected particle. The positive end of a dipole attracts the negative end of other dipoles; it repels the positive end of other dipoles. Hence, polar molecules tend to exhibit mutual attraction for one another. They interact only very weakly with nonpolar molecules. From this tendency we get the simple rule Like dissolves like. Using this rule, predict which of the molecules in this activity are soluble in H 2 O and/or C 6 H 14. Compare your predictions with the solubilities found on the Internet. Dipole Moment Order Formula Solubility (include units) H 2 O C 6 H 14 Least To Greatest
6 Questions: 1) What relationship do you see between the polarity of a substance and its solubility in water? (In the first six molecules.) What reasons can you give for this relationship. (Hint: think about what is happening in a polar molecule vs. a nonpolar molecule.) 2) Rank the substances in order of their polarity from least polar to most polar. Does the solubility of these molecules in water show a pattern? If you observe a general pattern, or relationship, between polarity and water solubility, describe the pattern. 3) Does the solubility and polarity of CO 2 fit the pattern? If not, what possible explanation can you offer? 4) Assuming you are able to locate the solubilities of the molecules in hexane, does the solubility of the molecules in hexane show a pattern? If you observe a general pattern, or relationship, between polarity and hexane solubility, describe the pattern. 5) In what ways has this activity helped you understand the relationship between solubility and polarity? 5
7 Teacher Notes: Determining Solubility: Keeping in mind that Like dissolves like, which of the following are soluble in H 2 O and/or CCl 4. Formula Name Dipole (Debye) Polarity Soluble in H 2 O Soluble in C 6 H 12 C 6 H 12 hexane nonpolar xxxxxxxxxx 20ºC H 2 O water polar xxxxxxxxxxx (1.85) CH 3 OCH 3 dimethyl ether polar 20ºC (1.30) CH 3 CH 2 OH ethanol polar miscible (1.69) HF Hydrogen fluoride polar miscible (1.91) CH 3 CH 2 CH 3 propane nonpolar 20ºC (0.08) CO 2 Carbon dioxide nonpolar ºC 6
POLAR COVALENT BONDS Ionic compounds form repeating. Covalent compounds form distinct. Consider adding to NaCl(s) vs. H 2 O(s):
 POLAR COVALENT BONDS Ionic compounds form repeating. Covalent compounds form distinct. Consider adding to NaCl(s) vs. H 2 O(s): Sometimes when atoms of two different elements form a bond by sharing an
POLAR COVALENT BONDS Ionic compounds form repeating. Covalent compounds form distinct. Consider adding to NaCl(s) vs. H 2 O(s): Sometimes when atoms of two different elements form a bond by sharing an
DCI for Electronegativity. Data Table:
 DCI for Electronegativity Data Table: Substance Ionic/covalent EN value EN Value EN NaCl ionic (Na) 0.9 (Cl) 3.0 2.1 KBr (K) 0.8 (Br) 2.8 MgO (Mg) 1.2 (O) 3.5 HCl (H) 2.1 (Cl) 3.0 HF (H) 2.1 (F) 4.0 Cl
DCI for Electronegativity Data Table: Substance Ionic/covalent EN value EN Value EN NaCl ionic (Na) 0.9 (Cl) 3.0 2.1 KBr (K) 0.8 (Br) 2.8 MgO (Mg) 1.2 (O) 3.5 HCl (H) 2.1 (Cl) 3.0 HF (H) 2.1 (F) 4.0 Cl
3/5/2014. iclicker Participation Question: A. MgS < AlP < NaCl B. MgS < NaCl < AlP C. NaCl < AlP < MgS D. NaCl < MgS < AlP
 Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Chapter 2 Polar Covalent Bonds; Acids and Bases
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE
 POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE LAB MOD4.COMP From Gannon University SIM INTRODUCTION Many physical properties of matter, such as boiling point and melting point, are the result of the
POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE LAB MOD4.COMP From Gannon University SIM INTRODUCTION Many physical properties of matter, such as boiling point and melting point, are the result of the
5. Structure, Geometry, and Polarity of Molecules
 5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
Reporting Continuing Education Credits on the CMTBC Online Portal
 Reporting Continuing Education Credits on the CMTBC Online Portal As part of CMTBC s Quality Assurance program, RMTs in BC receive continuing education credits (CECs) for completing approved continuing
Reporting Continuing Education Credits on the CMTBC Online Portal As part of CMTBC s Quality Assurance program, RMTs in BC receive continuing education credits (CECs) for completing approved continuing
In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and oxygen. [Include any charges or partial charges.
 Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
e-portal Web Orders Instructions:
 e-portal Web Orders Instructions: Login Page Please save the http://opa.uchc.edu/uchc_eportal_1.htm in your favorites. This is the page that you must Login from. Important information regarding the system
e-portal Web Orders Instructions: Login Page Please save the http://opa.uchc.edu/uchc_eportal_1.htm in your favorites. This is the page that you must Login from. Important information regarding the system
Molecular Geometry & Polarity
 Name AP Chemistry Molecular Geometry & Polarity Molecular Geometry A key to understanding the wide range of physical and chemical properties of substances is recognizing that atoms combine with other atoms
Name AP Chemistry Molecular Geometry & Polarity Molecular Geometry A key to understanding the wide range of physical and chemical properties of substances is recognizing that atoms combine with other atoms
Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134)
 Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134) 1. Helium atoms do not combine to form He 2 molecules, What is the strongest attractive
Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134) 1. Helium atoms do not combine to form He 2 molecules, What is the strongest attractive
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
EXPERIMENT 9 Dot Structures and Geometries of Molecules
 EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s)
 BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1. Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57, 61, 63, 67, 69, 71(a), 73, 75, 79
 Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1 Text: Petrucci, Harwood, Herring 8 th Edition Suggest text problems Review questions: 1, 5!11, 13!17, 19!23 Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57,
Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1 Text: Petrucci, Harwood, Herring 8 th Edition Suggest text problems Review questions: 1, 5!11, 13!17, 19!23 Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57,
How to Use JCWHosting Reseller Cloud Storage Solution
 How to Use JCWHosting Reseller Cloud Storage Solution Go to https://www.internetspace.co.za and log in with your Cloud Reseller account username and password. How to Use create a cloud account for your
How to Use JCWHosting Reseller Cloud Storage Solution Go to https://www.internetspace.co.za and log in with your Cloud Reseller account username and password. How to Use create a cloud account for your
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory
 EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
Chapter 2 Polar Covalent Bonds: Acids and Bases
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds: Acids and Bases Modified by Dr. Daniela R. Radu Why This Chapter? Description of basic ways chemists account for chemical
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds: Acids and Bases Modified by Dr. Daniela R. Radu Why This Chapter? Description of basic ways chemists account for chemical
Organic Functional Groups Chapter 7. Alcohols, Ethers and More
 Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
Joining an XP workstation to a domain Version 1.00
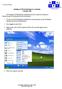 Joining an XP workstation to a domain Version 1.00 All Windows XP Professional workstations need to be joined to a domain to function as part of the domain security environment. Need to Know TM 1. To join
Joining an XP workstation to a domain Version 1.00 All Windows XP Professional workstations need to be joined to a domain to function as part of the domain security environment. Need to Know TM 1. To join
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Section Activity #1: Fill out the following table for biology s most common elements assuming that each atom is neutrally charged.
 LS1a Fall 2014 Section Week #1 I. Valence Electrons and Bonding The number of valence (outer shell) electrons in an atom determines how many bonds it can form. Knowing the number of valence electrons present
LS1a Fall 2014 Section Week #1 I. Valence Electrons and Bonding The number of valence (outer shell) electrons in an atom determines how many bonds it can form. Knowing the number of valence electrons present
Outlook Web Access. PRECEDED by v\
 Outlook Web Access Logging in to OWA (Outlook Web Access) from Home 1. Login page http://mail.vernonct.org/exchange 2. To avoid these steps each time you login, you can add the login page to your favorites.
Outlook Web Access Logging in to OWA (Outlook Web Access) from Home 1. Login page http://mail.vernonct.org/exchange 2. To avoid these steps each time you login, you can add the login page to your favorites.
Drip Marketing Campaign Manual
 Drip Marketing Campaign Manual Released May 2006 Manual for Drip Marketing Campaign: Getting Started 1. Log into www.graphicaldata.com. 2. Hold cursor over the Tools tab. 3. Click on Drip Marketing Campaign.
Drip Marketing Campaign Manual Released May 2006 Manual for Drip Marketing Campaign: Getting Started 1. Log into www.graphicaldata.com. 2. Hold cursor over the Tools tab. 3. Click on Drip Marketing Campaign.
VSEPR Model. The Valence-Shell Electron Pair Repulsion Model. Predicting Molecular Geometry
 VSEPR Model The structure around a given atom is determined principally by minimizing electron pair repulsions. The Valence-Shell Electron Pair Repulsion Model The valence-shell electron pair repulsion
VSEPR Model The structure around a given atom is determined principally by minimizing electron pair repulsions. The Valence-Shell Electron Pair Repulsion Model The valence-shell electron pair repulsion
Mobile: Getting Started with Workday for ipad
 Install and Log in to Workday From your ipad: 1. Tap the App Store Application icon. 2. Tap Search and enter Workday. 3. Select Workday for ipad from the search results. 4. Tap Free to install the app.
Install and Log in to Workday From your ipad: 1. Tap the App Store Application icon. 2. Tap Search and enter Workday. 3. Select Workday for ipad from the search results. 4. Tap Free to install the app.
AP* Bonding & Molecular Structure Free Response Questions page 1
 AP* Bonding & Molecular Structure ree Response Questions page 1 (1) AP is a registered trademark of the ollege Board. The ollege Board was not involved in the production of and does not endorse this product.
AP* Bonding & Molecular Structure ree Response Questions page 1 (1) AP is a registered trademark of the ollege Board. The ollege Board was not involved in the production of and does not endorse this product.
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
OSPI SFTP User Guide
 OSPI SFTP User Guide NOTE: Please contact OSPI to request an account BEFORE setting up this software. In order to configure the software you will need account information from OSPI. Here are some steps
OSPI SFTP User Guide NOTE: Please contact OSPI to request an account BEFORE setting up this software. In order to configure the software you will need account information from OSPI. Here are some steps
Vestal Central School District New Service Desk System: Service-Now Go live July 2, 2011
 435 Glenwood Road, Binghamton, NY 13905-1609 Service Desk Phone: (607) 766-3800 Vestal Central School District New Service Desk System: Service-Now Go live July 2, 2011 In order to help facilitate increased
435 Glenwood Road, Binghamton, NY 13905-1609 Service Desk Phone: (607) 766-3800 Vestal Central School District New Service Desk System: Service-Now Go live July 2, 2011 In order to help facilitate increased
Gravity Forms: Creating a Form
 Gravity Forms: Creating a Form 1. To create a Gravity Form, you must be logged in as an Administrator. This is accomplished by going to http://your_url/wp- login.php. 2. On the login screen, enter your
Gravity Forms: Creating a Form 1. To create a Gravity Form, you must be logged in as an Administrator. This is accomplished by going to http://your_url/wp- login.php. 2. On the login screen, enter your
Bonding Practice Problems
 NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
AVDC Document Management System Getting Started
 Page 1 of 7 AVDC Document Management System Getting Started Login Upon entering the AVDC document management system (http://www.avdc-dms.org/dms/ or via the link on the AVDC web site Home page), the initial
Page 1 of 7 AVDC Document Management System Getting Started Login Upon entering the AVDC document management system (http://www.avdc-dms.org/dms/ or via the link on the AVDC web site Home page), the initial
IB Chemistry. DP Chemistry Review
 DP Chemistry Review Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant Assessment statement Apply the mole concept to substances. Determine the number of particles and the amount
DP Chemistry Review Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant Assessment statement Apply the mole concept to substances. Determine the number of particles and the amount
Check current version of Remote Desktop Connection for Mac.. Page 2. Remove Old Version Remote Desktop Connection..Page 8
 CONTENTS SECTION 1 Check current version of Remote Desktop Connection for Mac.. Page 2 SECTION 2 Remove Old Version Remote Desktop Connection..Page 8 SECTION 3 Download and Install Remote Desktop Connection
CONTENTS SECTION 1 Check current version of Remote Desktop Connection for Mac.. Page 2 SECTION 2 Remove Old Version Remote Desktop Connection..Page 8 SECTION 3 Download and Install Remote Desktop Connection
Molecular Geometry and Chemical Bonding Theory
 Chapter 10 Molecular Geometry and Chemical Bonding Theory Concept Check 10.1 An atom in a molecule is surrounded by four pairs of electrons, one lone pair and three bonding pairs. Describe how the four
Chapter 10 Molecular Geometry and Chemical Bonding Theory Concept Check 10.1 An atom in a molecule is surrounded by four pairs of electrons, one lone pair and three bonding pairs. Describe how the four
Facilities and Safety How-To Guide: Accessing and Using Your UCF Webmail Account
 Launch Internet Explorer Click on the Internet Explorer icon at the bottom left of the computer screen. Go to the UCF Webmail Website 1. In the address bar at the top of the screen, type webmail.ucf.edu.
Launch Internet Explorer Click on the Internet Explorer icon at the bottom left of the computer screen. Go to the UCF Webmail Website 1. In the address bar at the top of the screen, type webmail.ucf.edu.
The elements of the second row fulfill the octet rule by sharing eight electrons, thus acquiring the electronic configuration of neon, the noble gas o
 2. VALENT BNDING, TET RULE, PLARITY, AND BASI TYPES F FRMULAS LEARNING BJETIVES To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its
2. VALENT BNDING, TET RULE, PLARITY, AND BASI TYPES F FRMULAS LEARNING BJETIVES To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY
 EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY Purpose: 1. To distinguish between different types of chemical bonds. 2. To predict the polarity of some common molecules from a knowledge of bond
EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY Purpose: 1. To distinguish between different types of chemical bonds. 2. To predict the polarity of some common molecules from a knowledge of bond
pre -TEST Big Idea 2 Chapters 8, 9, 10
 Name: AP Chemistry Period: Date: R.F. Mandes, PhD, NBCT Complete each table with the appropriate information. Compound IMF Compound IMF 1 NiCl 3 7 ClCH 2 (CH 2 ) 3 CH 3 2 Fe 8 H 2 CF 2 3 Ar 9 H 2 NCH 2
Name: AP Chemistry Period: Date: R.F. Mandes, PhD, NBCT Complete each table with the appropriate information. Compound IMF Compound IMF 1 NiCl 3 7 ClCH 2 (CH 2 ) 3 CH 3 2 Fe 8 H 2 CF 2 3 Ar 9 H 2 NCH 2
Using Outlook Web Access
 Using Outlook Web Access Log on JTSA Outlook Web Access 1. Enter the following URL into the address bar on your web browser (Internet Explorer recommended) and press enter http://exweb.jtsa.edu 2. The
Using Outlook Web Access Log on JTSA Outlook Web Access 1. Enter the following URL into the address bar on your web browser (Internet Explorer recommended) and press enter http://exweb.jtsa.edu 2. The
The Lynx A Step by Step guide
 The Lynx A Step by Step guide Section 1: Updating your organization and recruiting tips (pg.1) Section 2: For page managers (pg. 5) Section 3: Club Sports specific tasks (pg. 12) Section 4: New Users and
The Lynx A Step by Step guide Section 1: Updating your organization and recruiting tips (pg.1) Section 2: For page managers (pg. 5) Section 3: Club Sports specific tasks (pg. 12) Section 4: New Users and
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Using STAGES. Logging into STAGES. Verifying your User Profile
 Using STAGES Logging into STAGES 1. You will receive an email letting you know that your evaluation has been started. 2. Click on the link provided in the email to access the STAGES Website. 3. Enter your
Using STAGES Logging into STAGES 1. You will receive an email letting you know that your evaluation has been started. 2. Click on the link provided in the email to access the STAGES Website. 3. Enter your
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
NextGen Setup Guide First-time Workstation Setup & Logging In
 This guide will help you get setup on NextGen for the first time you log onto a City or SBC computer. It will also help if you are a first-time user and need to create your password. I. Setting up Desktop
This guide will help you get setup on NextGen for the first time you log onto a City or SBC computer. It will also help if you are a first-time user and need to create your password. I. Setting up Desktop
Accessing the Technology Help Desk Self-Service site:
 Technology Help Desk Self-Service Site Find Technology Solutions and Manage Work Ordersfor all current faculty, staff and students of Philadelphia University Requirements a personal computer connected
Technology Help Desk Self-Service Site Find Technology Solutions and Manage Work Ordersfor all current faculty, staff and students of Philadelphia University Requirements a personal computer connected
Chapter 4 Lecture Notes
 Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Element of same atomic number, but different atomic mass o Example: Hydrogen
 Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Quick Start Guide Students must wait until the first day of the semester to gain access to their online and hybrid course(s).
 Quick Start Guide Students must wait until the first day of the semester to gain access to their online and hybrid course(s). The following topics are covered in this guide: How to Login to Moodle Locating
Quick Start Guide Students must wait until the first day of the semester to gain access to their online and hybrid course(s). The following topics are covered in this guide: How to Login to Moodle Locating
Use the Force! Noncovalent Molecular Forces
 Use the Force! Noncovalent Molecular Forces Not quite the type of Force we re talking about Before we talk about noncovalent molecular forces, let s talk very briefly about covalent bonds. The Illustrated
Use the Force! Noncovalent Molecular Forces Not quite the type of Force we re talking about Before we talk about noncovalent molecular forces, let s talk very briefly about covalent bonds. The Illustrated
Tutorial of Deltek s Expense Report Domestic Travel Edition
 Tutorial of Deltek s Expense Report Domestic Travel Edition Please note that any expense incurred by an employee and to be reimbursed by BCF Solutions, Inc. has to be through an expense report. Step 1:
Tutorial of Deltek s Expense Report Domestic Travel Edition Please note that any expense incurred by an employee and to be reimbursed by BCF Solutions, Inc. has to be through an expense report. Step 1:
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models
 EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
Name: Class: Date: 2) Which one of the following exhibits dipole-dipole attraction between molecules? A) XeF 4 B) AsH 3 C) CO 2 D) BCl 3 E) Cl 2
 Name: Class: Date: IM Bonding 1) In liquids, the attractive intermolecular forces are. A) very weak compared with kinetic energies of the molecules B) strong enough to hold molecules relatively close together
Name: Class: Date: IM Bonding 1) In liquids, the attractive intermolecular forces are. A) very weak compared with kinetic energies of the molecules B) strong enough to hold molecules relatively close together
CI Financial Exception Dashboard. User Manual
 CI Financial Exception Dashboard User Manual CONTENTS THE CI FINANCIAL EXCEPTION DASHBOARD... 2 ACCESSING ANU INSIGHT... 2 LOGGING INTO ANU INSIGHT... 3 NAVIGATING TO THE DASHBOARD... 4 THE INSIGHT HOMEPAGE...
CI Financial Exception Dashboard User Manual CONTENTS THE CI FINANCIAL EXCEPTION DASHBOARD... 2 ACCESSING ANU INSIGHT... 2 LOGGING INTO ANU INSIGHT... 3 NAVIGATING TO THE DASHBOARD... 4 THE INSIGHT HOMEPAGE...
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1. A chemical equation. (C-4.4)
 Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
Intermolecular and Ionic Forces
 Intermolecular and Ionic Forces Introduction: Molecules are attracted to each other in the liquid and solid states by intermolecular, or attractive, forces. These are the attractions that must be overcome
Intermolecular and Ionic Forces Introduction: Molecules are attracted to each other in the liquid and solid states by intermolecular, or attractive, forces. These are the attractions that must be overcome
Creating an Event Registration Web Page with Special Features using regonline Page 1
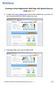 Creating an Event Registration Web Page with Special Features using regonline 1. To begin, enter www.regonline.com in your browser s address bar. A red arrow on each screen shot shows you where to place
Creating an Event Registration Web Page with Special Features using regonline 1. To begin, enter www.regonline.com in your browser s address bar. A red arrow on each screen shot shows you where to place
12-Steps: Searching & Registering for Classes
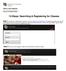 Office of the Registrar. Division of Student Affairs 12-Steps: Searching & Registering for Classes Step 1: Access your web browser and log on to www.ucdenver.edu/ucdaccess. Proceed to login with your User
Office of the Registrar. Division of Student Affairs 12-Steps: Searching & Registering for Classes Step 1: Access your web browser and log on to www.ucdenver.edu/ucdaccess. Proceed to login with your User
Chapter 4. Chemical Composition. Chapter 4 Topics H 2 S. 4.1 Mole Quantities. The Mole Scale. Molar Mass The Mass of 1 Mole
 Chapter 4 Chemical Composition Chapter 4 Topics 1. Mole Quantities 2. Moles, Masses, and Particles 3. Determining Empirical Formulas 4. Chemical Composition of Solutions Copyright The McGraw-Hill Companies,
Chapter 4 Chemical Composition Chapter 4 Topics 1. Mole Quantities 2. Moles, Masses, and Particles 3. Determining Empirical Formulas 4. Chemical Composition of Solutions Copyright The McGraw-Hill Companies,
Logging in to Google Chrome
 Logging in to Google Chrome By logging in to Google Chrome, you will be able to quickly access any saved applications, bookmarks, and resources from any location. Please remember...if you are using a lab
Logging in to Google Chrome By logging in to Google Chrome, you will be able to quickly access any saved applications, bookmarks, and resources from any location. Please remember...if you are using a lab
Unit 11 Practice. Name: Class: Date: Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Class: Date: Unit 11 Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Crystalline solids. A) have their particles arranged randomly B) have
Name: Class: Date: Unit 11 Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Crystalline solids. A) have their particles arranged randomly B) have
How To Use Webmail. Guiding you through the Universities online email
 How To Use Webmail Guiding you through the Universities online email Table of Contents LOGGING ON...2 VIEWING MESSAGES...2 SENDING A MESSAGE...3 Using the University s Address Book...3 To send a message
How To Use Webmail Guiding you through the Universities online email Table of Contents LOGGING ON...2 VIEWING MESSAGES...2 SENDING A MESSAGE...3 Using the University s Address Book...3 To send a message
Getting the most from this book...4 About this book...5
 Contents Getting the most from this book...4 About this book....5 Content Guidance Topic 1 Atomic structure and the periodic table...8 Topic 2 Bonding and structure...14 Topic 2A Bonding....14 Topic 2B
Contents Getting the most from this book...4 About this book....5 Content Guidance Topic 1 Atomic structure and the periodic table...8 Topic 2 Bonding and structure...14 Topic 2A Bonding....14 Topic 2B
Schools Remote Access Server
 Schools Remote Access Server This system is for school use only. Not for personal or private file use. Please observe all of the school district IT rules. 6076 State Farm Rd., Guilderland, NY 12084 Phone:
Schools Remote Access Server This system is for school use only. Not for personal or private file use. Please observe all of the school district IT rules. 6076 State Farm Rd., Guilderland, NY 12084 Phone:
Accessing the Illinois CRM Report Archive Database
 Accessing the Illinois CRM Report Archive Database Connecting to the Illinois CRM Report Archive Database The Illinois CRM Report Archive is a web-based database. Browsers supported are: Windows: Internet
Accessing the Illinois CRM Report Archive Database Connecting to the Illinois CRM Report Archive Database The Illinois CRM Report Archive is a web-based database. Browsers supported are: Windows: Internet
How to search for and view our school district's e-book/audio book collections on a computer
 How to search for and view our school district's e-book/audio book collections on a computer If you are logged into a computer anywhere in the world, you will be able to view and read e-books, and listen
How to search for and view our school district's e-book/audio book collections on a computer If you are logged into a computer anywhere in the world, you will be able to view and read e-books, and listen
(You will use the login ID and password below to login through the first two websites.)
 (You will use the login ID and password below to login through the first two websites.) https://providers.cvch.com Citrix Logon: Password: Press the SKIP TO LOG ON selection after
(You will use the login ID and password below to login through the first two websites.) https://providers.cvch.com Citrix Logon: Password: Press the SKIP TO LOG ON selection after
TBR System Office Performance Management Employee s Guide
 TBR System Office Performance Management Employee s Guide A Step-By-Step Employee Guide for completing performance evaluations in the PeopleAdmin Performance Management Suite PeopleAdmin 7.0 is a multi-functional
TBR System Office Performance Management Employee s Guide A Step-By-Step Employee Guide for completing performance evaluations in the PeopleAdmin Performance Management Suite PeopleAdmin 7.0 is a multi-functional
Piazza in Blackboard for Instructors
 Piazza in Blackboard for Instructors Piazza is an online platform designed to facilitate interaction among students and instructors and efficiently manage class Q&A s. Students can post questions and collaborate
Piazza in Blackboard for Instructors Piazza is an online platform designed to facilitate interaction among students and instructors and efficiently manage class Q&A s. Students can post questions and collaborate
Verified Volunteers. System User Guide 10/2014. For assistance while navigating through the system, please contact Client Services at:
 Verified Volunteers System User Guide 10/2014 For assistance while navigating through the system, please contact Client Services at: RCAN@verifiedvolunteers.com - (855) 326-1860 - Option 1 Welcome to Verified
Verified Volunteers System User Guide 10/2014 For assistance while navigating through the system, please contact Client Services at: RCAN@verifiedvolunteers.com - (855) 326-1860 - Option 1 Welcome to Verified
Microsoft OneDrive. How to login to OneDrive:
 Microsoft OneDrive The beauty of OneDrive is that it is accessible from anywhere you have an Internet connection. You can access it from a Mac or Windows computer. You can even access it on your Smartphone
Microsoft OneDrive The beauty of OneDrive is that it is accessible from anywhere you have an Internet connection. You can access it from a Mac or Windows computer. You can even access it on your Smartphone
How To Use Textbuster On Android (For Free) On A Cell Phone
 www.textbuster.com 1 Applications and Account Manager Dashboard User Guide For Android phones www.textbuster.com 2 Downloading the TextBuster applications After the TextBuster device is installed into
www.textbuster.com 1 Applications and Account Manager Dashboard User Guide For Android phones www.textbuster.com 2 Downloading the TextBuster applications After the TextBuster device is installed into
Name Lab #3: Solubility of Organic Compounds Objectives: Introduction: soluble insoluble partially soluble miscible immiscible
 Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Instructions to Add Faculty Exceptions and Justifications for Teaching Credentials Effective Fall 2015
 Our regional accreditor, the Southern Association of Colleges and Schools Commission on Colleges (SACSCOC), requires that we document the qualifications and credentials of ALL faculty members (irrespective
Our regional accreditor, the Southern Association of Colleges and Schools Commission on Colleges (SACSCOC), requires that we document the qualifications and credentials of ALL faculty members (irrespective
Education Solutions Development, Inc. APECS Navigation: Business Systems Getting Started Reference Guide
 Education Solutions Development, Inc. APECS Navigation: Business Systems Getting Started Reference Guide March 2013 Education Solutions Development, Inc. What s Inside The information in this reference
Education Solutions Development, Inc. APECS Navigation: Business Systems Getting Started Reference Guide March 2013 Education Solutions Development, Inc. What s Inside The information in this reference
Student Manager s Guide to the Talent Management System
 Department of Human Resources 50 Student Manager s Guide to the Talent Management System 1 Table of Contents Topic Page SYSTEM INTRODUCTION... 3 GETTING STARTED... 4 NAVIGATION WITHIN THE TALENT MANAGEMENT
Department of Human Resources 50 Student Manager s Guide to the Talent Management System 1 Table of Contents Topic Page SYSTEM INTRODUCTION... 3 GETTING STARTED... 4 NAVIGATION WITHIN THE TALENT MANAGEMENT
: : Solutions to Additional Bonding Problems
 Solutions to Additional Bonding Problems 1 1. For the following examples, the valence electron count is placed in parentheses after the empirical formula and only the resonance structures that satisfy
Solutions to Additional Bonding Problems 1 1. For the following examples, the valence electron count is placed in parentheses after the empirical formula and only the resonance structures that satisfy
INTERMOLECULAR FORCES
 INTERMOLECULAR FORCES Intermolecular forces- forces of attraction and repulsion between molecules that hold molecules, ions, and atoms together. Intramolecular - forces of chemical bonds within a molecule
INTERMOLECULAR FORCES Intermolecular forces- forces of attraction and repulsion between molecules that hold molecules, ions, and atoms together. Intramolecular - forces of chemical bonds within a molecule
The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride
 CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
University of Colorado Denver Anschutz Medical Campus Virtual EMS User s Guide
 University of Colorado Denver Anschutz Medical Campus Virtual EMS User s Guide Updated August 28, 2015 1 Table of Contents Getting Started... 3 Requesting an Account in Virtual EMS... 3 Logging in to Virtual
University of Colorado Denver Anschutz Medical Campus Virtual EMS User s Guide Updated August 28, 2015 1 Table of Contents Getting Started... 3 Requesting an Account in Virtual EMS... 3 Logging in to Virtual
Using the GroupWise Client
 Spring 2006 (Our appreciation to Jennifer Sherouse for her assistance in editing and improving this document) Page 1 of 15 What is the GroupWise Client The GroupWise client is a program that installs on
Spring 2006 (Our appreciation to Jennifer Sherouse for her assistance in editing and improving this document) Page 1 of 15 What is the GroupWise Client The GroupWise client is a program that installs on
Chapter 10 Molecular Geometry and Chemical Bonding Theory
 Chem 1: Chapter 10 Page 1 Chapter 10 Molecular Geometry and Chemical Bonding Theory I) VSEPR Model Valence-Shell Electron-Pair Repulsion Model A) Model predicts Predicts electron arrangement and molecular
Chem 1: Chapter 10 Page 1 Chapter 10 Molecular Geometry and Chemical Bonding Theory I) VSEPR Model Valence-Shell Electron-Pair Repulsion Model A) Model predicts Predicts electron arrangement and molecular
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
External Account Creation and Upload Instructions for the Local Government (LG) Audit Report Collection System
 External Account Creation and Upload Instructions for the Local Government (LG) Audit Report Collection System In order to submit data for any Department of Audits and Accounts (DOAA) web application,
External Account Creation and Upload Instructions for the Local Government (LG) Audit Report Collection System In order to submit data for any Department of Audits and Accounts (DOAA) web application,
End User Service Desk Guide
 435 Glenwood Road, Binghamton, NY 13905-1609 Service Desk Phone: (607) 766-3800 End User Service Desk Guide In order to help facilitate increased communication regarding user requests, the South Central
435 Glenwood Road, Binghamton, NY 13905-1609 Service Desk Phone: (607) 766-3800 End User Service Desk Guide In order to help facilitate increased communication regarding user requests, the South Central
Sample Exercise 8.1 Magnitudes of Lattice Energies
 Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the ionic compounds NaF, CsI, and CaO in order of increasing lattice energy. Analyze From the formulas for three
Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the ionic compounds NaF, CsI, and CaO in order of increasing lattice energy. Analyze From the formulas for three
10 The Mole. Section 10.1 Measuring Matter
 Name Date Class The Mole Section.1 Measuring Matter In your textbook, read about counting particles. In Column B, rank the quantities from Column A from smallest to largest. Column A Column B 0.5 mol 1.
Name Date Class The Mole Section.1 Measuring Matter In your textbook, read about counting particles. In Column B, rank the quantities from Column A from smallest to largest. Column A Column B 0.5 mol 1.
Sample Exercise 8.1 Magnitudes of Lattice Energies
 Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the following ionic compounds in order of increasing lattice energy: NaF, CsI, and CaO. Analyze: From the formulas
Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the following ionic compounds in order of increasing lattice energy: NaF, CsI, and CaO. Analyze: From the formulas
Virtual Office Remote Installation Guide
 Virtual Office Remote Installation Guide Table of Contents VIRTUAL OFFICE REMOTE INSTALLATION GUIDE... 3 UNIVERSAL PRINTER CONFIGURATION INSTRUCTIONS... 12 CHANGING DEFAULT PRINTERS ON LOCAL SYSTEM...
Virtual Office Remote Installation Guide Table of Contents VIRTUAL OFFICE REMOTE INSTALLATION GUIDE... 3 UNIVERSAL PRINTER CONFIGURATION INSTRUCTIONS... 12 CHANGING DEFAULT PRINTERS ON LOCAL SYSTEM...
THE CHILDREN S HEALTH NETWORK CONTRACTING TOOL TRAINING MANUAL
 THE CHILDREN S HEALTH NETWORK CONTRACTING TOOL TRAINING MANUAL 1 TCHN CONTRACTING TOOL TABLE OF CONTENTS 2 Overview 3 Step by Step Instructions 3 Logging In 4 The Main Menu Options 5 Creating Custom Lists
THE CHILDREN S HEALTH NETWORK CONTRACTING TOOL TRAINING MANUAL 1 TCHN CONTRACTING TOOL TABLE OF CONTENTS 2 Overview 3 Step by Step Instructions 3 Logging In 4 The Main Menu Options 5 Creating Custom Lists
SFSP Quick User Guide
 Page 1 of 11 SFSP Quick User Guide This guide will walk you through how to submit a SFSP application and a claim. Step 1 navigate to the CNPWeb Log into the CNPWeb (https://www.ade.az.gov/commonlogon/logon.aspx).
Page 1 of 11 SFSP Quick User Guide This guide will walk you through how to submit a SFSP application and a claim. Step 1 navigate to the CNPWeb Log into the CNPWeb (https://www.ade.az.gov/commonlogon/logon.aspx).
Classification of Chemical Substances
 Classification of Chemical Substances INTRODUCTION: Depending on the kind of bonding present in a chemical substance, the substance may be called ionic, molecular or metallic. In a solid ionic compound
Classification of Chemical Substances INTRODUCTION: Depending on the kind of bonding present in a chemical substance, the substance may be called ionic, molecular or metallic. In a solid ionic compound
FOR TEACHERS ONLY. The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING/CHEMISTRY
 FOR TEACHERS ONLY PS CH The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING/CHEMISTRY Wednesday, January 29, 2003 9:15 a.m. to 12:15 p.m., only SCORING KEY AND RATING
FOR TEACHERS ONLY PS CH The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING/CHEMISTRY Wednesday, January 29, 2003 9:15 a.m. to 12:15 p.m., only SCORING KEY AND RATING
Why? Intermolecular Forces. Intermolecular Forces. Chapter 12 IM Forces and Liquids. Covalent Bonding Forces for Comparison of Magnitude
 1 Why? Chapter 1 Intermolecular Forces and Liquids Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature for such a small molecule? Why does ice float on water?
1 Why? Chapter 1 Intermolecular Forces and Liquids Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature for such a small molecule? Why does ice float on water?
0 10 20 30 40 50 60 70 m/z
 Mass spectrum for the ionization of acetone MS of Acetone + Relative Abundance CH 3 H 3 C O + M 15 (loss of methyl) + O H 3 C CH 3 43 58 0 10 20 30 40 50 60 70 m/z It is difficult to identify the ions
Mass spectrum for the ionization of acetone MS of Acetone + Relative Abundance CH 3 H 3 C O + M 15 (loss of methyl) + O H 3 C CH 3 43 58 0 10 20 30 40 50 60 70 m/z It is difficult to identify the ions
GRS Advantage Website User Reference Guide
 GRS Advantage Website User Reference Guide This document describes how to use the GRS Advantage Website. Table of Contents GRS Advantage Website... 2 Accessing the Website... 2 Requesting Access to the
GRS Advantage Website User Reference Guide This document describes how to use the GRS Advantage Website. Table of Contents GRS Advantage Website... 2 Accessing the Website... 2 Requesting Access to the
