Mechanisms of ectodermal organogenesis
|
|
|
- Arron Hilary Greene
- 7 years ago
- Views:
Transcription
1 Available online at R Developmental Biology 262 (2003) Review Mechanisms of ectodermal organogenesis Johanna Pispa and Irma Thesleff* Developmental Biology Programme, Institute of Biotechnology, Viikki Biocenter, University of Helsinki, Helsinki, Finland Received for publication 3 March 2003, revised 9 April 2003, accepted 28 May 2003 Abstract All ectodermal organs, e.g. hair, teeth, and many exocrine glands, originate from two adjacent tissue layers: the epithelium and the mesenchyme. Similar sequential and reciprocal interactions between the epithelium and mesenchyme regulate the early steps of development in all ectodermal organs. Generally, the mesenchyme provides the first instructive signal, which is followed by the formation of the epithelial placode, an early signaling center. The placode buds into or out of the mesenchyme, and subsequent proliferation, cell movements, and differentiation of the epithelium and mesenchyme contribute to morphogenesis. The molecular signals regulating organogenesis, such as molecules in the FGF, TGF, Wnt, and hedgehog families, regulate the development of all ectodermal appendages repeatedly during advancing morphogenesis and differentiation. In addition, signaling by ectodysplasin, a recently identified member of the TNF family, and its receptor Edar is required for ectodermal organ development across vertebrate species. Here the current knowledge on the molecular regulation of the initiation, placode formation, and morphogenesis of ectodermal organs is discussed with emphasis on feathers, hair, and teeth Elsevier Inc. All rights reserved. Keywords: Tooth; Hair; Feather; Mammary gland; Salivary gland; Lacrimal gland; Epithelium; Mesenchyme; Placode Introduction Hair, feathers, scales, teeth, beaks, nails, horns, and several eccrine glands (e.g., mammary, sweat, salivary, and lacrimal glands) are all derivatives of the ectoderm. These ectodermal organs diverge greatly from each other in shape and form (Fig. 1). Although most ectodermal organogenesis is initiated during the embryonic period, morphogenesis does continue postnatally. Tooth eruption generally occurs after birth, and the second dentition in humans develops during the first 20 years of life. Ectodermal organs also have an ability, limited though, for regeneration. The mammary gland goes through growth and differentiation during puberty and pregnancy, and this is repeated at each new pregnancy. Cyclical growth is seen in hair and feathers, where a new follicle develops from the older one. Some * Corresponding author. University of Helsinki, Institute of Biotechnology, Development Biology Programme, P.O. Box 56 Viikki Biocenter 1, Helsinki, Finland. Fax: address: irma.thesleff@helsinki.fi (I. Thesleff). ectodermal organs grow continuously during adulthood, such as nails or the rodent incisor. Despite the diversity in form and function, ectodermal organs share several common features in development. They originate from adjacent layers of epithelial (ectodermal) and mesenchymal (mesodermal or neural crest derived) tissues (Fig. 1). The first visible sign of development in most organs is the local thickening of the epithelial layer in order to make an ectodermal placode. A condensation of mesenchymal cells, a papilla, forms under the placode, which then buds into or out of the mesenchyme. Subsequent morphogenesis involves continued growth of the epithelial and mesenchymal components associated with folding and branching of the epithelium, and will then result in the final shape and size of the organ. The cellular mechanisms of ectodermal organ development have not been systematically investigated using comparable molecular markers, so, e.g., the relative contribution of proliferation and cellular migration to placode formation is not well understood (Balinsky, 1950; Wessells, 1965; Magerl et al., 2001). Growth of the epithelial bud into the mesenchyme is generally considered to be driven by proliferation of epithelial cells, though /03/$ see front matter 2003 Elsevier Inc. All rights reserved. doi: /s (03)
2 196 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) Ectodermal organ development has been studied intensively during the last 50 years mainly using a small number of model systems such as feathers, hair, teeth, and mammary and salivary glands (reviews by Chuong, 1998; Millar, 2002; Thesleff and Mikkola, 2002a; Veltmaat et al., 2003). Tissue recombination studies have shown that organogenesis is directed by reciprocal and sequential interactions between the epithelium and mesenchyme. Also many endodermal organs, such as lung and pancreas, as well as mesodermal organs like kidney, share similar epithelialmesenchymal interactions during early development (reviews by Hogan and Yingling, 1998, and Kuure et al., 2000). Signaling molecules belonging to the fibroblast growth factor (FGF), hedgehog (Hh), transforming growth factor (TGF ), Wnt, and tumor necrosis factor (TNF) families are involved repeatedly at different stages of ectodermal organogenesis, and the target genes they regulate are often the same in different ectodermal organs (Table 1). In this review we will concentrate on embryonic development of these organs, and describe current knowledge on the molecular regulation of the early stages of development. Initiation of organs The mesenchyme seems to supply the first signals directing organogenesis in most organs studied. The recombination of epithelium and mesenchyme between species or between different body regions has shown that the pattern and shape of organs appear to be regulated by the mesenchyme. For example, feather-forming dermis from chicken directs scale-forming epidermis from lizards to produce small protruding buds similar to feather follicles rather than the large closely packed elevations seen in lizard skin (Dhouailly, 1975). Recent evidence also indicates that premigratory neural crest mesenchyme from mouse can induce tooth-like morphogenesis in chick oral epithelium (Mitsiadis et al., 2003) and that early mammary gland mesenchyme is instructive for mammary gland development (Veltmaat et al., 2003). Specification of the chicken feather mesenchyme In chick the first sign of feather formation in the dorsal skin is the condensation of mesenchymal cells immediately underneath the epithelium throughout the dermis. This happens before ectodermal placode and dermal papilla formation. The mesenchyme is now called dense dermis as opposed to loose mesenchyme (Wessells, 1965; Chuong and Widelitz, 1998). The mesenchymal cells of the dermis are derived from the dermomyotome and are already determined to form feathers by signals from the dorsal neural tube. Aggregates of Wnt1-expressing cells can substitute for the dorsal neural tube (Olivera-Martinez et al., 2001). Wnt1 induces Wnt11 in a subset of dermomyotome cells. Some of these cells will then migrate to form the dense dermis suggesting that specification of the feather mesenchyme depends on Wnt-mediated signals (Olivera-Martinez et al., 2002). Furthermore, specific Wnts can maintain the hairinducing ability of mouse dermal papilla cells in culture (Kishimoto et al., 2000). cdermo-1 is a transcription factor of the helix-loop-helix (HLH) family that is an early marker for the dense dermis in dorsal chick skin. BMP2, which is expressed in the overlying ectoderm, induces cdermo-1 and can induce ectopic feather buds in vitro indicating that, in addition to Wnts, BMPs may also be involved in the specification of the mesenchyme (Scaal et al., 2002). This early function in promotion of organ formation is in contrast to the later inhibitory function of BMP during feather development (see below). The nature of the first dermal message In hair and feather development the first signal from the mesenchyme is called the first dermal message, which directs the epithelium to make an appendage (Hardy, 1992). A reaction-diffusion model has been put forward that proposes that the molecular signal(s) of the first dermal message are uniformly expressed throughout the mesenchyme, and activate both positive and negative regulators of placodal fate. Local competition will then restrict placode formation to sites of the future organs (Turing, 1952; Koch and Meinhardt, 1994; Barsh, 1999; Jiang et al., 1999). Jiang and colleagues (1999) have developed an in vitro assay where mesenchymal cells from placode-stage dorsal chick skin are dissociated and replated together with an intact epithelium. New feather follicles form in culture, but their number is dependent on the amount and density of mesenchymal cells in the assay. Based on their results Jiang and colleagues (1999) have suggested that when the density of the dermis reaches a certain threshold level small cell aggregates form in the dense dermis by random collisions. Local competitions (in line with the reaction-diffusion model) will then cause some microaggregates to enlarge and some to disappear. The remaining aggregates induce placodes in the overlying epithelium, and placodes will then induce the dermal papilla (Jiang et al., 1999). In other organs these microaggregates have not been reported. Although the identity of the first dermal message is not known, it is suspected that Wnt family molecules may be involved. As already mentioned, Wnt11 is expressed in the dense dermis of chick dorsal skin and is a target of Wnt1, which can mimic the feather-inducing abilities of the neural tube (Olivera-Martinez et al., 2001, 2002). Inhibition of Wnt signaling by Dickkopf1 inhibits hair, tooth, and mammary gland formation (Andl et al., 2002). Canonical Wnt signaling is mediated by the coactivity of the transcription factors beta-catenin and LEF1. Expression of beta-catenin is transiently found in the dense dermis prior to placode formation (Noramly et al., 1999). Lef1 mutant mice lack vibris-
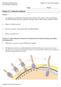
3 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) Fig. 1. Development of ectodermal organs. Morphologically very different organs, such as the tooth, hair, mammary gland, and feather, develop from two adjacent tissues: the epithelium (green) and the mesenchyme (blue). An epithelial placode is formed, which then buds either into or out of the mesenchyme. During morphogenesis the mesenchyme directs folding and branching of the epithelium, determining the shape of the organ. sae, teeth, mammary glands, and most hairs, and LEF1 has been shown to be required specifically in the vibrissa mesenchyme prior to placode formation (van Genderen et al., 1994; Kratochwil et al., 1996). In Lef1 mutant mice the first wave of hair follicles is nevertheless initiated. This may reflect a redundancy of LEF1 with other members of the LEF/TCF family. Wnt signaling is also required later in the formation of feather and hair placodes, and thus defining the exact roles of Wnts in organ initiation from mutational analysis is not easy. Specifying a dental field Mammalian teeth develop from the epithelia of the first branchial arch and the frontonasal process and neural crestderived mesenchyme (Imai et al., 1996; Chai et al., 2000). They form from the dental lamina, a U-shaped ectodermal ridge in the mandible and maxilla. Similarly, mammary glands develop only from the bilateral presumptive milk lines on the abdomen of mammals (Veltmaat et al., 2003). Recent studies have shed some light on how the placodes are patterned in the dental field. The patterning of the mandibular arch and consequently the definition of the dental placode locations in the lower jaws have been suggested to be determined by a balance of FGF and BMP signaling (Neubuser et al., 1997; Mandler and Neubuser, 2001). Conditional inactivation of Fgf8 in the first branchial arch ectoderm in transgenic mice or inhibition of all FGF signaling in vitro by FGFR-specific inhibitors results in loss of tooth development (Trumpp et
4 198 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) Table 1 Molecules implicated in the development of at least two different ectodermal organs a Molecule Hair/feather Tooth Mammary gland FGF2/4/8 x x FGF10 x x x x FGFR2b x x x x BMP2/4 x x Activin x x Wnt/Lef1 x x x Shh x x Eda/Edar/Edaradd x x x x Msx1/2 x x x PTHrP x x Lacrimal gland a Only molecules that have an ectodermal organ phenotype in transgenic animals or in other functional assays are listed here. For references, see reviews by Millar, 2002 (hair), Thesleff and Mikkola, 2002a (tooth), and Veltmaat et al., 2003 (mammary gland). al., 1999; Mandler and Neubuser, 2001). FGFs and BMPs regulate several targets in the dental mesenchyme or epithelium, some of them antagonistically (Vainio et al., 1993; Neubuser et al., 1997; Tucker et al., 1998; St. Amand et al., 2000; Mandler and Neubuser, 2001). Some of these target genes such as Pax9, Msx1, Msx2, and Pitx2 are essential for tooth development (Satokata and Maas, 1994; Bei and Maas, 1998; Peters et al., 1998; Lin et al., 1999; Lu et al., 1999). An early instructive signal in tooth development comes from the oral epithelium as shown by epithelial-mesenchymal recombinations (Mina and Kollar, 1987; Lumsden, 1988). A long-standing question has been whether the mesenchymal neural crest cells are prepatterned prior to the epithelial signal. The anterior-posterior body axis of most vertebrates is patterned by Hox genes, and it has been suggested that the Dlx homeobox transcription factors might provide similar cues for the patterning of the oral mesenchyme. Dlx1/Dlx2 mutant mice do not develop maxillary teeth, and Dlx5/Dlx6 mutant mandibles have maxillary identity (Thomas et al., 1997; Depew et al., 2002). Only maxillary mesenchyme is competent to express Dlx5 upon induction by the maxillary ectoderm suggesting that maxillary mesenchymal identity differs from that of the mandibular mesenchyme. On the other hand, Barx1, a molarspecific marker, can be induced throughout the mandible irrespective of location (Ferguson et al., 2000). Epithelial BMP signaling negatively regulates Barx1, since beads of Noggin can enlarge the Barx1 expression domain, and result in an apparent transformation of incisors into molars (Tucker et al., 1998). These findings imply that the mandibular mesenchyme may not be prepatterned into incisor and molar areas. Placodes as signaling centers An epithelial placode can be considered a basal unit of ectodermal organogenesis. In addition to ectodermal appendages, e.g., the olfactory, lens and otic placodes mark the early development of the corresponding sensory organs. Several signaling factors are coexpressed in the placodes, Fig. 2. Placodes as signaling centers. (A) Signaling at the hair and feather placode. Positive signaling (activators, red) promotes placode development, whereas negative signaling (inhibitors, black) represses it. The activity of the inhibitors is believed to be prevented inside the developing placode, whereas they can diffuse outside the placode to mediate lateral inhibition. (B) Placodes can be visualized with whole mount in situ hybridization detecting the restricting expression of many signaling molecules. Molar (arrows) and incisor (arrowheads) tooth placodes express Shh (E12 mouse mandible; t, tongue). Vibrissa and mammary gland placodes (arrows) are positive for Edar mrna (E12 mouse embryo). Hair placodes express Patched (E14 mouse embryo, expression can also be seen at nails and joints).
5 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) and their interplay is presumably required for morphogenesis. Lessons from feathers and hair The reaction-diffusion model presumes that the initial mesenchymal signals regulate molecules that either promote or repress placode formation. The activators and inhibitors may affect either the initiation and/or the growth of placodes, they can act either from the mesenchyme or can be induced in the preplacode itself, and their local competition is required for placode formation. At placodal sites the relative contribution of the activators is higher and at interplacodal sites the amount of negative regulators is higher, thus inhibiting placode initiation (Fig. 2). In feathers additional complexity is created by the formation of a hexagonal pattern of placodes, which is formed in precise spatial and temporal order. The mechanisms of reaction-diffusion and lateral inhibition together with cellular proliferation, migration, and cell-cell communication must be exactly integrated to achieve the regular pattern (Jung and Chuong, 1998). In addition to its potential role in generating the first dermal message, Wnt signaling seems also to act downstream of the first dermal message as a promoter of placode formation. The use of a reporter construct that mimics LEF1 activation and therefore possibly also Wnt activation has revealed LEF1 signaling both in the vibrissa placode itself and in the underlying mesenchyme (DasGupta and Fuchs, 1999). The relative intensities between the epithelial and mesenchymal expressions vary suggesting that they are activated sequentially. The lack of beta-catenin in the developing mouse epidermis results in lack of hair follicle formation, and conversely epithelially expressed constitutively active beta-catenin can induce ectopic feathers and hair (Gat et al., 1998; Noramly et al., 1999; Widelitz et al., 2000; Huelsken et al., 2001). These experiments cannot of course differentiate between the requirement for Wnt as a mesenchymal inducer and it role later as a placode promoter. Wnts may act as activators also in mammary placodes, as the expression of Lef1 in the mammary epithelium is one of the earliest markers for placode development (van Genderen et al., 1994; Mailleux et al., 2002). FGFs can also promote placode growth. For example, FGF2 can induce feathers both in wild-type skin as well as in the chick mutant scaleless, which lacks feather follicles despite having at least a partially formed dense dermis (Goetinck and Sekellick, 1970; Song et al., 1996; Widelitz et al., 1996). FGF receptor mutants, Fgfr2b mice, have dysgenic hair formation (Revest et al., 2001). TGF 2 and follistatin can both increase the number of hair follicles in vitro. In line with this, Tgf 2 null mice have a reduced amount of hair follicles, and follistatin null mice show retarded hair follicle development (Foitzik et al., 1999; Nakamura et al., 2003). BMPs are currently the best characterized candidates for placode inhibitors, and mediators of lateral inhibition. Ectopic expression of BMP2, BMP4, or constitutively active BMPR1 in chick skin disrupts feather formation (Noramly and Morgan, 1998; Ashique et al., 2002). Similarly, BMP4 releasing beads inhibit hair and feather follicle formation (Jung et al., 1998; Botchkarev et al., 1999). Although they are expressed in the placode itself, their activity within the placode is believed to be negated by BMP inhibitors, such as Noggin. Increasing or decreasing Noggin levels has the same effect on follicle formation as decreasing or increasing BMP activity, respectively (Botchkarev et al., 1999, 2002). BMPs and FGFs seem to antagonize each other s activity by, e.g., differentially regulating the same target genes (Jung et al., 1998; Noramly and Morgan, 1998). One of their targets is probably TGF -stimulated clone 22 (TSC-22), a leucine zipper transcription factor, which is differentially regulated by FGF and other RTK signaling, and by BMPs in chick skin. Beads of FGF2, EGF, and TGF all stimulate TSC-22 expression, and BMP4 beads or transfection with BMP4 suppress it (Dohrmann et al., 2002). In addition to BMPs, TGF 1 also inhibits hair follicle development in vitro, and Tgf 1 null mice have slightly advanced hair follicle formation. The negative effect may be based on inhibition of proliferation (Foitzik et al., 1999). Signaling at the tooth placode In teeth early epithelial signals such as FGF8 and BMP4 regulate the expression of mesenchymal transcription factors. Mutations in several of these factors (or, more precisely, double mutations of Msx1/2 or Dlx1/2 or Gli2/3) arrest tooth development to the dental lamina stage and the dental placode does not form (Bei and Maas, 1998; Thomas et al., 1997; Hardcastle et al., 1998). It is plausible that these transcription factors regulate signals, e.g., activin, that stimulate formation of the placodes (Ferguson et al., 1998; Laurikkala et al., 2001). The dental placode, which forms in the dental lamina, is a transient epithelial signaling center, and in mouse embryos it expresses at least Bmp2, Bmp4, Fgf8, Shh, Wnt10b, Msx2, Lef1, and p21 (Jernvall and Thesleff, 2000; Thesleff and Mikkola, 2002a). Comparative studies between the mouse and a related rodent, the sibling vole, which has a very different molar shape, show that the molecules of the signaling center are conserved between species (Keränen et al., 1998). Epithelial BMPs upregulate mesenchymal Bmp4 via Msx1 and Msx2, and mesenchymal BMP4 further upregulates Lef1 in the mesenchyme (Vainio et al., 1993; Chen et al., 1996; Kratochwil et al., 1996; Dassule and McMahon, 1998). Shh has been associated with promoting proliferation but its role in the dental placode is not clear. Ectopic infection of the oral ectoderm with Wnt7b expressing virus represses placodal Shh (Sarkar et al., 2000). This inhibits tooth formation but whether this is due to the lack of Shh is not certain. Reduction of Shh in the teeth of transgenic mice does not result in complete inhibition of tooth formation (Dassule et al., 2000; Zhang et al., 2000).
6 200 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) FGF10 promotes gland development The interplay of promoting and inhibiting signals has been proposed as a regulatory mechanism also in gland development (Hogan, 1999). FGF10 seems to act as a promoter for epithelial budding. In an endodermal organ, the lung, Fgf10 is expressed in the lung mesenchyme at sites where epithelial buds will form, and lack of FGF10 results in no budding. In addition, FGF10 can act as chemoattractant for the lung endothelium (Bellusci et al., 1997; Min et al., 1998; Sekine et al., 1999). Current data indicate that FGF10 is also involved in the development of the mammary gland, and lacrimal and Harderian glands of the eye. First, in all three glands, Fgf10 is expressed in the mesenchyme underneath the epithelial bud (Makarenkova et al., 2000; Mailleux et al., 2002). In the mammary epithelium, expression of the receptor for FGF10, Fgfr2b, is seen throughout placode and bud development (Spencer-Dene et al., 2001; Mailleux et al., 2002). Second, the lack of FGF10 or its receptor results in loss of most mammary, lacrimal, or Harderian placodes (Govindarajan et al., 2000; Makarenkova et al., 2000; Mailleux et al., 2002). Third, misexpression of FGF10 or FGF7 in the lens is sufficient to cause ectopic ocular gland formation in the overlying corneal epithelium (Lovicu et al., 1999; Govindarajan et al., 2000). Similarly, beads of FGF10 or FGF7 can induce lacrimal glands in vitro (Makarenkova et al., 2000). It is probable that mesenchymal FGFs are required for development of most, if not all, ectodermal organs. Defects in salivary glands, mammary glands, teeth, hair follicles, and nails have been reported in mice lacking Fgf10 or Fgfr2b, and in mice misexpressing a dominant negative FGFR2 (Celli et al., 1998; De Moerlooze et al., 2000; Ohuchi et al., 2000; Mailleux et al., 2002). A new player in the ectodermal organ field: ectodysplasin, a TNF family member Ectodermal dysplasias are developmental syndromes that specifically affect ectodermal organs. Hypohidrotic ectodermal dysplasia patients (HED) and their mouse models Tabby, downless, and crinkled (Ta/dl/cr) have defects in teeth, hair, and, e.g., salivary, lacrimal, and sweat glands (OMIM, 2001; Blake et al., 2002). The mutated genes belong to the tumor necrosis factor (TNF) signaling pathway: ectodysplasin (Eda), a TNF ligand, its receptor Edar, and the intracellular adapter protein Edaradd (Thesleff and Mikkola, 2002b). These molecules are expressed during hair and tooth development first in the epithelium prior to placode formation, then in the placode, and also later during morphogenesis and cell differentiation. The early expression of the ligand, ectodysplasin, and its receptor Edar is overlapping, but when placodes are formed the patterns separate so that ectodysplasin is expressed in the interplacodal ectoderm and Edar in the placode itself (Headon and Overbeek, 1999; Laurikkala et al., 2001, 2002; Tucker et al., 2000). Although activation of the transcription factor NF B by Edar has been shown, the actual genes regulated by Edar signaling are not known (Yan et al., 2000; Koppinen et al., 2001; Kumar et al., 2001). The first hair placodes are missing in Ta/dl/cr skin and consequently all placode markers so far analyzed are missing (Headon and Overbeek, 1999; Andl et al., 2002; Laurikkala et al., 2002; Nishioka et al., 2002). When Edar is overexpressed in the epithelium, upregulation of BMP4 is seen in the skin mesenchyme suggesting that BMP4 is an indirect target of Edar signaling (Pispa, J., unpublished results). Accumulating evidence suggests that ectodysplasin-edar signaling is an early and necessary component of placode formation in all ectodermal organs. First, Edar is one of the earliest markers of forming placodes. When beta-catenin activity is conditionally ablated in mouse epidermis Edar is expressed in a punctated placodal manner even if no hair follicles develop, and several other placode markers are missing (Huelsken et al., 2001). Second, Ta/dl/cr mice lack the first hair placodes (Headon and Overbeek, 1999; Laurikkala et al., 2002). Third, overexpression of ectodysplasin induces ectopic teeth and mammary glands, stimulates hair and nail growth, and increases the activity of sweat glands (Mustonen et al., 2003). Edar is also evolutionarily conserved as Edar mutant medaka fish lack scales (Kondo et al., 2001). Nevertheless, since lack of ectodysplasin-edar signaling does not inhibit organogenesis completely it is likely that this signaling pathway can either be compensated for by a different molecular pathway or is redundant with other TNF and TNFR molecules. This is supported by overlapping expression of another TNF receptor, TN- FRSF19, at the same sites as Edar (Pispa et al., 2003). Morphogenesis After the initial mesenchymal signals epithelial signals induce the condensation of mesenchymal cells, the papilla, around the placode. Later the mesenchyme directs morphogenesis, i.e., the organ-specific folding and branching of the epithelium (Dhouailly, 1975; Mina and Kollar, 1987; Lumsden, 1988; Hardy, 1992). For example, salivary gland mesenchyme can cause the mammary gland epithelium to branch in a salivary-like dense pattern (Kratochwil, 1969). It is noteworthy, however, that ectodermal cells in such recombinations retain their mammary gland-specific differentiation program. This indicates that morphogenesis and cell differentiation are at least partially independent processes. The enamel knot regulates tooth shape When the epithelial tooth bud has reached its full size it invaginates and folds at its tip. A new signaling center, the enamel knot, which is a transient structure of condensed epithelial cells, forms at the tip of the folding bud. This
7 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) Fig. 3. Regulation of tooth morphogenesis by the signaling center, the enamel knot. More than 10 signaling molecules are locally expressed in the enamel knot (dark green), and regulate the growth and morphogenesis of tooth crown. The function of the enamel knot is regulated by at least Edar and LEF1. Wnt signaling mediated by LEF1 in the enamel knot upregulates FGF, which then induces mesenchymal FGFs, promoting proliferation in the cervical loops. SHH from the enamel knot acts via the mesenchyme to regulate epithelial growth specifically on the lingual side of the tooth germ. Lunatic fringe (L-fng) presumably contributes to the modulation of enamel knot signaling. marks the initiation of tooth crown development. The epithelium flanking the enamel knot proliferates forming cervical loops, which grow to surround the mesenchymal dental papilla. The enamel knot cells do not proliferate, and it has been suggested that this is a mechanism for the differential growth of the tooth epithelium. The enamel knot is then removed by apoptosis (Jernvall et al., 1994; Vaahtokari et al., 1996a,b; Thesleff and Jernvall, 1997). Currently four Fgfs, three Bmps, three Wnts, Shh, Edar, Msx2, Lef1, and p21 are known to be specifically expressed in the enamel knot (Fig. 3) (Thesleff and Mikkola, 2002a). Studies by Kratochwil and colleagues have shown that LEF1 is required specifically in the enamel knot during the bud-to-cap stage transition for the transduction of Wnt signals inducing Fgf4 in the enamel knot (Kratochwil et al., 1996; Kratochwil et al., 2002). Lef1 mutant teeth lack mesenchymal Fgfs, and their development can be rescued by beads releasing either epithelial or mesenchymal FGFs (Kratochwil et al., 2002). The transcription factor Runx2 is required in the mesenchyme for mediation of the same pathway (Åberg, T., Wang, X., personal communication). Mesenchymal FGFs stimulate proliferation in the epithelium, and are presumably required for the growth of the cervical loops. The FGF receptors for them, Fgfr1b and Fgfr2b, are expressed in the epithelial cervical loops, and mice carrying Fgfr2b mutations cease tooth development at an early stage (De Moerlooze et al., 2000). Lack of two of the molecules expressed in the enamel knot, Edar and Shh, causes defects in the patterning of cusps in molar teeth thus establishing a direct link between the enamel knot and tooth shape (Pispa et al., 1999; Dassule et al., 2000). Shh is required specifically for the growth of cervical loop at the lingual side, but this is not a direct effect, as ablation of Smo, the receptor of Shh, in epithelium does not inhibit cervical loop growth. Hence, Shh exerts its effect on epithelial growth via the mesenchyme (Fig. 3) (Gritli-Linde et al., 2002). Mutations in some mesenchymal transcription factors such as Msx1 or Pax9 arrest tooth development at the bud stage prior to enamel knot formation (Satokata and Maas, 1994; Peters et al., 1998). It is therefore likely that mesenchymal signals regulate the formation of the enamel knot, and it has indeed been shown that exogenous BMP4 can rescue the Msx1 phenotype and induce the expression of two enamel knot markers, Msx2 and p21 (Jernvall et al., 1998; Bei et al., 2000). Modulation of the enamel knot may also be achieved by signals within the dental epithelium. Lunatic fringe (L-fng), a modifier of the Notch signaling pathway, is expressed in the epithelial cervical loops flanking the enamel knot. Since L-fng is important in establishing tissue boundaries in other systems it possibly assists in defining the enamel knot area. It is not indispensable for tooth development though, since the L-fng null mice have no tooth phenotype (Mustonen et al., 2002). Later in tooth development when the epithelial morphogenesis has progressed to the bell stage in molars, new signaling centers, the secondary enamel knots, are formed at sites of the future cusps where they will induce further folding of the epithelium (Jernvall et al., 2000). The shape of feathers and hair The molecular nature of the second dermal message that regulates epithelial downgrowth and proliferation in hair development is not known, although several molecules that are expressed in the dermal condensate exhibit a hair phenotype when mutated (Hardy, 1992; Millar, 2002). Shh is probably involved in activating this dermal message; it is expressed in the epithelium and mice lacking Shh or its transcriptional mediator Gli2 have immature hair follicles with a reduced amount of proliferation (St. Jacques et al., 1998; Mill, 2003). Despite the existence of several spontaneous and transgenic mouse models in which hair differentiation is affected, little is known about the signaling events regulating terminal differentiation of hair (Sundberg, 1994; Millar, 2002). Wnt signaling, mediated by LEF1, is likely to regulate the expression of the hair keratins, as the hair keratin promoters have LEF1 binding sites and Lef1 is expressed in the hair matrix (Zhou et al., 1995). BMPs may be involved, as perturbations in their levels cause hair shaft differentiation defects (Blessing et al., 1993; Kulessa et al., 2000). Feathers come in diverse combinations of the rachis ( trunk ) and barbs ( branches ). Recent evidence suggests that the interplay and periodicity of BMP, Noggin, and Shh is involved in the shaping of the feather filament. Suppression of BMP activity promotes rachis and barb branching,
8 202 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) whereas overexpression of BMPs results in repression of Shh and in increased rachis formation and fusion of barbs (Harris et al., 2002; Yu et al., 2002). Future prospects One problem in past research has been that analysis of mutant phenotypes has been hindered by the use of the same molecules reiteratively during the formation of one organ. Identifying the function of a gene in morphogenesis is difficult if it is also required in initiation of organogenesis. Combining conditional and inducible transgenic approaches to more traditional tissue recombination experiments should overcome some of these difficulties. Microarray techniques are likely to speed up the study of downstream target genes such as those that regulate cellular and structural changes such as proliferation, polarization, adhesion, and migration. In this review we have omitted the discussion on the role of stem cells in ectodermal organs due to lack of space. Recent years have witnessed increasing interest in this line of research (Fuchs et al., 2001). The skin and its appendages are remarkable in respect that they can, to a certain degree, renew themselves. New hair follicles are initiated in the bulge region of the older degenerating follicles, where the epithelial stem cells reside, and the same stem cells appear to supply progenitors also for the sebaceous gland and the epidermis. Wnt signaling mediated by LEF1 and TCF3 is involved in their regulation (Merrill et al., 2001; Niemann et al., 2002). The rodent incisor grows continuously, which has been attributed to stem cells located in the cervical loops. Mesenchymal FGF10 and Notch pathway signals have been implicated in this process (Harada et al., 1999). The elucidation of the molecular regulation of stem cell maintenance and differentiation may obviously open up possibilities for regeneration of ectodermal organs. If the same signals are used to make all ectodermal organs, where does the specificity come from? Global regulation by signals patterning the body axes could contribute to organ-specific development. In the control of identity of different subgroups of the same organ type, e.g., incisor and molar teeth or maxillary and mandibular molars, differential expression of transcription factors such as BarxI and Dlx genes has been implicated (Thomas et al., 1997; Tucker et al., 1998). There may also exist molecules that specify a certain type of organ. Evidence from Caenorhabditis elegans supports this possibility (Gaudet and Mango, 2002), but no regulatory molecules specific for individual ectodermal appendages have been discovered to date. Subtle changes in the amount and location of signaling molecules are potentially important. Manipulations in the level of beta-catenin in chick skin have shown that the identity of a feather or a scale placode may depend on the amount of beta-catenin received (Widelitz et al., 2000). The sequencing of the genomes and the development of technologies for global analysis of gene expression can be expected to reveal novel genes associated with the development of specific organs or organ types. Variation in shape and structure is seen within ectodermal organs. For example, hair of different individuals can be curly or straight or of different color, and different species have species-specific composition of fur. The molecular basis for this variation is not well understood. Salazar-Ciudad and Jernvall (2002) have explained variation in molar tooth shape with a model based on activators and inhibitors that are expressed in the secondary enamel knots. The location of these knots will determine the location of the future cusps, and changes in the activator/inhibitor signaling will cause changes in tooth shape. With their mathematical model they have managed to predict the shape differences of the mouse and vole molars by using differential signaling parameters (Salazar-Ciudad and Jernvall, 2002). This implies that similar models may be useful in the future in determining molecular and cellular factors regulating shape variability in other ectodermal organs as well. Acknowledgments We thank Marja Mikkola and Tim Mitsiadis for critical reading of the manuscript, and Tuija Mustonen and Aapo Kangas for help with figures. Financial support was received from the Academy of Finland and the Sigrid Juselius Foundation. References Andl, T., Reddy, S.T., Gaddapara, T., Millar, S.E., WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, Ashique, A.M., Fu, K., Richman, J.M., Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int. J. Dev. Biol. 46, Balinsky, B.I., On the prenatal growth of the mammary gland rudiment in the mouse. J. Anat. 84, Barsh, G., Of ancient tales and hairless tails. Nat. Genet. 22, Bei, M., Kratochwil, K., Mass, R.L., BMP4 rescues a non-cellautonomous function of Msx1 in tooth development. Development 127, Bei, M., Maas, R., FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 125, Bellusci, S., Furuta, Y., Rush, M.G., Henderson, R., Winnier, G., Hogan, B.L.M., Involvement of Sonic Hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 124, Blake, J.A., Richardson, J.E., Bult, C.J., Kadin, J.A., Eppig, J.T., the mouse database group, The Mouse Genome Database (MGD): the model organism database for the laboratory mouse. Nucleic Acids Res. 30, Blessing, M., Nanney, L.B., King, L.E., Jones, C.M., Hogan, B.L., Transgenic mice as a model to study the role of TGF-beta-related molecules in hair follicles. Genes Dev. 7, Botchkarev, V.A., Botchkareva, N.V., Roth, W., Nakamura, M., Chen, L.H., Herzog, W., Lindner, G., McMahon, J.A., Peters, C., Lauster,
9 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) McMahon, A.P., Paus, R., Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol. 1, Botchkarev, V.A., Botchkareva, N.V., Sharov, A.A., Funa, K., Huber, O., Gilchrest, B.A., Modulation of BMP signaling by noggin is required for induction of the secondary (Nontylotrich) hair follicles. J. Invest. Dermatol. 118, Celli, G., Larochelle, W.J., Mackem, S., Sharp, R., Merlino, G., Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J. 17, Chai, Y., Jiang, X., Ito, Y., Bringas, P.J., Han, J., Rowitch, D.H., Soriano, P., McMahon, A.P., Sucov, H.M., Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, Chen, Y., Bei, M., Woo, I., Satokata, I., Maas, R., Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122, Chuong, C.-M., Molecular Basis of Epithelial Appendage Morphogenesis. R.G. Landes Company, Austin, TX, USA. Chuong, C.-M., Widelitz, R.B., Feather morphogenesis: a model of the formation of epithelial appendages, in: Chuong, C.-M. (Ed.), Molecular Basis of Epithelial Appendage Morphogenesis. R.G. Landes Company, Austin, TX, USA, pp DasGupta, R., Fuchs, E., Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, Dassule, H.R., Lewis, P., Bei, M., Maas, R., McMahon, A.P., Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, Dassule, H.R., McMahon, A.P., Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev. Biol. 202, De Moerlooze, L., Spencer-Dene, B., Revest, J., Hajihosseini, M., Rosewell, I., Dickson, C., An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127, Depew, M.J., Lufkin, T., Rubenstein, J.L., Specification of jaw subdivisions by Dlx genes. Science 298, Dhouailly, D., Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds and mammals. Wilhelm Roux Arch. 177, Dohrmann, C.E., Noramly, S., Raftery, L.A., Morgan, B.A., Opposing effects on TSC- 22 expression by BMP and receptor tyrosine kinase signals in the developing feather tract. Dev. Dyn. 223, Ferguson, C.A., Tucker, A.S., Christensen, L., Lau, A.L., Matzuk, M.M., Sharpe, P.T., Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 12, Ferguson, C.A., Tucker, A.S., Sharpe, P.T., Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development 127, Foitzik, K., Paus, R., Doetschman, T., Dotto, G.P., The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev. Biol. 212, Fuchs, E., Merrill, B.J., Jamora, C., DasGupta, R., At the roots of a never-ending cycle. Dev. Cell 1, Gat, U., DasGupta, R., Degenstein, L., Fuchs, E., De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95, Gaudet, J., Mango, S.E., Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 295, Goetinck, P.F., Sekellick, M.J., Early morphogenetic events in normal and mutant skin development in the chick embryo and their relationship to alkaline phosphatase activity. Dev. Biol. 21, Govindarajan, V., Ito, M., Makarenkova, H.P., Lang, R.A., Overbeek, P.A., Endogenous and ectopic gland induction by FGF-10. Dev. Biol. 225, Gritli-Linde, A., Bei, M., Maas, R., Zhang, XM., Linde, A., McMahon, A.P., Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129, Harada, H., Kettunen, P., Jung, H.S., Mustonen, T., Wang, Y.A., Thesleff, I., Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J. Cell Biol. 147, Hardcastle, Z., Mo, R., Hui, C.C., Sharpe, P.T., The Shh signalling pathway in tooth development defects in Gli2 and Gli3 mutants. Development 125, Hardy, M.H., The secret life of the hair follicle. Trends Genet. 8, Harris, M.P., Fallon, J.F., Prum, R.O., Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J. Exp. Zool. 294, Headon, D.J., Overbeek, P.A., Involvement of a novel TNF receptor homologue in hair follicle induction. Nat. Genet. 22, Hogan, B.L., Morphogenesis. Cell 96, Hogan, B.L., Yingling, J.M., Epithelial/mesenchymal interactions and branching morphogenesis of the Jung. Curr. Opin. Genet. Dev. 8, Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G., Birchmeier, W., Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, Imai, H., Osumi-Yamashita, N., Ninomiya, Y., Eto, K., Contribution of early-emigrating midbrain crest cells to the dental mesenchyme of mandibular molar teeth in rat embryos. Dev. Biol. 176, Jernvall, J., ÅAberg, T., Kettunen, P., Keränen, S., Thesleff, I., The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125, Jernvall, J., Keränen, S.V., Thesleff, I., Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc. Natl. Acad. Sci. USA 97, Jernvall, J., Kettunen, P., Karavanova, I., Martin, L.B., Thesleff, I., Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int. J. Dev. Biol. 38, Jernvall, J., Thesleff, I., Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 92, Jiang, T.X., Jung, H.S., Widelitz, R.B., Chuong, C.M., Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development 126, Jung, H.S., Chuong, C.-M., Periodic pattern formation of the feathers, in: Chuong, C.-M. (Ed.), Molecular Basis of Epithelial Appendage Morphogenesis. R.G. Landes Company, Austin, TX, USA, pp Jung, H.S., Francis-West, P.H., Widelitz, R.B., Jiang, T.X., Ting-Berreth, S., Tickle, C., Wolpert, L., Chuong, C.M., Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev. Biol. 196, Keränen, S.V.E., ÅAberg, T., Kettunen, P., Thesleff, I., Jernvall, J., Association of developmental regulatory genes with the development of different molar tooth shapes in two species of rodents. Dev. Genes Evol. 208, Kishimoto, J., Burgeson, R.E., Morgan, B.A., Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 14, Koch, A.J., Meinhardt, H., Biological pattern formation: from basic mechanisms to complex structures. Rev. Mod. Phys. 66, Kondo, S., Kuwahara, Y., Kondo, M., Naruse, K., Mitani, H., Wakamatsu, Y., Ozato, K., Asakawa, S., Shimizu, N., Shima, A., The medaka
10 204 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) rs-3 locus required for scale development encodes ectodysplasin-a receptor. Curr. Biol. 11, Koppinen, P., Pispa, J., Laurikkala, J., Thesleff, I., Mikkola, M.L., Signalling and subcellular localization of the TNF receptor Edar. Exp. Cell Res. 269, Kratochwil, K., Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev. Biol. 20, Kratochwil, K., Dull, M., Fariñas, I., Galceran, J., Grosschedl, R., Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 10, Kratochwil, K., Galceran, J., Tontsch, S., Roth, W., Grosschedl, R., FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1 ( / ) mice. Genes Dev. 16, Kulessa, H., Turk, G., Hogan, B.L., Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 19, Kumar, A., Eby, M.T., Sinha, S., Jasmin, A., Chaudhary, P.M., Ectodermal dysplasia receptor activates the nuclear factor kappa B, c-jun N-terminal kinase and cell death pathways and binds to ectodysplasin A. J. Biol. Chem. 276, Kuure, S., Vuolteenaho, R., Vainio, S., Kidney morphogenesis: cellular and molecular regulation. Mech. Dev. 92, Laurikkala, J., Mikkola, M., Mustonen, T., ÅAberg, T., Koppinen, P., Pispa, J., Nieminen, P., Galceran, J., Grosschedl, R., Thesleff, I., TNF signaling via the ligand-receptor pair ectodysplasin and edar controls the function of epithelial signaling centers and is regulated by Wnt and activin during tooth organogenesis. Dev. Biol. 229, Laurikkala, J., Pispa, J., Jung, H.S., Nieminen, P., Mikkola, M., Wang, X., Saarialho-Kere, U., Galceran, J., Grosschedl, R., Thesleff, I., Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development 129, Lin, C.R., Kioussi, C., O Connell, S., Briata, P., Szeto, D., Liu, F., Izpisua- Belmonte, J.C., Rosenfeld, M.G., Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 401, Lovicu, F.J., Kao, W.W., Overbeek, P.A., Ectopic gland induction by lens-specific expression of keratinocyte growth factor (FGF-7) in transgenic mice. Mech. Dev. 88, Lu, M.F., Pressman, C., Dyer, R., Johnson, R.L., Martin, J.F., Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature 401, Lumsden, A.G., Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Dev. Suppl. 103, Magerl, M., Tobin, D.J., Muller-Rover, S., Hagen, E., Lindner, G., McKay, I.A., Paus, R., Patterns of proliferation and apoptosis during murine hair follicle morphogenesis. J. Invest. Dermatol. 116, Mailleux, A.A., Spencer-Dene, B., Dillon, C., Ndiaye, D., Savona-Baron, C., Itoh, N., Kato, S., Dickson, C., Thiery, J.P., Bellusci, S., Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 129, Makarenkova, H.P., Ito, M., Govindarajan, V., Faber, S.C., Sun, L., Mc- Mahon, G., Overbeek, P.A., Lang, R.A., FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development 127, Mandler, M., Neubuser, A., FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev. Biol. 240, Merrill, B.J., Gat, U., DasGupta, R., Fuchs, E., Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15, Mill, P.M., Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 17, Millar, S.E., Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 118, Min, H., Danilenko, D.M., Scully, S.A., Bolon, B., Ring, B.D., Tarpley, J.E., DeRose, M., Simonet, W.S., Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, Mina, M., Kollar, E.J., The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral. Biol. 32, Mitsiadis, T.A., Cheraud, Y., Sharpe, P., Fontaine-Perus, J., Development of teeth in chick embryos after mouse neural crest trans. Proc. Natl. Acad. Sci. USA 100, Mustonen, T., Pispa, J., Mikkola, M.L., Pummila, M., Kangas, A.T., Jaatinen, R., Thesleff, I., Stimulation of ectodermal organ development by ectodysplasin-a1. Dev. Biol. 253, Mustonen, T., Tummers, M., Mikami, T., Itoh, N., Zhang, N., Gridley, T., Thesleff, I., Lunatic fringe, FGF, and BMP regulate the Notch pathway during epithelial morphogenesis of teeth. Dev. Biol. 248, Nakamura, M., Matzuk, M.M., Gerstmayer, B., Bosio, A., Lauster, R., Miyachi, Y., Werner, S., Paus, R., Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J. 17, Neubuser, A., Peters, H., Balling, R., Martin, G.R., Antagonistic interactions between FGF and BMP signaling pathways a mechanism for positioning the sites of tooth formation. Cell 90, Niemann, C., Owens, D.M., Hulsken, J., Birchmeier, W., Watt, F.M., Expression of NLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129, Nishioka, E., Tanaka, T., Yoshida, H., Matsumura, K., Nishikawa, S., Naito, A., Inoue, J., Funasaka, Y., Ichihashi, M., Miyasaka, M., Mucosal addressin cell adhesion molecule 1 plays an unexpected role in the development of mouse guard hair. J. Invest. Dermatol. 119, Noramly, S., Freeman, A., Morgan, B.A., Catenin signaling can initiate feather bud development. Development 126, Noramly, S., Morgan, B.A., BMPs mediate lateral inhibition at successive stages in feather tract development. Development 125, Ohuchi, H., Hori, Y., Yamasaki, M., Harada, H., Sekine, K., Kato, S., Itoh, N., FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, Olivera-Martinez, I., Missier, S., Fraboulet, S., Thelu, J., Dhouailly, D., Differential regulation of the chick dorsal thoracic dermal progenitors from the medial dermomyotome. Development 129, Olivera-Martinez, I., Thelu, J., Teillet, M.A., Dhouailly, D., Dorsal dermis development depends on a signal from the dorsal neural tube, which can be substituted by Wnt-1. Mech. Dev. 100, OMIM, Online Mendelian Inheritance in Man, nlm.nih.gov/omim/. Peters, H., Neubuser, A., Kratochwil, K., Balling, R., Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12, Pispa, J., Mikkola, M.L., Mustonen, T., Thesleff, I., Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr. Patterns (in press). Pispa, J., Jung, H.S., Jernvall, J., Kettunen, P., Mustonen, T., Tabata, M.J., Kere, J., Thesleff, I., Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Dev. Biol. 216, Revest, J.M., Spencer-Dene, B., Kerr, K., De Moerlooze, L., Rosewell, I., Dickson, C., Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev. Biol. 231,
11 J. Pispa, I. Thesleff / Developmental Biology 262 (2003) Salazar-Ciudad, I., Jernvall, J., A gene network model accounting for development and evolution of mammalian teeth. Proc. Natl. Acad. Sci. USA 99, Sarkar, L., Cobourne, M., Naylor, S., Smalley, M., Dale, T., Sharpe, P.T., Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc. Natl. Acad. Sci. USA 97, Satokata, I., Maas, R., Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 6, Scaal, M., Prols, F., Fuchtbauer, E.M., Patel, K., Hornik, C., Kohler, T., Christ, B., Brand-Saberi, B., BMPs induce dermal markers and ectopic feather tracts. Mech. Dev. 110, Sekine, K., Ohuchi, H., Fujiwara, M., Yamasaki, M., Yoshizawa, T., Sato, T., Yagishita, N., Matsui, D., Koga, Y., Itoh, N., Kato, S., Fgf10 is essential for limb and lung formation. Nat. Genet. 21, Song, H., Wang, Y., Goetinck, P.F., Fibroblast growth factor 2 can replace ectodermal signaling for feather development. Proc. Natl. Acad. Sci. USA 93, Spencer-Dene, B., Dillon, C., Fantl, V., Kerr, K., Petiot, A., Dickson, C., Fibroblast growth factor signalling in mouse mammary gland development. Endocr. Relat. Cancer 8, St. Amand, T.R., Zhang, Y., Semina, E.V., Zhao, X., Hu, Y., Nguyen, Murray, J.C., Chen, Y., Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev. Biol. 217, St. Jacques, B., Dassule, H.R., Karavanova, I., Botchkarev, V.A., Li, J., Danielian, P.S., McMahon, J.A., Lewis, P.M., Paus, R., McMahon, A.P., Sonic hedgehog signaling is essential for hair development. Curr. Biol. 8, Sundberg, J.P., Handbook of Mouse Mutations with Skin and Hair Abnormalities. CRC Press, Boca Raton, FL, USA. Thesleff, I., Jernvall, J., The enamel knot a putative signaling center regulating tooth development. Cold Spring Harb. Symp. Quant. Biol. 62, Thesleff, I., Mikkola, M.L., 2002a. The role of growth factors in tooth development. Int. Rev. Cytol. 217, Thesleff, I., Mikkola, M.L., 2002b. Death receptor signaling giving life to ectodermal organs. Science s STKE content/full/oc_sigtrans;2002/131/pe22. Thomas, B.L., Tucker, A.S., Qui, M., Ferguson, C.A., Hardcastle, Z., Rubenstein, J.L., Sharpe, P.T., Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development 124, Trumpp, A., Depew, M.J., Rubenstein, J.L., Bishop, J.M., Martin, G.R., Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 13, Tucker, A.S., Headon, D.J., Schneider, P., Ferguson, B.M., Overbeek, P., Tschopp, J., Sharpe, P.T., Edar/Eda interactions regulate enamel knot formation in tooth morphogenesis. Development 127, Tucker, A.S., Matthews, K.L., Sharpe, P.T., Transformation of tooth type induced by inhibition of BMP signaling. Science 282, Turing, A.M., The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 237, Vaahtokari, A., ÅAberg, T., Jernvall, J., Keränen, S., Thesleff, I., 1996a. The enamel knot as a signaling center in the developing mouse tooth. Mech. Dev. 54, Vaahtokari, A., ÅAberg, T., Thesleff, I., 1996b. Apoptosis in the developing tooth: association with an embryonic signaling center and suppression by EGF and FGF-4. Development 122, Vainio, S., Karavanova, I., Jowett, A., Thesleff, I., Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75, van Genderen, C., Okamura, R.M., Farinas, I., Quo, R.G., Parslow, T.G., Bruhn, L., Grosschedl, R., Development of several organs that require inductive epithelial- mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8, Veltmaat, J.M., Mailleux, A.A., Thiery, J.P., Bellusci, S., Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 71, Wessells, N.K., Morphology and proliferation during early feather development. Dev. Biol. 12, Widelitz, R.B., Jiang, T.X., Lu, J., Chuong, C.M., beta-catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated beta-catenin. Dev. Biol. 219, Widelitz, R.B., Jiang, T.X., Noveen, A., Chen, C.W., Chuong, C.M., FGF induces new feather buds from developing avian skin. J. Invest. Dermatol. 107, Yan, M.H., Wang, L.C., Hymowitz, S.G., Schilbach, S., Lee, J., Goddard, A., de Vos, A.M., Gao, W.Q., Dixit, V.M., Two-amino acid molecular switch in an epithelial morphogen that regulates binding to two distinct receptors. Science 290, Yu, M., Wu, P., Widelitz, R.B., Chuong, C.M., The morphogenesis of feathers. Nature 420, Zhang, Y., Zhang, Z., Zhao, X., Yu, X., Hu, Y., Geronimo, B., Fromm, S.H., Chen, Y.P., A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 127, Zhou, P., Byrne, C., Jacobs, J., Fuchs, E., Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 9,
Tooth Formation. Chapter 1
 Chapter Tooth Formation Teeth develop from epithelial cells from the mucosal lining of the oral cavity and cranial neural crest-derived ectomesenchymal cells. These latter cells originate at the ectodermal/neuroectodermal
Chapter Tooth Formation Teeth develop from epithelial cells from the mucosal lining of the oral cavity and cranial neural crest-derived ectomesenchymal cells. These latter cells originate at the ectodermal/neuroectodermal
Limb Development. Limb Development. Limb Axes. Limb Development. Clinical Terms
 Limb Development Overview of Limb Formation Initiation of Limb Development Limb Field Outgrowth of the Mesoderm Morphogenetic Signaling Development of Limb Tissues Skeleton Musculature Innervation Vasculature
Limb Development Overview of Limb Formation Initiation of Limb Development Limb Field Outgrowth of the Mesoderm Morphogenetic Signaling Development of Limb Tissues Skeleton Musculature Innervation Vasculature
Development of Teeth
 Development of Teeth Dr. Khaldoun Darwich Specialist in Oral and Maxillo-Facial Surgery Hamburg University PhD Hamburg University Academic Teacher - Department of OMF Surgery in Damascus University Instructor
Development of Teeth Dr. Khaldoun Darwich Specialist in Oral and Maxillo-Facial Surgery Hamburg University PhD Hamburg University Academic Teacher - Department of OMF Surgery in Damascus University Instructor
STEM CELL FELLOWSHIP
 Module I: The Basic Principles of Stem Cells 1. Basics of Stem Cells a. Understanding the development of embryonic stem cells i. Embryonic stem cells ii. Embryonic germ cells iii. Differentiated stem cell
Module I: The Basic Principles of Stem Cells 1. Basics of Stem Cells a. Understanding the development of embryonic stem cells i. Embryonic stem cells ii. Embryonic germ cells iii. Differentiated stem cell
DEVELOPMENT AND DISEASE Cyclic alopecia in Msx2 mutants: defects in hair cycling and hair shaft differentiation
 Development 130, 379-389 2003 The Company of Biologists Ltd doi:10.1242/dev.00201 379 DEVELOPMENT AND DISEASE Cyclic alopecia in Msx2 mutants: defects in hair cycling and hair shaft differentiation Liang
Development 130, 379-389 2003 The Company of Biologists Ltd doi:10.1242/dev.00201 379 DEVELOPMENT AND DISEASE Cyclic alopecia in Msx2 mutants: defects in hair cycling and hair shaft differentiation Liang
Secondary dentition permanent teeth - 32. Primary dentition deciduous teeth - 20
 Department of Histology and Embryology, P. J. Šafárik University, Medical Faculty, Košice DEVELOPMENT OF TEETH: Sylabus for foreign students Dental medicine Author: doc. MVDr. Iveta Domoráková, PhD. Primary
Department of Histology and Embryology, P. J. Šafárik University, Medical Faculty, Košice DEVELOPMENT OF TEETH: Sylabus for foreign students Dental medicine Author: doc. MVDr. Iveta Domoráková, PhD. Primary
Trasposable elements: P elements
 Trasposable elements: P elements In 1938 Marcus Rhodes provided the first genetic description of an unstable mutation, an allele of a gene required for the production of pigment in maize. This instability
Trasposable elements: P elements In 1938 Marcus Rhodes provided the first genetic description of an unstable mutation, an allele of a gene required for the production of pigment in maize. This instability
FGF-1 as Cosmetic Supplement
 FGF-1 as Cosmetic Supplement Ing-Ming Chiu, Ph.D. Professor, Internal Medicine and Molecular and Cellular Biochemistry The Ohio State University Columbus, Ohio, U.S.A. GENTEON USA Fibroblast Growth Factor
FGF-1 as Cosmetic Supplement Ing-Ming Chiu, Ph.D. Professor, Internal Medicine and Molecular and Cellular Biochemistry The Ohio State University Columbus, Ohio, U.S.A. GENTEON USA Fibroblast Growth Factor
Hair Chemistry. Chapter 1. Hair Relaxers Science, Design, and Application www.alluredbooks.com
 Hair Relaxers Science, Design, and Application www.alluredbooks.com Chapter 1 Hair Chemistry We all know that the hair on our head is dead, but underneath the scalp, within the hair follicle, is a surprisingly
Hair Relaxers Science, Design, and Application www.alluredbooks.com Chapter 1 Hair Chemistry We all know that the hair on our head is dead, but underneath the scalp, within the hair follicle, is a surprisingly
BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN. Third Examination April 2, 2009
 BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Third Examination April 2, 2009 Please put your name at the top of each page. Read each question carefully before answering- i.e. read the instructions. Be
BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Third Examination April 2, 2009 Please put your name at the top of each page. Read each question carefully before answering- i.e. read the instructions. Be
The Making of the Fittest: Evolving Switches, Evolving Bodies
 OVERVIEW MODELING THE REGULATORY SWITCHES OF THE PITX1 GENE IN STICKLEBACK FISH This hands-on activity supports the short film, The Making of the Fittest:, and aims to help students understand eukaryotic
OVERVIEW MODELING THE REGULATORY SWITCHES OF THE PITX1 GENE IN STICKLEBACK FISH This hands-on activity supports the short film, The Making of the Fittest:, and aims to help students understand eukaryotic
Chapter 47: Animal Development
 Name Period Overview 1. An organism s development is controlled by the genome of the zygote as well as by molecules from the mother that are in the cytoplasm of the egg. What are these proteins and RNAs
Name Period Overview 1. An organism s development is controlled by the genome of the zygote as well as by molecules from the mother that are in the cytoplasm of the egg. What are these proteins and RNAs
Influence of the skin mechanical and microbial properties on hair growth
 Call for Interdisciplinary Projects Sevres 2014 A General Information Project title Influence of the skin mechanical and microbial properties on hair growth Acronym TADDEI: The Ambiguous Dupond and Dupont
Call for Interdisciplinary Projects Sevres 2014 A General Information Project title Influence of the skin mechanical and microbial properties on hair growth Acronym TADDEI: The Ambiguous Dupond and Dupont
A. DEVELOPMENT OF THE DENTAL ORGAN (ENAMEL ORGAN):
 A. DEVELOPMENT OF THE DENTAL ORGAN (ENAMEL ORGAN): AS EARLY AS THE SECOND MONTH OF FETAL LIFE, THE DEVELOPMENT OF THE DECIDUOUS TEETH MAY FIRST BECOME EVIDENT. 1. Dental lamina and Bud stage At about six
A. DEVELOPMENT OF THE DENTAL ORGAN (ENAMEL ORGAN): AS EARLY AS THE SECOND MONTH OF FETAL LIFE, THE DEVELOPMENT OF THE DECIDUOUS TEETH MAY FIRST BECOME EVIDENT. 1. Dental lamina and Bud stage At about six
Plant Growth & Development. Growth Stages. Differences in the Developmental Mechanisms of Plants and Animals. Development
 Plant Growth & Development Plant body is unable to move. To survive and grow, plants must be able to alter its growth, development and physiology. Plants are able to produce complex, yet variable forms
Plant Growth & Development Plant body is unable to move. To survive and grow, plants must be able to alter its growth, development and physiology. Plants are able to produce complex, yet variable forms
Vertebrate Development Chapter 60
 Vertebrate Development Chapter 60 Copyright McGraw-Hill Companies Permission required for reproduction or display Raven - Johnson - Biology: 6th Ed. - All Rights Reserved - McGraw Hill Companies Stages
Vertebrate Development Chapter 60 Copyright McGraw-Hill Companies Permission required for reproduction or display Raven - Johnson - Biology: 6th Ed. - All Rights Reserved - McGraw Hill Companies Stages
How To Understand How Gene Expression Is Regulated
 What makes cells different from each other? How do cells respond to information from environment? Regulation of: - Transcription - prokaryotes - eukaryotes - mrna splicing - mrna localisation and translation
What makes cells different from each other? How do cells respond to information from environment? Regulation of: - Transcription - prokaryotes - eukaryotes - mrna splicing - mrna localisation and translation
The first steps to forming a new organism Descriptive embryology 2. Cleavage, Gastrulation, Neurulation and Organogenesis
 Developmental Biology BY1101 P. Murphy Lectures 4 and 5 The first steps to forming a new organism Descriptive embryology 2 Cleavage, Gastrulation, Neurulation and Organogenesis Early animal development
Developmental Biology BY1101 P. Murphy Lectures 4 and 5 The first steps to forming a new organism Descriptive embryology 2 Cleavage, Gastrulation, Neurulation and Organogenesis Early animal development
Von Mäusen und Menschen E - 1
 Von Mäusen und Menschen E - 1 Mus musculus: Genetic Portrait of the House Mouse E - 3 Outline Mouse genome Mouse life cycle Transgenic protocols Addition of genes by nuclear injection Removal of genes
Von Mäusen und Menschen E - 1 Mus musculus: Genetic Portrait of the House Mouse E - 3 Outline Mouse genome Mouse life cycle Transgenic protocols Addition of genes by nuclear injection Removal of genes
Molecular Histology in Skin Appendage Morphogenesis
 MICROSCOPY RESEARCH AND TECHNIQUE 38:452 465 (1997) Molecular Histology in Skin Appendage Morphogenesis RANDALL B. WIDELITZ, 1 TING-XIN JIANG, 1 ALEXANDER NOVEEN, 1 SHEREE A. TING-BERRETH, 1 ERIC YIN,
MICROSCOPY RESEARCH AND TECHNIQUE 38:452 465 (1997) Molecular Histology in Skin Appendage Morphogenesis RANDALL B. WIDELITZ, 1 TING-XIN JIANG, 1 ALEXANDER NOVEEN, 1 SHEREE A. TING-BERRETH, 1 ERIC YIN,
Embryonic & and induced pluripotent Stem Cells. May 2010 Dipl. Biol. Dr. Kurt Pfannkuche
 Embryonic & and induced pluripotent Stem Cells May 2010 Dipl. Biol. Dr. Kurt Pfannkuche Seite 2 What makes a stem cell? open chromatin structure plasticity! Seite 3 self-renewal Plasticity Seite 4 Culture
Embryonic & and induced pluripotent Stem Cells May 2010 Dipl. Biol. Dr. Kurt Pfannkuche Seite 2 What makes a stem cell? open chromatin structure plasticity! Seite 3 self-renewal Plasticity Seite 4 Culture
Causes of Birth Defects
 Causes of Birth Defects Some medical / genetic terms: congenital defects: visible defects present at birth (due to any cause (genetic, developmental error ). syndrome: the symptoms that characterize any
Causes of Birth Defects Some medical / genetic terms: congenital defects: visible defects present at birth (due to any cause (genetic, developmental error ). syndrome: the symptoms that characterize any
Two main classes: Epithelial Connective (synovial) Epithelial. Cutaneous Mucous Serous
 Two main classes: Epithelial Connective (synovial) Epithelial Cutaneous Mucous Serous Epithelial Membranes = sheet of epithelia + connective tissue base 1. Cutaneous membrane: outer skin layer (stratified
Two main classes: Epithelial Connective (synovial) Epithelial Cutaneous Mucous Serous Epithelial Membranes = sheet of epithelia + connective tissue base 1. Cutaneous membrane: outer skin layer (stratified
Anatomic Anomalies. Anomalies. Anomalies. Anomalies. Supernumerary Teeth. Supernumerary Teeth. Steven R. Singer, DDS 212.305.5674 srs2@columbia.
 Anatomic Anomalies Steven R. Singer, DDS 212.305.5674 srs2@columbia.edu Anomalies! Anomalies are variations in the:! Size! Morphology! Number! Eruption of the teeth Anomalies Anomalies There are two categories:!
Anatomic Anomalies Steven R. Singer, DDS 212.305.5674 srs2@columbia.edu Anomalies! Anomalies are variations in the:! Size! Morphology! Number! Eruption of the teeth Anomalies Anomalies There are two categories:!
THE UNIVERSITY OF THE WEST INDIES, ST. AUGUSTINE FACULTY OF SCIENCE & TECHNOLOGY DEPARTMENT OF LIFE SCIENCES COURSE DOCUMENTATION
 THE UNIVERSITY OF THE WEST INDIES, ST. AUGUSTINE COURSE CODE: BIOL 2061 FACULTY OF SCIENCE & TECHNOLOGY DEPARTMENT OF LIFE SCIENCES COURSE DOCUMENTATION COURSE TITLE: Cell & Developmental Biology CREDITS:
THE UNIVERSITY OF THE WEST INDIES, ST. AUGUSTINE COURSE CODE: BIOL 2061 FACULTY OF SCIENCE & TECHNOLOGY DEPARTMENT OF LIFE SCIENCES COURSE DOCUMENTATION COURSE TITLE: Cell & Developmental Biology CREDITS:
CONTRACTING ORGANIZATION: University of Alabama at Birmingham Birmingham, AL 35294
 AD Award Number: W81XWH-08-1-0030 TITLE: Regulation of Prostate Cancer Bone Metastasis by DKK1 PRINCIPAL INVESTIGATOR: Gregory A. Clines, M.D., Ph.D. CONTRACTING ORGANIZATION: University of Alabama at
AD Award Number: W81XWH-08-1-0030 TITLE: Regulation of Prostate Cancer Bone Metastasis by DKK1 PRINCIPAL INVESTIGATOR: Gregory A. Clines, M.D., Ph.D. CONTRACTING ORGANIZATION: University of Alabama at
T Cell Maturation,Activation and Differentiation
 T Cell Maturation,Activation and Differentiation Positive Selection- In thymus, permits survival of only those T cells whose TCRs recognize self- MHC molecules (self-mhc restriction) Negative Selection-
T Cell Maturation,Activation and Differentiation Positive Selection- In thymus, permits survival of only those T cells whose TCRs recognize self- MHC molecules (self-mhc restriction) Negative Selection-
In developmental genomic regulatory interactions among genes, encoding transcription factors
 JOURNAL OF COMPUTATIONAL BIOLOGY Volume 20, Number 6, 2013 # Mary Ann Liebert, Inc. Pp. 419 423 DOI: 10.1089/cmb.2012.0297 Research Articles A New Software Package for Predictive Gene Regulatory Network
JOURNAL OF COMPUTATIONAL BIOLOGY Volume 20, Number 6, 2013 # Mary Ann Liebert, Inc. Pp. 419 423 DOI: 10.1089/cmb.2012.0297 Research Articles A New Software Package for Predictive Gene Regulatory Network
Biotechnology. Srivatsan Kidambi, Ph.D.
 Stem Stem Cell Cell Engineering-What, Biology and it Application Why, How?? to Biotechnology Srivatsan Kidambi, Ph.D. Assistant Professor Department of Chemical & Biomolecular Engineering University of
Stem Stem Cell Cell Engineering-What, Biology and it Application Why, How?? to Biotechnology Srivatsan Kidambi, Ph.D. Assistant Professor Department of Chemical & Biomolecular Engineering University of
The Need for a PARP in vivo Pharmacodynamic Assay
 The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
No Disclosures. Learning Objectives 10/25/13
 No Disclosures Gregory A. Brent, MD Departments of Medicine and Physiology David Geffen School of Medicine at UCLA VA Greater Los Angeles Healthcare System Learning Objectives Describe the pathways that
No Disclosures Gregory A. Brent, MD Departments of Medicine and Physiology David Geffen School of Medicine at UCLA VA Greater Los Angeles Healthcare System Learning Objectives Describe the pathways that
PART 3.3: MicroRNA and Cancer
 BIBM 2010 Tutorial: Epigenomics and Cancer PART 3.3: MicroRNA and Cancer Dec 18, 2010 Sun Kim at Indiana University Outline of Part 3.3 Background on microrna Role of microrna in cancer MicroRNA pathway
BIBM 2010 Tutorial: Epigenomics and Cancer PART 3.3: MicroRNA and Cancer Dec 18, 2010 Sun Kim at Indiana University Outline of Part 3.3 Background on microrna Role of microrna in cancer MicroRNA pathway
Osteoblast Differentiation and Mineralization
 Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
GeneCopoeia Genome Editing Tools for Safe Harbor Integration in. Mice and Humans. Ed Davis, Liuqing Qian, Ruiqing li, Junsheng Zhou, and Jinkuo Zhang
 G e n e C o p o eia TM Expressway to Discovery APPLICATION NOTE Introduction GeneCopoeia Genome Editing Tools for Safe Harbor Integration in Mice and Humans Ed Davis, Liuqing Qian, Ruiqing li, Junsheng
G e n e C o p o eia TM Expressway to Discovery APPLICATION NOTE Introduction GeneCopoeia Genome Editing Tools for Safe Harbor Integration in Mice and Humans Ed Davis, Liuqing Qian, Ruiqing li, Junsheng
Reproductive System & Development: Practice Questions #1
 Reproductive System & Development: Practice Questions #1 1. Which two glands in the diagram produce gametes? A. glands A and B B. glands B and E C. glands C and F D. glands E and F 2. Base your answer
Reproductive System & Development: Practice Questions #1 1. Which two glands in the diagram produce gametes? A. glands A and B B. glands B and E C. glands C and F D. glands E and F 2. Base your answer
AP Biology Essential Knowledge Student Diagnostic
 AP Biology Essential Knowledge Student Diagnostic Background The Essential Knowledge statements provided in the AP Biology Curriculum Framework are scientific claims describing phenomenon occurring in
AP Biology Essential Knowledge Student Diagnostic Background The Essential Knowledge statements provided in the AP Biology Curriculum Framework are scientific claims describing phenomenon occurring in
Development of Multicellular Organisms
 Chapter 22 Development of Multicellular Organisms 22 An animal or plant starts its life as a single cell a fertilized egg. During development, this cell divides repeatedly to produce many different cells
Chapter 22 Development of Multicellular Organisms 22 An animal or plant starts its life as a single cell a fertilized egg. During development, this cell divides repeatedly to produce many different cells
Timeline for development. Facial and palatal development. Contributions to external face. Contributions to the external face
 Timeline for development Facial and palatal development L.Moss-Salentijn External face Pharyngeal arches Primary palate Secondary palate Completion of soft palate 4 wks 6 wks 8 wks 12 wks Decrease of severity
Timeline for development Facial and palatal development L.Moss-Salentijn External face Pharyngeal arches Primary palate Secondary palate Completion of soft palate 4 wks 6 wks 8 wks 12 wks Decrease of severity
An Overview of Cells and Cell Research
 An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
Zebrafish Chemical Screens
 Zebrafish Chemical Screens 1 Historical look at zebrafish chemical screens 2 The effects of AAF on embryonic development in relation to its carcinogenic role 3 First generation Screens HISAOKA, K. K. (1958).
Zebrafish Chemical Screens 1 Historical look at zebrafish chemical screens 2 The effects of AAF on embryonic development in relation to its carcinogenic role 3 First generation Screens HISAOKA, K. K. (1958).
Neurotrophic factors and Their receptors
 Neurotrophic factors and Their receptors Huang Shu-Hong Institute of neurobiology 1 For decades, scientists believed that brain cells of the central nervous system could not regrow following damage due
Neurotrophic factors and Their receptors Huang Shu-Hong Institute of neurobiology 1 For decades, scientists believed that brain cells of the central nervous system could not regrow following damage due
Transforming growth factor βs (TGF-βs)
 ORIGINAL PAPER Temporal and spatial expression of TGF-β 2 in tooth crown development in mouse first lower molar J.Y. Li, 1 B. Hu, 1 X.J. Wang, 1 S.L. Wang 1,2 1 Salivary Gland Disease Center and the Molecular
ORIGINAL PAPER Temporal and spatial expression of TGF-β 2 in tooth crown development in mouse first lower molar J.Y. Li, 1 B. Hu, 1 X.J. Wang, 1 S.L. Wang 1,2 1 Salivary Gland Disease Center and the Molecular
!!!!!!!!!!!!!!!!!!!!!!!!!!
 Figure S7 cdl-1 3'UTR gld-1 binding site (4..10) let-7 binding site (326..346) Figure Legends Figure S1. gld-1 genetically interacts with nhl-2 and vig-1 during germline development. (A) nhl-2(ok818) enhances
Figure S7 cdl-1 3'UTR gld-1 binding site (4..10) let-7 binding site (326..346) Figure Legends Figure S1. gld-1 genetically interacts with nhl-2 and vig-1 during germline development. (A) nhl-2(ok818) enhances
Cysts of the Jaws. Cyst. Types. Effects on adjacent structures. Non-Odontogenic cysts. Odontogenic Cysts
 Cyst A Cyst is a benign pathologic cavity filled with fluid, lined by epithelium, and surrounded by a connective tissue wall A = connective tissue wall Cysts of the Jaws B = epithelium Effects on adjacent
Cyst A Cyst is a benign pathologic cavity filled with fluid, lined by epithelium, and surrounded by a connective tissue wall A = connective tissue wall Cysts of the Jaws B = epithelium Effects on adjacent
DNA Fingerprinting. Unless they are identical twins, individuals have unique DNA
 DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
DEVELOPMENT AND GROWTH OF THE MANDIBLE
 2012-2013 ORAL BIOLOGY DEVELOPMENT AND GROWTH OF THE MANDIBLE Ass. Prof. Dr. Heba M. Elsabaa Development and Growth of the Mandible DEVELOPMENT OF THE MANDIBLE The Mandible Is the largest and strongest
2012-2013 ORAL BIOLOGY DEVELOPMENT AND GROWTH OF THE MANDIBLE Ass. Prof. Dr. Heba M. Elsabaa Development and Growth of the Mandible DEVELOPMENT OF THE MANDIBLE The Mandible Is the largest and strongest
Developmental Cysts. Non-odontogenic Cysts. Nasopalatine duct cyst. Palatal and gingival cysts of newborns
 Non-odontogenic Cysts Dr. Ioannis G. Koutlas Division of Oral Pathology Developmental Cysts a.k.a. fissural cysts Exact pathogenesis of some of them uncertain Generally, slow increase May be identified
Non-odontogenic Cysts Dr. Ioannis G. Koutlas Division of Oral Pathology Developmental Cysts a.k.a. fissural cysts Exact pathogenesis of some of them uncertain Generally, slow increase May be identified
Cell Biology Questions and Learning Objectives
 Cell Biology Questions and Learning Objectives (with hypothetical learning materials that might populate the objective) The topics and central questions listed here are typical for an introductory undergraduate
Cell Biology Questions and Learning Objectives (with hypothetical learning materials that might populate the objective) The topics and central questions listed here are typical for an introductory undergraduate
Lecture 3: Mutations
 Lecture 3: Mutations Recall that the flow of information within a cell involves the transcription of DNA to mrna and the translation of mrna to protein. Recall also, that the flow of information between
Lecture 3: Mutations Recall that the flow of information within a cell involves the transcription of DNA to mrna and the translation of mrna to protein. Recall also, that the flow of information between
4.1 3T12 and 312 are immortalized cell lines with transforming potential:
 DISCUSSION CHAPTER 4 4.1 3T12 and 312 are immortalized cell lines with transforming potential: Transformation of a normal cell with finite number of divisions into a tumorigenic cell of potentially infinite
DISCUSSION CHAPTER 4 4.1 3T12 and 312 are immortalized cell lines with transforming potential: Transformation of a normal cell with finite number of divisions into a tumorigenic cell of potentially infinite
High throughput screening, high content screening, primary and stem cells. new techniques now converging
 High throughput screening:layout 1 26/3/09 13:32 Page 25 High throughput screening, high content screening, primary and stem cells new techniques now converging Over the past decade, the use of cell-based
High throughput screening:layout 1 26/3/09 13:32 Page 25 High throughput screening, high content screening, primary and stem cells new techniques now converging Over the past decade, the use of cell-based
Chetek-Weyerhaeuser High School
 Chetek-Weyerhaeuser High School Anatomy and Physiology Units and Anatomy and Physiology A Unit 1 Introduction to Human Anatomy and Physiology (6 days) Essential Question: How do the systems of the human
Chetek-Weyerhaeuser High School Anatomy and Physiology Units and Anatomy and Physiology A Unit 1 Introduction to Human Anatomy and Physiology (6 days) Essential Question: How do the systems of the human
Antibody Function & Structure
 Antibody Function & Structure Specifically bind to antigens in both the recognition phase (cellular receptors) and during the effector phase (synthesis and secretion) of humoral immunity Serology: the
Antibody Function & Structure Specifically bind to antigens in both the recognition phase (cellular receptors) and during the effector phase (synthesis and secretion) of humoral immunity Serology: the
Development of pharyngeal pouches endoderm-derived glands Thymus: from organogenesis to involution
 Development of pharyngeal pouches endoderm-derived glands Thymus: from organogenesis to involution Unidade de Organogénese (IHBD-FMUL/IMM) Rita Zilhão ENCONTRO FCUL, 4 February, 2013 Faculdade de Medicina
Development of pharyngeal pouches endoderm-derived glands Thymus: from organogenesis to involution Unidade de Organogénese (IHBD-FMUL/IMM) Rita Zilhão ENCONTRO FCUL, 4 February, 2013 Faculdade de Medicina
CHAPTER 6: INTEGUMENTARY SYSTEM. 1. Explain why the skin is called the cutaneous membrane.
 OBJECTIVES: 1. Explain why the skin is called the cutaneous membrane. 2. Name the layers of the skin, describe the structure (tissues) of each, and name a general function of each. 3. Discuss the four
OBJECTIVES: 1. Explain why the skin is called the cutaneous membrane. 2. Name the layers of the skin, describe the structure (tissues) of each, and name a general function of each. 3. Discuss the four
Skeletal Development Multiple Cellular Origins
 Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
Summary of Ph.D. thesis. Tamás Matusek. Supervisor: József Mihály Ph.D.
 Summary of Ph.D. thesis GENETIC, CELL BIOLOGICAL AND BIOCHEMICAL ANALYSIS OF THE TISSUE SPECIFIC FUNCTIONS OF A NEW DROSOPHILA FORMIN Tamás Matusek Supervisor: József Mihály Ph.D. Biological Research Center
Summary of Ph.D. thesis GENETIC, CELL BIOLOGICAL AND BIOCHEMICAL ANALYSIS OF THE TISSUE SPECIFIC FUNCTIONS OF A NEW DROSOPHILA FORMIN Tamás Matusek Supervisor: József Mihály Ph.D. Biological Research Center
Organizing cell renewal in the intestine: stem cells, signals and combinatorial control
 Organizing cell renewal in the intestine: stem cells, signals and combinatorial control Cécile Crosnier, Despina Stamataki and Julian Lewis Abstract The lining of the intestine is renewed at an extraordinary
Organizing cell renewal in the intestine: stem cells, signals and combinatorial control Cécile Crosnier, Despina Stamataki and Julian Lewis Abstract The lining of the intestine is renewed at an extraordinary
Lesson 3 Reading Material: Oncogenes and Tumor Suppressor Genes
 Lesson 3 Reading Material: Oncogenes and Tumor Suppressor Genes Becoming a cancer cell isn t easy One of the fundamental molecular characteristics of cancer is that it does not develop all at once, but
Lesson 3 Reading Material: Oncogenes and Tumor Suppressor Genes Becoming a cancer cell isn t easy One of the fundamental molecular characteristics of cancer is that it does not develop all at once, but
Chapter 32: An Introduction to Animal Diversity
 Name Period Concept 32.1 Animals are multicellular, heterotrophic eukaryotes with tissues that develop from embryonic layers 1. Like the fungi, animals are multicellular heterotrophs. How do they feed?
Name Period Concept 32.1 Animals are multicellular, heterotrophic eukaryotes with tissues that develop from embryonic layers 1. Like the fungi, animals are multicellular heterotrophs. How do they feed?
CELL-INTRINSIC MECHANISMS GOVERNING STEM CELL QUIESCENCE AND REGULATING CANCER PROGRESSION IN MAMMALIAN SKIN
 CELL-INTRINSIC MECHANISMS GOVERNING STEM CELL QUIESCENCE AND REGULATING CANCER PROGRESSION IN MAMMALIAN SKIN by LI WANG B.A., Fudan University, 2009 M.A., University of Colorado, 2015 A thesis submitted
CELL-INTRINSIC MECHANISMS GOVERNING STEM CELL QUIESCENCE AND REGULATING CANCER PROGRESSION IN MAMMALIAN SKIN by LI WANG B.A., Fudan University, 2009 M.A., University of Colorado, 2015 A thesis submitted
Biological Sciences Initiative. Human Genome
 Biological Sciences Initiative HHMI Human Genome Introduction In 2000, researchers from around the world published a draft sequence of the entire genome. 20 labs from 6 countries worked on the sequence.
Biological Sciences Initiative HHMI Human Genome Introduction In 2000, researchers from around the world published a draft sequence of the entire genome. 20 labs from 6 countries worked on the sequence.
Chapter 7. Physical interaction between Jamb and Jamc is necessary for myoblast fusion. Summary
 Chapter 7 Physical interaction between Jamb and Jamc is necessary for myoblast fusion Summary In this chapter I describe a series of transplant experiments designed to further elucidate the function and
Chapter 7 Physical interaction between Jamb and Jamc is necessary for myoblast fusion Summary In this chapter I describe a series of transplant experiments designed to further elucidate the function and
 no!no! Thermicon: A Novel, Home-based Hair Removal Device Dr. Mira Barki Yavne, Israel Introduction Lasers and intense pulsed light sources have become a popular method for long-term removal of unwanted
no!no! Thermicon: A Novel, Home-based Hair Removal Device Dr. Mira Barki Yavne, Israel Introduction Lasers and intense pulsed light sources have become a popular method for long-term removal of unwanted
Graying of the human hair follicle
 J. Cosmet. Sci., 62, 121 125 (March/April 2011) Graying of the human hair follicle EVA M. J. PETERS, DOMINIK IMFELD, and REMO GRÄUB, University-Medicine Charité, CharitéCenter 12 (CC12) for Internal Medicine
J. Cosmet. Sci., 62, 121 125 (March/April 2011) Graying of the human hair follicle EVA M. J. PETERS, DOMINIK IMFELD, and REMO GRÄUB, University-Medicine Charité, CharitéCenter 12 (CC12) for Internal Medicine
Cell Division Mitosis and the Cell Cycle
 Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Sickle cell anemia: Altered beta chain Single AA change (#6 Glu to Val) Consequence: Protein polymerizes Change in RBC shape ---> phenotypes
 Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
Specialized Tissues, Stem Cells, and Tissue Renewal
 Chapter 23 Specialized Tissues, Stem Cells, and Tissue Renewal 23 Cells evolved originally as free-living individuals, but the cells that matter most to us, as human beings, are specialized members of
Chapter 23 Specialized Tissues, Stem Cells, and Tissue Renewal 23 Cells evolved originally as free-living individuals, but the cells that matter most to us, as human beings, are specialized members of
S1 Text. Modeling deterministic single-cell microrna-p53-mdm2 network Figure 2 Figure 2
 S1 Text. Modeling deterministic single-cell microrna-p53-mdm2 network The schematic diagram of the microrna-p53-mdm2 oscillator is illustrated in Figure 2. The interaction scheme among the mrnas and the
S1 Text. Modeling deterministic single-cell microrna-p53-mdm2 network The schematic diagram of the microrna-p53-mdm2 oscillator is illustrated in Figure 2. The interaction scheme among the mrnas and the
Biochemistry Major Talk 2014-15. Welcome!!!!!!!!!!!!!!
 Biochemistry Major Talk 2014-15 August 14, 2015 Department of Biochemistry The University of Hong Kong Welcome!!!!!!!!!!!!!! Introduction to Biochemistry A four-minute video: http://www.youtube.com/watch?v=tpbamzq_pue&l
Biochemistry Major Talk 2014-15 August 14, 2015 Department of Biochemistry The University of Hong Kong Welcome!!!!!!!!!!!!!! Introduction to Biochemistry A four-minute video: http://www.youtube.com/watch?v=tpbamzq_pue&l
9. PHARYNGEAL ARCHES. READING ASSIGNMENT: Larsen 3 rd Edition. Chapter 12: pp. 352; 358-365; 405-412 SUMMARY: LEARNING OBJECTIVES:
 9. PHARYNGEAL ARCHES Letty Moss-Salentijn DDS, PhD Dr. Edwin S. Robinson Professor of Dentistry (in Anatomy and Cell Biology) E-mail: lm23@columbia.edu READING ASSIGNMENT: Larsen 3 rd Edition. Chapter
9. PHARYNGEAL ARCHES Letty Moss-Salentijn DDS, PhD Dr. Edwin S. Robinson Professor of Dentistry (in Anatomy and Cell Biology) E-mail: lm23@columbia.edu READING ASSIGNMENT: Larsen 3 rd Edition. Chapter
TERATOGENESIS ONTOGENESIS
 TERATOGENESIS ONTOGENESIS Inborn developmental defects Occured during prenatal development Are present by delivery At about 3-5 % newborns are affected. Inborn developmental defects 1. CHROMOSOMAL ABERRATIONS
TERATOGENESIS ONTOGENESIS Inborn developmental defects Occured during prenatal development Are present by delivery At about 3-5 % newborns are affected. Inborn developmental defects 1. CHROMOSOMAL ABERRATIONS
Classification of Malocclusion
 Classification of Malocclusion What s going on here? How would you describe this? Dr. Robert Gallois REFERENCE: Where Do We Begin? ESSENTIALS FOR ORTHODONTIC PRACTICE By Riolo and Avery Chapter 6 pages
Classification of Malocclusion What s going on here? How would you describe this? Dr. Robert Gallois REFERENCE: Where Do We Begin? ESSENTIALS FOR ORTHODONTIC PRACTICE By Riolo and Avery Chapter 6 pages
Introduction to Dental Anatomy
 Introduction to Dental Anatomy Vickie P. Overman, RDH, MEd Continuing Education Units: N/A This continuing education course is intended for dental students and dental hygiene students. Maintaining the
Introduction to Dental Anatomy Vickie P. Overman, RDH, MEd Continuing Education Units: N/A This continuing education course is intended for dental students and dental hygiene students. Maintaining the
The Tissue Level of Organization
 The Tissue Level of Organization Tissues A groups of similar cells, usually having similar embryonic origin and specialized function Histology: the study of tissues Four general types Epithelial Muscle
The Tissue Level of Organization Tissues A groups of similar cells, usually having similar embryonic origin and specialized function Histology: the study of tissues Four general types Epithelial Muscle
Chapter 8. Summary and Perspectives
 Chapter 8 Summary and Perspectives 131 Chapter 8 Summary Overexpression of the multidrug resistance protein MRP1 confer multidrug resistance (MDR) to cancer cells. The contents of this thesis describe
Chapter 8 Summary and Perspectives 131 Chapter 8 Summary Overexpression of the multidrug resistance protein MRP1 confer multidrug resistance (MDR) to cancer cells. The contents of this thesis describe
Mitotic Membrane Turnover Coordinates Differential Induction of the Heart Progenitor Lineage
 Developmental Cell Supplemental Information Mitotic Membrane Turnover Coordinates Differential Induction of the Heart Progenitor Lineage Christina D. Cota and Brad Davidson Supplemental Figures S1-S7 S1.
Developmental Cell Supplemental Information Mitotic Membrane Turnover Coordinates Differential Induction of the Heart Progenitor Lineage Christina D. Cota and Brad Davidson Supplemental Figures S1-S7 S1.
Developmental Disorders
 Developmental Disorders General Principles Causes: Genetic Environmental (Maternal, Physical, Chemical) Mechanisms (Retinoic Acid) General Principles Teratology Study of Monsters Teratogen agent that produces
Developmental Disorders General Principles Causes: Genetic Environmental (Maternal, Physical, Chemical) Mechanisms (Retinoic Acid) General Principles Teratology Study of Monsters Teratogen agent that produces
The world of non-coding RNA. Espen Enerly
 The world of non-coding RNA Espen Enerly ncrna in general Different groups Small RNAs Outline mirnas and sirnas Speculations Common for all ncrna Per def.: never translated Not spurious transcripts Always/often
The world of non-coding RNA Espen Enerly ncrna in general Different groups Small RNAs Outline mirnas and sirnas Speculations Common for all ncrna Per def.: never translated Not spurious transcripts Always/often
Stem Cell:The Promise and The Challenge
 Stem Cell:The Promise and The Challenge Chia-Cheng Cheng Chang, Ph.D. Department of Pediatrics and Human Development Michigan State University Stem cells are undifferentiated cells with the capacity for
Stem Cell:The Promise and The Challenge Chia-Cheng Cheng Chang, Ph.D. Department of Pediatrics and Human Development Michigan State University Stem cells are undifferentiated cells with the capacity for
Molecule Shapes. support@ingenuity.com www.ingenuity.com 1
 IPA 8 Legend This legend provides a key of the main features of Network Explorer and Canonical Pathways, including molecule shapes and colors as well as relationship labels and types. For a high-resolution
IPA 8 Legend This legend provides a key of the main features of Network Explorer and Canonical Pathways, including molecule shapes and colors as well as relationship labels and types. For a high-resolution
Product: Expression Arrest TM egfp control shrna vector
 Product: Expression Arrest TM egfp control vector Catalog #: RHS1702 Product Description The laboratory of Dr. Greg Hannon at Cold Spring Harbor Laboratory (CSHL) has created an RNAi Clone Library comprised
Product: Expression Arrest TM egfp control vector Catalog #: RHS1702 Product Description The laboratory of Dr. Greg Hannon at Cold Spring Harbor Laboratory (CSHL) has created an RNAi Clone Library comprised
Understanding the dynamics and function of cellular networks
 Understanding the dynamics and function of cellular networks Cells are complex systems functionally diverse elements diverse interactions that form networks signal transduction-, gene regulatory-, metabolic-
Understanding the dynamics and function of cellular networks Cells are complex systems functionally diverse elements diverse interactions that form networks signal transduction-, gene regulatory-, metabolic-
Stem cells are cells that have the unique ability to selfrenew
 Wnt signalling in stem cells and cancer Tannishtha Reya 1 & Hans Clevers 2 1 Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, North Carolina 27710, USA 2 Hubrecht
Wnt signalling in stem cells and cancer Tannishtha Reya 1 & Hans Clevers 2 1 Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, North Carolina 27710, USA 2 Hubrecht
Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochemistry Journal. August 1, 2007 405, pp.
 Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies 1 Biochemistry Journal August 1, 2007 405, pp. 559 568 Joseph Friedman, Sarah Kraus, Yirmi Hauptman, Yoni Schiff
Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies 1 Biochemistry Journal August 1, 2007 405, pp. 559 568 Joseph Friedman, Sarah Kraus, Yirmi Hauptman, Yoni Schiff
STEM CELL FACTS. The ISSCR is an independent, nonproft organization providing a global forum for stem cell research and regenerative medicine.
 STEM CELL FACTS The ISSCR is an independent, nonproft organization providing a global forum for stem cell research and regenerative medicine. WHAT ARE STEM CELLS? Stem cells are the foundation cells for
STEM CELL FACTS The ISSCR is an independent, nonproft organization providing a global forum for stem cell research and regenerative medicine. WHAT ARE STEM CELLS? Stem cells are the foundation cells for
CHARACTERISTIC FEATURES OF STEM CELLS. CLONING TECHNOLOGIES
 CHARACTERISTIC FEATURES OF STEM CELLS. CLONING TECHNOLOGIES 1 Science is discovering the unknown Stem cell field is still in its infancy Human embryonic stem cell research is a decade old, adult stem cell
CHARACTERISTIC FEATURES OF STEM CELLS. CLONING TECHNOLOGIES 1 Science is discovering the unknown Stem cell field is still in its infancy Human embryonic stem cell research is a decade old, adult stem cell
AP Biology 2015 Free-Response Questions
 AP Biology 2015 Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online home
AP Biology 2015 Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online home
Vertebrate Body Organization
 Vertebrate Body Organization Digestive tube suspended in coelom from mouth to anus Body supported by internal skeleton of jointed bones Vertebrae and Cranium protects nervous system Diaphragm divides coelom
Vertebrate Body Organization Digestive tube suspended in coelom from mouth to anus Body supported by internal skeleton of jointed bones Vertebrae and Cranium protects nervous system Diaphragm divides coelom
Qualitative networks: A symbolic approach to analyze biological signaling networks
 Qualitative networks: A symbolic approach to analyze biological signaling networks Marc A Schaub 1, Thomas A Henzinger 1 and Jasmin Fisher 1 1 School of Computer and Communication Sciences EPFL, 1015 Lausanne,
Qualitative networks: A symbolic approach to analyze biological signaling networks Marc A Schaub 1, Thomas A Henzinger 1 and Jasmin Fisher 1 1 School of Computer and Communication Sciences EPFL, 1015 Lausanne,
Activity: Can You Identify the Age?
 Activity: Can You Identify the Age? Skeletons are good age markers because teeth and bones mature at fairly predictable rates. How Teeth Reveal Age For toddler to age 21, teeth are the most accurate age
Activity: Can You Identify the Age? Skeletons are good age markers because teeth and bones mature at fairly predictable rates. How Teeth Reveal Age For toddler to age 21, teeth are the most accurate age
Fig. S1. Visualizing and manipulating peripheral MEs and SAs in zebrafish. (A) Lateral wholemount view of peripheral MEs (magenta: TgNBT:dsRed) and
 Fig. S1. Visualizing and manipulating peripheral MEs and SAs in zebrafish. (A) Lateral wholemount view of peripheral MEs (magenta: TgNBT:dsRed) and SAs (yellow: Tg- 8.4neurog1:GFP) in 72 hpf zebrafish
Fig. S1. Visualizing and manipulating peripheral MEs and SAs in zebrafish. (A) Lateral wholemount view of peripheral MEs (magenta: TgNBT:dsRed) and SAs (yellow: Tg- 8.4neurog1:GFP) in 72 hpf zebrafish
The Integumentary System Dr. Ali Ebneshahidi
 The Integumentary System Dr. Ali Ebneshahidi The Skin The integument system consists of the skin (cutaneous membrane) and its accessory organs. The skin is composed of three layers of tissue: the outer
The Integumentary System Dr. Ali Ebneshahidi The Skin The integument system consists of the skin (cutaneous membrane) and its accessory organs. The skin is composed of three layers of tissue: the outer
Smads and early developmental signaling by the TGF superfamily
 REVIEW Smads and early developmental signaling by the TGF superfamily Malcolm Whitman 1 Department of Cell Biology, Harvard Medical School, Boston, Massachusetts 02115 USA 1 E-MAIL mwhitman@warren.med.harvard.edu;
REVIEW Smads and early developmental signaling by the TGF superfamily Malcolm Whitman 1 Department of Cell Biology, Harvard Medical School, Boston, Massachusetts 02115 USA 1 E-MAIL mwhitman@warren.med.harvard.edu;
germ layers in a vertebrate
 germ layers and animal evolution ecdysozoa (e.g., nematodes, flies) lophotrochozoa deuterostomes diploblasts sponges choanoflagellates mesoderm ectoderm + endoderm multicellularity germ layers in a vertebrate
germ layers and animal evolution ecdysozoa (e.g., nematodes, flies) lophotrochozoa deuterostomes diploblasts sponges choanoflagellates mesoderm ectoderm + endoderm multicellularity germ layers in a vertebrate
The Role of Hedgehog Signaling in Enthesis Healing
 The Role of Hedgehog Signaling in Enthesis Healing Andrea G. Schwartz, PhD, Leesa M. Galatz, MD, Stavros Thomopoulos, PhD. Washington University School of Medicine, Saint Louis, MO, USA. Disclosures: A.G.
The Role of Hedgehog Signaling in Enthesis Healing Andrea G. Schwartz, PhD, Leesa M. Galatz, MD, Stavros Thomopoulos, PhD. Washington University School of Medicine, Saint Louis, MO, USA. Disclosures: A.G.
John Eriksson Department of Biosciences Åbo Akademi University Turku, Finland. john.eriksson@abo.fi www.btk.fi www.bioimaging.fi
 John Eriksson Department of Biosciences Åbo Akademi University Turku, Finland john.eriksson@abo.fi www.btk.fi www.bioimaging.fi Three cytoskeletal polymer systems Intermediate filaments Mikrotubuli Mikrofilaments
John Eriksson Department of Biosciences Åbo Akademi University Turku, Finland john.eriksson@abo.fi www.btk.fi www.bioimaging.fi Three cytoskeletal polymer systems Intermediate filaments Mikrotubuli Mikrofilaments
Animal Tissues. I. Epithelial Tissue
 Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
BIOLOGY REDISCOVERING. Genes and Development. Molecular to Global Perspectives. Differentiation and Genetic Cascades
 REDISCOVERING BIOLOGY Molecular to Global Perspectives Animals that look nothing like each other develop by using much the same basic toolkit of molecules and often in much the same ways. M. PALOPOLI AND
REDISCOVERING BIOLOGY Molecular to Global Perspectives Animals that look nothing like each other develop by using much the same basic toolkit of molecules and often in much the same ways. M. PALOPOLI AND
Introduction to Anatomy and Physiology: Tissues and Integumentary System. Biology 105 Lecture 7 Chapter 4
 Introduction to Anatomy and Physiology: Tissues and Integumentary System Biology 105 Lecture 7 Chapter 4 Outline I. Tissues A. Epithelial B. Connective C. Muscle D. Nervous tissues II. Cell-to-cell contact
Introduction to Anatomy and Physiology: Tissues and Integumentary System Biology 105 Lecture 7 Chapter 4 Outline I. Tissues A. Epithelial B. Connective C. Muscle D. Nervous tissues II. Cell-to-cell contact
